Experimental
1H-NMR spectra were recorded on a General Electric QE-300 (300 MHz) instrument. The spectral data are reported in the following format: chemical shift (all relative to Me4Si as an internal reference standard unless otherwise indicated), multiplicity (s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet, b = broad ), integration, coupling constants, exchangeability after D2O addition, and assignment of resonances. Elemental Microanalyses were performed by Atlantic Microlab, Inc., Norcross, Georgia. The mass spectra were recorded at the Mass Spectral Facility, Department of Biochemistry, Michigan State University. Thin layer chromatography was performed on Merck Kieselgel 60 GF254 plates (0.2 mm thickness). Melting points were determined on a Thomas- Hoover capillary melting point apparatus, and are uncorrected.
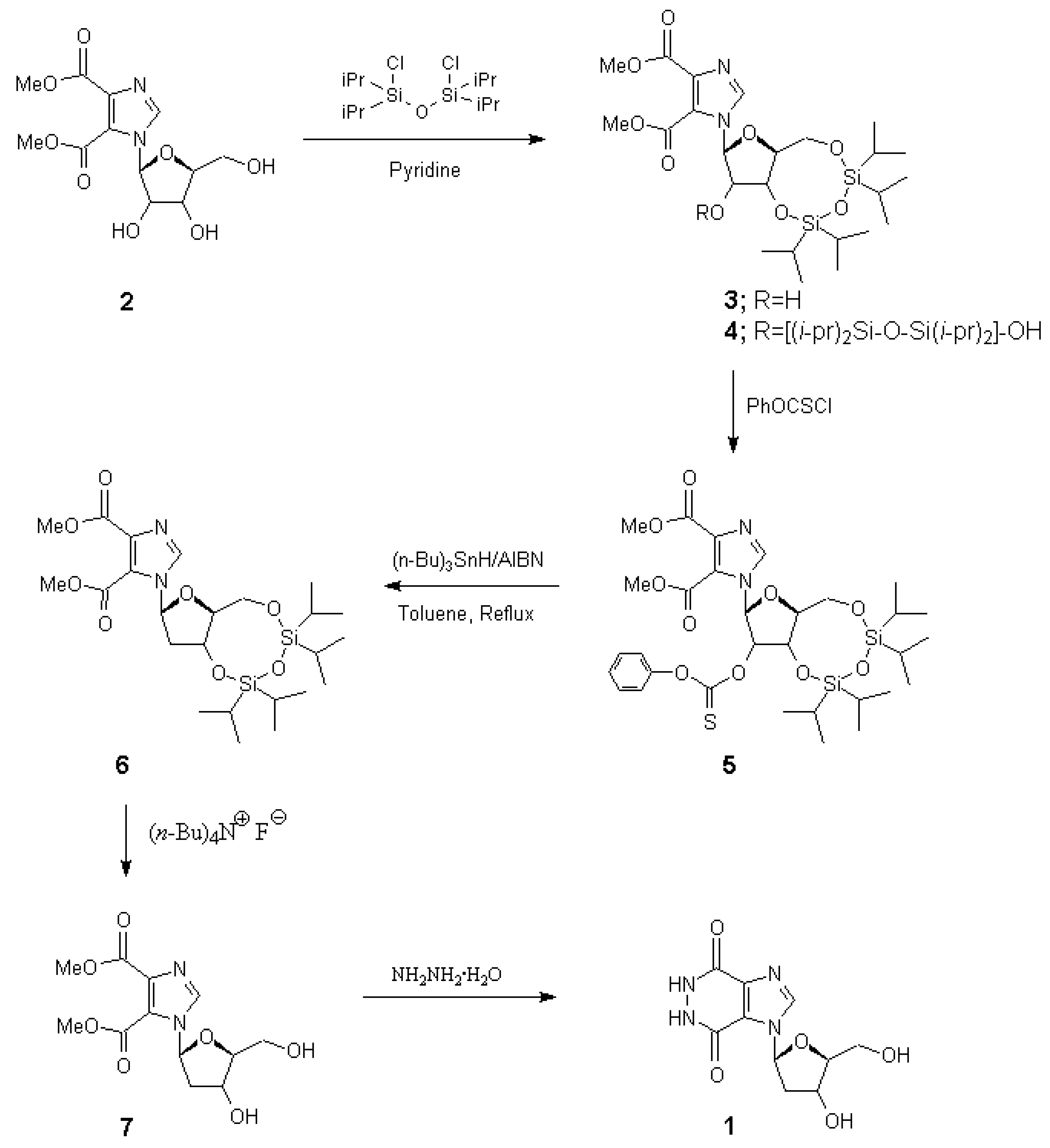
Methyl 1-[(3',5'-O-(1,1,3,3-tetraisopropyldisiloxan-1,3-diyl))-ß-d-ribofuranosyl]-4,5-imidazole- dicarboxylate (3) and Methyl 1-[((2'-O-(3-hydroxy-1,1,3,3-tetraisopropyldisiloxyl)-3',5'-O- (1,1,3,3-tetraisopropyldisiloxan-1,3-diyl))-ß-d-ribofuranosyl]-4,5-imidazoledicarboxylate (4)
To a solution of dry methyl 1-β-
d-ribofuranosyl-4,5-imidazoledicarboxylate [
2] (
2) (500 mg, 1.58 mmol) in dry pyridine (10 mL) was added 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane [
3] (550 mg, 1.75 mmol), and the mixture was stirred for 4 h at ambient temperature with protection from moisture. Volatile materials were evaporated
in vacuo, and the residue was dissolved in chloroform. The chloroform solution was washed twice with cold water and dried over anhydrous sodium sulfate. The residue after evaporation was purified by silica gel flash chromatography, eluting with chloroform to give
3 and
4 as a colorless liquids, whose yield, spectral and analytical data are given below.
