Effect of Mesoporosity on Structural, Textural, and Optical Characteristics of Fe(III) Ion-Exchanged ZSM-5 Zeolites
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
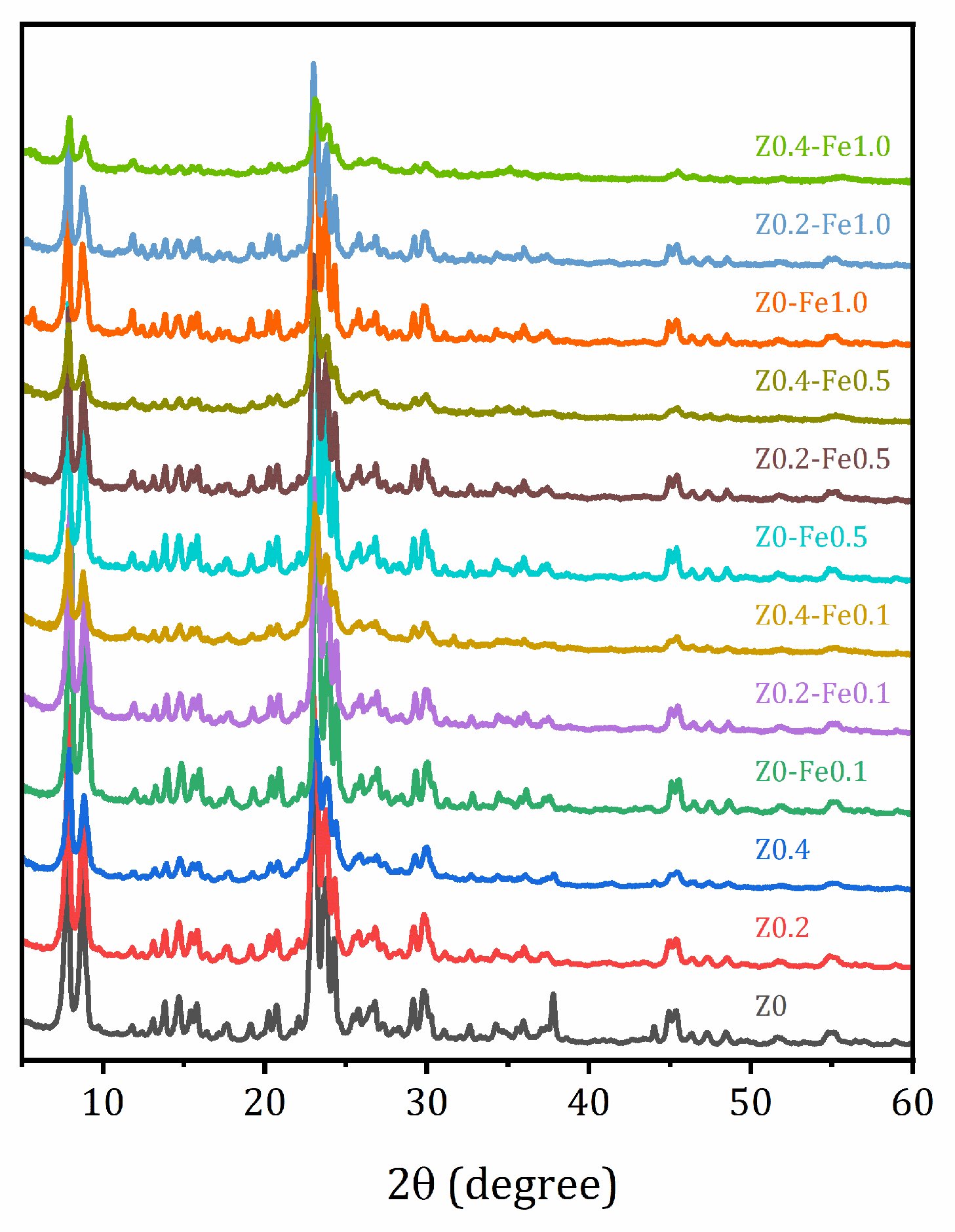
2.2. Structure and Morphology Studied by XRD and SEM
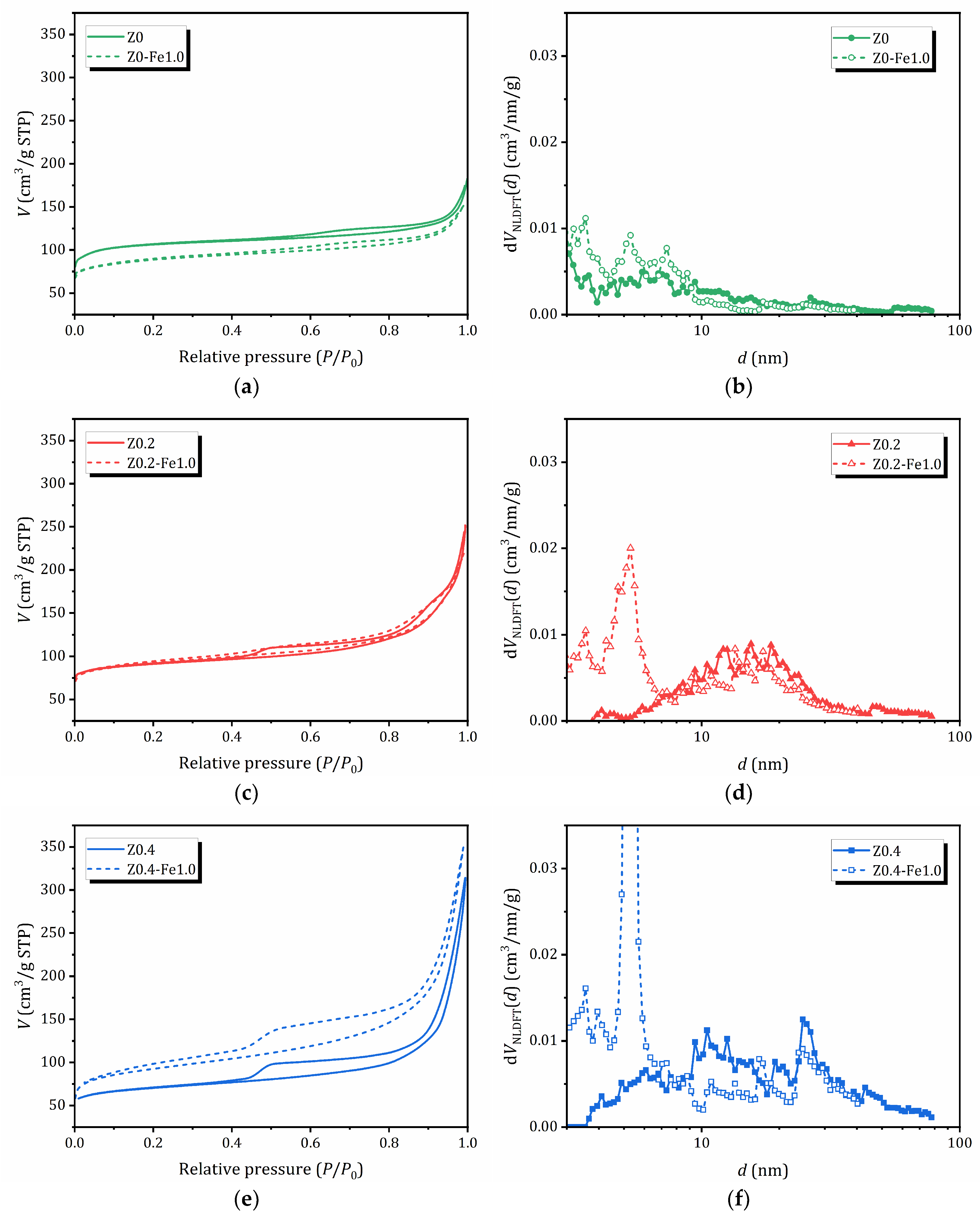
2.3. N2 Adsorption/Desorption
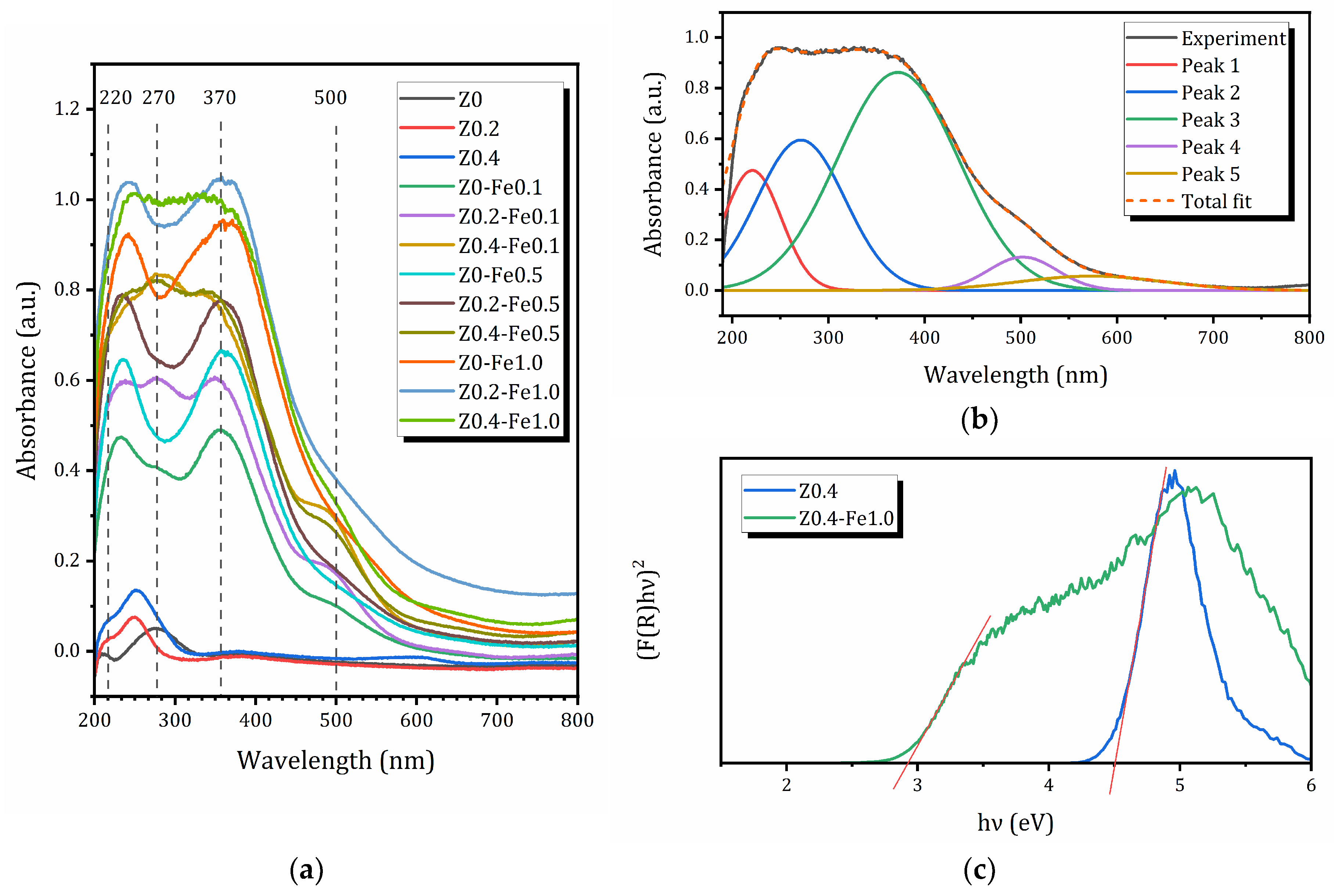
2.4. UV-Vis Studies
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller (method) |
| BJH | Barrett–Joyner–Halenda (method) |
| DRS | Diffuse reflectance spectroscopy |
| EDXRF | Energy-dispersive X-ray fluorescence spectroscopy |
| NLDFT | Nonlocal density functional theory |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Čejka, J.; Millini, R.; Opanasenko, M.; Serrano, D.P.; Roth, W.J. Advances and Challenges in Zeolite Synthesis and Catalysis. Catal. Today 2020, 345, 2–13. [Google Scholar] [CrossRef]
- Bateni, H.; Able, C. Development of Heterogeneous Catalysts for Dehydration of Methanol to Dimethyl Ether: A Review. Catal. Ind. 2019, 11, 7–33. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. On the Location of Lewis Acidic Aluminum in Zeolite Mordenite and the Role of Framework-Associated Aluminum in Mediating the Switch between Brønsted and Lewis Acidity. Chem. Sci. 2021, 12, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, G.; Migliori, M.; Catizzone, E. Recent Trends in Tailoring External Acidity in Zeolites for Catalysis. ACS Omega 2024, 9, 29072–29087. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Krylova, E.A.; Mazur, A.S.; Tsyganenko, A.A.; Shergin, Y.V.; Satikova, E.; Petranovskii, V. Active Sites in H-Mordenite Catalysts Probed by NMR and FTIR. Catalysts 2022, 13, 344. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Mineva, T.; van Daele, S.; Valtchev, V.; Giordano, G. New Synthesis Routes and Catalytic Applications of Ferrierite Crystals. Part 2: The Effect of OSDA Type on Zeolite Properties and Catalysis. Microporous Mesoporous Mater. 2020, 296, 109988. [Google Scholar] [CrossRef]
- Giordano, G.; Migliori, M.; Ferrarelli, G.; Giorgianni, G.; Dalena, F.; Peng, P.; Debost, M.; Boullay, P.; Liu, Z.; Guo, H.; et al. Passivated Surface of High Aluminum Containing ZSM-5 by Silicalite-1: Synthesis and Application in Dehydration Reaction. ACS Sustain. Chem. Eng. 2022, 10, 4839–4848. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Krylova, E.A.; Zhukov, Y.M.; Zvereva, I.A.; Rodriguez-Iznaga, I.; Petranovskii, V.; Fuentes-Moyado, S. Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods. Molecules 2019, 24, 4216. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Paris, C.; Boronat, M.; Corma, A. Selective Active Site Placement in Lewis Acid Zeolites and Implications for Catalysis of Oxygenated Compounds. Chem. Sci. 2020, 11, 10225–10235. [Google Scholar] [CrossRef]
- Townsend, R.P.; Coker, E.N. Ion Exchange in Zeolites. In Studies in Surface Science and Catalysis 137; van Bekkum, H., Flanigen, E.M., Jacobs, P.A., Jansen, J.C., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; pp. 467–524. [Google Scholar]
- Rodríguez-iznaga, I.; Shelyapina, M.G.; Petranovskii, V. Ion Exchange in Natural Clinoptilolite: Aspects Related to Its Structure and Applications. Minerals 2022, 12, 1628. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Cui, Q.; Wang, T.; Wang, C.; Yang, J.; Shi, J.; Bao, X.; Yue, Y. Ion Exchange: An Essential Piece in the Fabrication of Zeolite Adsorbents. Phys. Chem. Chem. Phys. 2025, 27, 15819–15834. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Khramov, E.; Chowdari, R.K.; Estrada, M.A.; Berlier, G.; Zubavichus, Y.; Fuentes, S.; Petranovskii, V.; Chávez-Rivas, F. Properties of Iron-Modified-by-Silver Supported on Mordenite as Catalysts for Nox Reduction. Catalysts 2020, 10, 1156. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhu, Y.; Chen, M.; Zhang, Z.; Shangguan, W. Efficient Fe-ZSM-5 Catalyst with Wide Active Temperature Window for NH3 Selective Catalytic Reduction of NO: Synergistic Effect of Isolated Fe3+ and Fe2 O3. J. Catal. 2019, 378, 17–27. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Active Iron Sites Associated with the Reaction Mechanism of N2O Conversions over Steam-Activated FeMFI Zeolites. J. Catal. 2004, 227, 512–522. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Y.; Tong, N.; Zhang, Z.; Wang, Y.; Zhang, X.; Zhu, S.; Lia, F.; Wanga, X. HZSM-5 Zeolites Containing Impurity Iron Species for the Photocatalytic Reduction of CO2 with H2O. Catal. Sci. Technol. 2016, 6, 7579–7585. [Google Scholar] [CrossRef]
- Nezamzadeh-ejhieh, A.; Shahriari, E. Photocatalytic Decolorization of Methyl Green Using Fe (II)-o-Phenanthroline as Supported onto Zeolite Y. J. Ind. Eng. Chem. 2014, 20, 2719–2726. [Google Scholar] [CrossRef]
- Kessouri, A.; Boukoussa, B.; Bengueddach, A. Synthesis of Iron-MFI Zeolite and Its Photocatalytic Application for Hydroxylation of Phenol. Res. Chem. Intermed. 2017, 44, 2475–2487. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.; Meng, Q.; Feng, X.; Wang, J.; Li, Y.; Li, J. Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance. Molecules 2025, 30, 4030. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 30 November 2025).
