Construction of S-Scheme BiVO4/Bi2O2S Heterojunction for Highly Effective Photocatalysis of Antibiotic Pollutants
Abstract
1. Introduction
2. Results and Discussion
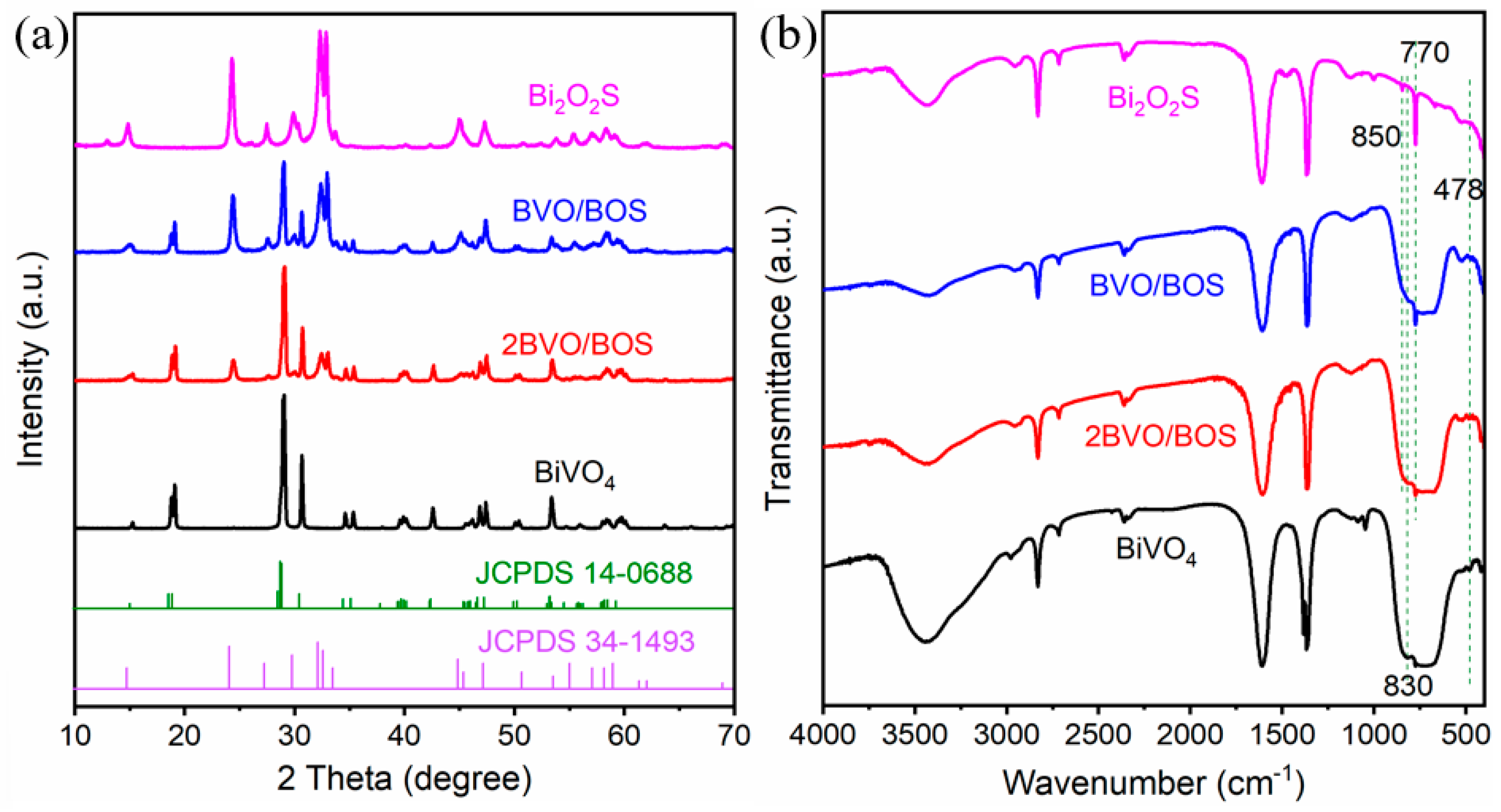
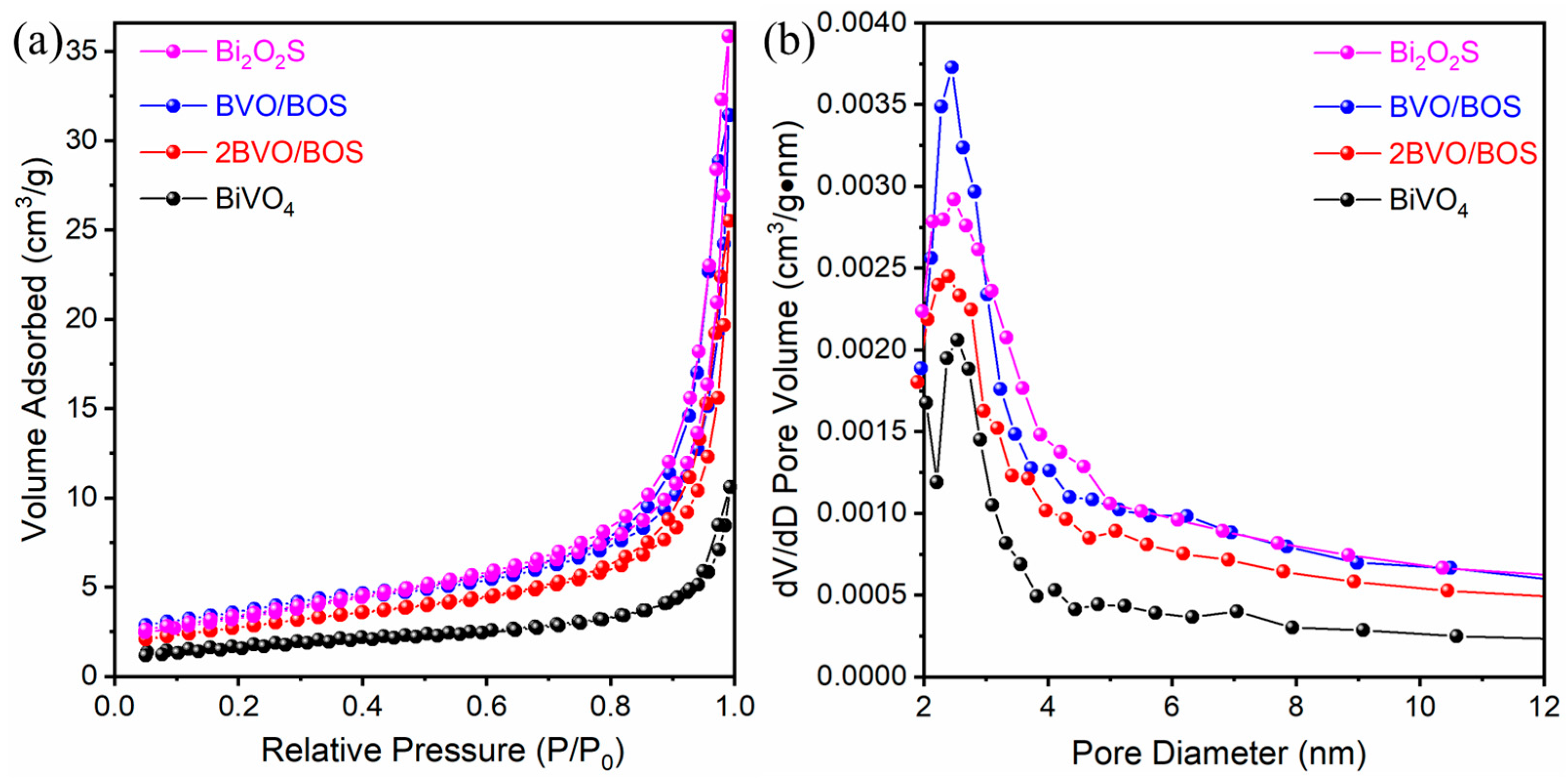
