Rubia cordifolia L. Dichloromethane Extract Ameliorates Contrast-Induced Acute Kidney Injury by Activating Autophagy via the LC3B/p62 Axis
Abstract
1. Introduction
2. Results
2.1. UPLC-Q-TOF-MS Analysis of Rubia cordifolia L. Dichloromethane Extract
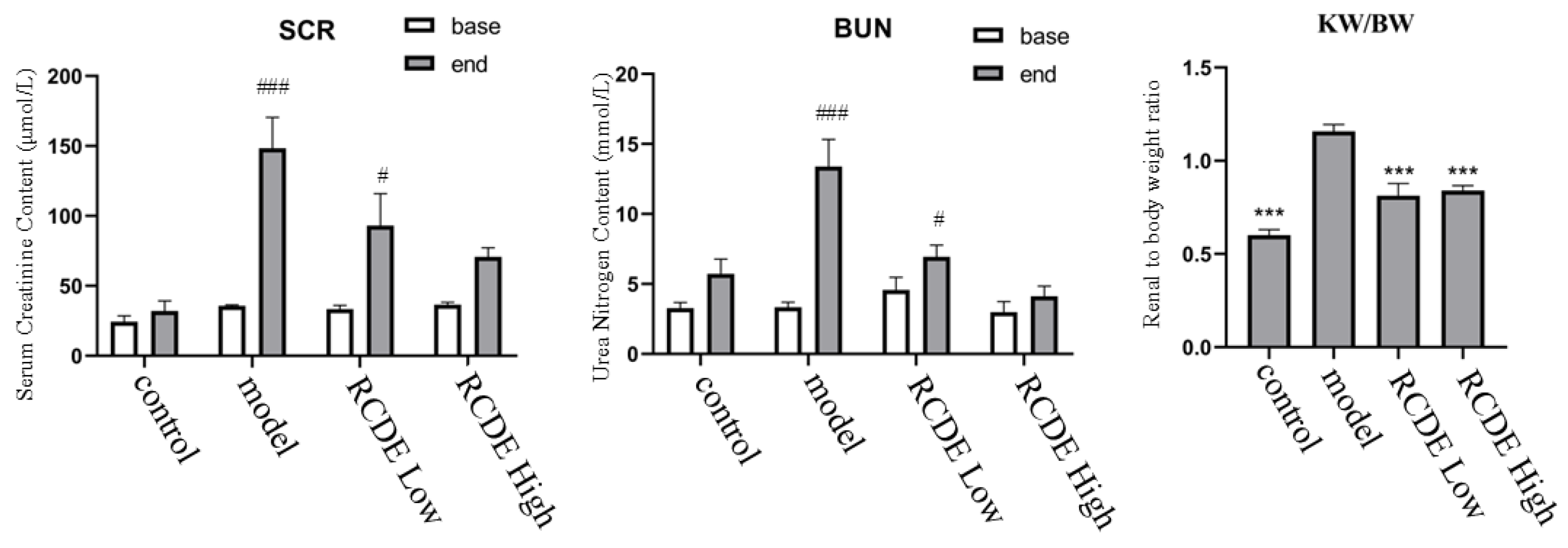
2.2. Effects of RCDE on Serum BUN, SCr, and Kidney/Body Weight Ratio in CIAKI Rats
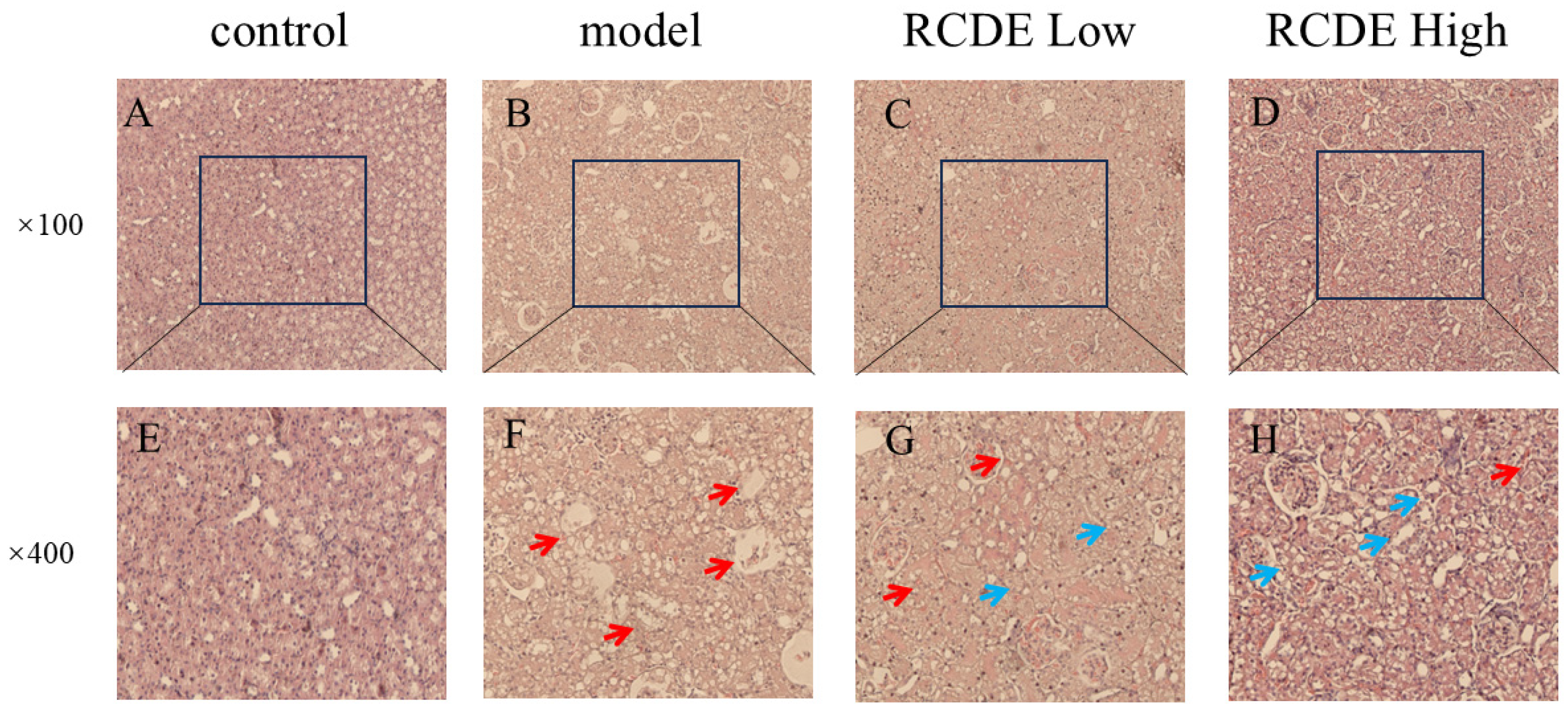
2.3. HE Staining
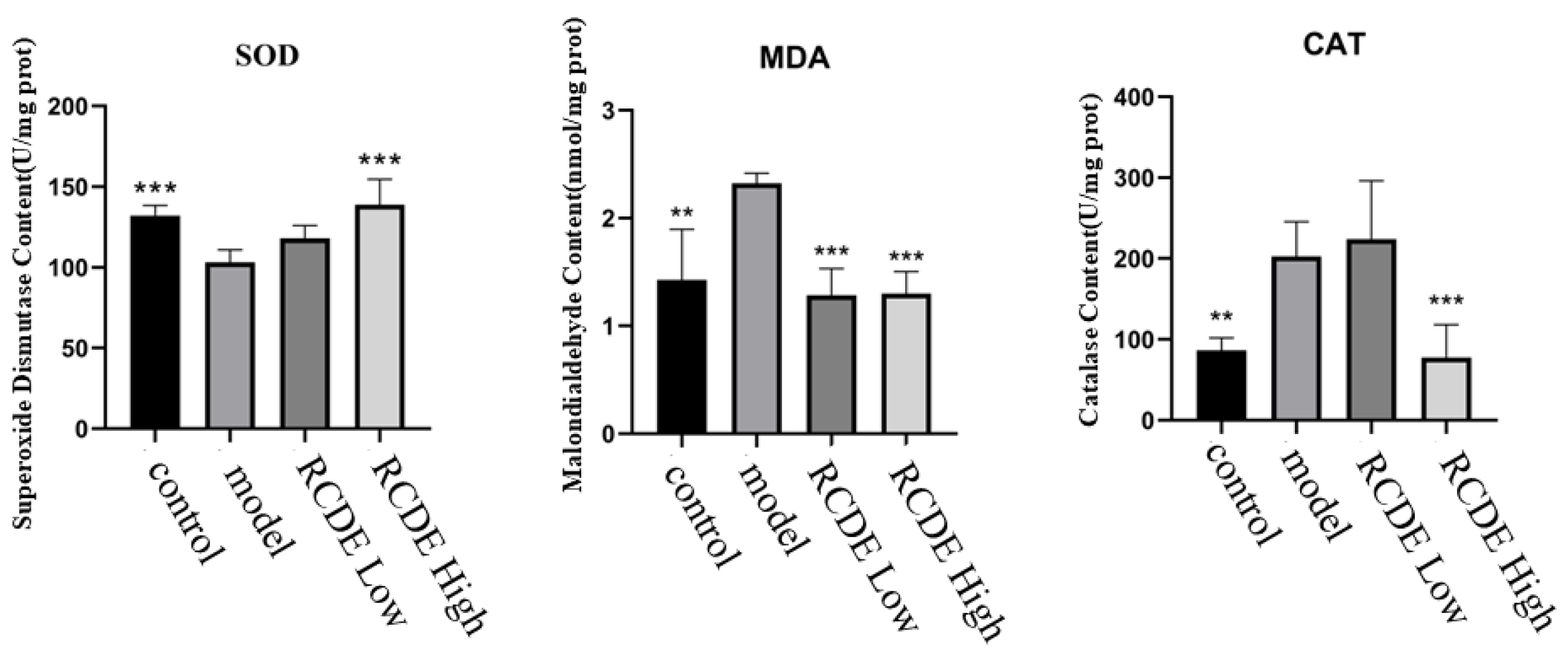
2.4. RCDE Attenuates Renal Oxidative Damage in Contrast-Induced Acute Kidney Injury
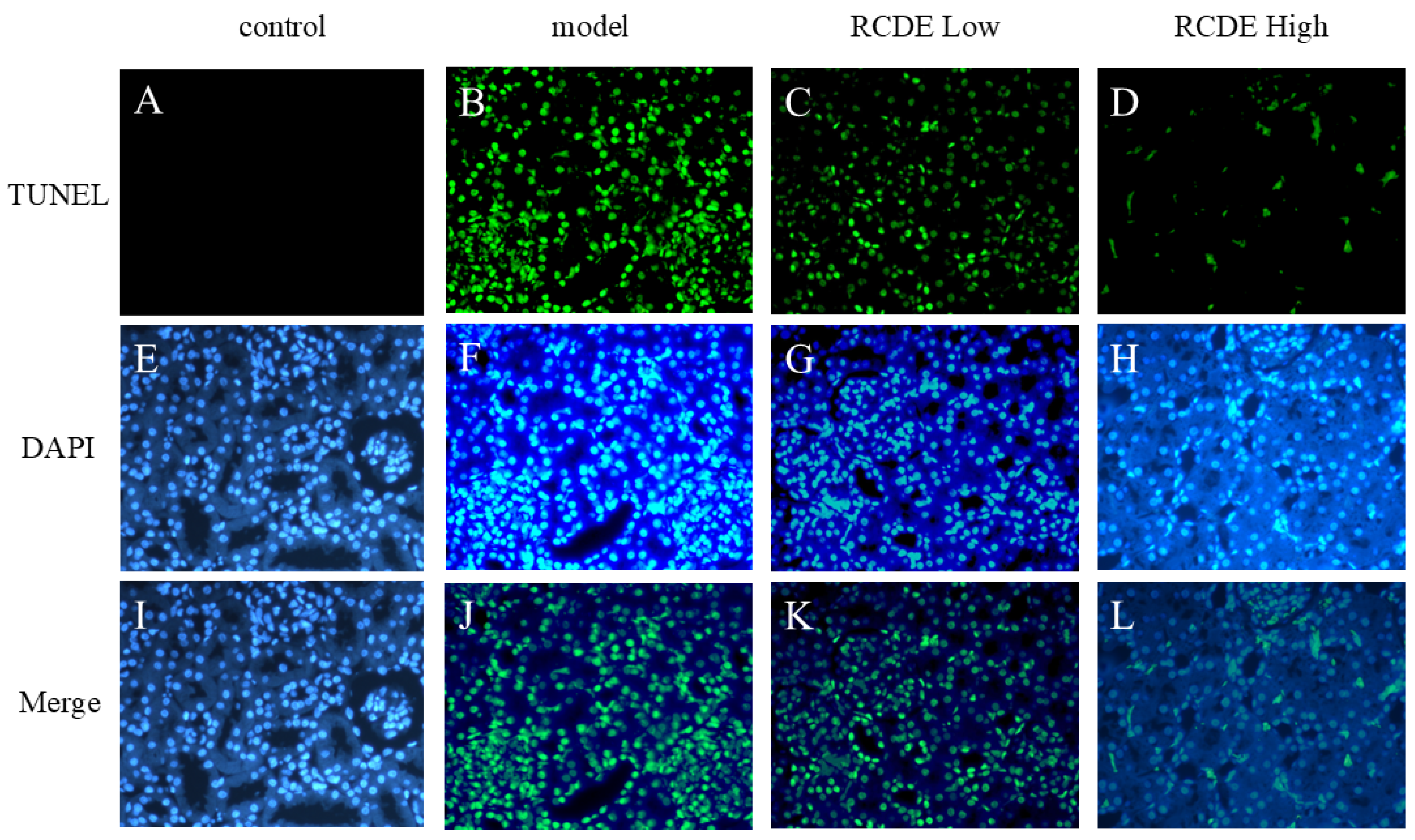
2.5. TUNEL
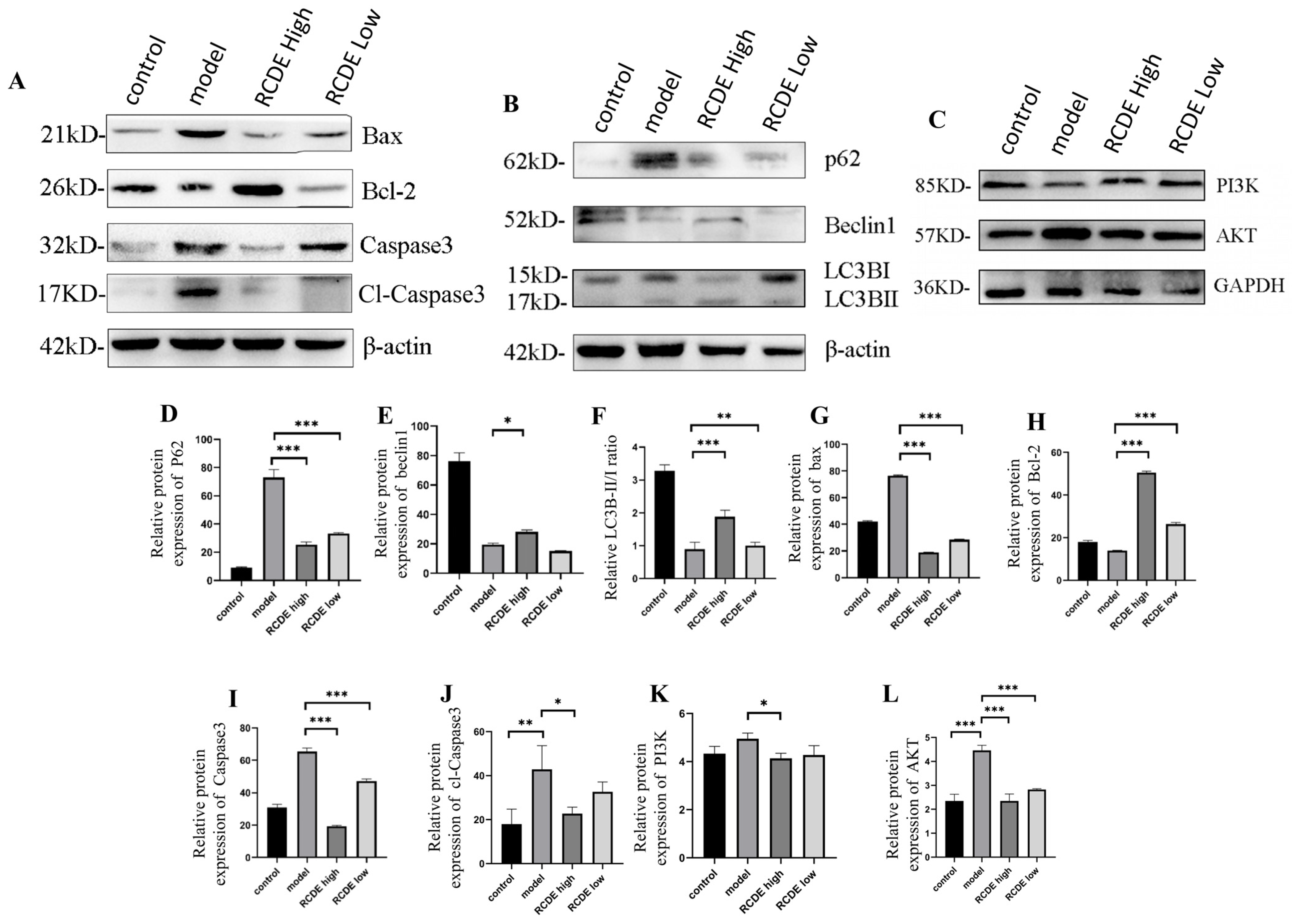
2.6. Western Blot
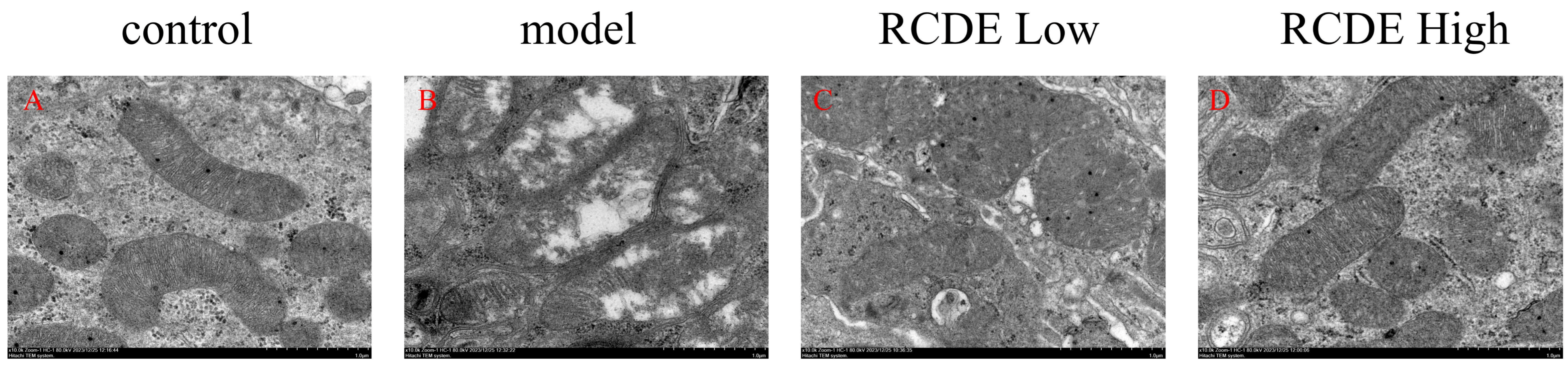
2.7. Observation of Mitochondria via TEM
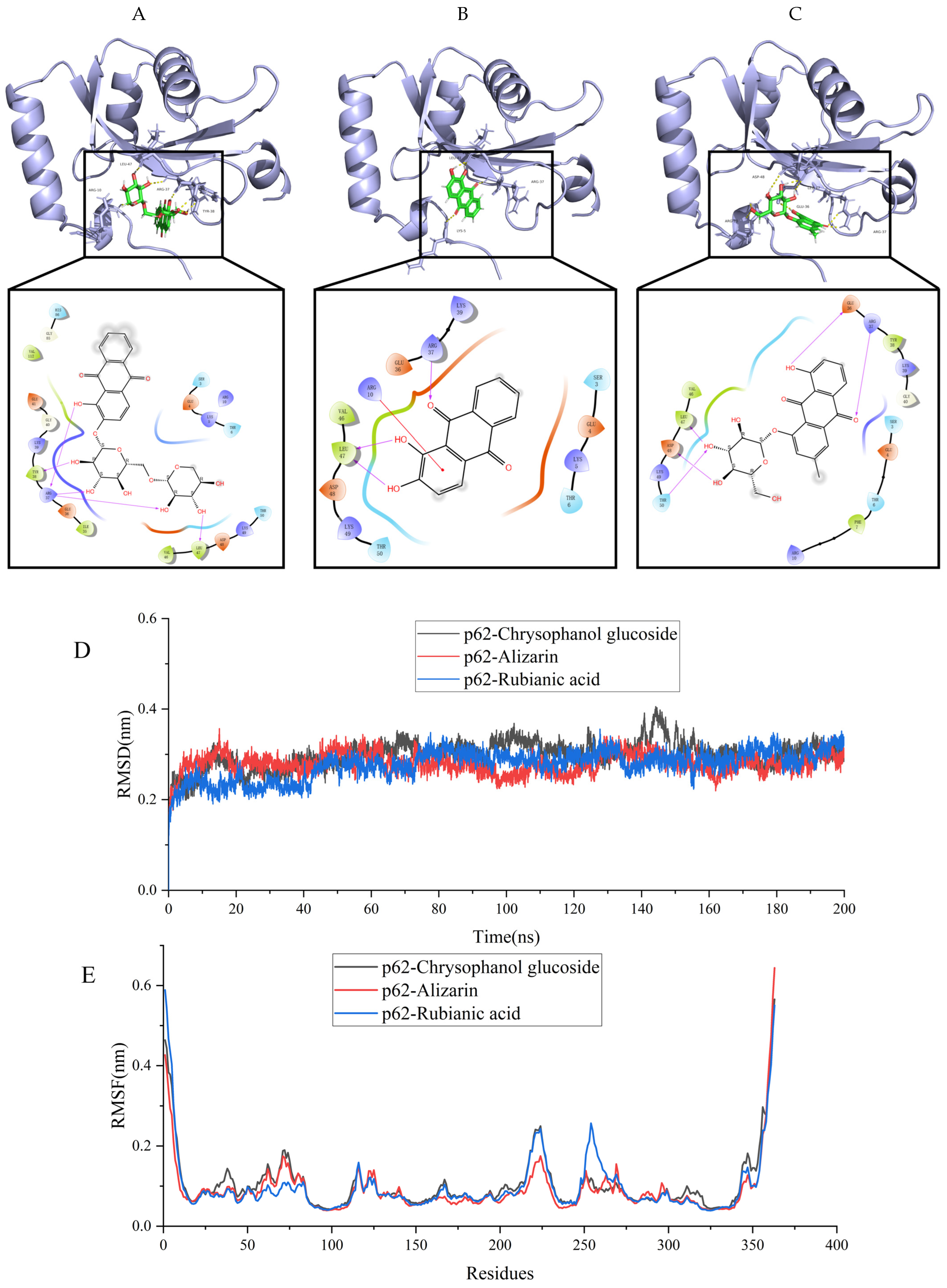
2.8. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Regents
4.3. Extraction of Dichloromethane-Soluble Compounds from Rubia cordifolia L. (Madder Root)
4.4. UPLC-Q-TOF-MS Analysis
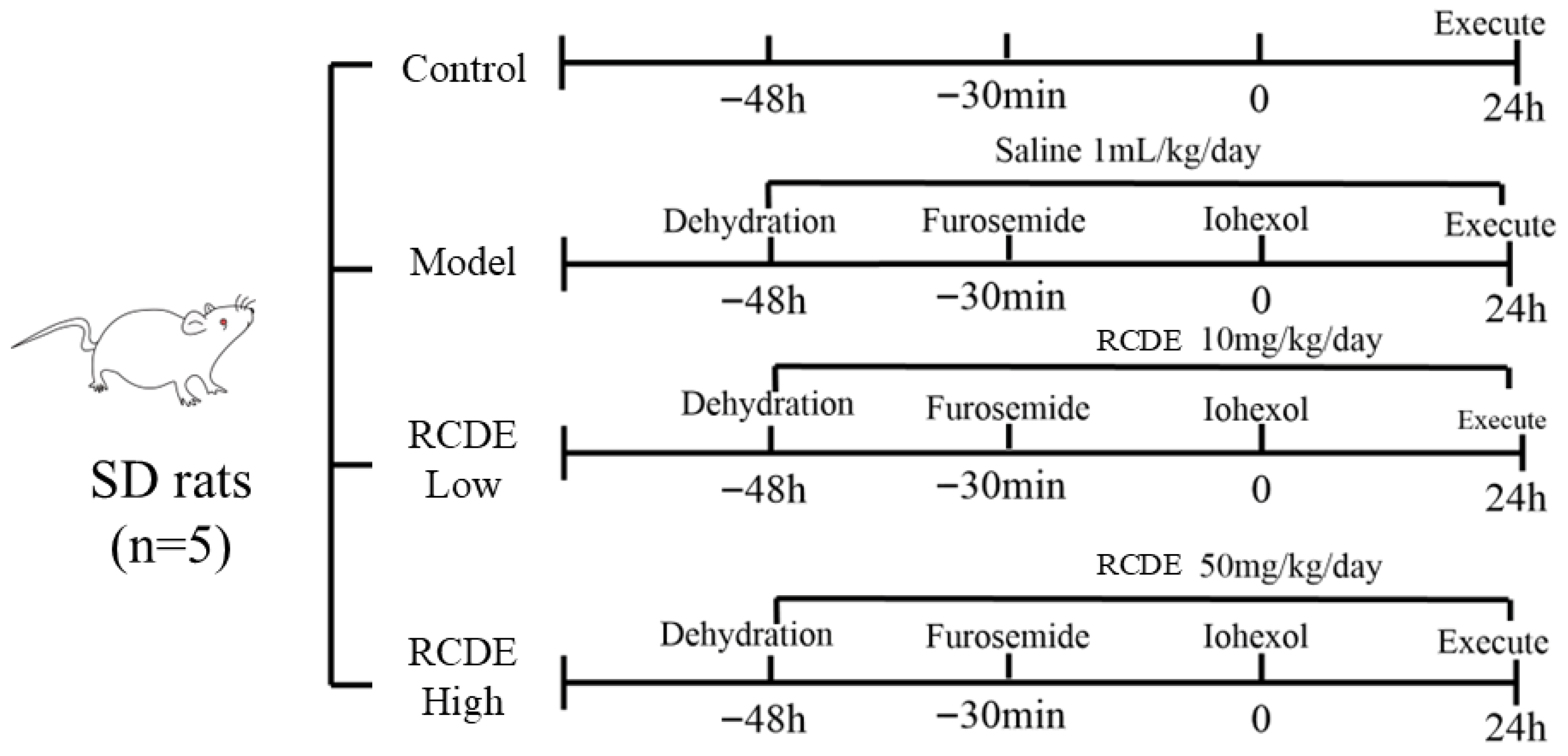
4.5. Establishment and Grouping of Rat Models of CIAKI
4.6. Determination of Kidney-to-Body Weight Ratio
4.7. Determination of Renal Function Indicators
4.8. HE Staining of Renal Tissues
4.9. TUNEL Staining
4.10. Detection of Oxidative Stress Levels in Renal Tissues
4.11. Western Blot Analysis
4.12. Molecular Docking
4.13. Molecular Dynamics Simulations
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIAKI | Contrast-induced acute kidney injury |
| RCDE | Rubia cordifolia L. dichloromethane extract |
| UPLC-Q-TOF-MS | Ultra-high performance liquid chromatography quadrupole time-of-flight tandem mass |
| SD | Sprague–Dawley |
| MDA | Malondialdehyde |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| BCA | Bicinchoninic acid |
| RIPA | Radio-Immunoprecipitation Assay Lysis Buffer |
| SCr | Serum Creatinine |
| BUN | Blood Urea Nitrogen |
| HE | Hematoxylin and Eosin |
| TUNEL | TdT-mediated dUTP Nick-End Labeling |
References
- Fähling, M.; Seeliger, E.; Patzak, A.; Persson, P.B. Understanding and preventing contrast-induced acute kidney injury. Nat. Rev. Nephrol. 2017, 13, 169–180. [Google Scholar] [CrossRef]
