A Concise Asymmetric Synthesis of the Aggregation Pheromone of Cryptolestes ferrugineus, Ferrulactone II, and Its Enantiomer
Abstract
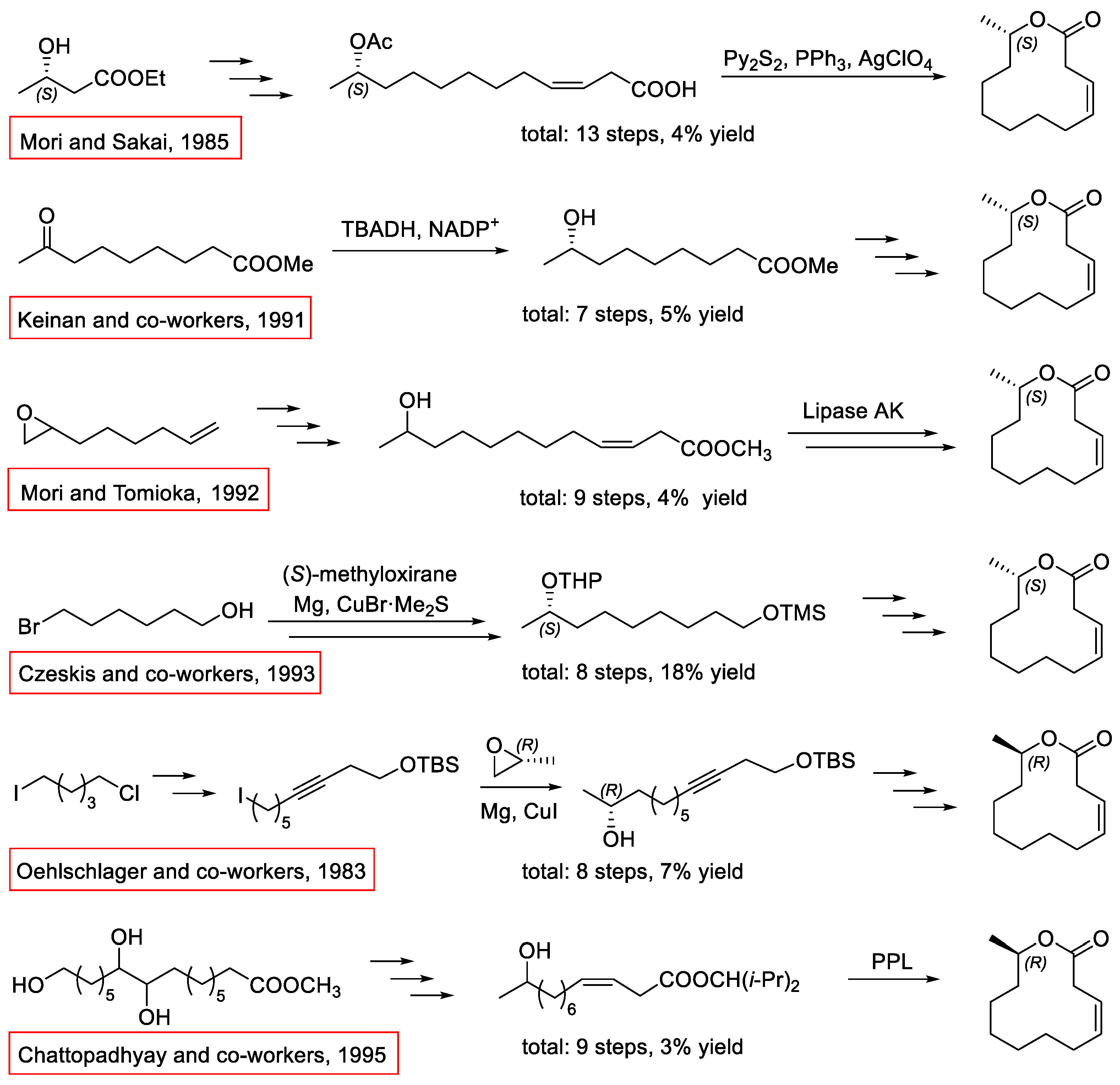
1. Introduction
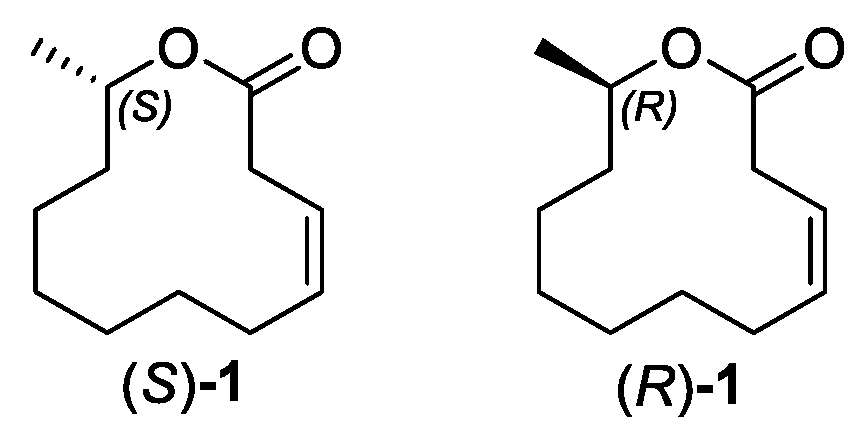
2. Results and Discussion
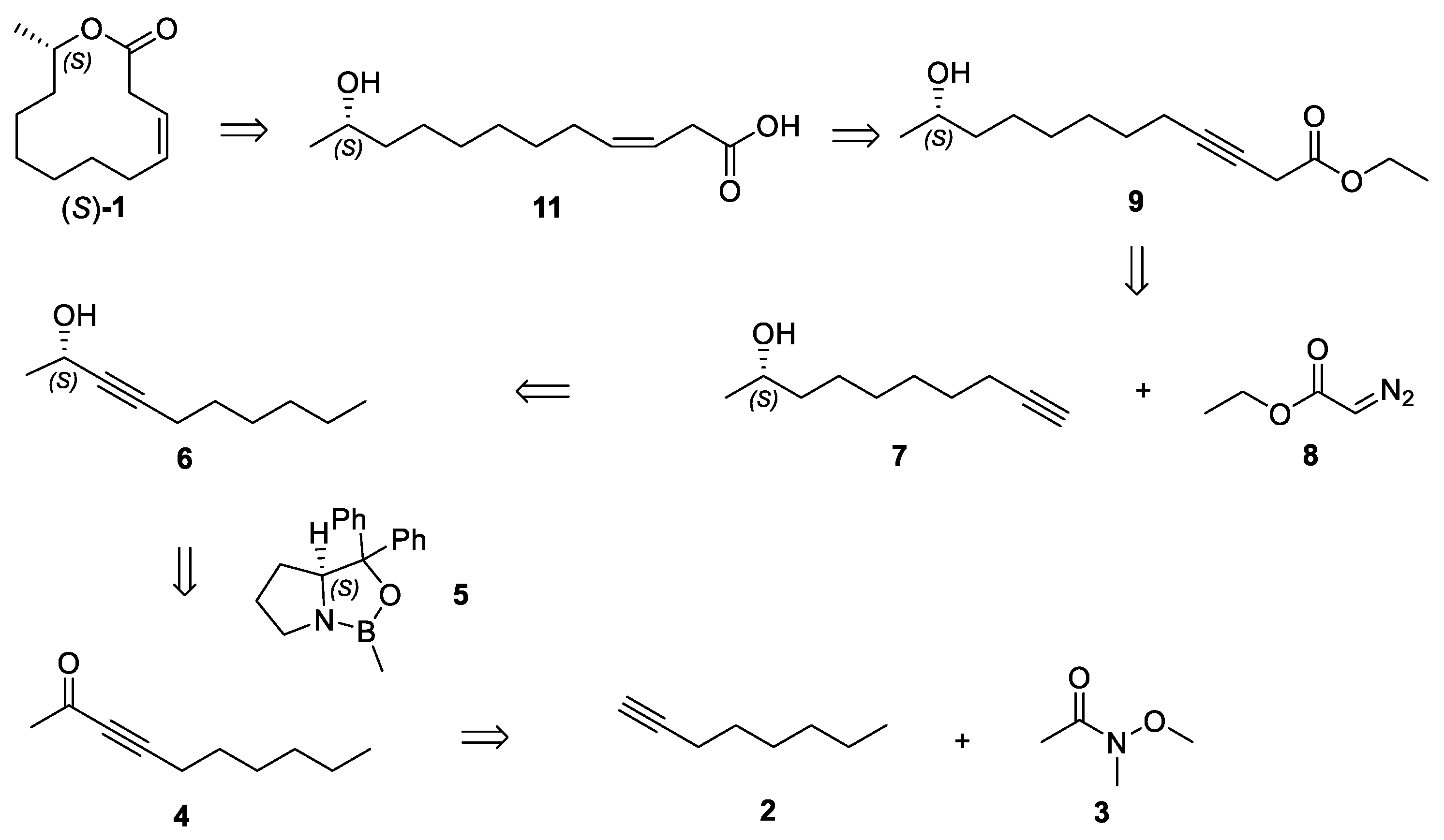
2.1. Retrosynthetic Analysis
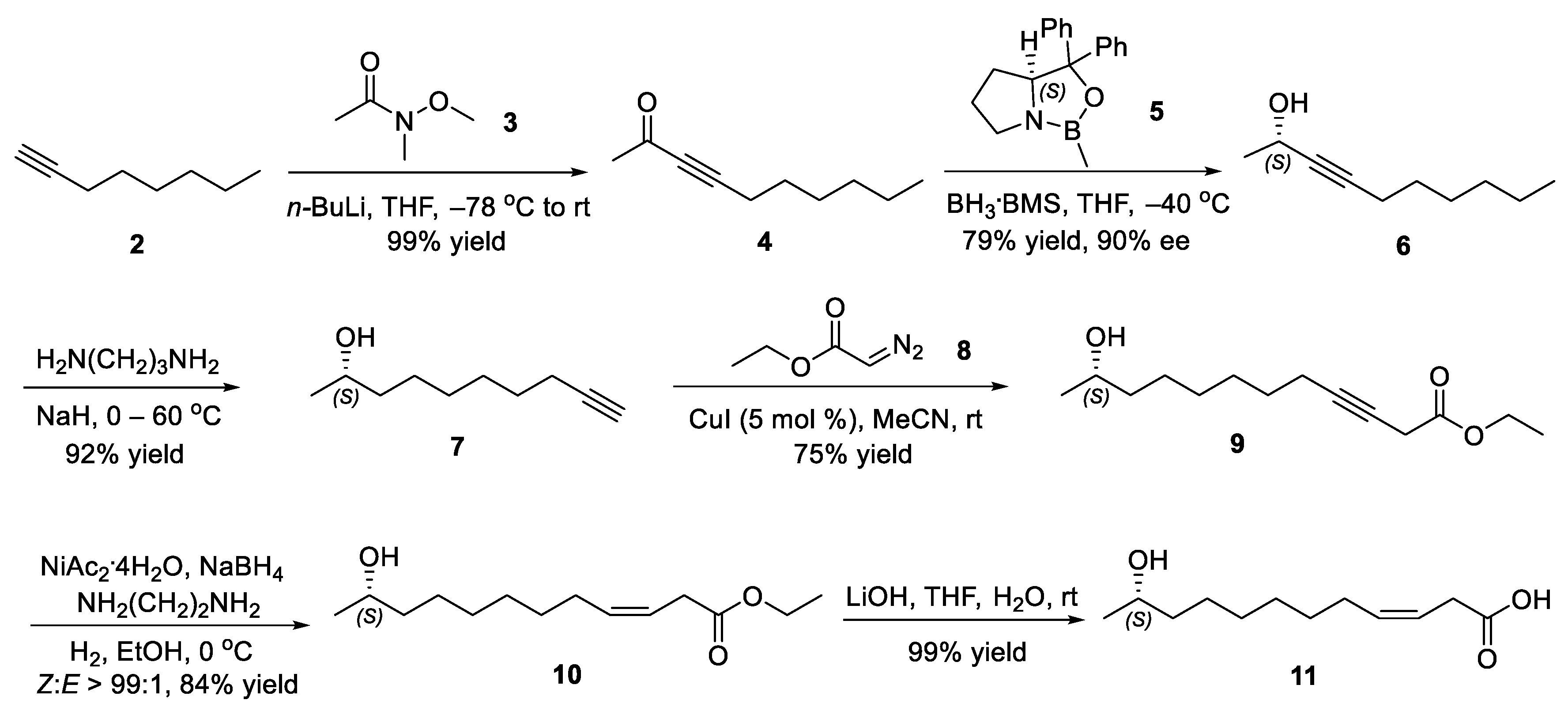
2.2. Synthesis of (S,Z)-11-Hydroxydodec-3-enoic Acid (11)
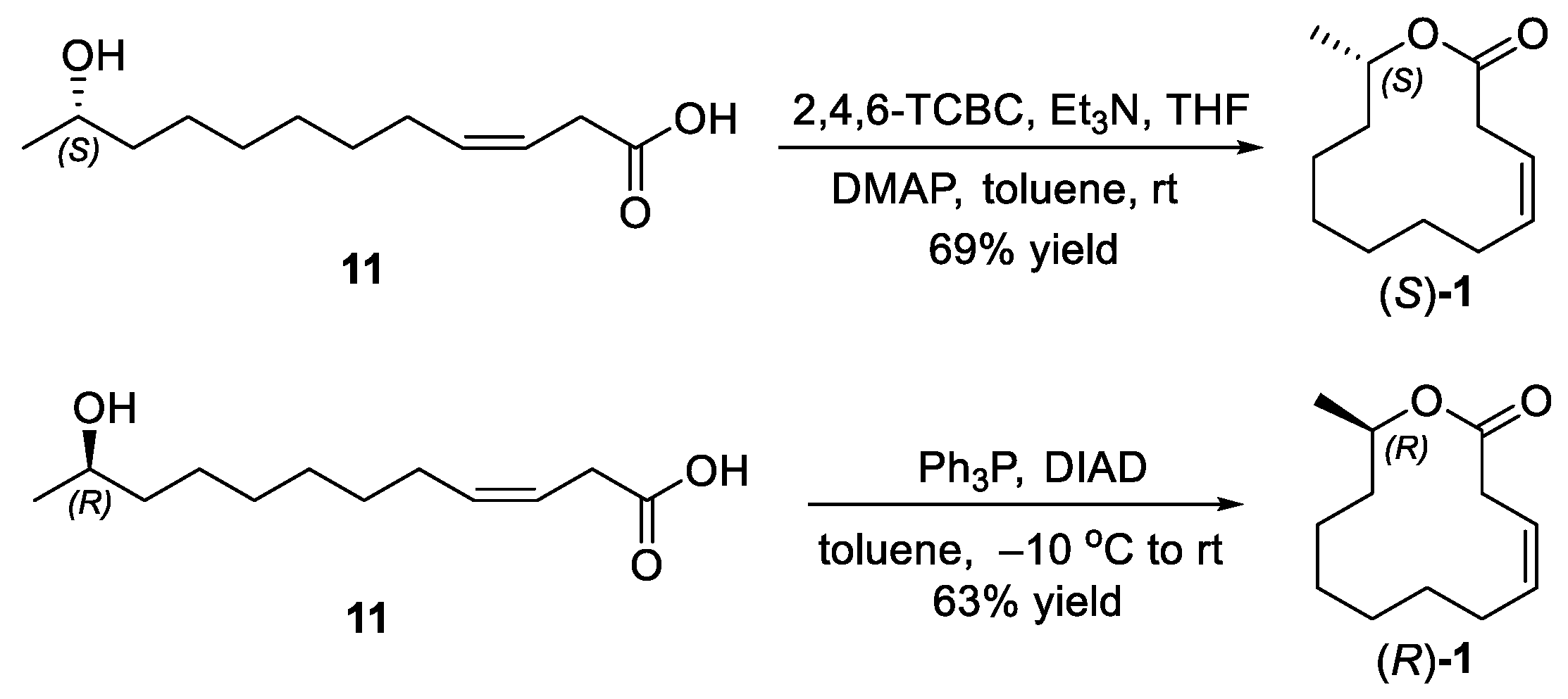
2.3. Synthesis of Ferrulactone II (S)-1 and Its Enantiomer (R)-1
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Dec-3-yn-2-one (4) (CAS 91658-50-3)
3.3. Synthesis of (S)-Dec-3-yn-2-ol (6) (CAS 72132-11-7)
3.4. Synthesis of (S)-Dec-9-yn-2-ol (7) (CAS 107351-67-7)
3.5. Synthesis of Ethyl (S)-11-Hydroxydodec-3-ynoate (9) (New Compound)
3.6. Synthesis of Ethyl (S,Z)-11-Hydroxydodec-3-enoate (10) (CAS 3054699-79-2)
3.7. Synthesis of (S,Z)-11-Hydroxydodec-3-enoic Acid (11) (CAS 87583-43-5)
3.8. Synthesis of (S,Z)-12-Methyloxacyclododec-4-en-2-one ((S)-1) (CAS 86578-99-6)
3.9. Synthesis of (R,Z)-12-Methyloxacyclododec-4-en-2-one ((R)-1) (CAS 87583-38-8)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boopathy, H.; Bharathi, V.S.K.; Jayas, D.S.; Jian, F. Three-dimensional movement and distribution of Tribolium castaneum (Coleoptera: Tenebrionidae) and Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) in stored wheat at different temperatures and at different times. Environ. Entomol. 2025, 54, 15–26. [Google Scholar] [CrossRef]
- Bharathi, V.S.K.; Jian, F.; Jayas, D.S. Biology, ecology, and behavior of rusty grain beetle (Cryptolestes ferrugineus (Stephens)). Insects 2023, 14, 590. [Google Scholar] [CrossRef]