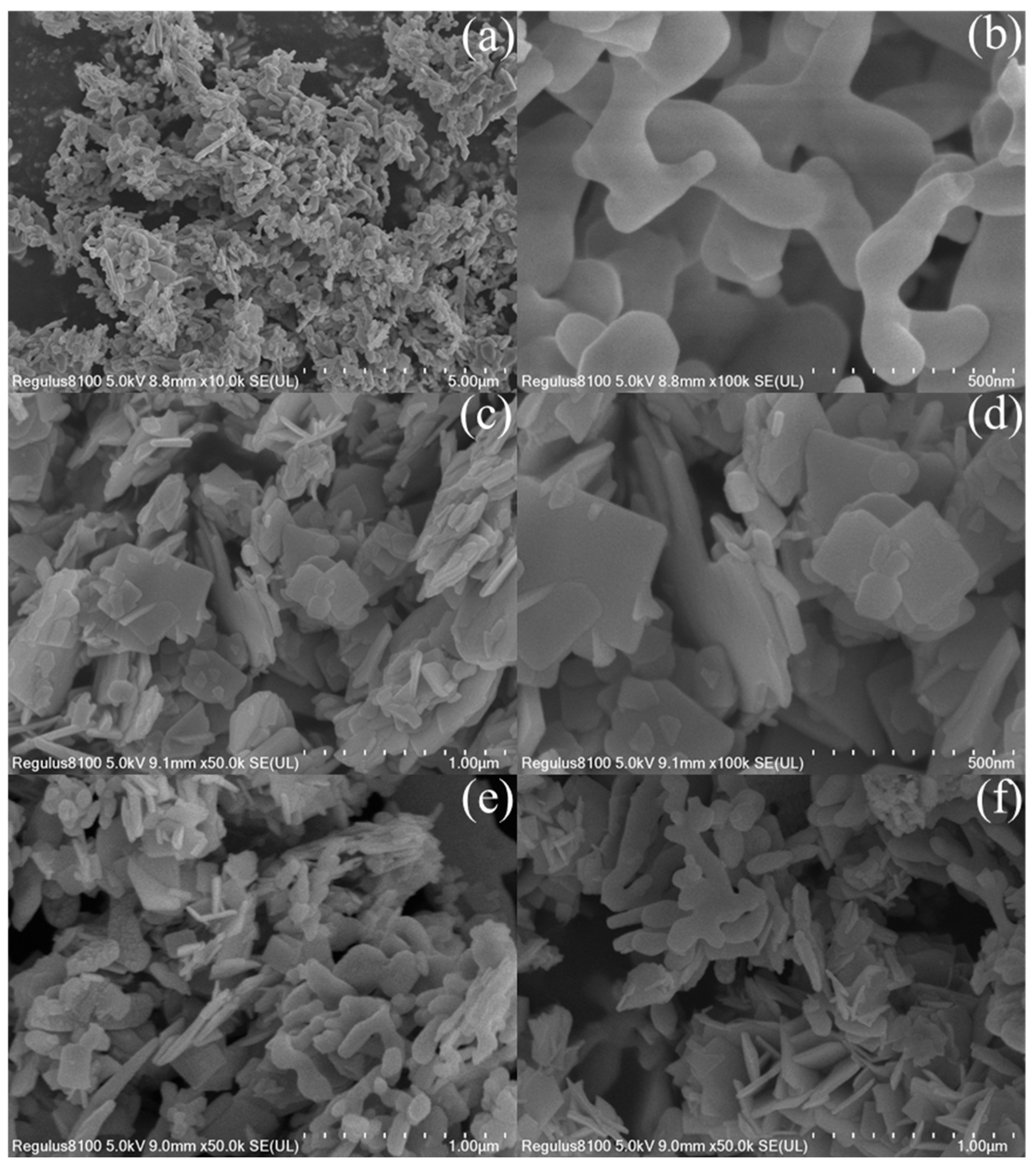
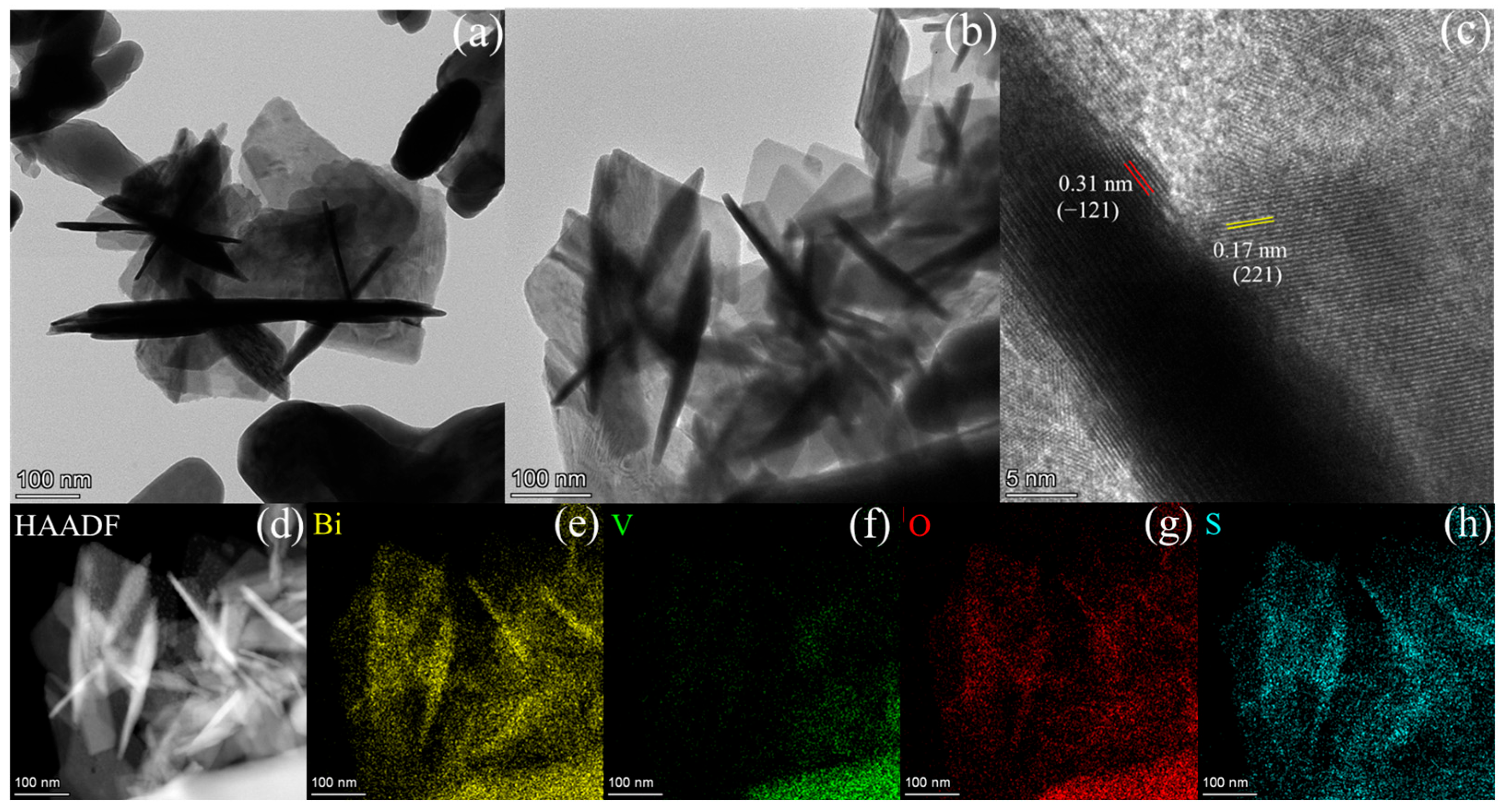
