An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones Catalyzed by KSF Montmorillonite
Abstract
:Introduction
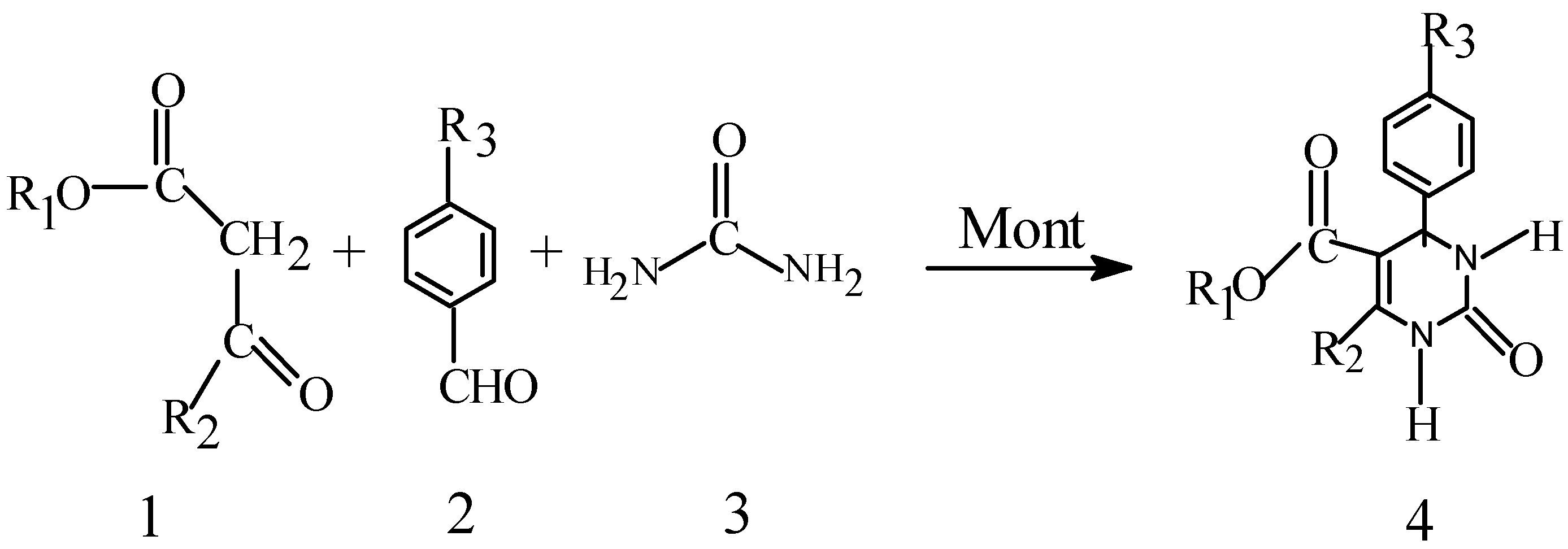
Results and Discussion
Conclusions
Experimental
General
Syntheses
References
- McKinstry, D. W.; Reading, E. H. J. Frankin Inst. 1944, 237, 422–431.
- Matsuda, T.; Hirao, I. Nippon Kagaku Zasshi 1965, 86, 1195–1197.
- Kato, T. Japn. Kokai Tokkyo Koho JP 1984, 59, 190,974.
- Takatani, T.; Takasugi, H.; Kuno, A.; Inoue, Z. Japn. Kokai Tokkyo koho JP 1987, 62, 252,775.
- Overman, L. E.; Rabinowitz, M. H.; Renhowe, P. A. J. Am. Chem. Soc. 1995, 117, 1657–2658.
- Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360.
- Hu, E. H.; Sidler, D. R.; Dolling, U. H. J. Org. Chem. 1998, 63, 3454–3457.
- Kappe, C. O.; Falsone, F. S. Synlett 1998, 718.
- Kappe, C. O.; Kumar, D.; Varma, R. S. Synthesis 1999, 10, 1799–1803.
- Lu, J.; Ma, H. Synlett 2000, 1, 63–64.
- O’Reilly, B. C.; Atwal, K. S. Heterocycles 1987, 26, 1185.
- Shutalev, A. D.; Kishko, E. A.; Sivova, N.; Kuznetsov, A. Y. Molecules 1998, 3, 100.
- Studer, A.; Hadida, S.; Ferrito, R.; Kim, S. Y.; Jeger, P.; Wipf, P.; Curran, D. P. Science 1997, 275, 823. [PubMed]
- Lewandowski, K.; Murer, P.; Svec, F.; Frechet, J. M. J. Chem. Soc. Chem. Commun. 1998, 2237.
- Bigi, F.; Carloni, S.; Frullanti, B.; Maggi, R.; Sartori, G. Tetrahedron Letters 1999, 40, 3465–3468.
- Lin, H. X.; Zhang, L. X.; Cheng, L. S. Chin. Chem. Lett. 1999, 10(11), 915–916.
- Samples Availability: Samples are available from the authors.
| entry | R1 | R2 | R3 | Yield(%) | m.p. (°C) (literature) [7] | |||
| Aa | Bb | Cc | Dd | |||||
| 1 | Et | Me | Me | 88 | 213-4 | |||
| 2 | Et | Me | Cl | 93 | 56 | 92 | 76 | 214-5(213-5) |
| 3 | Et | Me | NO2 | 89 | 54 | 91 | 209-11(208-11) | |
| 4 | Et | Me | OMe | 82 | 37 | 85 | 79 | 200-2(201-3) |
| 5 | Et | Me | H | 92 | 71 | 94 | 82 | 205-6(202-4) |
| 6 | Et | Ph | H | 80 | 10 | 70 | 75 | 158-9(157-9) |
| 7 | Me | Me | H | 90 | 42 | 88 | 211-3(209-12) | |
© 2000 by MDPI (http://www.mdpi.org)
Share and Cite
Lin, H.; Ding, J.; Chen, X.; Zhang, Z. An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones Catalyzed by KSF Montmorillonite. Molecules 2000, 5, 1240-1243. https://doi.org/10.3390/51201240
Lin H, Ding J, Chen X, Zhang Z. An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones Catalyzed by KSF Montmorillonite. Molecules. 2000; 5(12):1240-1243. https://doi.org/10.3390/51201240
Chicago/Turabian StyleLin, Haixia, Jinchang Ding, Xianten Chen, and Ziyi Zhang. 2000. "An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones Catalyzed by KSF Montmorillonite" Molecules 5, no. 12: 1240-1243. https://doi.org/10.3390/51201240
APA StyleLin, H., Ding, J., Chen, X., & Zhang, Z. (2000). An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones Catalyzed by KSF Montmorillonite. Molecules, 5(12), 1240-1243. https://doi.org/10.3390/51201240




