Mass Spectra of Some 4- and 5-Substituted Derivatives of Benzoselenadiazoles
Abstract
:Introduction
Results and Discussion
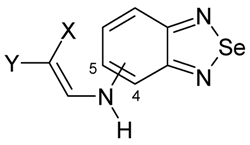
| 4-subst. | 5-subst. | X | Y |
|---|---|---|---|
| 1 | 9 | COCH3 | COCH3 |
| 2 | 10 | COOCH3 | COOCH3 |
| 3 | 11 | COOC2H5 | COOC2H5 |
| 4 | 12 | COOC(CH3)2OCO | |
| 5 | 13 | CN | COOCH3 |
| 6 | 14 | CN | COOC2H5 |
| 7 | 15 | COCH33 | COOCH3 |
| 8 | 16 | COCH3 | COOC2H5 |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 | 309 M+. (27), 307 (14), 268 (12), 266 (62), 264 (31), 263 (12), 262 (11), 224 (12), 112 (11), 43 (100). 341 M+. (35), 309 (80), 307 (40), 282 (70), 250 (35), 249 (46), 143 (53),103 (36), 59 (57), 53 (100). 369 M+. (50), 323 (92), 321 (48), 296 (100), 294 (49), 249 (32), 223 (58), 221 (28), 143 (93), 53 (51). 353 M+. (8), 295 (29), 225 (18) ,223 (94), 221 (47), 143 (100), 116 (15),103 (17), 53 (52),43 (76). 308 M+. (82), 306 (42), 276 (36), 249 (61), 248 (100), 246 (55), 168 (57), 103 (50), 76 (45), 52 (54). 322 M+. (64), 320 (32), 276 (31), 249 (62), 248 (100), 247 (31), 246 (47),168 (31), 103 (28), 52 (35), 325 M+. (20), 293 (16), 282 (62), 280 (31), 250 (34), 248 (18), 223 (17), 143 (46), 53 (35), 43 (100). 339 M+. (41), 296 (100), 294 (59), 293 (54), 265 (44), 250 (71), 248 (37) 223 (32), 143 (80), 53 (53). 309 M+. (77), 307 (36), 266 (28), 252 (42), 250 (28), 224 (27), 172 (20), 143 (27), 112 (100), 70 (26). 341 M+. (78), 339 (39), 309 (100), 307 (50), 250 (51), 157 (50), 143 (32), 103 (36), 59 (66). 369 M+. (71), 367 (35), 323 (100), 321 (49), 267 (42), 250 (50), 223 (44),170 (28), 143 (68), 103 (28). 353 M+. (11), 295 (36), 251 (27), 250 (59), 248 (32), 223 (33), 143 (100), 76 (23), 53 (50), 43 (99). 308 M+. (95), 306 (46), 276 (54), 249 (71), 248 (42), 169 (69), 168 (100),103 (76), 76 (65), 52 (63). 322 M+. (93), 320 (45), 276 (66), 249 (95), 247 (49), 169 (100),168 (95), 103 (72), 76 (65), 52 (92). 325 M+. (51), 323 (26), 293 (35), 291 (18), 265 (69), 263 (34), 250 (35),248 (19), 143 (33), 43 (100). 339 M+. (44), 337 (21), 293 (36), 291 (17), 265 (71), 263 (35), 250 (35), 248 (17), 143 (32), 43 (100). |
- a
- Ten the most abundant ions in the mass spectra are presented.
- b
- M+. for the most abundant isotope 80Se.