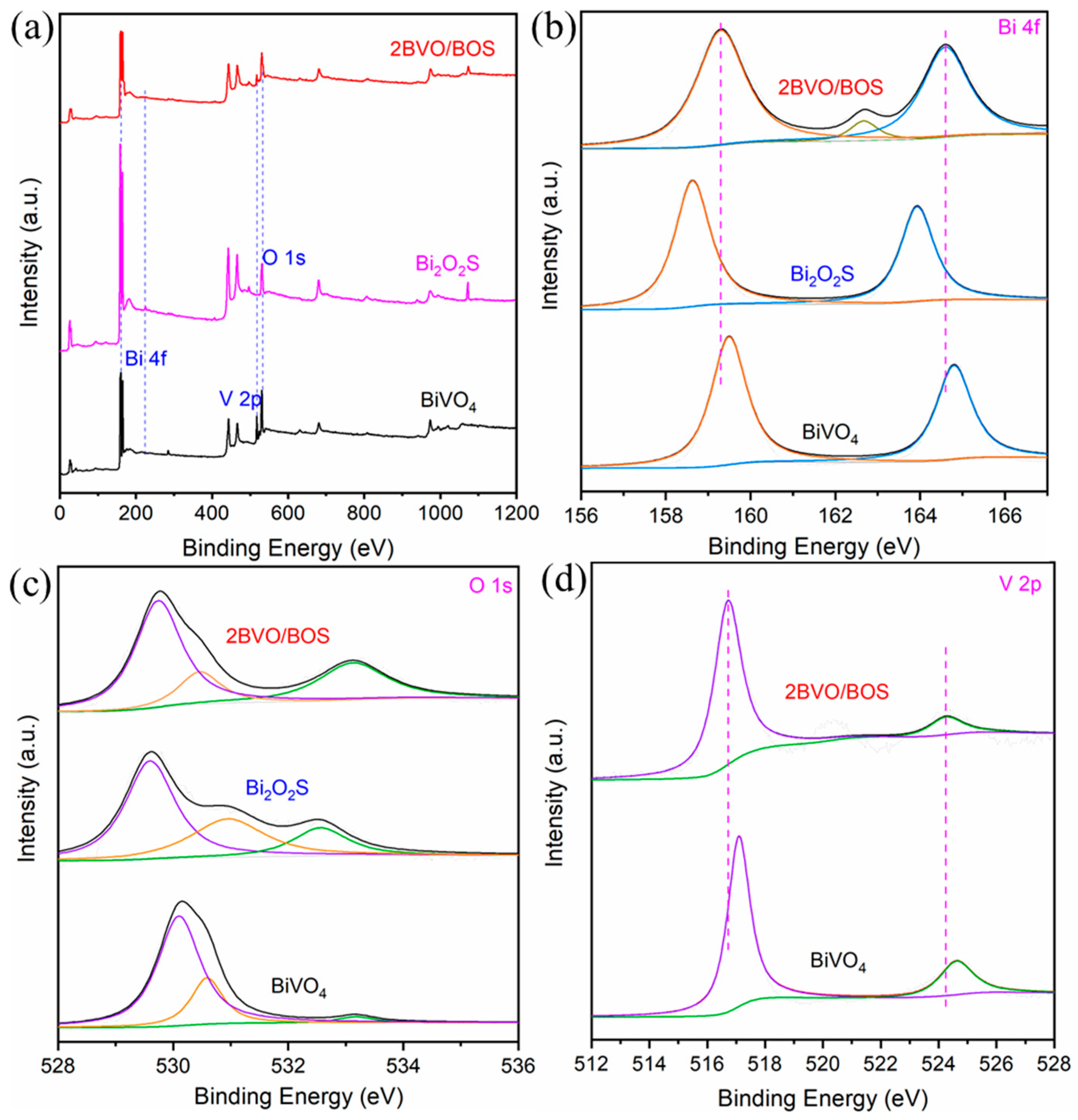
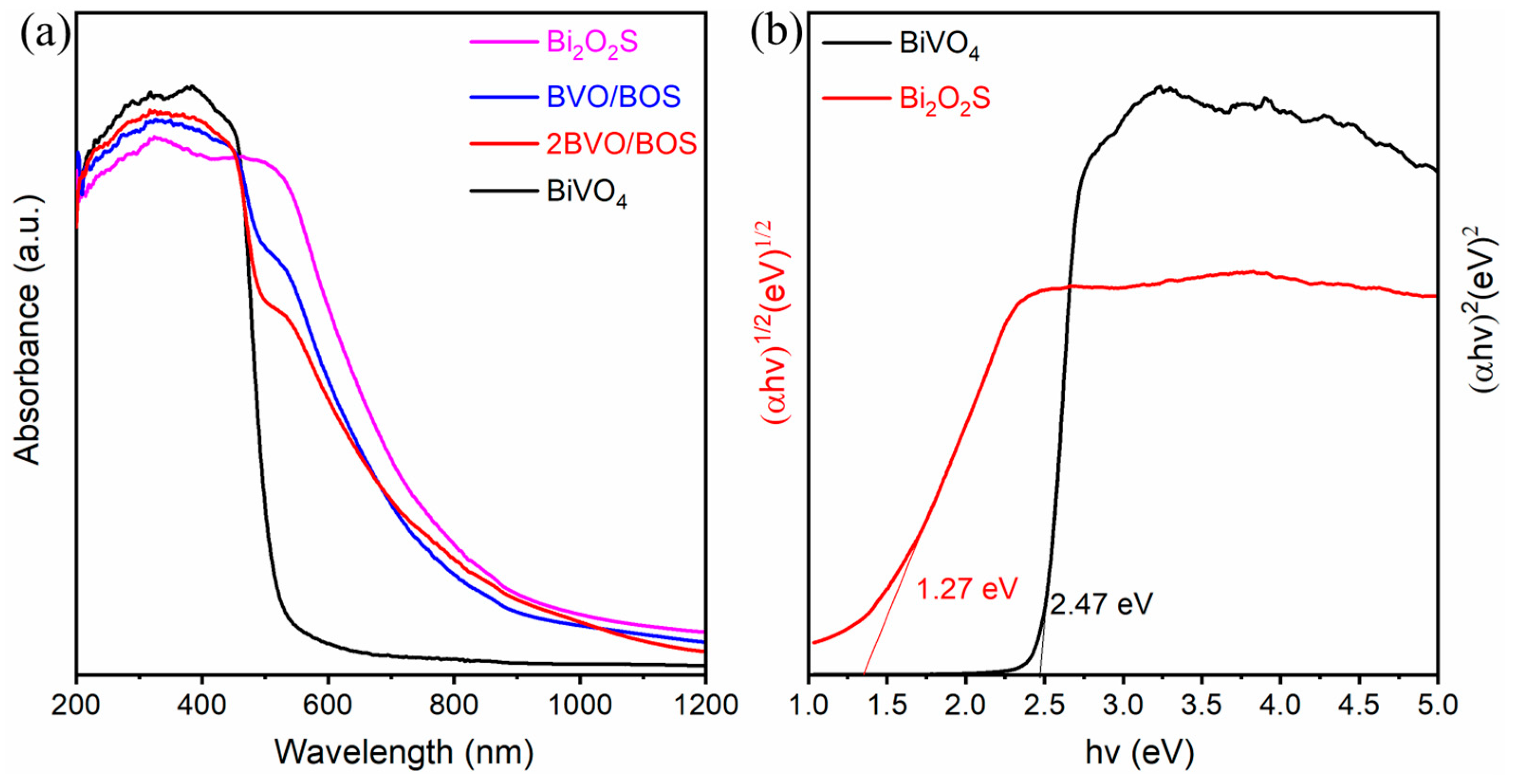
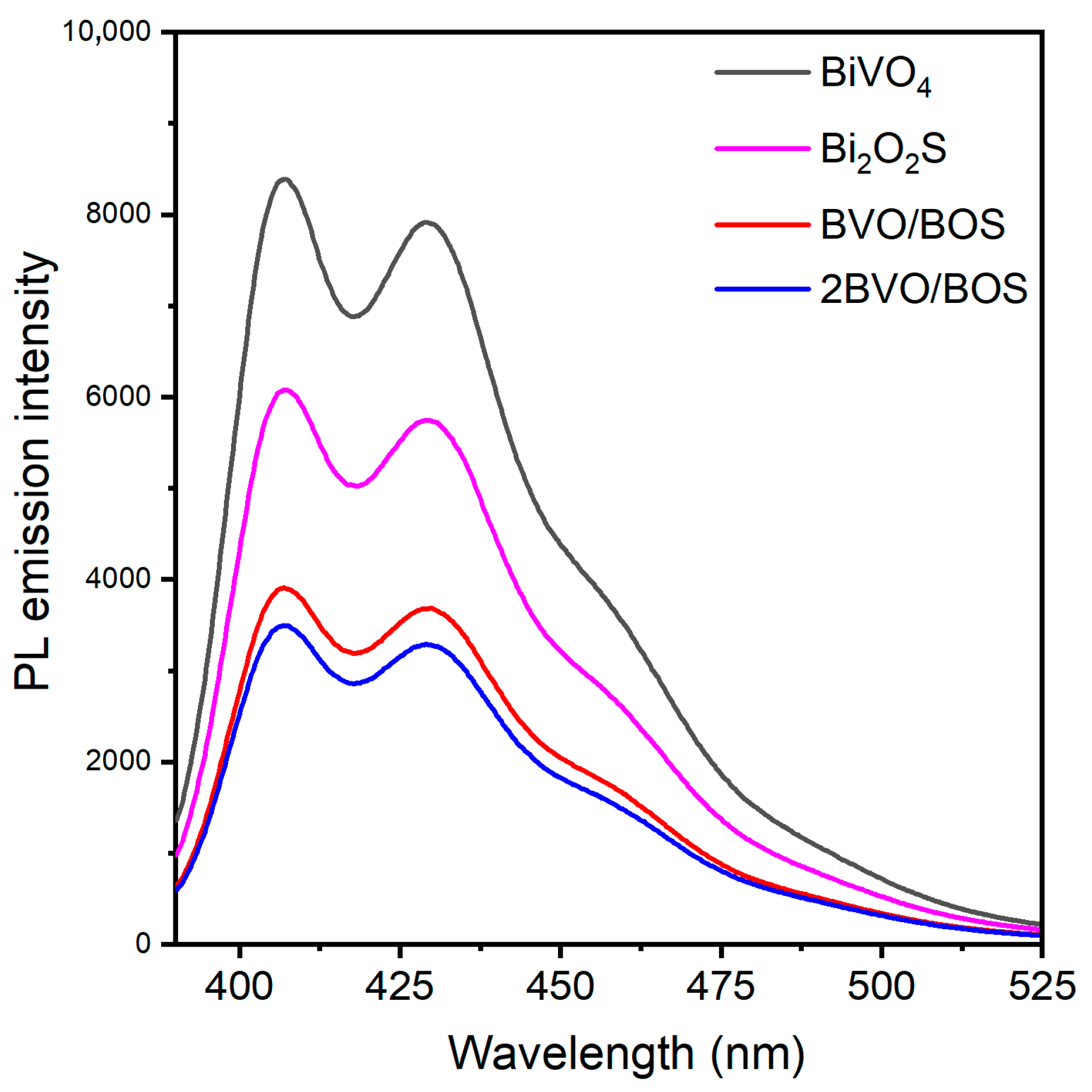