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; et al. Mechanism of Formation of Framework Fe3+ in Bimetallic Ag-Fe Mordenites—Effective Catalytic Centers for DeNOx Reaction. Microporous Mesoporous Mater. 2019, 299, 109841. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kapteijn, F.; Groen, J.C.; Doménech, A.; Mul, G.; Moulijn, J.A. Steam-Activated FeMFI Zeolites. Evolution of Iron Species and Activity in Direct N2O Decomposition. J. Catal. 2003, 214, 33–45. [Google Scholar] [CrossRef]
- Sazama, P.; Moravkova, J.; Sklenak, S.; Vondrova, A.; Tabor, E.; Sadovska, G.; Pilar, R. Effect of the Nuclearity and Coordination of Cu and Fe Sites in β Zeolites on the Oxidation of Hydrocarbons. ACS Catal. 2020, 10, 3984–4002. [Google Scholar] [CrossRef]
- Zhao, G.; Chodyko, K.; Benhelal, E.; Adesina, A.; Kennedy, E.; Stockenhuber, M. Methane Oxidation by N2O over Fe-FER Catalysts Prepared by Different Methods: Nature of Active Iron Species, Stability of Surface Oxygen Species and Selectivity to Products. J. Catal. 2021, 400, 10–19. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Yang, J.; Rui, P.; Fan, N.; Liao, W.; Shu, X. Zeolite Fe-MFI as Catalysts in the Selective Liquid-Phase Dehydration of 1-Phenylethanol. Catal. Commun. 2018, 110, 97–101. [Google Scholar] [CrossRef]
- Diallo, M.M.; Laforge, S.; Pouilloux, Y.; Mijoin, J. Influence of the Preparation Procedure and Crystallite Size of Fe-MFI Zeolites in the Oxidehydration of Glycerol to Acrolein and Acrylic Acid. Catal. Commun. 2019, 126, 21–25. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, Y.; Tang, X.; Yi, H.; Zhao, S. A Novel Ferrisilicate MEL Zeolite with Bi-Functional Adsorption/Catalytic Oxidation Properties for Non-Methane Hydrocarbon Removal from Cooking Oil Fumes. Microporous Mesoporous Mater. 2020, 309, 110509. [Google Scholar] [CrossRef]
- Jamalluddin, N.A.; Abdullah, A.Z. Low Frequency Sonocatalytic Degradation of Azo Dye in Water Using Fe-Doped Zeolite Y Catalyst. Ultrason. Sonochem. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Wu, P.; Komatsu, T.; Yashima, T. Isomorphous Substitution of Fe3+ in the Framework of Aluminosilicate Mordenite by Hydrothermal Synthesis. Microporous Mesoporous Mater. 1998, 20, 139–147. [Google Scholar] [CrossRef]
- Uddin, M.A.; Komats, T.; Yashima, T. MFI-Fype Ferrisilicate Catalysts for the Oxidative Dehydrogenation of Alkanes. J. Catal. 1994, 150, 439–441. [Google Scholar] [CrossRef]
- Shah, D.R.; Nezam, I.; Zhou, W.; Proaño, L.; Jones, C.W. Isomorphous Substitution in ZSM-5 in Tandem Methanol/Zeolite Catalysts for the Hydrogenation of CO2 to Aromatics. Energy Fuels 2024, 38, 2224–2234. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, Characterization and Application of Fe-Zeolite: A Review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Lima, R.B.; Pergher, S.B.C.; Caldeira, V.P.S. Hierarchical Zeolite Synthesis by Alkaline Treatment: Advantages and Applications. Catalysts 2023, 13, 316. [Google Scholar] [CrossRef]