- Bharathi, V.S.K.; Jayas, D.S.; Jian, F. Effects of insect density, movement period, and temperature on three-dimensional movement and distribution of adult Cryptolestes ferrugineus (Coleoptera: Laemophloeidae). J. Insect Sci. 2022, 22, 3. [Google Scholar] [CrossRef]
- Bharathi, V.S.K.; Jayas, D.S.; Jian, F. Study on 300 t of wheat stored in corrugated steel bin for two years in Canada. Part II—Movement and distribution of Cryptolestes ferrugineus (Stephens) and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2023, 100, 102062. [Google Scholar] [CrossRef]
- Venkidusamy, M.; Jagadeesan, R.; Nayak, M.K.; Subbarayalu, M.; Subramaniam, C.; Collins, P.J. Relative tolerance and expression of resistance to phosphine in life stages of the rusty grain beetle, Cryptolestes ferrugineus. J. Pest Sci. 2018, 91, 277–286. [Google Scholar] [CrossRef]
- Konemann, C.E.; Hubhachen, Z.; Opit, G.P.; Gautam, S.; Bajracharya, N.S. Phosphine resistance in Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) collected from grain storage facilities in Oklahoma, USA. J. Econ. Entomol. 2017, 110, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, V.; Sonai, R.T.; Chandrasekaran, S.; Mohankumar, S. Phosphine resistance in rusty grain beetle Cryptolestes ferrugineus (Stephens) from south India. Ind. J. Entomol. 2018, 80, 935–941. [Google Scholar] [CrossRef]
- Toon, A.; Daglish, G.J.; Ridley, A.W.; Emery, R.N.; Holloway, J.C.; Walter, G.H. Significant population structure in Australian Cryptolestes ferrugineus and interpreting the potential spread of phosphine resistance. J. Stored Prod. Res. 2018, 77, 219–224. [Google Scholar] [CrossRef]