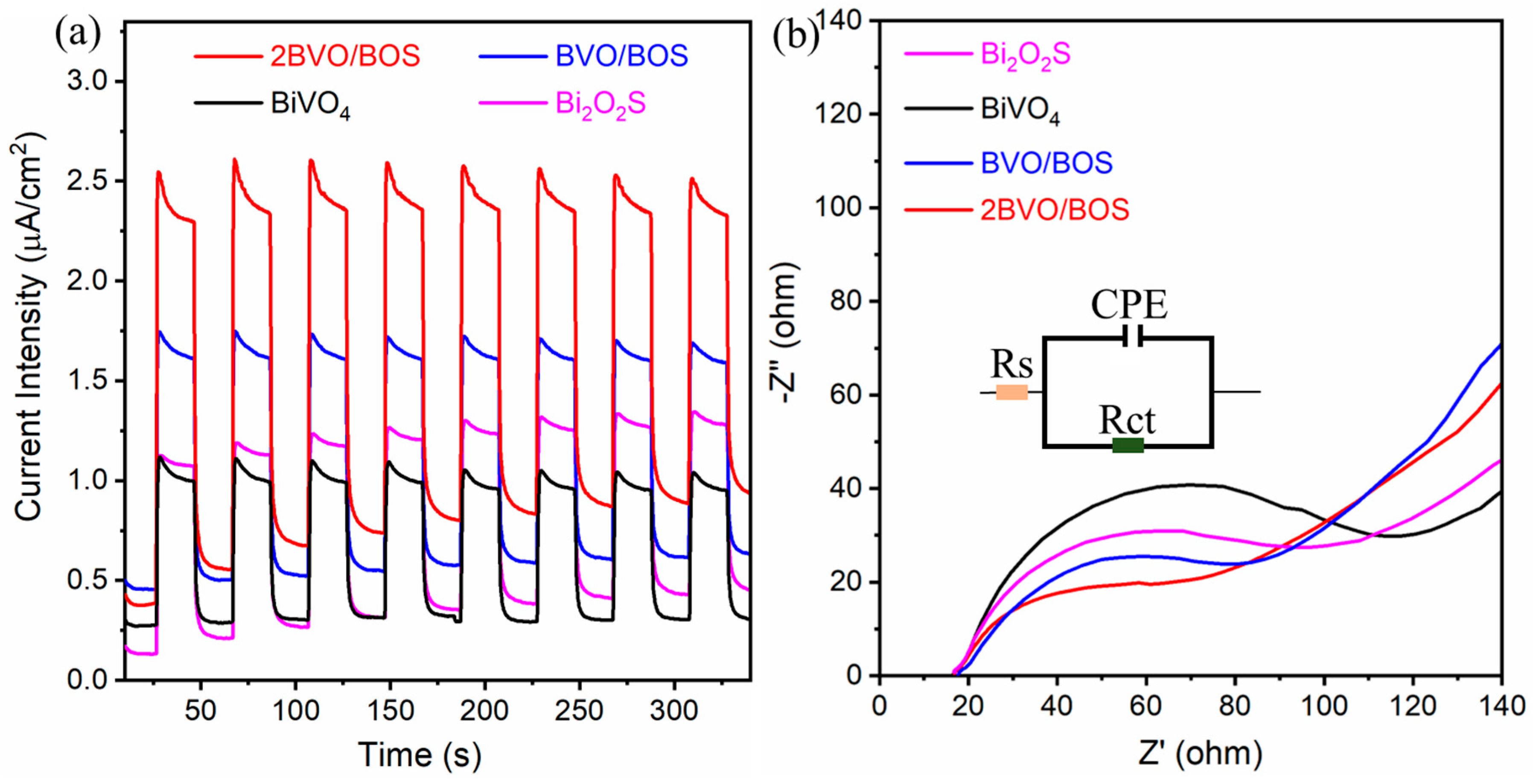
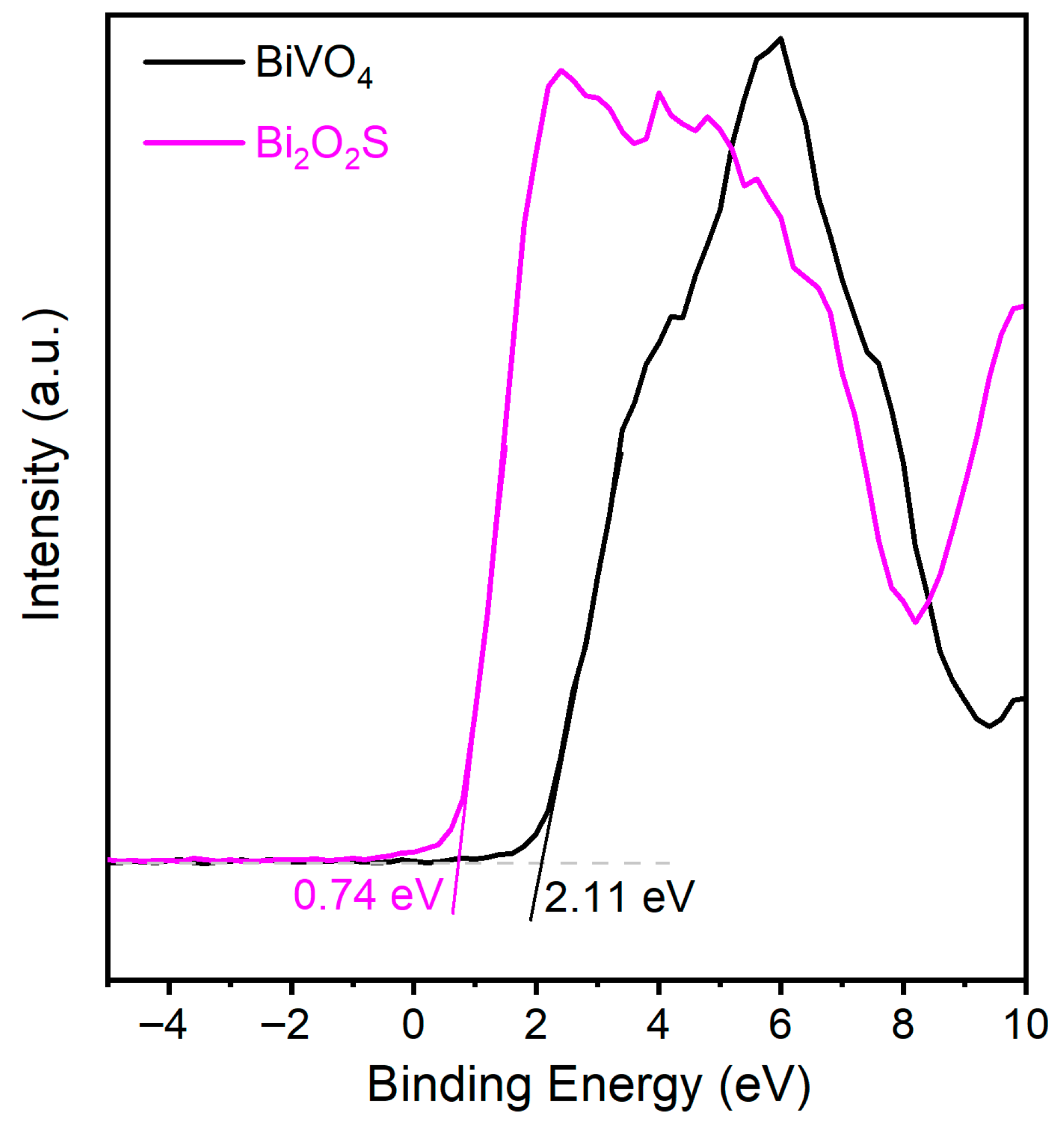