- Goldfarb, S.; McCullough, P.A.; McDermott, J.; Gay, S.B. Contrast-induced acute kidney injury: Specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin. Proc. 2009, 84, 170–179. [Google Scholar] [CrossRef]
- Bartels, E.D.; Brun, G.C.; Gammeltoft, A.; GjØRup, P.A. Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Medica Scand. 1954, 150, 297–302. [Google Scholar] [CrossRef]
- Azzalini, L.; Kalra, S. Contrast-Induced Acute Kidney Injury-Definitions, Epidemiology, and Implications. Interv. Cardiol. Clin. 2020, 9, 299–309. [Google Scholar] [CrossRef]
- Brar, S.S.; Aharonian, V.; Mansukhani, P.; Moore, N.; Shen, A.Y.; Jorgensen, M.; Dua, A.; Short, L.; Kane, K. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: The POSEIDON randomised controlled trial. Lancet 2014, 383, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J. Contrast-induced acute kidney injury: A review of definition, pathogenesis, risk factors, prevention and treatment. BMC Nephrol. 2024, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, M.R.; Mitchell, J.; Levin, S.; Smith, A.; Menez, S.; Hinson, J.S.; Klein, E.Y. Renal outcomes following intravenous contrast administration in patients with acute kidney injury: A multi-site retrospective propensity-adjusted analysis. Intensive Care Med. 2023, 49, 205–215. [Google Scholar] [CrossRef]
- Davenport, M.S.; Perazella, M.A.; Yee, J.; Dillman, J.R.; Fine, D.; McDonald, R.J.; Rodby, R.A.; Wang, C.L.; Weinreb, J.C. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020, 294, 660–668. [Google Scholar] [CrossRef]
- McDonald, J.S.; McDonald, R.J. Risk of Acute Kidney Injury Following IV Iodinated Contrast Media Exposure: 2023 Update, From the AJR Special Series on Contrast Media. AJR. Am. J. Roentgenol. 2024, 223, e2330037. [Google Scholar] [CrossRef]
- Ostermann, M.; Lumlertgul, N.; Jeong, R.; See, E.; Joannidis, M.; James, M. Acute kidney injury. Lancet 2025, 405, 241–256. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Q.; Yang, Y.; Li, S.; Shao, X.; Zhang, W.; Cai, H.; Li, J.; Wu, J.; Zhang, K.; et al. αKlotho modulates BNIP3-mediated mitophagy by regulating FoxO3 to decrease mitochondrial ROS and apoptosis in contrast-induced acute kidney injury. Cell. Mol. Life Sci. CMLS 2024, 81, 454. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, S.B.; Wang, L.; Luo, X.Q.; Wang, H.S.; Deng, Y.H.; Wu, X.; Wu, T.; Yan, P.; Kang, Y.X. Apelin-13 alleviates contrast-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Ren. Fail. 2023, 45, 2179852. [Google Scholar] [CrossRef]
- González-Nicolás, M.; González-Guerrero, C.; Goicoechea, M.; Boscá, L.; Valiño-Rivas, L.; Lázaro, A. Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective. Int. J. Mol. Sci. 2024, 25, 3438. [Google Scholar] [CrossRef]
- Tian, F.Y.; Liu, K.; Tang, Z.Y.; Zhou, G.; Zhou, G.L.; Chen, R.F.; Liu, H.B.; Fang, W.J.; Zuo, X.C.; Zhou, L.Y. Glycyrrhizin alleviates contrast-induced acute kidney injury via inhibiting HMGB1-mediated renal tubular epithelial cells ferroptosis. Ren. Fail. 2025, 47, 2548613. [Google Scholar] [CrossRef]
- Perrotta, A.M.; Gigante, A.; Rotondi, S.; Menè, P.; Notturni, A.; Schiavetto, S.; Tanzilli, G.; Pellicano, C.; Guaglianone, G.; Tinti, F.; et al. Contrast-Induced Acute Kidney Injury and Endothelial Dysfunction: The Role of Vascular and Biochemical Parameters. J. Pers. Med. 2023, 13, 701. [Google Scholar] [CrossRef]
- Peng, L.; Luo, Y.; Tan, F.; Chen, Q.; Wang, J.; Ouyang, X.; Wu, B.; Tang, X.; Li, S. microRNA-30c attenuates contrast-induced acute kidney injury by reducing renal tubular epithelial cell apoptosis via targeting SOCS1. Exp. Cell Res. 2025, 446, 114456. [Google Scholar] [CrossRef]
- Chen, W.; Lu, H.; Dai, W.; Li, H.; Chen, Y.; Liu, G.; He, L. PUM2 Lowers HDAC9 mRNA Stability to Improve Contrast-Induced Acute Kidney Injury through Attenuating Oxidative Stress and Promoting Autophagy. Diabetes Metab. J. 2025; ahead of print. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Kim, J.H.; Jung, M.H.; Jang, S.J.; Lee, T.W.; Jung, S.; Lee, S.; Jang, H.N.; Chang, S.H.; Park, D.J. Paricalcitol Attenuates Contrast-Induced Acute Kidney Injury by Regulating Mitophagy and Senescence. Oxidative Med. Cell. Longev. 2020, 2020, 7627934. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, X.; Chen, S.; Lv, M.; Tan, J.; Yang, D. Ox-LDL aggravates contrast-induced injury of renal tubular epithelial cells. J. Biochem. Mol. Toxicol. 2023, 37, e23379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wei, S.; Xiao, T.; Li, G. LC3B/p62-mediated mitophagy protects A549 cells from resveratrol-induced apoptosis. Life Sci. 2021, 271, 119139. [Google Scholar] [CrossRef]
- Duan, M.; Dong, S.; Wang, K.; Shang, P.; Li, C. Ammonia-induced testicular tissue damage: Apoptosis and autophagy pathways mediated by regulating Cyt C/Bcl-2 and p62/LC3B pathways. Environ. Toxicol. Pharmacol. 2025, 116, 104716. [Google Scholar] [CrossRef]
- Kim, J.W.; Jun, S.Y.; Kim, J.M.; Oh, Y.H.; Yoon, G.; Hong, S.M.; Chung, J.Y. Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma. J. Clin. Med. 2021, 10, 5398. [Google Scholar] [CrossRef]
- Qin, W.; Luo, H.; Yang, L.; Hu, D.; Jiang, S.P.; Peng, D.Y.; Hu, J.M.; Liu, S.J. Rubia cordifolia L. ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of NLRP3 inflammasome and IL-6/JAK2/STAT3 pathways. Heliyon 2022, 8, e10314. [Google Scholar] [CrossRef]
- Xia, M.; Li, Z.; Yang, C.; Wang, Y.; Zhang, J.; Zhong, G.; Ouyang, H.; Feng, Y. Rubia cordifolia L. extract ameliorates vitiligo by inhibiting the CXCL10/CXCL9/STAT1 signaling pathway. J. Ethnopharmacol. 2025, 350, 120027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, W.T.; Li, Y.; Kong, Q.H.; Fang, C.W.; Luo, H.; Liu, S.J. Chemical constituents from the aerial parts of Rubia cordifolia L. with their NO inhibitory activity. Nat. Prod. Res. 2024, 38, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Deng, J.; Zhao, Y.; Zhang, Y.; Wang, Z.; Zhang, L.; Wang, K. Combined network pharmacology and metabolomics reveal that Rubia cordifolia L. ameliorates exhaustive exercise-induced myocardial injury in rats via the BCAA degradation pathway. Fitoterapia 2025, 184, 106617. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Ye, Z. Madder (Rubia cordifolia L.) Alleviates Myocardial Ischemia-Reperfusion Injury by Protecting Endothelial Cells from Apoptosis and Inflammation. Mediat. Inflamm. 2023, 2023, 5015039. [Google Scholar] [CrossRef]