Compound 3: Yield 800 mg (91%), Rf 0.38 (chloroform/methanol, 30:1); 1H-NMR (CDCl3) δ 8.10 (s, 1H, imidazole), 6.11 (s, 1H, 1'-H), 4.44 (dd, 1H, J=4.2 and 9.0 Hz, 3'-H), 4.26 (d, 1H, Jgem=13.5 Hz, 5'-H), 4.18 (d, 1H, J=4.2 Hz, 2'-H), 4.16 (dd, 1H, J=9.0 and 2.4 Hz, 4'-H), 4.03 (dd, 1H, Jgem=13.5 Hz, J5',4'=2.4 Hz, 5'-H), 3.95 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 2.89 (brs, 1H, 2'-OH, exchangeable with D2O), 1.05 (m, 28H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) δ 12.45 (CHSi), 12.82 (CHSi), 12.90 (CHSi), 13.35 (CHSi), 16.81 (CCH3), 16.87 (CCH3), 16.97 (CCH3), 16.97 (CCH3), 17.26 (CCH3), 17.26 (CCH3), 17.32 (CCH3), 17.42 (CCH3), 52.50 (OCH3), 52.78 (OCH3), 59.91 (C-5'), 68.43 (C-3'), 76.82 (C-2'), 81.87 (C-4'), 91.54 (C-1'), 122.83 (C-4 or 5), 137.26 (C-2), 138.57 (C-5 or 4), 160.26 (C=O), 162.91 (C=O); Anal. Calcd. for C24H42N2O9Si2 (MW 558.78): C, 51.59; H, 7.58; N, 5.01. Found: C, 51.50; H, 7.57; N, 5.04.
Compound 4: Yield 120 mg (9%), Rf 0.45 (chloroform/methanol, 30:1); 1H-NMR (CDCl3) δ 8.37 (s, 1H, imidazole), 6.06 (s, 1H, 1'-H), 4.48 (d, 1H, J=3.3 Hz, 2'-H), 4.35 (dd, 1H, J=3.3 and 9.6 Hz, 3'-H), 4.31 (d, 1H, Jgem=13.8 Hz, 5'-H), 4.27 (dd, 1H, J=9.6 and 2.4 Hz, 4'-H), 4.02 (dd, 1H, Jgem=13.8 Hz, J5',4'=2.4 Hz, 5'-H), 3.93 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 1.03 (m, 56H, isopropyl groups); 13C- NMR (CDCl3, 75.48 MHz) δ 12.51 (CHSi), 12.91 (CHSi), 13.05 (CHSi), 13.05 (CHSi), 13.24 (CHSi), 13.35 (CHSi), 13.39 (CHSi), 13.48 (CHSi), 16.59 (CCH3), 16.78 (CCH3), 16.81 (CCH3), 16.81 (CCH3), 17.06 (CCH3), 17.06 (CCH3), 17.06 (CCH3), 17.23 (CCH3), 17.26 (CCH3), 17.26 (CCH3), 17.30 (CCH3), 17.37 (CCH3), 17.42 (CCH3), 17.51 (CCH3), 17.55 (CCH3), 17.65 (CCH3), 52.56 (OCH3), 53.17 (OCH3), 59.56 (C-5'), 68.47 (C-3'), 77.95 (C-2'), 81.44 (C-4'), 92.15 (C-1'), 121.16 (C-4 or 5), 138.64 (C-2), 139.48 (C-5 or 4), 161.49 (C=O), 163.11 (C=O); Anal. Calcd. for C36H70N2O11Si4 (MW 819.30): C, 52.78; H, 8.61; N, 3.42. Found: C, 52.45; H, 8.89; N, 2.93.