- Soltani, A.; Djebbi, T.; Jemaa, J.M.B. Encapsulating Myrtus communis essential oil in date kernel polysaccharides: An innovative approach to controlling Cryptolestes ferrugineus (Coléoptères: Laemophloeidés) (Stephens 1831). J. Plant Dis. Prot. 2025, 132, 111. [Google Scholar] [CrossRef]
- Mahmoud, H.A.; Azab, M.M.; Sleem, F.M.A. Bioactivity of Cinnamomum verum powder and extract against Cryptolestes ferrugineus S., Rhyzopertha dominica F. and Sitophilus granarius L. (Coleoptera). Int. J. Trop. Insect Sci. 2023, 43, 629–636. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Jayas, D.S. Chemical cues in grain storage: A review on semiochemical types, pest behavior, and control strategies. J. Stored Prod. Res. 2025, 113, 102674. [Google Scholar] [CrossRef]
- Yu, L.; Cao, X.-L.; Zheng, W.; Li, J.; Xie, F.-Y.; Zheng, X.-L. Aggregation pheromones of bostrichidae: A review. J. Stored Prod. Res. 2025, 114, 102767. [Google Scholar] [CrossRef]
- Wong, J.W.; Verigin, V.; Oehlschlager, A.C.; Borden, J.H.; Pierce, H.D.; Pierce, A.M.; Chong, L. Isolation and identification of two macrolide pheromones from the frass of Cryptolestes ferrugineus (Coleoptera: Cucujidae). J. Chem. Ecol. 1983, 9, 451–474. [Google Scholar] [CrossRef] [PubMed]
- Loschiavo, S.R.; Wong, J.; White, N.D.G.; Pierce, H.D.; Borden, J.H.; Oehlschlager, A.C. Field-evaluation of a pheromone to detect adult rusty grain beetles, Cryptolestes ferrugineus (Coleoptera, Cucujidae), in stored grain. Can. Entomol. 1986, 118, 1–8. [Google Scholar] [CrossRef]
- Sakai, T.; Mori, K. Pheromone synthesis. Part 82. New synthesis of the macrolide pheromones of the rusty grain beetle, Cryptolestes ferrugineus Stephens. Agric. Biol. Chem. 1986, 50, 177–183. [Google Scholar] [CrossRef]