- Chandrashekar, B.S.; Prabhakara, S.; Mohan, T.; Shabeer, D.; Bhandare, B.; Nalini, M.; Sharmila, P.S.; Meghana, D.L.; Reddy, B.K.; Hanumantha Rao, H.M.; et al. Characterization of Rubia cordifolia L. root extract and its evaluation of cardioprotective effect in Wistar rat model. Indian J. Pharmacol. 2018, 50, 12–21. [Google Scholar] [CrossRef]
- Wen, M.; Chen, Q.; Chen, W.; Yang, J.; Zhou, X.; Zhang, C.; Wu, A.; Lai, J.; Chen, J.; Mei, Q.; et al. A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front. Pharmacol. 2022, 13, 965390. [Google Scholar] [CrossRef]
- Min, S.; Fang, Y.; Zhang, M.; Shen, H.; Zhu, L. The potential mechanism of co-administration of Scutellaria baicalensis Georgi and Rubia cordifolia L. ameliorating ulcerative colitis: Integration of metabolomics, network pharmacology, and molecular docking. J. Pharm. Biomed. Anal. 2025, 263, 116948. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.; Xu, J.; Zhang, H.; Ma, Z.; Wu, H. Isolation of anthraquinone derivatives from Rubia cordifolia (Rubiaceae) and their bioactivities against plant pathogenic microorganisms. Pest Manag. Sci. 2024, 80, 4617–4627. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, P.H.; Jung, A.Y.; Lee, J.S.; Cho, Y.A.; Suh, C.H.; Jung, J.; Yoon, H.M. Risk of acute kidney injury after contrast-enhanced MRI examinations in a pediatric population. Eur. Radiol. 2025, 35, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, B. Contrast-Induced Acute Kidney Injury in Radiology: Recent Insights and Advances. J. Pharm. Bioallied Sci. 2024, 16, S3862–S3864. [Google Scholar] [CrossRef] [PubMed]
- Bana, S.; Kumar, N.; Sartaj, A.; Alhalmi, A.; Qurtam, A.A.; Nasr, F.A.; Al-Zharani, M.; Singh, N.; Gaur, P.; Mishra, R.; et al. Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats. Pharmaceuticals 2023, 16, 1586. [Google Scholar] [CrossRef]
- Marković, Z.; Komolkin, A.V.; Egorov, A.V.; Milenković, D.; Jeremić, S. Alizarin as a potential protector of proteins against damage caused by hydroperoxyl radical. Chem. -Biol. Interact. 2023, 373, 110395. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Dangas, G.D.; Weisbord, S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019, 380, 2146–2155. [Google Scholar] [CrossRef]