2.1. Characterization of the Photocatalysts
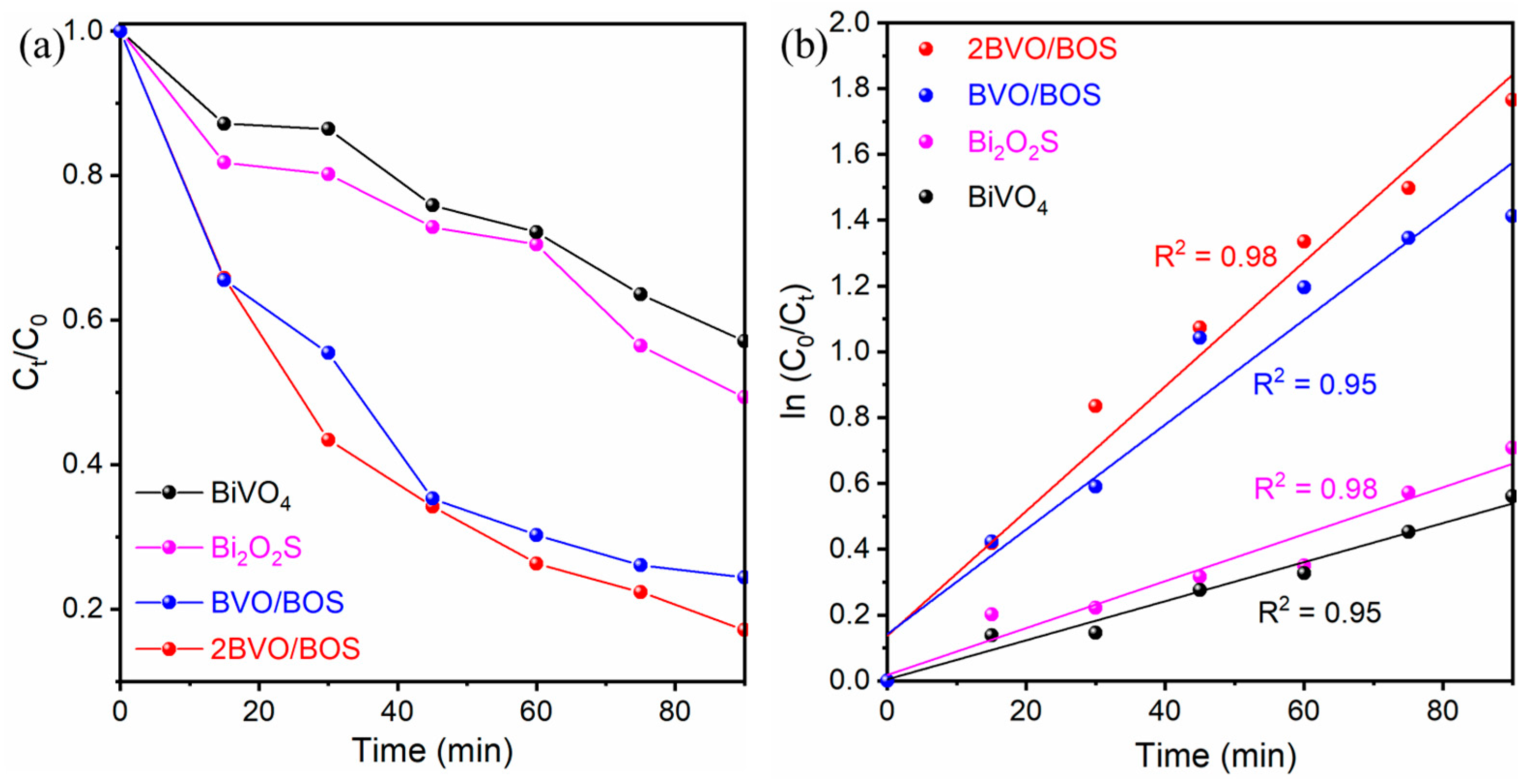