- Czeskis, B.A.; Shpiro, N.A.; Moiseenkov, A.M. Efficient synthesis of (S,Z)-dodec-3-en-11-olide (Ferrulactone II) using (2-carboxyethyl)triphenylphosphonium bromide. Mendeleev Commun. 1993, 3, 96. [Google Scholar] [CrossRef]
- Oehlschlager, A.C.; Wong, J.W.; Verigin, V.G.; Pierce, H.D., Jr. Synthesis of two macrolide pheromones of the rusty grain beetle, Cryptolestes ferrugineus (Stephens). J. Org. Chem. 1983, 48, 5009–5017. [Google Scholar] [CrossRef]
- Keinan, E.; Sinha, S.C.; Singh, S.P. Thermostable enzymes in organic synthesis. 5. Total synthesis of S-(+)-Z-dodec-3-en-11-olide (Ferrulactone II) using a Thermoanaerobium brockii alcohol dehydrogenase-generated bifunctional chiron. Tetrahedron 1991, 47, 4631–4638. [Google Scholar] [CrossRef]
- Mori, K.; Tomioka, H. Synthesis of four macrolide pheromones to define the scope and limitation of enzymatic macrolactonization. Liebigs Ann. Chem. 1992, 1992, 1011–1017. [Google Scholar] [CrossRef]
- Pawar, A.S.; Sankaranarayanan, S.; Chattopadhyay, S. Chemoenzymic synthesis of Ferrulactone II and (2E)-9-hydroxydecenoic acid. Tetrahedron Asymmetry 1995, 6, 2219–2226. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Liu, Z.-J.; Huang, P.-Q. Enantioselective total syntheses of marine natural products (+)-cylindricines C, D, E and their diastereomers. Org. Chem. Front. 2022, 9, 58–63. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, H.; Ma, Z. Concise synthesis of (±)-myrioneurinol enabled by sequential [2 + 2] cycloaddition/retro-Mannich fragmentation/Mannich reaction. Angew. Chem. Int. Ed. 2022, 61, e202200085. [Google Scholar] [CrossRef]
