Synthesis Of Some Novel Silver-Cysteamine Complexes
Abstract
:Introduction
Results and Discussion
1) Microanalysis results for 16 different silver-cysteamine complexes
| No. | Mole ratio of AgNO3 to cysteamine(taken) | pH | Microanalytical data (experimental) | Metal to ligand ratio (observed) | |||||
|---|---|---|---|---|---|---|---|---|---|
| %C | %H | %N | %S | %Ag | empirical formulas | ||||
| 1 | 1 : 1 | 7 | 9.1 | 2.6 | 5.2 | 12.0 | 52 | (C2H7NS), Ag1.3 | 4 : 3 |
| 2 | 1 : 1 | 8 | 9.1 | 2.7 | 5.5 | 12.3 | 53.1 | (C1.98H7NS), Ag1.28 | 4 : 3 |
| 3 | 1 : 1 | 9 | 8.5 | 2.5 | 5.1 | 11.7 | 52.0 | (C1.95H6.9NS), Ag1.32 | 4 : 3 |
| 4 | 1 : 1 | 10 | 9.2 | 2.6 | 5.2 | 12.0 | 51.9 | (C2H7NS), Ag1.29 | 4 : 3 |
| 5 | 1 : 2 | 7 | 9.1 | 2.6 | 5.2 | 12.1 | 52.4 | (C2H7NS), Ag1.3 | 4 : 3 |
| 6 | 1 : 2 | 8 | 9.2 | 2.8 | 5.6 | 12.8 | 54.3 | (C1.92H7NS), Ag1.25 | 4 : 3 |
| 7 | 1 : 2 | 9 | 9.4 | 2.8 | 5.6 | 12.7 | 55.0 | (C1.97H7NS), Ag1.28 | 4 : 3 |
| 8 | 1 : 2 | 10 | 9.1 | 2.6 | 5.4 | 12.0 | 53.2 | (C2H6.9NS), Ag1.31 | 4 : 3 |