Methyl 1-[(2'-O-phenoxythiocarbonyl)-3',5'-O-(1,1,3,3-tetraisopropyldisiloxan-1,3-diyl)-ß-d-erythro- pentofuranosyl)]-4,5-imidazoledicarboxylate (5)
To a solution of methyl 1-(3',5'-O-(1,1,3,3,-tetraisopropyldisiloxan-1,3-diyl)-β-d-erythropento- furanosyl)-4,5-imidazoledicarboxylate (3) (560 mg, 1 mmol) and DMAP (250 mg, 2.05 mmol) in 15 mL of dried acetonitrile was added phenoxythiocarbonyl chloride (200 μL, 250 mg, 1.45 mmol). The solution was stirred for 16 h at ambient temperature, and evaporated to dryness in vacuo. The residue was applied to column chromatography, eluted with chloroform to give a colorless oily product (660 mg, 95%). Rf 0.60, chloroform/ methanol (30:1); 1H-NMR (CDCl3) δ 8.16 (s, 1H, imidazole), 7.43 (t, 2H, J=7.8 Hz, Phmeta-H), 7.31 (t, 1H, J=7.8 Hz, Phpara-H), 7.13 (d, 2H, J=7.8 Hz, Phortho-H), 6.38(s, 1H, 1'-H), 6.01 (d, 1H, J=4.5 Hz, 2'-H), 4.61 (dd, 1H, J=4.5 and 9.3 Hz, 3'-H), 4.31 (d, 1H, Jgem=13.5 Hz, 5'-H), 4.16 (dd, 1H, J=9.3 and 2.4 Hz, 4'-H), 4.05 (dd, 1H, Jgem=13.5 Hz, J5',4'=2.4 Hz, 5'-H), 3.93 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 1.06 (m, 28H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) δ 12.80 (CHSi), 12.91 (CHSi), 13.37 (CHSi), 13.49 (CHSi), 16.87 (CCH3), 16.94 (CCH3), 16.96 (CCH3), 17.08 (CCH3), 17.24 (CCH3), 17.27 (CCH3), 17.34 (CCH3), 17.44 (CCH3), 52.49 (OCH3), 52.74 (OCH3), 59.29 (C-5'), 67.69 (C-3'), 82.39 (C-4'), 85.10 (C-2'), 89.40 (C-1'), 121.68 (Ph-C2,6), 126.69 (Ph-C4), 129.57 (Ph-C3,5), 137.13 (C-2), 138.93 (C-5 or 4), 139.24 (C-4 or 5), 153.47 (Ph-C1), 159.79 (C=O), 162.76 (C=O), 193.70 (C=S); Anal. Calcd. for C31H46N2O10SSi2 (MW 694.94): C, 53.58; H, 6.67; N, 4.03; S, 4.61. Found: C, 53.88; H, 7.47; N, 3.53; S, 3.27.
Methyl 1-(2'-Deoxy-3',5'-O-(1,1,3,3-tetraisopropyldisiloxan-1,3-diyl)-ß-d-erythropentofuranosyl)-4,5- imidazoledicarboxylate (6)
A solution of methyl1-(2'-O-phenoxythiocarbonyl-3',5'-O-(1,1,3,3-tetraisopropyldisiloxan-1,3-diyl)- β-d-erythropentofuranosyl)-4,5-imidazoledicarboxylate (5) (695 mg, 1 mmol) and AIBN (32 mg, 0.2 mmol) in 30 mL of dried toluene was purged with oxygen-free nitrogen for 30 min. Tributylstannane (400 μL, 433 mg, 1.49 mmol) was added and the solution was refluxed for 3 h. Tlc showed a single spot with very slightly lower Rf value than that of starting material. The solvent was evaporated and the pure product was obtained as a colorless liquid by column chromatography (hexane/ethyl acetate, 3:1) in almost quantitative yield. Rf 0.12, hexane/ethyl acetate (3:1); 1H-NMR (CDCl3) δ 8.09 (s, 1H, imidazole), 6.33 (dd, 1H, J=7.4 and 1.2 Hz, 1'-H), 4.54 (dt, 1H, J=10.2 and 7.4 Hz, 3'-H), 4.11 (dd, 1H, Jgem=13.2 Hz, J5',4'=2.4 Hz, 5'-H), 4.06 (dd, 1H, Jgem=13.2 Hz, J5',4'=3.0 Hz, 5'-H), 3.93 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.82 (dt, 1H, J=8.2 and 2.6 Hz, 4'-H), 2.64 (ddd, 1H, J=13.2, 10.2 and 7.4 Hz, 2'- H), 2.35 (ddd, 1H, J=13.2, 7.4 and 1.2 Hz, 2'-H), 1.06 (m, 28H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) δ 12.54 (CHSi), 12.94 (CHSi), 13.04 (CHSi), 13.43 (CHSi), 16.86 (CCH3), 16.97 (CCH3), 16.97 (CCH3), 17.09 (CCH3), 17.31 (CCH3), 17.31 (CCH3), 17.39 (CCH3), 17.49 (CCH3), 41.70 (C-2'), 52.34 (OCH3), 52.50 (OCH3), 60.48 (C-5'), 67.52 (C-3'), 85.25 (C-4'), 86.02 (C-1'), 136.41 (C-4 or 5), 137.12 (C-2), 138.41 (C-5 or 4), 160.37 (C=O), 163.04 (C=O); Anal. Calcd. for C24H42N2O8Si2 (MW 542.78): C, 53.11; H, 7.80; N, 5.16. Found: C, 53.39; H, 7.83; N, 4.91.
