UV Spectral Properties of Benzophenone. Influence of Solvents and Substituents
Abstract
:Introduction
Experimental
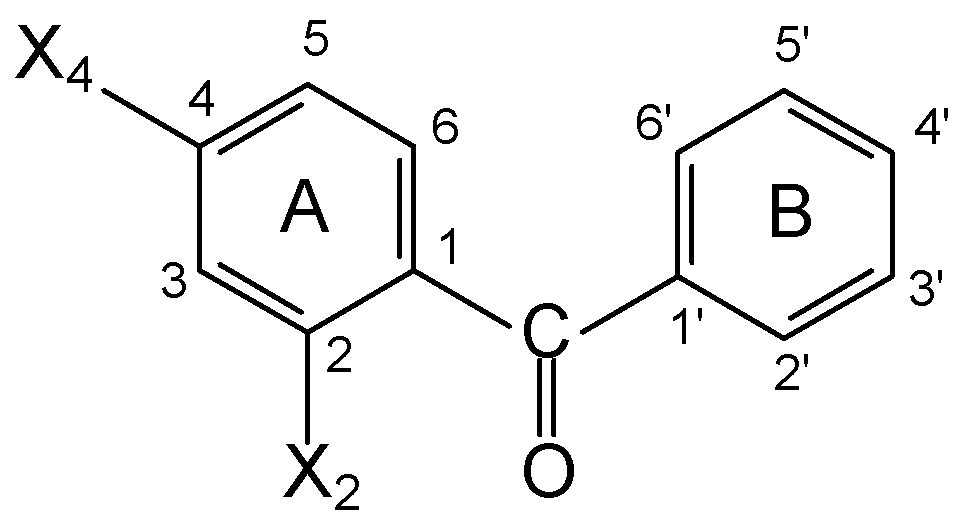
Results and Discussion
Acknowledgements
References and Notes
- Freedlander, B.L. Proc. Soc. Exptl. Biol. Med. 1942, 51, 153. [CrossRef]
- Freedlan-der, B.L. Am. Rev. Tuberc. 1944, 49, 543.
- Kato, H.; Takashashi, A.; Tamuya, H.; Kono, M.; Shimo, M. Shokuhin Eiseigku Zasshi 1966, 7, 60.
- Collins, P.; Ferguson, J. British J. Dermat. 1994, 131, 124.
- Bremard, C.; Buntinx, G.; Ginestet, G. J. Mol. Struct. 1997, 410, 379.
- Olszanowski, A.; Krzyzanowska, E.; Alejski, K. J. Chem. Tech. Biotech. 1997, 68, 236.
- Terazima, M. J. Phys. Chem. A 1998, 102, 545.
- Kasha, M. Disc. Faraday Soc. 1950, 9, 14. [CrossRef]
- McConnell, H. J. Chem. Phys. 1952, 20, 700.
- Brealy, G.J.; Kasha, M. J. Amer. Chem. Soc. 1955, 77, 4462.
Share and Cite
Castro, G.T.; Blanco, S.E.; Giordano, O.S. UV Spectral Properties of Benzophenone. Influence of Solvents and Substituents. Molecules 2000, 5, 424-425. https://doi.org/10.3390/50300424
Castro GT, Blanco SE, Giordano OS. UV Spectral Properties of Benzophenone. Influence of Solvents and Substituents. Molecules. 2000; 5(3):424-425. https://doi.org/10.3390/50300424
Chicago/Turabian StyleCastro, G. T., S. E. Blanco, and O. S. Giordano. 2000. "UV Spectral Properties of Benzophenone. Influence of Solvents and Substituents" Molecules 5, no. 3: 424-425. https://doi.org/10.3390/50300424
APA StyleCastro, G. T., Blanco, S. E., & Giordano, O. S. (2000). UV Spectral Properties of Benzophenone. Influence of Solvents and Substituents. Molecules, 5(3), 424-425. https://doi.org/10.3390/50300424



