Abstract
An improved selective ipso-nitration of the tripropoxy derivative of tert-butylcalix[4]arene at the upper rim is described. The synthesized products are key interme-diates for construction of molecular receptors based on calixarenes.
Introduction
Calix[4]arenes have attracted considerable interest as building blocks for constructing selective host molecules [1]. Upper rim functionalised compounds can be obtained in a multi-step procedure from suitably substituted precursors [2]. Mono and dinitrated calix[4]arene are especially useful for this pur-pose. Several methods have been reported for selective substitution of nitro groups at the upper rim [3,4,5]. Selective ipso-nitration of partial ethers has only been described for a few ethers of tert-butylcalix[4]arene [6,7,8,9]. In this paper we report the selective substitution of one or two nitro group(s) by direct replacement of tert-butyl group(s) via an ipso-aromatic nitration. These compounds afford important starting materials for the construction of molecular receptors based on calixarenes.
Results and Discussion
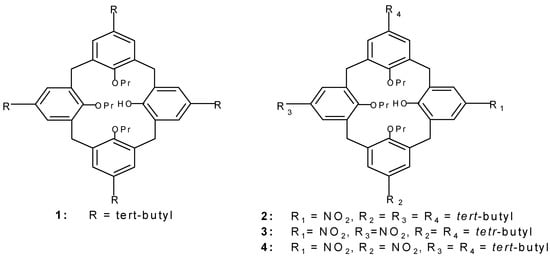
The cone conformation of 5,11,17,23-tetra-tert-butyl-25-hydroxy-26,27,28-tripropoxycalix[4]arene (1) reacts with 10 eq. of 63% HNO3 in a mixture of dichloromethane and glacial acetic acid in less than 5 min. to afford the 23-mononitro calix(4)arene 2 in 85% yield. Compared to the published procedure [7], the present method offers both milder reaction conditions and an improved yield. In the 1H-NMR spectrum of 2, the aromatic meta protons in the nitrated phenolic ring shift downfield from δ 5.41 (in 1) to δ 7.22 (in 2), and the protons ortho to the nitro group appears at δ 8.06. The 1H-NMR spectrum of 2 exhibits two doubles of doublets signals for the bridge methylene protons, indicating the symmetrical structure of 2.
Treatment of 1 with 10 equivalents of 63% HNO3 for 5 min. in dichloromethane and in presence of Ac2O, afforded the dinitro compounds 3 and 4 in a ratio of 3:1, judged from the 1H-NMR of the crude reaction mixture. The spectrum of 3 exhibits two doublets of doublets for the bridge methylene pro-tons, indicating a central plane of symmetry. The spectrum of 4 exhibits four doublets of doublets sig-nals for the bridge methylene protons indicating an asymmetric molecule. Symmetrical dinitro deriva-tives of calix[4]arene have been synthesized from the diether precursors [3], however by the present procedure the chiral dinitro derivative 4 is obtained. This compound could be used as a starting mate-rial to build up chiral hosts based on calix[4]arene to mimic the enzyme functions [1].
Experimental
General
Melting points are taken on a Büchi SMP-20 apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on a Bruker AM-400 MHz in CDCl3 with Me4Si as an internal standard. Ele-mental analysis were carried out on Carlo-Erba-Analyser Model 1104. IR spectra were recorded on a Bruker IFS-25 spectrophotometer. The calix(4)arene 1 was prepared according to the published proce-dure [10].
Procedure for the preparation of 2
To a solution of 10g (12.9 mmol) 1 in dichloromethane (50mL) and glacial acetic acid (30 mL), 63% HNO3 (5 mL) was added dropwise at -15°C in 2 min and the reaction mixture was then stirred at room temperature for 4 min and poured into water (250 mL). The organic layer was extracted with di-chloromethane (2x100mL), the solvent was removed and solid residue was dissolved in methanol (100 mL), concentration of solution to a volume of ca. 50 mL led to the appearance of a pale yellow pre-cipitate (yield 85%).The product thus obtained was pure enough for subsequent reactions but could be further purified by recrystallization from a mixture of dichloromethane and methanol; mp 182-184°C.