- Aggarwal, S.; Wang, Z.; Rincon Fernandez Pacheco, D.; Rinaldi, A.; Rajewski, A.; Callemeyn, J.; Van Loon, E.; Lamarthée, B.; Covarrubias, A.E.; Hou, J.; et al. SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys. Science 2024, 383, eadd6371. [Google Scholar] [CrossRef]
- Bao, N.; Wang, J.; Yue, Q.; Cao, F.; Gu, X.; Wen, K.; Kong, W.; Gu, M. Chrysophanol-mediated trx-1 activation attenuates renal fibrosis through inhibition of the JNK/Cx43 signaling pathway. Ren. Fail. 2024, 46, 2398710. [Google Scholar] [CrossRef]
- Gu, M.; Zhou, Y.; Liao, N.; Wei, Q.; Bai, Z.; Bao, N.; Zhu, Y.; Zhang, H.; Gao, L.; Cheng, X. Chrysophanol, a main anthraquinone from Rheum palmatum L. (rhubarb), protects against renal fibrosis by suppressing NKD2/NF-κB pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 105, 154381. [Google Scholar] [CrossRef]
- Qazi, N.G.; Khan, A.U.; Khan, A.; Ali, F.; Minhas, A.M.; Alvi, A.M. In vivo, in vitro and in silico evaluation of Rumex nepalensis Spreng. and its active phytoconstituent (Chrysophanol) in gastrointestinal disorders. J. Ethnopharmacol. 2025, 352, 120177. [Google Scholar] [CrossRef]
- Chen, J.; Ding, W.; Zhang, Z.; Li, Q.; Wang, M.; Feng, J.; Zhang, W.; Cao, L.; Ji, X.; Nie, S.; et al. Shenfu injection targets the PI3K-AKT pathway to regulate autophagy and apoptosis in acute respiratory distress syndrome caused by sepsis. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 129, 155627. [Google Scholar] [CrossRef]
- Kong, Z.; Xiao, M.; Wang, B.; Zhang, W.; Che, K.; Lv, W.; Wang, Y.; Huang, Y.; Zhao, H.; Zhao, Y.; et al. Renoprotective Effect of Isoorientin in Diabetic Nephropathy via Activating Autophagy and Inhibiting the PI3K-AKT-TSC2-mTOR Pathway. Am. J. Chin. Med. 2023, 51, 1269–1291. [Google Scholar] [CrossRef]
- Chang, S.J.; Chen, Y.C.; Chang, Y.C.; Cheng, C.C.; Chan, Y.C. ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality. Pharmaceuticals 2025, 18, 1243. [Google Scholar] [CrossRef]