Methyl 1-(2'-Deoxy-ß-d-erythropentofuranosyl)-4,5-imidazoledicarboxylate (7)
A solution of 1M tetrabutylammonium fluoride in THF (2 mL, 2 mmol) was added to an ice-cooled solution of methyl 1-(2'-deoxy-3',5'-O-(1,1,3,3,-tetraisopropyldisiloxan-1,3-diyl)-ß-d-erythropento- furanosyl)-4,5-imidazoledicarboxylate (6) (543 mg, 1 mmol) in 10 mL of dried THF. The reaction was stopped after 45 min stirring at 0 °C. The solvent was evaporated in vacuo and the pure product was obtained as a foam by column chromatography (chloroform/methanol, 10:1) in 75 % yield. Rf 0.20, chloroform/methanol (10:1); 1H-NMR (CDCl3) δ 8.51 (s, 1H, imidazole), 6.38 (dd, 1H, J=6.2 and 3.4 Hz, 1'-H), 4.82 (brs, 1H, OH, exchangeable with D2O), 4.54 (dd 1H, J=12.8 and 6.6 Hz, 3'-H), 4.15 (brs, 1H, OH, exchangeable with D2O), 3.98 (m, 3H, 4',5'-H), 3.91 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 2.56 (ddd, 1H, J=13.6, 12.8 and 6.2 Hz, 2'-H), 2.35 (ddd, 1H, J=13.6, 6.6 and 3.4 Hz, 2'-H); 13C-NMR (CDCl3, 75.48 MHz) δ 42.49 (C-2'), 52.42 (OCH3), 52.73 (OCH3), 60.62 (C-5'), 68.91 (C-3'), 87.03 (C-4'), 87.21 (C-1'), 136.50 (C-4 or 5), 136.86 (C-5 or 4), 137.68 (C-2), 160.40 (C=O), 163.05 (C=O); Anal. Calcd. for C12H16N2O7 (MW 300.27): C, 48.00; H, 5.37; N, 9.33. Found: C, 48.21; H, 5.68; N, 9.45.
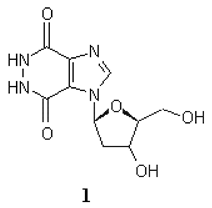
1-(2'-Deoxy-ß-d-ribofuranosyl)-1H-imidazo[4,5-d]pyridazine-4,7(5H,6H)-dione (1)
A solution of methyl 1-(2'-deoxy-β-d-ribofuranosyl)imidazole-4,5-dicarboxylate (7) (0.15 g, 0.5 mmol) and 99% hydrazine (10 mL) was refluxed for 6 hours. The excess hydrazine was removed by distillation in vacuo and the residue coevaporated several times with water. The crystalline residue was recrystallized from methanol to give white crystals (0.1g, 75%). mp. >250 °C; Rf, 0.34 (chloroform/methanol/30% ammonium hydroxide, 2:2:1); 1H NMR (DMSO-d6) δ 8.62 (s, 1H, imidazole), 6.76 (t, 1H, J=6.2 Hz, 1'-H), 5.31 (brs, 1H, OH, exchangeable with D2O), 5.06 (brs, 1H, OH, exchangeable with D2O), 4.33 (m, 1H, 4'-H), 3.87 (m, 1H, 3’-H), 3.57 (m, 2H, 5'-H), 2.34 (dt, 1H, J=13.6 and 6.3 Hz, 2'-H), 2.08 (d, 1H, J=13.6 Hz, 2'-H). ms: (FAB) m/z 310 (MH+).; Anal. Calcd. For C10H12N4O5.H2O (286.24): C, 41.96; H, 4.93; N, 19.57. Found: C, 41.98; H, 4.92; N, 19.56.