- Kaneko, H.; Takahashi, S.; Kogure, N.; Kitajima, M.; Takayama, H. Asymmetric total synthesis of fawcettimine-type lycopodium alkaloid, lycopoclavamine-A. J. Org. Chem. 2019, 84, 5645–5654. [Google Scholar] [CrossRef]
- Corey, E.J.; Bakshi, R.K.; Shibata, S.; Chen, C.P.; Singh, V.K. A stable and easily prepared catalyst for the enantioselective reduction of ketones. Applications to multistep syntheses. J. Am. Chem. Soc. 1987, 109, 7925–7926. [Google Scholar] [CrossRef]
- Claesson, A.; Olsson, L.-I. Allenes and acetylenes. 22. Mechanistic aspects of the allene-forming reductions (SN2’ reaction) of chiral propargylic derivatives with hydride reagents. J. Am. Chem. Soc. 1979, 101, 7302–7311. [Google Scholar] [CrossRef]
- Brown, C.A.; Yamashita, A. Saline hydrides and superbases in organic reactions. Ix. Acetylene zipper. Exceptionally facile contrathermodynamic multipositional isomeriazation of alkynes with potassium 3-aminopropylamide. J. Am. Chem. Soc. 1975, 97, 891–892. [Google Scholar] [CrossRef]
- Brown, C.A.; Yamashita, A. Exceptionally easy isomerization of acetylenic alcohols with potassium 3-aminopropylamide. A new high yield synthesis of functionally differentiated αω-difunctional structures. J. Chem. Soc. Chem. Commun. 1976, 959–960. [Google Scholar] [CrossRef]
- Steib, P.; Breit, B. Enantioselective rhodium-catalyzed dimerization of ω-allenyl carboxylic acids: Straightforward synthesis of C2-symmetric macrodiolides. Angew. Chem. Int. Ed. 2018, 57, 6572–6576. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, J.; Li, S.; Li, M.; Wu, L.; Wang, Q.; Mao, J.; Liu, S.; Zheng, B.; Wang, M.; et al. Total syntheses of (R)-strongylodiols C and D. J. Nat. Prod. 2016, 79, 244–247. [Google Scholar] [CrossRef]