- Omrane, M.; Ben M’Barek, K.; Santinho, A.; Nguyen, N.; Nag, S.; Melia, T.J.; Thiam, A.R. LC3B is lipidated to large lipid droplets during prolonged starvation for noncanonical autophagy. Dev. Cell 2023, 58, 1266–1281.e1267. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ha, H.; Lee, B.S.; Kim, B.H.; Song, H.K.; Kim, Y.K. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, J.; Yao, J.; Mi, N.; Yang, A. Phase separation of p62: Roles and regulations in autophagy. Trends Cell Biol. 2025, 35, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, Y.K. The role of LC3B in autophagy as an RNA-binding protein. Autophagy 2023, 19, 1028–1030. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Wei, Q.; Xu, J.; Zhao, Z.; Sun, Y.; Lu, Q. Suppression of Inflammation by Si Miao San in Experimental Rheumatoid Arthritis Through Modulation of the AKT/ROS/Autophagy Axis. J. Inflamm. Res. 2025, 18, 9459–9476. [Google Scholar] [CrossRef]
- Stepchenko, A.G.; Georgieva, S.G.; Pankratova, E.V. XMU-MP-1, Inhibitor of STE20-like MST1/2 Kinases of the Hippo Signaling Pathway, Suppresses the Cell Cycle, Activates Apoptosis and Autophagy, and Induces Death of Hematopoietic Tumor Cells. Pharmaceuticals 2025, 18, 874. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jayaprakash, P.; Marwan, N.; Aziz, E.; Kuder, K.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice. Pharmaceuticals 2024, 17, 1293. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2021, 17, 2975–2990. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Li, Q.; Zhou, S.; Yu, R.; Wu, C.; Chen, J.; Xiao, Y.; Chen, H.; Song, J.; Pan, Y.; et al. Berberine ameliorates contrast-induced acute kidney injury by regulating HDAC4-FoxO3a axis-induced autophagy: In vivo and in vitro. Phytother. Res. PTR 2024, 38, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
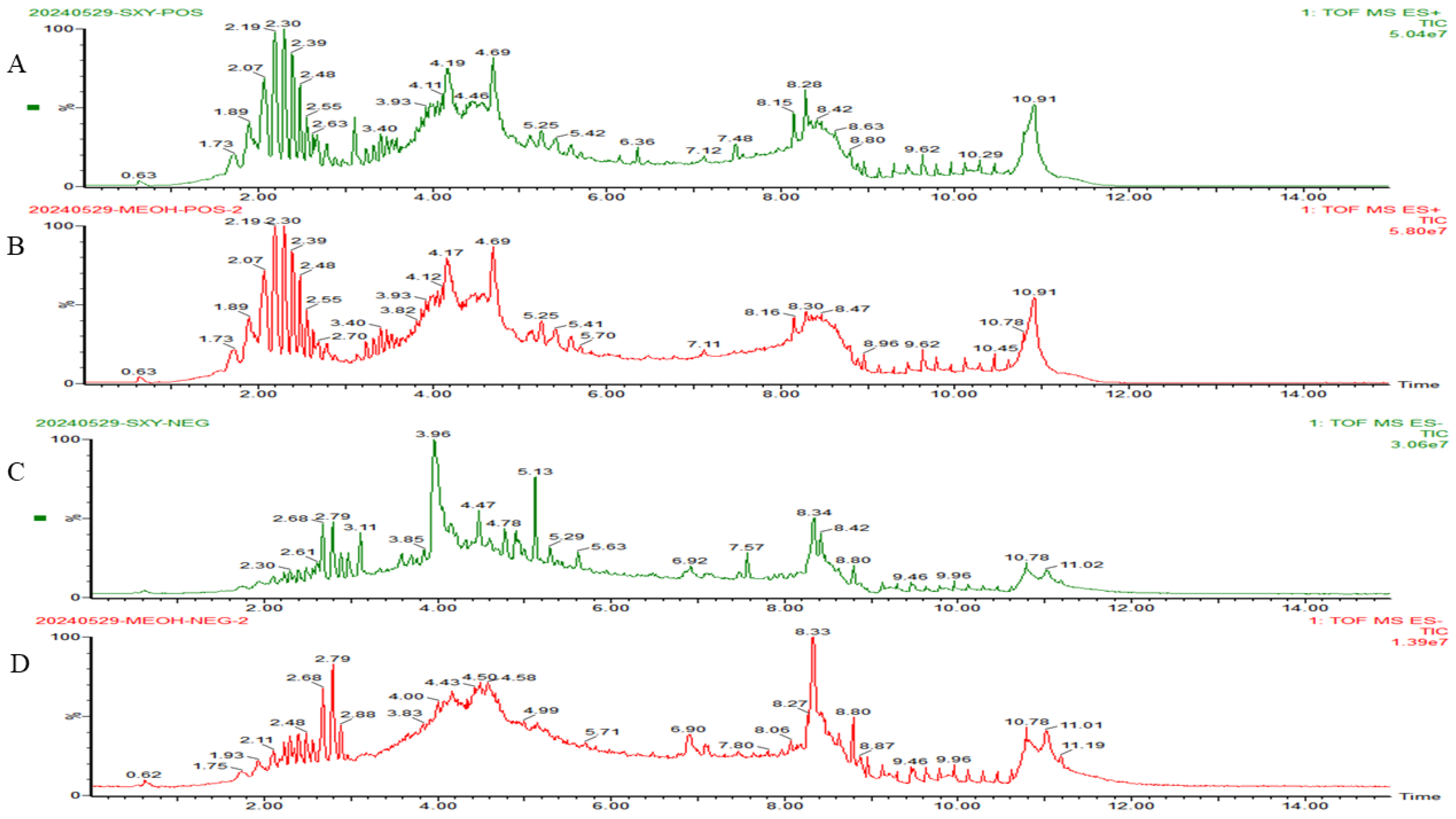
| Peal | RT/min | Identified Anthraquinone Compound | Molecular Formula | [M+H]+ | [M+H]+ Calculated | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 2.63 | d-Corydaline | C22H27NO4 | 369.1940 | 369.1933 | −1.68 |
| 2 | 6.36 | Xantholide A | C15H18O2 | 230.1307 | 230.1307 | 0.00 |
| 3 | 7.48 | Chrysophanol glucoside a | C21H20O9 | 416.1107 | 416.1077 | −6.73 |
| 4 | 8.63 | Cimicifugadine a | C35H51NO8 | 613.3614 | 613.3622 | 1.19 |