- Lago, C.D.; Decolatti, H.P.; Tonutti, L.G.; Dalla Costa, B.O.; Querini, C.A. Gas Phase Glycerol Dehydration over H-ZSM-5 Zeolite Modified by Alkaline Treatment with Na2CO3. J. Catal. 2018, 366, 16–27. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Crivelli, P.; Cooke, D.; Pérez-Ramírez, J. Mesopore Quality Determines the Lifetime of Hierarchically Structured Zeolite Catalysts. Nat. Commun. 2014, 5, 3922. [Google Scholar] [CrossRef]
- Al-ani, A.; Darton, R.J.; Sneddon, S.; Zholobenko, V. Nanostructured Zeolites: The Introduction of Intracrystalline Mesoporosity in Basic Faujasite-Type Catalysts. ACS Appl. Nano Mater. 2018, 1, 310–318. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Raja, D.; Khusni, N.B.; Liu, J.; Zhang, J.; Abdulridha, S.; Xiang, H.; Jiang, S.; Guan, Y.; et al. On the Effect of Mesoporosity of FAU Y Zeolites in the Liquid-Phase Catalysis. Microporous Mesoporous Mater. 2019, 278, 297–306. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Heracleous, E.; Pachatouridou, E.; Horvat, A.; Hernando, H.; Serrano, D.P.; Lappas, A.A.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Effect of Mesoporosity, Acidity and Crystal Size of Zeolite ZSM-5 on Catalytic Performance during the Ex-Situ Catalytic Past Pyrolysis of Biomass. ChemCatChem 2021, 13, 1207–1219. [Google Scholar] [CrossRef]
- Liu, Q.; He, P.; Qian, X.; Fei, Z.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X.; Shi, Y. Enhanced CO2 Adsorption Performance on Hierarchical Porous ZSM-5 Zeolite. Energy Fuels 2017, 31, 13933–13941. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Mesoporosity—A New Dimension for Zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Nefedov, D.Y.; Antonenko, A.O.; Valkovskiy, G.A.; Yocupicio-gaxiola, R.I.; Petranovskii, V. Nanoconfined Water in Pillared Zeolites Probed by 1H Nuclear Magnetic Resonance. Int. J. Mol. Sci. 2023, 24, 15898. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Kimura, M.; Sugino, M.; Horikawa, T.; Nakagawa, K.; Sugiyama, S. Modification of Commercial NaY Zeolite to Give High Water Diffusivity and Adsorb a Large Amount of Water. J. Colloid Interface Sci. 2015, 455, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, H.; Leng, S.; Yu, J.; Feng, K.; Cao, L. Regulation of Hydrophilicity/Hydrophobicity of Aluminosilicate Zeolites: A Review. Crit. Rev. Solid State Mater. Sci. 2020, 46, 330–348. [Google Scholar] [CrossRef]
- Cakicioglu-Ozkan, F.; Ulku, S. The Effect of HCl Treatment on Water Vapor Adsorption Characteristics of Clinoptilolite Rich Natural Zeolite. Microporous Mesoporous Mater. 2005, 77, 47–53. [Google Scholar] [CrossRef]
- Senderov, E.; Halasz, I.; Olson, D.H. On Existence of Hydroxyl Nests in Acid Dealuminated Zeolite Y. Microporous Mesoporous Mater. 2014, 186, 94–100. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Yocupicio-Gaxiola, R.I.; Zhelezniak, I.V.; Chislov, M.V.; Antúnez-García, J.; Murrieta-Rico, F.N.; Galván, D.H.; Petranovskii, V.; Fuentes-Moyado, S. Local Structures of Two-Dimensional Zeolites—Mordenite and ZSM-5—Probed by Multinuclear NMR. Molecules 2020, 25, 4678. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Lopes, C.W.; Meira, D.M.; Díaz, U.; Corma, A.; Pergher, S. Lamellar MWW Zeolite with Silicon and Niobium Oxide Pillars—A Catalyst for the Oxidation of Volatile Organic Compounds. Chem.