| 9 | 1 : 3 | 7 | 9.1 | 2.6 | 5.2 | 11.9 | 53.2 | (C2H7NS), Ag1.32 | 4 : 3 |
| 10 | 1 : 3 | 8 | 9.2 | 2.6 | 5.2 | 12.0 | 52.0 | (C2H7NS), Ag1.3 | 4 : 3 |
| 11 | 1 : 3 | 9 | 10.0 | 2.6 | 5.2 | 12.1 | 52.8 | (C2.2H7NS), Ag1.31 | 4 : 3 |
| 12 | 1 : 3 | 10 | 9.2 | 2.7 | 5.2 | 12.0 | 53.0 | (C2H7.2NS), Ag1.32 | 4 : 3 |
| 13 | 1 : 4 | 7 | 10.0 | 2.8 | 5.7 | 13.0 | 54.0 | (C2H7NS), Ag1.27 | 4 : 3 |
| 14 | 1 : 4 | 8 | 9.2 | 2.7 | 5.7 | 13.0 | 54.0 | (C1.9H6.8NS), Ag1.25 | 4 : 3 |
| 15 | 1 : 4 | 9 | 9.1 | 2.6 | 5.2 | 11.9 | 52.9 | (C2H7.1NS), Ag1.32 | 4 : 3 |
| 16 | 1 : 4 | 10 | 9.1 | 2.6 | 5.2 | 12.0 | 52.7 | (C2H7NS), Ag1.31 | 4 : 3 |
2) Electrochemical behavior of the cation, the ligand, and the complexes by cyclic voltammetry
- a)
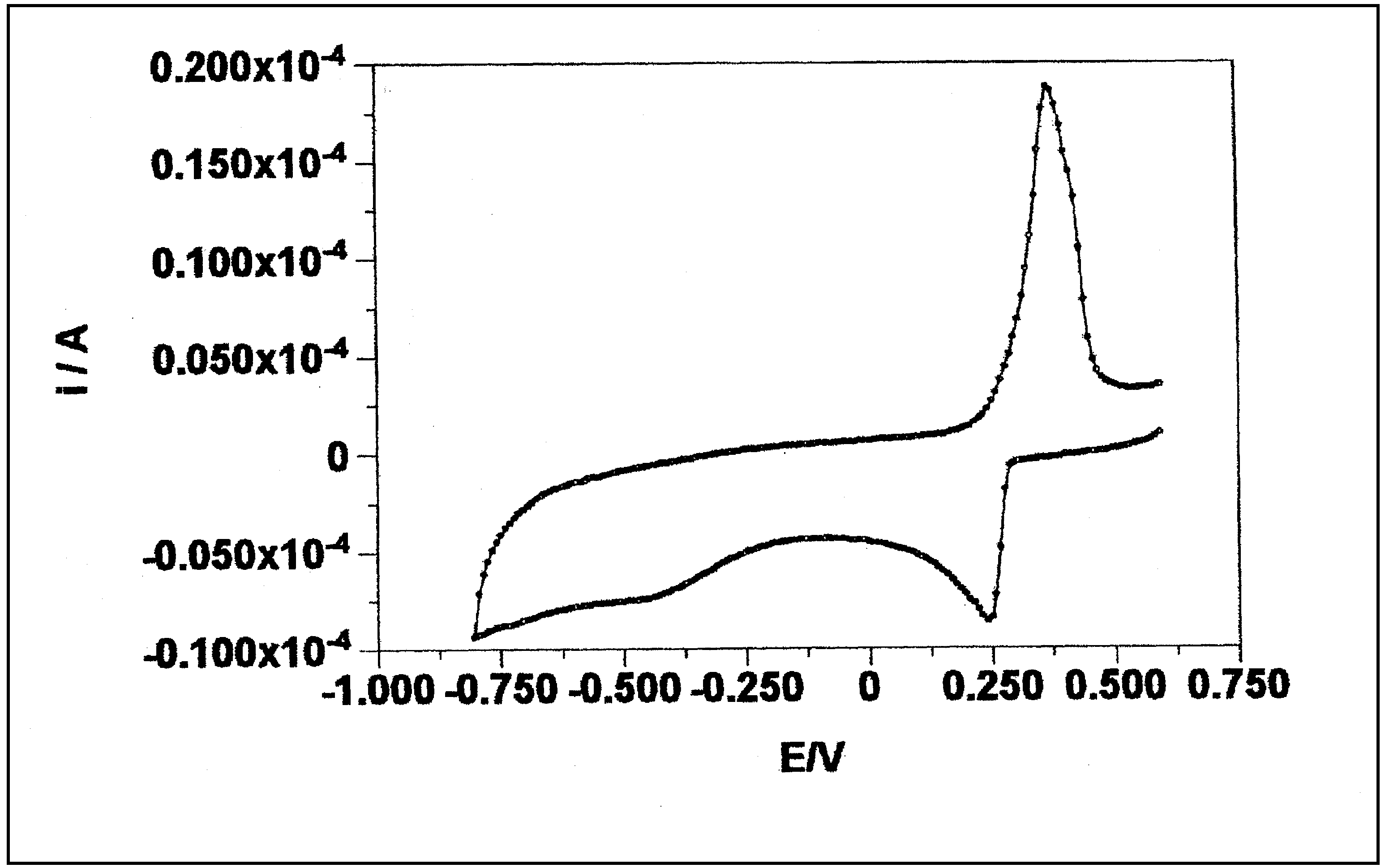
- Electrochemical behavior of the silver cation: According to figure A, the voltammogram has a cathodic peak at 240, and an anodic peak at 500 mv versus SCE. The cathodic peak belongs to the reduction of Ag+ to metallic silver. The anodic counterpart is related to the oxidation of metallic silver.
