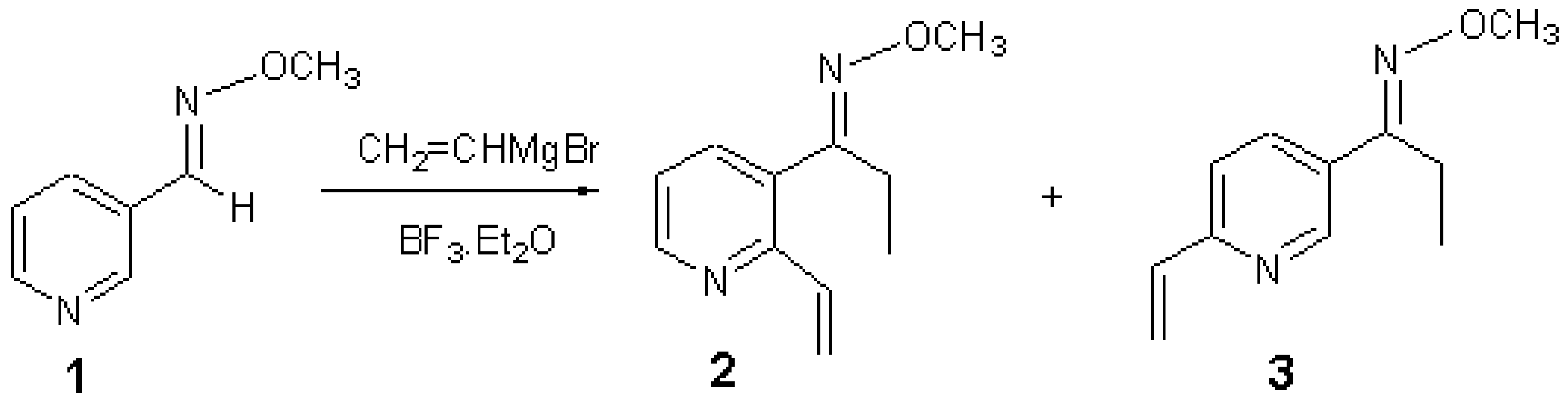
The general part of the experimental section [1] has been presented elsewhere. Boron trifluoride diethyl etherate (313 mg, 2.21 mmol) was added dropwise to a solution of oxime 1 (100 mg, 0.735 mmol) in toluene (8 mL) at -78oC under nitrogen and the mixture stirred for 5 min. Vinylmagnesium bromide (1 M in THF) (2.2 mL, 2.20 mmol) was added dropwise over 10 min and the resultant orange coloured solution stirred at -78°C for 1.5 h [2]. Water (1 mL) was added and the reaction warmed to room temperature. The mixture was extracted with ethyl acetate (3 x 15 mL), dried over magnesium sulfate and concentrated under reduced pressure to give a dark red solid. Further purification by flash chromatography using diethyl ether-hexane (1:9) gave the title compounds 2 (9 mg, 8%) and (3) (14 mg, 10%) as pale yellow oils.
1-(2-Vinyl-pyridin-3-yl)propanal O-methyloxime 2
IR (neat): 2917s, 2847m, 1696m, 1627bm, 1460m, 1054m.
1H NMR (200 MHz, CDCl3): 1.23 (3H, t, J 7.8 Hz, H3), 2.67 (2H, q, J 7.8 Hz, H2), 4.08 (3H, s, OCH3), 5.54 (1H, dd, J 1.9 Hz, J 10.8 Hz, H2″A), 6.26 (1H, dd, J 1.9 Hz, J 17.4 Hz, H2″B), 6.82 (1H, dd, J 10.8 Hz, J 17.4 Hz, H1″), 7.35 (1H, m, H5'), 8.06 (1H, bs, H4'), 8.66 (1H, d, J 2.0 Hz, H6').
EI-MS: 190 (M+, 23%), 189 ((M-H)+, 28%), 159 (100%), 144 (87%), 136 (10%), 91 (12%), 77 (15%).
Anal. Calc. For C11H14N2O, 190.1106; found M+ 190.1103.
1-(6-Vinyl-pyridin-3-yl)propanal O-methyloxime 3
IR (cm-1, CH2Cl2): 3053m, 2972m, 2938m, 1452m, 1422m, 1265s, 1054s, 739b, 705s.
1H NMR (200 MHz, CDCl3): 1.24 (3H, t, J 7.6 Hz, H3), 2.67 (2H, q, J 7.6 Hz, H2), 4.01 (3H, s, OCH3), 5.56 (1H, dd, J 1.9 Hz, J 10.8 Hz, H2″A), 6.28 (1H, dd, J 1.9 Hz, J 17.0 Hz, H2″B), 7.06 (1H, dd, J 10.8 Hz, J 17.0 Hz, H1″), 7.87 (1H, d, J4',5' 2.2 Hz, H5'), 8.39 (1H, s, H2'), 8.45 (1H, d, J4',5' 2.2 Hz, H4').
EI-MS: 190 (M+, 26%), 189 ((M-H)+, 28%), 159 (100%), 144 (84%), 130 (11%), 117 (10%), 77 (15%).
Anal. calc. for C11H14N2O (M+), 190.1106; found M+ 190.1099.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4References
- Brimble, M. A.; Duncalf, L. J. Molecules 2000, 5, 162–166. [CrossRef]
- Dieter, R.K.; Datar, R. Can. J. Chem. 1993, 71, 814–823.
- Sample availability: available from the authors.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/