| 5 | 8.80 | Meranzin hydrate | C15H18O5 | 278.1154 | 278.1178 | 8.70 |
| Peal | RT/min | Identified Anthraquinone Compound | Molecular Formula | [M+H]− | [M+H]− Calculated | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 2.61 | Psoralen a | C11H6O3 | 186.0317 | 186.0327 | 4.50 |
| 2 | 3.11 | Isaindigodione a | C20H18N2O4 | 350.1267 | 350.1291 | 6.06 |
| 3 | 3.96 | Alizarin | C14H8O4 | 240.0423 | 240.0437 | 6.04 |
| 4 | 5.13 | Rubianic acid | C25H26O13 | 534.1373 | 534.1324 | −9.02 |
| 5 | 5.29 | Catenarin | C15H10O6 | 286.0477 | 286.0491 | 4.70 |
| 6 | 5.63 | 1-Hydroxy-2-hydromethylanthraquinone | C15H10O4 | 254.0579 | 254.0591 | 4.56 |
| 7 | 7.57 | 3β-Acetoxy-16α-hydroxylanosta-8,24-dien-21-oic acid | C32H50O5 | 514.3658 | 514.3669 | 2.19 |
| 8 | 8.42 | Ganolactone a | C27H36O6 | 456.2512 | 456.2486 | −5.07 |
| Time (min) | A (%) | B (%) | Curve |
|---|---|---|---|
| 0 | 5 | 95 | - |
| 10 | 95 | 5 | 6 |
| 12 | 95 | 5 | 6 |
| 13 | 5 | 95 | 6 |
| 15 | 5 | 95 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sun, X.; He, K.; Chen, G.; Yang, X.; Pan, X.; Liao, K. Rubia cordifolia L. Dichloromethane Extract Ameliorates Contrast-Induced Acute Kidney Injury by Activating Autophagy via the LC3B/p62 Axis. Molecules 2026, 31, 316. https://doi.org/10.3390/molecules31020316
Sun X, He K, Chen G, Yang X, Pan X, Liao K. Rubia cordifolia L. Dichloromethane Extract Ameliorates Contrast-Induced Acute Kidney Injury by Activating Autophagy via the LC3B/p62 Axis. Molecules. 2026; 31(2):316. https://doi.org/10.3390/molecules31020316
Chicago/Turabian StyleSun, Xiaoying, Kangxu He, Guanzhong Chen, Xiaoda Yang, Xinhui Pan, and Kai Liao. 2026. "Rubia cordifolia L. Dichloromethane Extract Ameliorates Contrast-Induced Acute Kidney Injury by Activating Autophagy via the LC3B/p62 Axis" Molecules 31, no. 2: 316. https://doi.org/10.3390/molecules31020316
APA StyleSun, X., He, K., Chen, G., Yang, X., Pan, X., & Liao, K. (2026). Rubia cordifolia L. Dichloromethane Extract Ameliorates Contrast-Induced Acute Kidney Injury by Activating Autophagy via the LC3B/p62 Axis. Molecules, 31(2), 316. https://doi.org/10.3390/molecules31020316