- b)
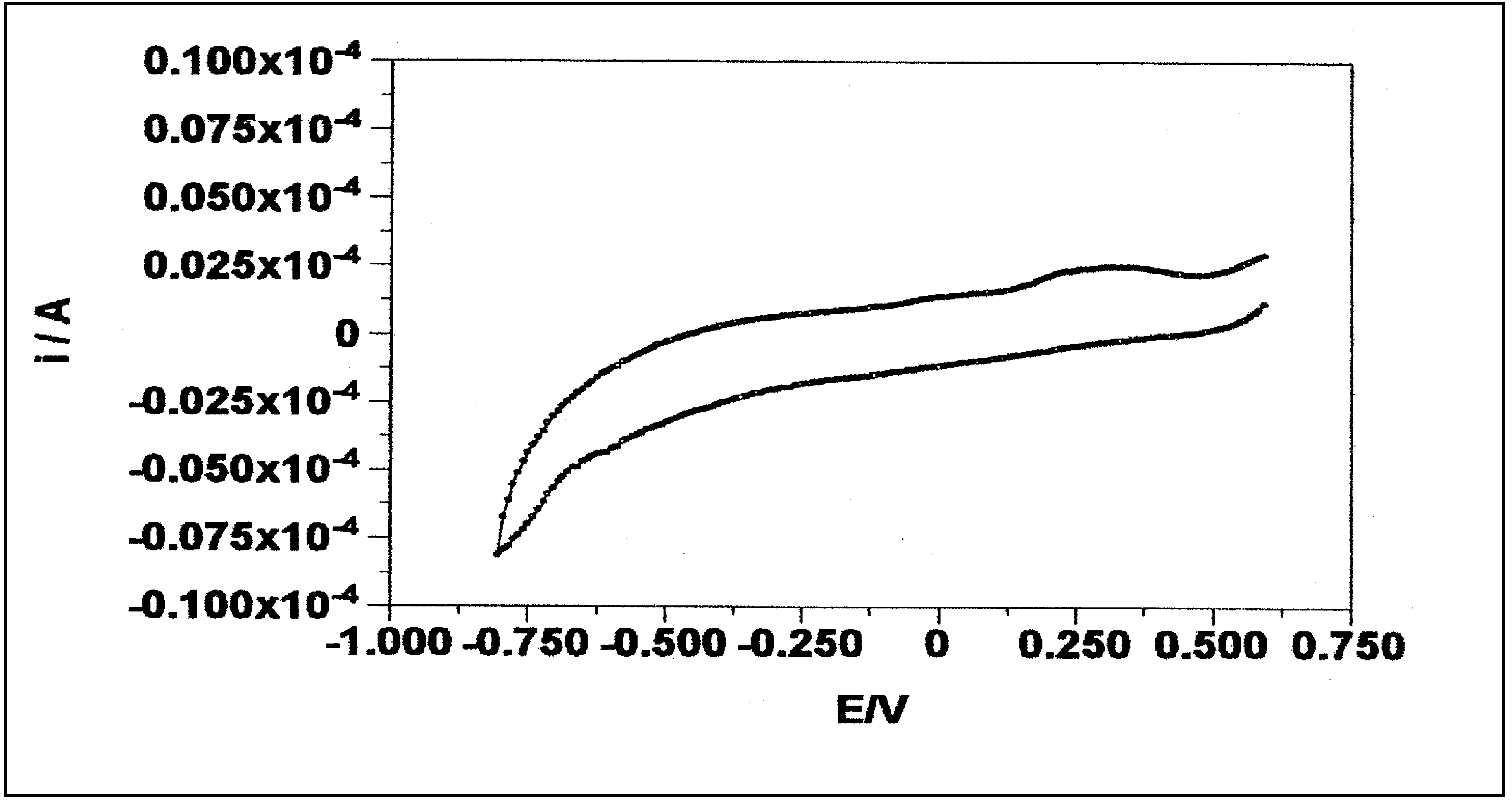
- Electrochemical behavior of the cysteamine ligand: According to figure B, the cysteamine ligand does not participate in any electron transfer in scanned region.