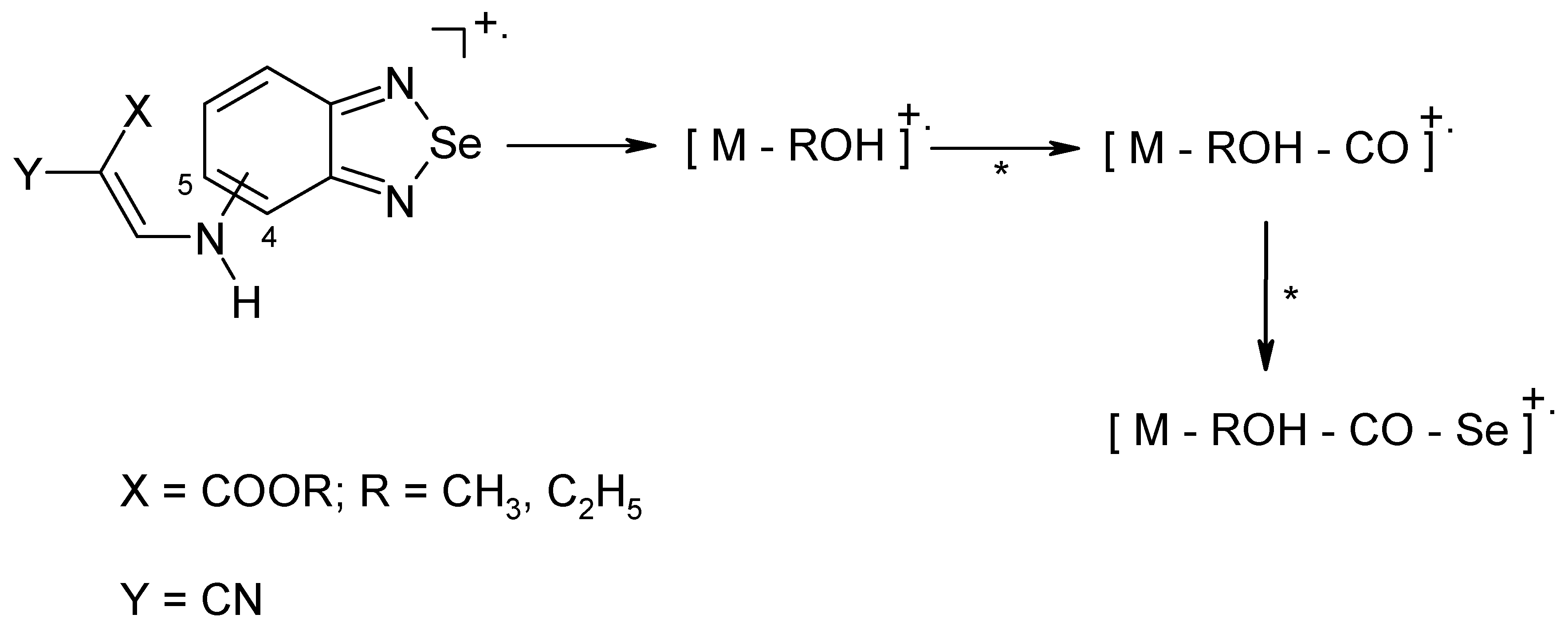
Conclusion
Experimental
References and Notes
- Porter, Q. N. Mass Spectrometry of Heterocyclic Compounds, 2nd Ed. ed; Wiley: New York, 1985. [Google Scholar]
- Pedersen, Ch. L.; Moller, J. Acta Chem. Scand. 1975, B 29, 483.
- Arshadi, M. R. Org. Mass Spectrom. 1978, 13, 379.
- Mills, I.; Cvitaš, T.; Homann, K.; Kallay, N.; Kuchitsu, K. IUPAC, Quantities, Units and Symbols in Physical Chemistry; Black Sci. Publ.: Oxford, 1988. [Google Scholar]
- Milata, V.; Ilavský, D.; Goljer, I.; Leško, J.; Henry-Basch, E.; Barry, J. Bull. Soc. Chim. Fr. 1996, 133, 897.
- Milata, V.; Schultz, M.; Duddeck, H.; Prónayová, N.; Leško, J. (prepared for the press).
- Sample Availability: Available from the authors.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Leško, J.; Milata, V.; Schultz, M. Mass Spectra of Some 4- and 5-Substituted Derivatives of Benzoselenadiazoles. Molecules 2000, 5, 937-940. https://doi.org/10.3390/50700937
Leško J, Milata V, Schultz M. Mass Spectra of Some 4- and 5-Substituted Derivatives of Benzoselenadiazoles. Molecules. 2000; 5(7):937-940. https://doi.org/10.3390/50700937
Chicago/Turabian StyleLeško, Ján, Viktor Milata, and Marcel Schultz. 2000. "Mass Spectra of Some 4- and 5-Substituted Derivatives of Benzoselenadiazoles" Molecules 5, no. 7: 937-940. https://doi.org/10.3390/50700937
APA StyleLeško, J., Milata, V., & Schultz, M. (2000). Mass Spectra of Some 4- and 5-Substituted Derivatives of Benzoselenadiazoles. Molecules, 5(7), 937-940. https://doi.org/10.3390/50700937