- Suarez, A.; Fu, G.C. A straightforward and mild synthesis of functionalized 3-alkynoates. Angew. Chem. Int. Ed. 2004, 43, 3580–3582. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Ahuja, V.K. P-2 nickel catalyst with ethylenediamine, a novel system for highly stereospecific reduction of alkynes to cis-olefins. J. Chem. Soc. Chem. Commun. 1973, 553–554. [Google Scholar] [CrossRef]
- Oehlschlager, A.C.; Czyzewska, E.; Aksela, R.; Pierce, H.D., Jr. Improved syntheses of hydroxy acid precursors of macrolide pheromones of cucujid grain beetles. Can. J. Chem. 1986, 64, 1407–1413. [Google Scholar] [CrossRef]
- Morandi, S.; Pellati, F.; Ori, C.; Adinolfi, B.; Nieri, P.; Benvenuti, S.; Prati, F. Isolation, structure elucidation and total synthesis of a cytotoxic dienone from Echinacea pallida. Org. Biomol. Chem. 2008, 6, 4333–4339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Y.; Li, S.; Yang, P.; Zhong, J.; Yin, J.; Ji, K.; Yang, Y.; Ye, N.; Wang, L.; et al. Total syntheses of 9-epoxyfalcarindiol and its diastereomer. Tetrahedron Asymmetry 2017, 28, 288–295. [Google Scholar] [CrossRef]
- Wang, H.; Jie, X.; Su, T.; Wu, Q.; Kuang, J.; Sun, Z.; Zhao, Y.; Chong, Q.; Guo, Y.; Zhang, Z.; et al. Cobalt-catalyzed chemo- and stereoselective transfer semihydrogenation of 1,3-dienes with water as a hydrogen source. J. Am. Chem. Soc. 2024, 146, 23476–23486. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghalavand, N.; Ghanbarian, M.; Mohammadkhani, L. Applications of Mitsunobu reaction in total synthesis of natural products. Appl. Organomet. Chem. 2018, 32, e4464. [Google Scholar] [CrossRef]