- c)
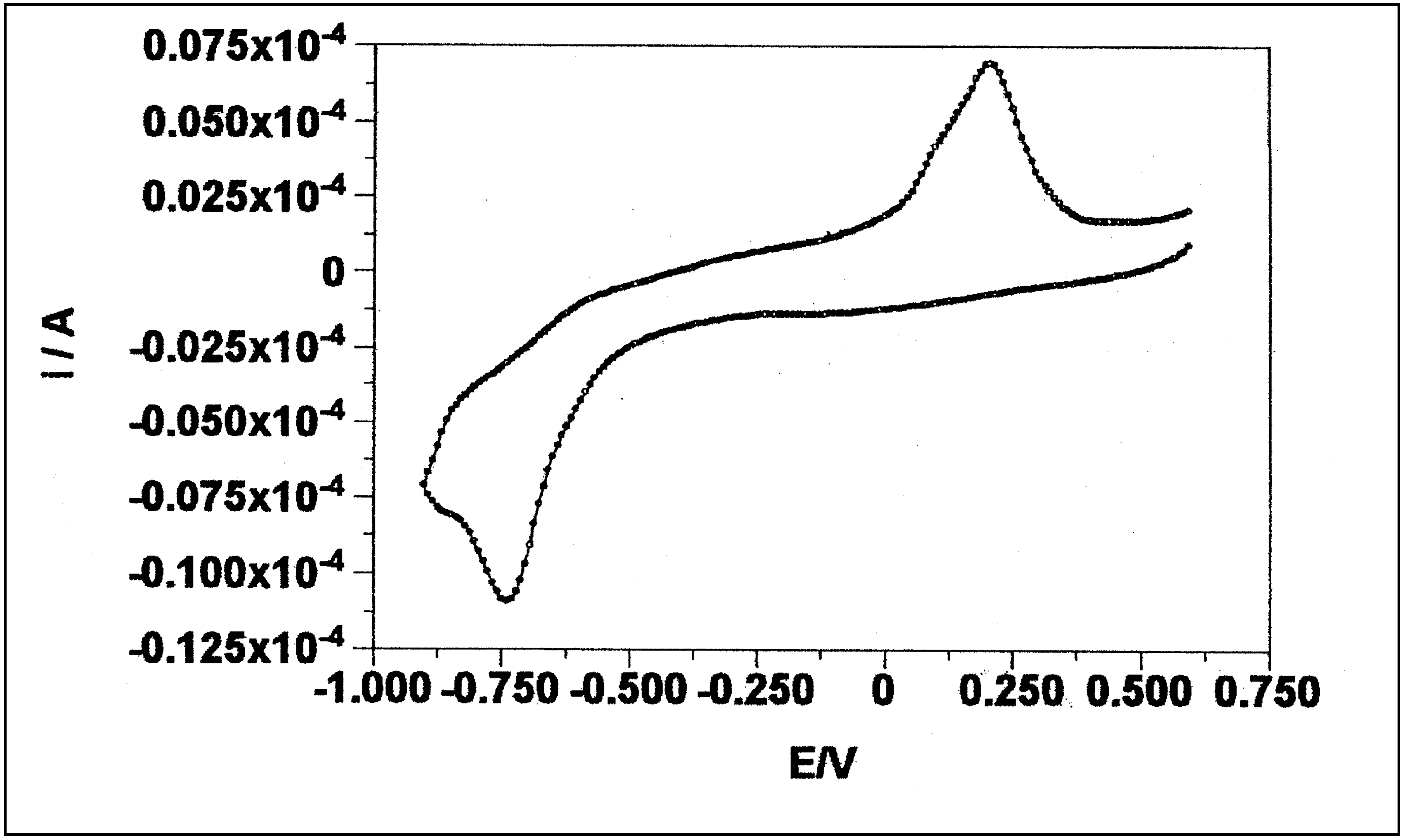
- Electrochemical behavior of the silver-cysteamine complex: According to figure C, and its comparison with the figure A, it can be seen that the anodic and the cathodic peaks of the complex were shifted to a more negative potential. The cathodic peak belongs to the reduction of the silver ion in the presence of ligand. Due to participation of the silver in formation of the complex, its reduction is more difficult, and thus it is reduced at a more negative potential. The anodic peak in the voltammogram belongs to the oxidation of metallic silver in the presence of the relevant ligand, and thus it is oxidized at a more negative potential in comparison with the simple oxidation of the silver (i.e. oxidation of the silver has been facilitated in the presence of the ligand).