—Eur. J. 2020, 26, 10459–10470. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Yocupicio-gaxiola, R.I.; Valkovsky, G.A.; Petranovskii, V. TiO2 Immobilized on 2D Mordenite: Effect of Hydrolysis Conditions on Structural, Textural, and Optical Characteristics of the Nanocomposites. Beilstein J. Nanotechnol. 2025, 16, 128–140. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Tarach, K.A.; Gora-Marek, K. Top-down Engineering of Zeolite Porosity. Chem. Soc. Rev. 2025, 54, 7484–7560. [Google Scholar] [CrossRef]
- Gu, J.; Lin, J.; Smith, A.J.; Soontaranon, S.; Rugmai, S.; Kongmark, C.; Coppens, M.; Sankar, G. Towards Understanding Mesopore Formation in Zeolite Y Crystals Using Alkaline Additives via in Situ Small-Angle X-Ray Scattering. Microporous Mesoporous Mater. 2022, 338, 111867. [Google Scholar] [CrossRef]
- Pilar, R.; Moravkova, J.; Sadovska, G.; Sklenak, S.; Brabec, L.; Pastvova, J.; Sazama, P. Controlling the Competitive Growth of Zeolite Phases without Using an Organic Structure-Directing Agent. Synthesis of Al-Rich *BEA. Microporous Mesoporous Mater. 2022, 333, 111726. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Cui, W.; Tan, J.; Tian, P.; Liu, Z. High-Silica Zeolite Y: Seed-Assisted Synthesis, Characterization and Catalytic Properties. Inorg. Chem. Front. 2022, 9, 2213–2220. [Google Scholar] [CrossRef]
- Zhang, R.; Zou, R.; Li, W.; Chang, Y.; Fan, X. On Understanding the Sequential Post-Synthetic Microwave-Assisted Dealumination and Alkaline Treatment of Y Zeolite. Microporous Mesoporous Mater. 2022, 333, 111736. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Fan, C.; Do, D.D.; Nicholson, D. On the Cavitation and Pore Blocking in Slit-Shaped Ink-Bottle Pores. Langmuir 2011, 27, 3511–3526. [Google Scholar] [CrossRef]
- Bläker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of Activated Carbon Adsorbents—State of the Art and Novel Approaches. ChemBioEng Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Javier, P. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Nedyalkova, R.; Shwan, S.; Skoglundh, M.; Olsson, L. Improved Low-Temperature SCR Activity for Fe-BEA Catalysts by H2 -Pretreatment. Appl. Catal. B Environ. 2013, 138–139, 373–380. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Zheng, W.; Zhang, W.; Guo, L.; Wu, X. Excellent Performance of One-Pot Synthesized Fe-Containing MCM-22 Zeolites for the Selective Catalytic Reduction of NOx with NH3. Catal. Sci. Technol. 2020, 10, 6583–6598. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]

| Sample | XPS | EDXRF | |||||
|---|---|---|---|---|---|---|---|
| Si/Al | Na/Al | Fe/Al | Si/Al | Na/Al | Fe/Al | Fe (wt%) | |
| Z0 | 14.39 | - | - | 10.15 | - | - | |
| Z0.2 | 9.05 | 2.31 | - | 7.77 | 1.49 | - | |
| Z0.4 | 5.49 | 2.2 | - | 4.96 | 1.79 | - | |
| Z0-Fe0.1 | 14.56 | - | 0.20 | 9.3 | - | 0.21 | 0.9 |
| Z0.2-Fe0.1 | 14.98 | 0.44 | 0.48 | 8.5 | 0.28 | 0.39 | 1.8 |
| Z0.4-Fe0.1 | 13.13 | 0.36 | 0.98 | 7.8 | 0.38 | 0.92 | 3.1 |
| Z0-Fe0.5 | 13.19 | - | 0.22 | 9.34 | - | 0.57 | 2.5 |
| Z0.2-Fe0.5 | 12.18 | 2.09 | 0.42 | 8.83 | 0.11 | 1.33 | 5.7 |