IR ν max (KBr)/cm-1 3456, 1592, 1472, 1334, 1201, 1006; δ H( 400 MHz; CDCl3 ) 0.83[18H, s, C(CH3 )3 )], 0.95[3H, t, CH3 ], 1.10 [6H, t, CH3 ], 1.35[9H, s, C(CH3)3 ], 1.93[4H, m, CH2 ], 2.2[2H, m, CH2 ], 3.19 and 4.34[4H, d of d, J=12.6 Hz, ArCH2 Ar], 3.75[2H, t, OCH2 ], 3.81[ 4H, t, O CH2 ], 3.39 and 4.31[4H, d of d, J=13.9 Hz, ArCH2 Ar], 6.45[ 2H, d, J=2.3 Hz, ArH], 6.60[2H, d, J=2.3Hz, ArH], 7.16[2H, s, ArH], 7.22[1H, s, OH], 8.06[2H, s, ArH]; δ C( 100 MHz) 9.56, 10.70, 22.55, 23.37, 31.10, 31.36, 31.47, 31.68, 33.74, 34.15, 58.46, 76.15, 78.01, 124.31, 125.68, 125.70,129.80, 132.65, 135.76, 139.34, 145.82, 151.75, 153.78, 159.98; M/Z (FD) 764(m+ , 100%). Anal. Calcd for C49 H65 NO6 : C, 77.06%; H, 8.52%; N, 1.84%. Found C, 76.8%, H, 8.7%; N, 2.2%.
Procedure for the preparation of 3 and 4
To a solution of 2g (2.58 mmol) 1 in dichloromethane (25mL) and acetic anhydride (4mL) at -10°C, 63% HNO3 (1 mL) was added in 2 min and the reaction mixture was then stirred at room temperature for 5 min and poured into water (250mL). The organic layer was extracted by dichloromethane (2x100mL), the solvent was removed and the solid residue was dissolved in methanol (100mL), con-centration of solution to ca. 50 mL led to a white precipitate. 3 is obtained as a white crystals in 63% separated yield (crystallized from a mixture of CH2Cl2 and CH3OH). mp, 214-217°C; 4 was purified by column chromatography of the mother liquors (silicagel, CH2Cl2 , n-hexane, 1:4); yield 22% mp, 225-228°C.
Spectral data for 3
IR ν max (KBr)/cm-1 3510, 1598, 1513, 1343 and 1014. δ H( 400 MHz; CDCl3 ) 0.86[18H, s, C(CH3)3 ], 0.97[3H, t, CH3 ], 1.11 [6H, t, CH3 ],1.94 [4H, m, CH2 ], 2.22[2H, m, CH2 ], 3.35 and 4.42[4H, d of d, J=12.9 Hz, ArCH2Ar], 3.78[4H, t, OCH2 ], 3.96[2H, t, OCH2 ], 3.43, 4.29[4H, d of d, J=13.8 Hz, ArCH2Ar], 6.56[4H, s, ArH], 7.37[1H, s, OH], 8.07[2H, s, ArH], 8.10[2H, s, ArH]; δ C( 100 MHz) 9.48, 10.66, 22.44, 23.40, 33.01, 31.13, 31.43, 33.87, 76.67, 78.15, 124.12, 124.46, 125.28, 125.46, 129.43, 130.30, 130.98, 138.06, 140.10, 143.05, 146.64; 151.83, 159.70, 164.05; M/Z (FD) 753(m+, 100%). Anal. Calcd for C45H56N2O8 : C, 71.79%; H, 7.50%; N, 3.72%. Found C, 71.5%, H, 7.5%; N, 3.1%.