- Iwasaki, T.; Tajimi, Y.; Kameda, K.; Kingwell, C.; Wcislo, W.; Osaka, K.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Synthesis of 23-, 25-, 27-, and 29-membered (Z)-selective unsaturated and saturated macrocyclic lactones from 16- and 17-membered macrocyclic lactones and bromoalcohols by Wittig reaction, Yamaguchi macrolactonization, and photoinduced decarboxylative radical macrolactonization. J. Org. Chem. 2019, 84, 8019–8026. [Google Scholar] [CrossRef]
- Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. A rapid esterification by mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Yamada, M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- Evans, D.A.; Ratz, A.M.; Huff, B.E.; Sheppard, G.S. Total synthesis of the polyether antibiotic lonomycin A (Emericid). J. Am. Chem. Soc. 1995, 117, 3448–3467. [Google Scholar] [CrossRef]
- Cui, J.; Hillman, P.F.; Kim, G.J.; Bui, T.T.M.; Moon, K.; Nam, S.-J.; Choi, H.; Oh, D.-C. Configurational assignments of type-I polyketide synthase (PKS)-derived natural products based on spectroscopic and chemical analysis: Methodologies and case studies. Nat. Prod. Rep. 2025, 42, 1136–1174. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Dai, X.-Y.; Zhu, Y.-J.; Wang, M.-Y.; Gao, G.; Zhang, M.-Y.; Feng, W.-S.; Xie, Z.-S.; Chen, H. Structure revision of lyciumamides A-C and their hypoglycemic activities. ACS Omega 2025, 10, 55932–55941. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Hamamoto, H.; Mori, K. Pheromone synthesis. Part 85. New synthesis of macrolide pheromones of the flat grain beetle, Cryptolestes pusillus Schoenherr. Agric. Biol. Chem. 1986, 50, 1621–1627. [Google Scholar] [CrossRef]