| Z0.4-Fe0.5 | 16.68 | 0.45 | 0.84 | 9.44 | 0.06 | 2.61 | 10.0 |
| Z0-Fe1.0 | 13.49 | 0.19 | 0.69 | 9.05 | - | 2.15 | 8.8 |
| Z0.2-Fe1.0 | 13.52 | 0.32 | 0.61 | 8.84 | 0.21 | 3.36 | 13.0 |
| Z0.4-Fe1.0 | 15.36 | 0.42 | 1.12 | 9.29 | 0.34 | 8.62 | 25.0 |
| Sample | SBET (m2/g) | SBJH (m2/g) | SNLDFT (m2/g) | VBJH (cm3/g) | VNLDFT (cm3/g) | DBJH (nm) | DNLDFT (nm) |
|---|---|---|---|---|---|---|---|
| Z0 | 409 | 33 | 747 | 0.127 | 0.24 | 5 | 3–7 |
| Z0.2 | 353 | 32 | 635 | 0.277 | 0.30 | 17 | 15 |
| Z0.4 | 263 | 102 | 362 | 0.418 | 0.40 | 28 | 6/10/25 |
| Z0-Fe1.0 | 333 | 35 | 574 | 0.103 | 0.19 | 5.5/17 | 3–7 |
| Z0.2-Fe1.0 | 349 | 62 | 576 | 0.212 | 0.27 | 12 | 3.5/6/25 |
| Z0.4-Fe1.0 | 338 | 135 | 435 | 0.434 | 0.36 | 28 | 3.5/6/30 |
| Sample | 220 nm | 270 nm | 370 nm | 400–650 | ||||
|---|---|---|---|---|---|---|---|---|
| I (%) | Fe3+T (wt.%) | I (%) | Fe3+O (wt.%) | I (%) | Fe3+oli (wt.%) | I (%) | Fe3+oxi (wt.%) | |
| Z0-Fe0.1 | 18 | 0.16 | 19 | 0.17 | 38 | 0.34 | 25 | 0.23 |
| Z0.2-Fe0.1 | 20 | 0.36 | 11 | 0.21 | 57 | 1.03 | 12 | 0.21 |
| Z0.4-Fe0.1 | 8 | 0.26 | 11 | 0.35 | 71 | 2.16 | 10 | 0.32 |
| Z0-Fe0.5 | 18 | 0.44 | 17 | 0.42 | 42 | 1.06 | 23 | 0.57 |
| Z0.2-Fe0.5 | 15 | 0.86 | 23 | 1.3 | 47 | 2.7 | 15 | 0.83 |
| Z0.4-Fe0.5 | 8 | 0.75 | 19 | 1.96 | 62 | 6.2 | 11 | 1.09 |
| Z0-Fe1.0 | 14 | 1.25 | 24 | 2.1 | 47 | 4.12 | 15 | 1.33 |
| Z0.2-Fe1.0 | 19 | 2.41 | 14 | 1.81 | 50 | 6.53 | 17 | 2.25 |
| Z0.4-Fe1.0 | 14 | 3.39 | 26 | 6.54 | 52 | 12.91 | 8 | 2.15 |
| Sample | Eg (eV) | λmax (nm) |
|---|---|---|
| Z0 | 4.13 | 300 |
| Z0.2 | 4.66 | 266 |
| Z0.4 | 4.50 | 276 |
| Z0-Fe0.1 | 3.06 | 405 |
| Z0.2-Fe0.1 | 3.06 | 405 |
| Z0.4-Fe0.1 | 3.08 | 403 |
| Z0-Fe0.5 | 3.01 | 412 |
| Z0.2-Fe0.5 | 3.03 | 409 |
| Z0.4-Fe0.5 | 3.04 | 408 |
| Z0-Fe1.0 | 2.96 | 419 |
| Z0.2-Fe1.0 | 2.96 | 419 |
| Z0.4-Fe1.0 | 2.93 | 423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zvereva, I.A.; Samadov, A.; Kurnosenko, S.A.; Kirichenko, S.O.; Shelyapina, M.G.; Petranovskii, V. Effect of Mesoporosity on Structural, Textural, and Optical Characteristics of Fe(III) Ion-Exchanged ZSM-5 Zeolites. Molecules 2026, 31, 23. https://doi.org/10.3390/molecules31010023
Zvereva IA, Samadov A, Kurnosenko SA, Kirichenko SO, Shelyapina MG, Petranovskii V. Effect of Mesoporosity on Structural, Textural, and Optical Characteristics of Fe(III) Ion-Exchanged ZSM-5 Zeolites. Molecules. 2026; 31(1):23. https://doi.org/10.3390/molecules31010023
Chicago/Turabian StyleZvereva, Irina A., Azamat Samadov, Sergey A. Kurnosenko, Sergey O. Kirichenko, Marina G. Shelyapina, and Vitalii Petranovskii. 2026. "Effect of Mesoporosity on Structural, Textural, and Optical Characteristics of Fe(III) Ion-Exchanged ZSM-5 Zeolites" Molecules 31, no. 1: 23. https://doi.org/10.3390/molecules31010023
APA StyleZvereva, I. A., Samadov, A., Kurnosenko, S. A., Kirichenko, S. O., Shelyapina, M. G., & Petranovskii, V. (2026). Effect of Mesoporosity on Structural, Textural, and Optical Characteristics of Fe(III) Ion-Exchanged ZSM-5 Zeolites. Molecules, 31(1), 23. https://doi.org/10.3390/molecules31010023