3) Formation of complexes by pH investigations
| Volume of Ag+ | pH | Volume of Ag+ | pH | Volume of Ag+ | pH | Volume of Ag+ | pH | Volume of Ag+ | pH |
|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 3.62 | 5.0 | 2.98 | 9.0 | 2.46 | 13.0 | 2.31 | 17.0 | 2.26 |
| 1.5 | 3.48 | 5.5 | 2.92 | 9.5 | 2.42 | 13.5 | 2.30 | 17.5 | 2.26 |
| 2.0 | 3.40 | 6.0 | 2.85 | 10.0 | 2.40 | 14.0 | 2.29 | 18.0 | 2.26 |
| 2.5 | 3.31 | 6.5 | 2.78 | 10.5 | 2.37 | 14.5 | 2.28 | 18.5 | 2.26 |
| 3.0 | 3.24 | 7.0 | 2.72 | 11.0 | 2.35 | 15.0 | 2.28 | 19.0 | 2.26 |
| 3.5 | 3.18 | 7.5 | 2.65 | 11.5 | 2.34 | 15.5 | 2.27 | 19.5 | 2.26 |
| 4.0 | 3.12 | 8.0 | 2.57 | 12.0 | 2.33 | 16.0 | 2.27 | 20.0 | 2.26 |
| 4.5 | 3.05 | 8.5 | 2.51 | 12.5 | 2.32 | 16.5 | 2.26 |

Experimental
Preparation of silver-cysteamine complexes
Formation of silver-cysteamine complexes for pH investigations
Electrochemical behavior of the cation, the ligand, and the complexes by cyclic voltammetry
References
- Su, W.; Cao, R.; Hong, M.; Chen, J.; Lu, J. J. Chem. Soc., Chem. Commun. 1998, 1389.
- Jassem, M. D.; Spek, A. L.; Grove, D. M.; van Koten, G. J. Am. Chem. Soc. 1996, 35, 4078.
- Salehi, Z.; Parish, R. V.; Pritchard, R. G. J. Chem. Soc., Dalton Trans. 1997, 4241.
- Parish, R. V.; Salehi, Z.; Pritchard, R. G. Angew. Chem. Int. Ed, Rngl. 1997, 36, 251.
- Pritchard, R. G.; Saleh, Z.; Parish, R. V. J. Chem. Soc., Chem. in press.
- Sample Availability: Samples are available from the authors.
© 2000 by MDPI (http://www.mdpi.org). All rights reserved
Share and Cite
Habibi, D.; Ghaemi, E.; Nematollahi, D. Synthesis Of Some Novel Silver-Cysteamine Complexes. Molecules 2000, 5, 1194-1200. https://doi.org/10.3390/51201194
Habibi D, Ghaemi E, Nematollahi D. Synthesis Of Some Novel Silver-Cysteamine Complexes. Molecules. 2000; 5(12):1194-1200. https://doi.org/10.3390/51201194
Chicago/Turabian StyleHabibi, Davood, Ezat Ghaemi, and Davood Nematollahi. 2000. "Synthesis Of Some Novel Silver-Cysteamine Complexes" Molecules 5, no. 12: 1194-1200. https://doi.org/10.3390/51201194
APA StyleHabibi, D., Ghaemi, E., & Nematollahi, D. (2000). Synthesis Of Some Novel Silver-Cysteamine Complexes. Molecules, 5(12), 1194-1200. https://doi.org/10.3390/51201194