Spectral data for 4
IR ν max (KBr)/cm-1 3508, 1592, 1521, 1341 and 1005. δ H( 400 MHz; CDCl3 ) 0.74[9H, s, C(CH3)3], 0.92[3H, t, CH3 ], 1.12 [3H, t, CH3 ], 1.13[3H, t, CH3 ], 1.40[9H, s, C(CH3)3 ], 1.90 [2H, m, CH2 ], 2.08[2H, m, CH2 ], 2.23[2H, m,CH2 ], 3.21 and 4.40[2H, d of d, J=13.1 Hz, ArCH2Ar], 3.25 and 4.30[2H, d of d, J=13.0 Hz, ArCH2Ar], 3.42 and 4.46[2H, d of d, J=13.7 Hz, ArCH2Ar], 3.51 and 4.20[2H, d of d, J=14.5 Hz, ArCH2Ar], 3.77[6H, m, OCH2 ], 6.17[1H, s, OH], 6.45[1H, d, J=2.1 Hz, ArH], 6.51[1H, d, J=2.1 Hz, ArH], 7.21[1H, d, J=2.3 Hz, ArH], 7.22[1H, d, J=2.6 Hz, ArH], 7.24[1H, d, J=2.2 Hz, ArH], 7.27[1H, d, J=2.6 Hz, ArH], 8.11[1H, d, J=2.5 Hz, ArH], 8.14[1H, d, J=2.5 Hz, ArH]; δ C( 100 MHz ) 9.43, 10.69, 10.71, 22.52, 23.34, 23.46, 30.63, 30.80, 31.05, 31.08, 31.63, 34.01, 34.30, 76.45, 77.63, 78.24, 122.31, 123.51, 124.30, 124.66, 124.77, 125.88, 126.72, 126.77,128.64, 128.77, 129.94, 133.56, 133.78, 134.80, 135.08, 136.00, 139.93, 142.81, 146.78, 147.54, 150.68, 153.79, 159.31, 161.73; M/Z (FD) 753(m+, 100%). Anal. Calcd for C45H56N2O8: C, 71.79%; H, 7.50%; N, 3.72%. Found C, 71.6%, H, 7.5%; N, 3.1%.
Acknowledgements:
Parviz Rashidi-Ranjbar is grateful to the Research Council of Tehran University for financial support. Generous support from Prof. Dr. J. Ipaktschi, Institute of Organic Chemistry, Gi-essen University is highly acknowledged. The Ministry of Culture and Higher Education of Iran is ac-knowledged for a grant to S. T-G.
References and Notes
- Gutsche, C. D.; Levine. J. Am. Chem. Soc. 1982, 104, 2652.
- Merkushev, E. B. Synthesis 1988, 923.
- Mogch, L.; Böhmer, B.; Fergusen, G.; Vogt, W. J. Chem. Soc, Perkin Trans, 1 1996, 1711.
- No, K.; Noh, Y. Bull. Korean Chem. Soc. 1986, 7, 314.
- Van Loon, J. D.; Arduini, A.; Coppi, L.; Verboom, W.; Pochini, A.; Ungaro, R.; Harkema, S.; Reinhoudt, D. N. J. Org. Chem. 1990, 55, 5639.
- Verboom, W.; Durie, A.; Egberink, R. J. M.; Asfari; Reinhoudt, D. N. J. Org. Chem. 1992, 57, 1313. [CrossRef]
- Bitter, I.; Gruen, A.; Toth, G.; Szoellosy, A.; Horvath, G.; Agai, B.; Toke, L. Tetrahedron 1996, 52, 636.
- Mogck, O.; Parzuchowski, P.; Nissinen, M.; Boehmer, V.; Rokicki, G.; Rissanen, K. Tetrahedron 1998, 54, 10053.
- Fischer, C.; Nieger, M.; Mogck, O.; Boehmer, V.; Ungaro, R.; Voegtle, F. Eur. J. Org. Chem. 1998, 155.
- Iwamoto, K.; Araki, K.; Shinkai, S. Tetrahedron 1991, 47, 4325.
- Sample Availability: Available from the authors.
© 2000 by MDPI (http://www.mdpi.org).