- Miura, K.; Wang, D.; Matsumoto, Y.; Hosomi, A. Highly regio- and stereoselective hydrostannylation of alkynols with a new lewis acidic hydrostannane. Org. Lett. 2005, 7, 503–505. [Google Scholar] [CrossRef]
- Lin, C.; Zhu, W.; Wu, S.; Bian, Q.; Zhong, J. Asymmetric synthesis and biological activity of contact pheromone of western flower thrips, frankliniella occidentalis. Int. J. Mol. Sci. 2024, 25, 11699. [Google Scholar] [CrossRef]
- Harada, S.; Takita, R.; Ohshima, T.; Matsunaga, S.; Shibasaki, M. Ligand accelerated indium(III)-catalyzed asymmetric alkynylation of aldehydes with 2-methyl-3-butyn-2-ol as an ethyne equivalent donor. Chem. Commun. 2007, 948–950. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tang, H.; An, B.; Huang, Y.; Liu, D.; Bian, Q.; Zhong, J. A Concise Asymmetric Synthesis of the Aggregation Pheromone of Cryptolestes ferrugineus, Ferrulactone II, and Its Enantiomer. Molecules 2026, 31, 404. https://doi.org/10.3390/molecules31030404
Tang H, An B, Huang Y, Liu D, Bian Q, Zhong J. A Concise Asymmetric Synthesis of the Aggregation Pheromone of Cryptolestes ferrugineus, Ferrulactone II, and Its Enantiomer. Molecules. 2026; 31(3):404. https://doi.org/10.3390/molecules31030404
Chicago/Turabian StyleTang, Hong, Biyu An, Yiwen Huang, Dan Liu, Qinghua Bian, and Jiangchun Zhong. 2026. "A Concise Asymmetric Synthesis of the Aggregation Pheromone of Cryptolestes ferrugineus, Ferrulactone II, and Its Enantiomer" Molecules 31, no. 3: 404. https://doi.org/10.3390/molecules31030404
APA StyleTang, H., An, B., Huang, Y., Liu, D., Bian, Q., & Zhong, J. (2026). A Concise Asymmetric Synthesis of the Aggregation Pheromone of Cryptolestes ferrugineus, Ferrulactone II, and Its Enantiomer. Molecules, 31(3), 404. https://doi.org/10.3390/molecules31030404