Synthesis of Aromatic Vinyl Esters by Exchange Reaction Catalyzed with Pd(II)
Abstract
:Introduction
Results and Discussion
Experimental
Acknowledgements
References and Notes
- Tsuji, J. Synthesis 1990, 739–749.
- Henry, P. M. Palladium Catalyzed Oxidation of Hydrocarbons; Reidel: Dordrecht, 1980; pp. 41–84. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts; Wiley: New York, 1996; pp. 19–108. [Google Scholar]
- Jira, R.; Freiesleben, W. Organomet. React. 1972, 3, 1–190.
- Larock, R. C.; Hightower, T. R. J. Org. Chem. 1993, 58, 5298–5300.
- Kaneda, K.; Uchiyama, T.; Fujiwara, Y.; Imanaka, T.; Teranishi, S. J. Org. Chem. 1979, 44, 55–63.
- Smidt, J.; Hafner, W.; Jira, R.; Sieber, R.; Sedlmeier, J.; Sabel, A. Angew. Chem. 1962, 74, 93–102.
- McKeon, J. E.; Fitton, P. Tetrahedron 1972, 28, 233–238.
- Keith, D. D.; Tortora, J. A.; Ineichen, K.; Leimgruber, W. Tetrhedron 1975, 31, 2633–2636.
- Divers, G. A.; Berchtold, G. A. Synth. Commun. 1977, 7, 43–48.
- Henry, P. M. Acc. Chem. Res. 1973, 6, 16–24.
- Henry, P. M. J. Am. Chem. Soc. 1972, 94, 7316–7322.
- Bjorkquist, D. W.; Bush, R. D.; Ezra, F. S.; Keough, T. J. Org. Chem. 1986, 51, 3192–3196.
- Kharasch, M. S.; Seyler, R. C.; Mayo, R. R. J. Am. Chem. Soc. 1938, 60, 882–884.
- Samples Availability: Available from the authors.
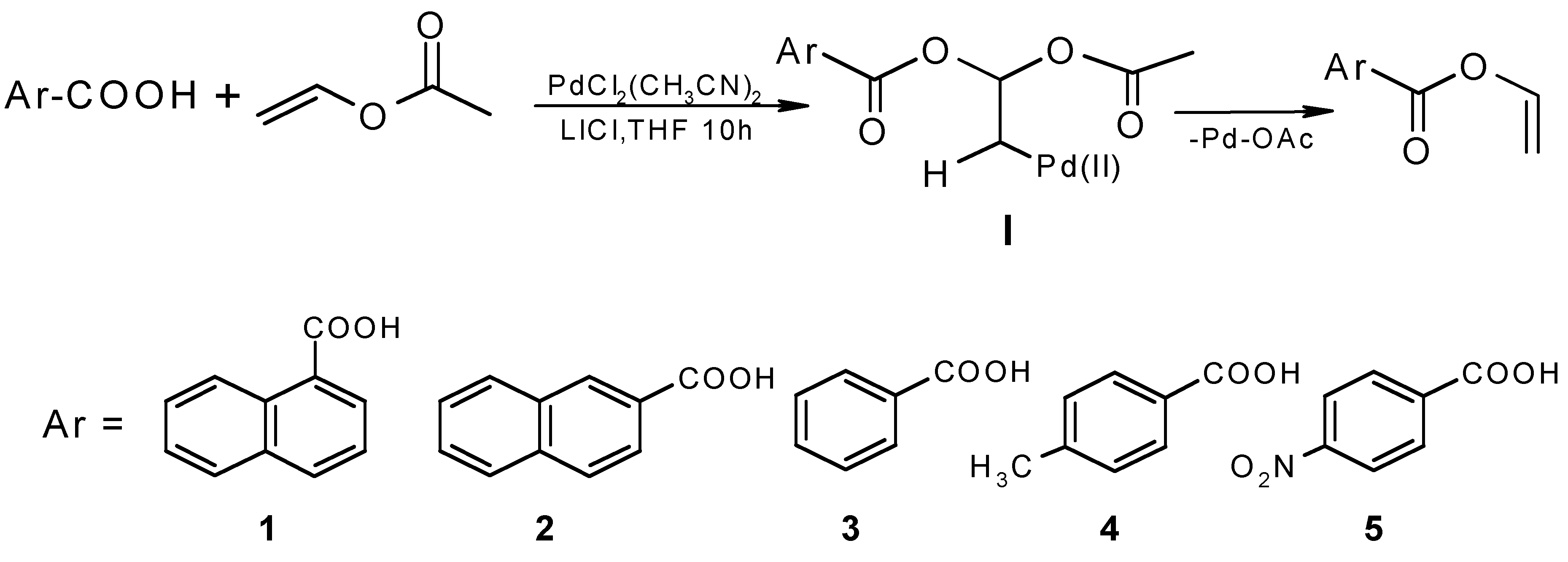
| Aromatic acid | Product | Yield |
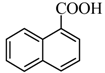 | 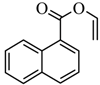 | 53% |
 | 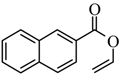 | 51% |
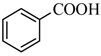 | 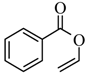 | 43% |
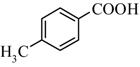 | 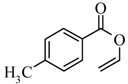 | 47% |
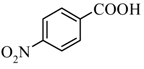 | 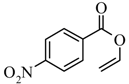 | 39% |
© 1999 MDPI. All rights reserved.
Share and Cite
Rashidi-Ranjbar, P.; Piri, F. Synthesis of Aromatic Vinyl Esters by Exchange Reaction Catalyzed with Pd(II). Molecules 1999, 4, 135-138. https://doi.org/10.3390/40500135
Rashidi-Ranjbar P, Piri F. Synthesis of Aromatic Vinyl Esters by Exchange Reaction Catalyzed with Pd(II). Molecules. 1999; 4(5):135-138. https://doi.org/10.3390/40500135
Chicago/Turabian StyleRashidi-Ranjbar, Parviz, and Farideh Piri. 1999. "Synthesis of Aromatic Vinyl Esters by Exchange Reaction Catalyzed with Pd(II)" Molecules 4, no. 5: 135-138. https://doi.org/10.3390/40500135
APA StyleRashidi-Ranjbar, P., & Piri, F. (1999). Synthesis of Aromatic Vinyl Esters by Exchange Reaction Catalyzed with Pd(II). Molecules, 4(5), 135-138. https://doi.org/10.3390/40500135




