Determination of the pKa of Benzophenones in Ethanol-Water
Abstract
:Introduction
Experimental
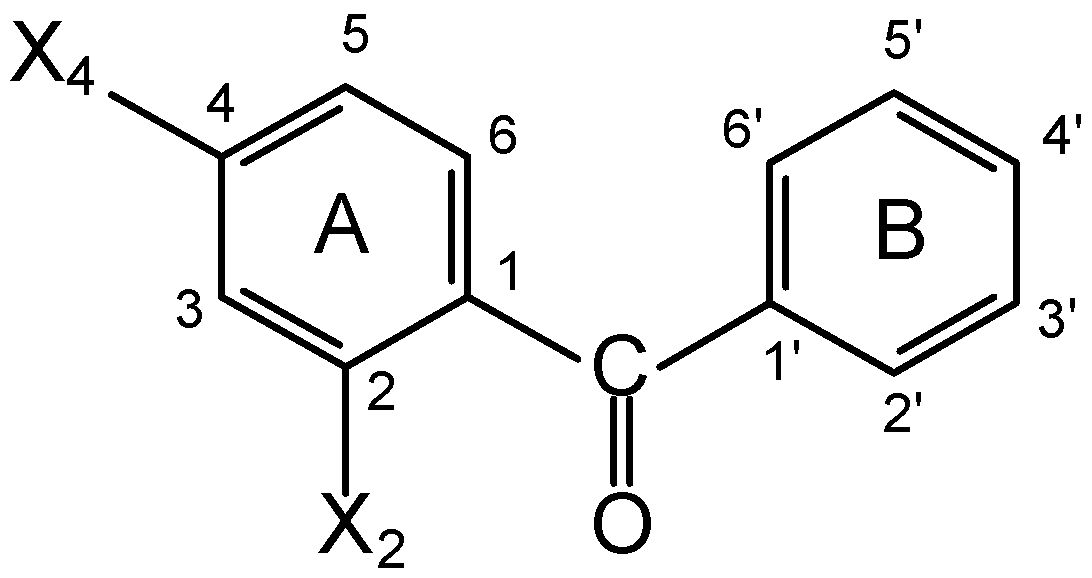
Results and Discussion
Acknowledgements
References and Notes
- Isaacs, N.I. Physical Organic Chemistry, 2nd ed.; Addison Wesley Longman: England, 1996; pp. 235–286. [Google Scholar]
- Wells, J.I. Pharmaceutical Preformulation, 1st ed.; Ellis Horwood: NY, 1988; pp. 25–28, 70–80, 106–108. [Google Scholar]
- Collins, P.; Ferguson, J. British J. Dermat. 1994, 131, 124–129.
- Albert, A.; Serjeant, E. P. The Determination of Ionization Constants; Chapman and Hall: USA, 1984; pp. 70–101. [Google Scholar]
- Gasull, E.I.; Blanco, S.E.; Ferretti, F.H. An. Asoc. Quím. Argent. 1999, 87, 73–82.
Share and Cite
Castro, G.T.; Blanco, S.E.; Giordano, O.S. Determination of the pKa of Benzophenones in Ethanol-Water. Molecules 2000, 5, 426-427. https://doi.org/10.3390/50300426
Castro GT, Blanco SE, Giordano OS. Determination of the pKa of Benzophenones in Ethanol-Water. Molecules. 2000; 5(3):426-427. https://doi.org/10.3390/50300426
Chicago/Turabian StyleCastro, G. T., S. E. Blanco, and O. S. Giordano. 2000. "Determination of the pKa of Benzophenones in Ethanol-Water" Molecules 5, no. 3: 426-427. https://doi.org/10.3390/50300426
APA StyleCastro, G. T., Blanco, S. E., & Giordano, O. S. (2000). Determination of the pKa of Benzophenones in Ethanol-Water. Molecules, 5(3), 426-427. https://doi.org/10.3390/50300426