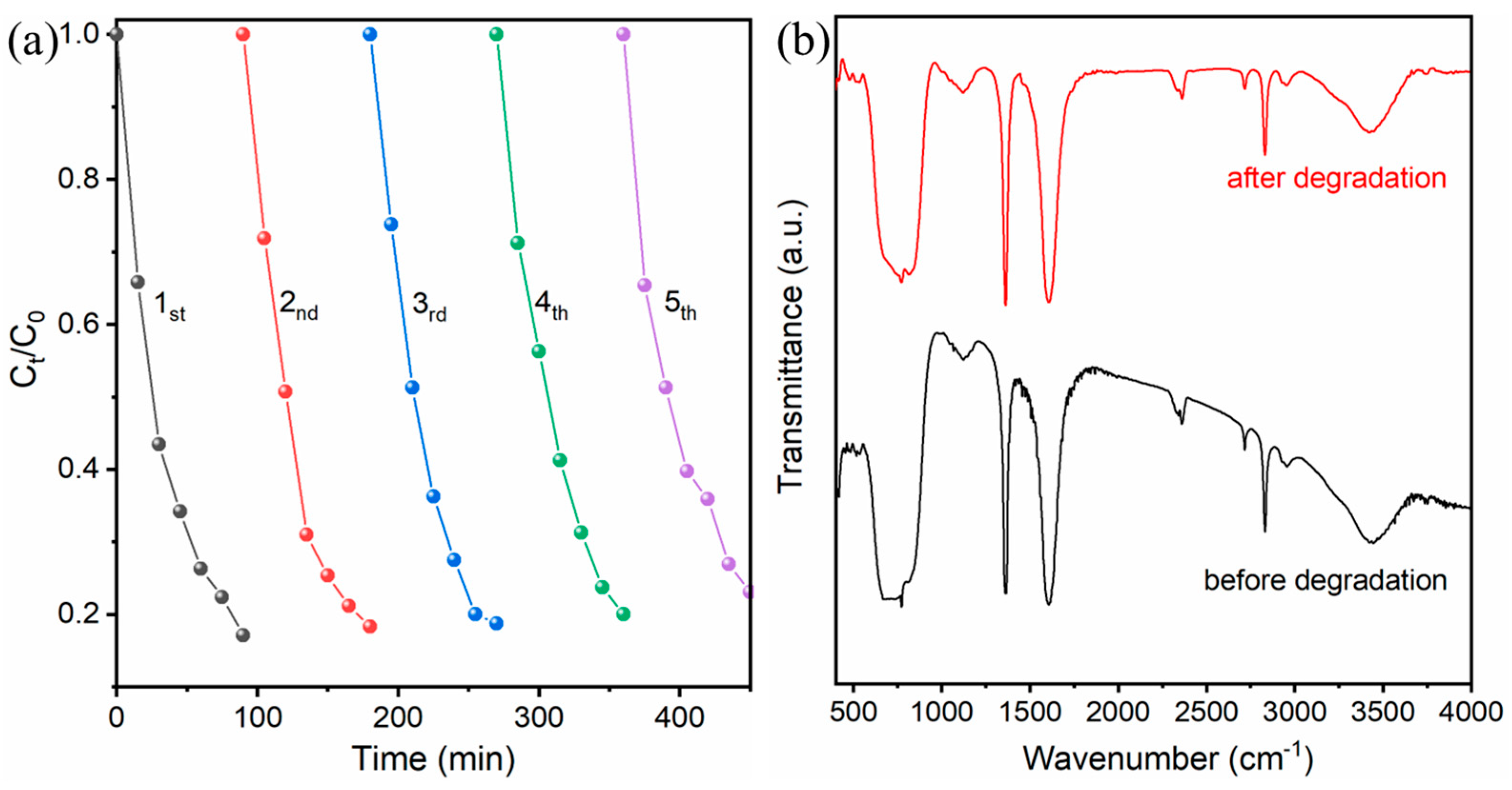
2.2. Photocatalytic Degradation of TC
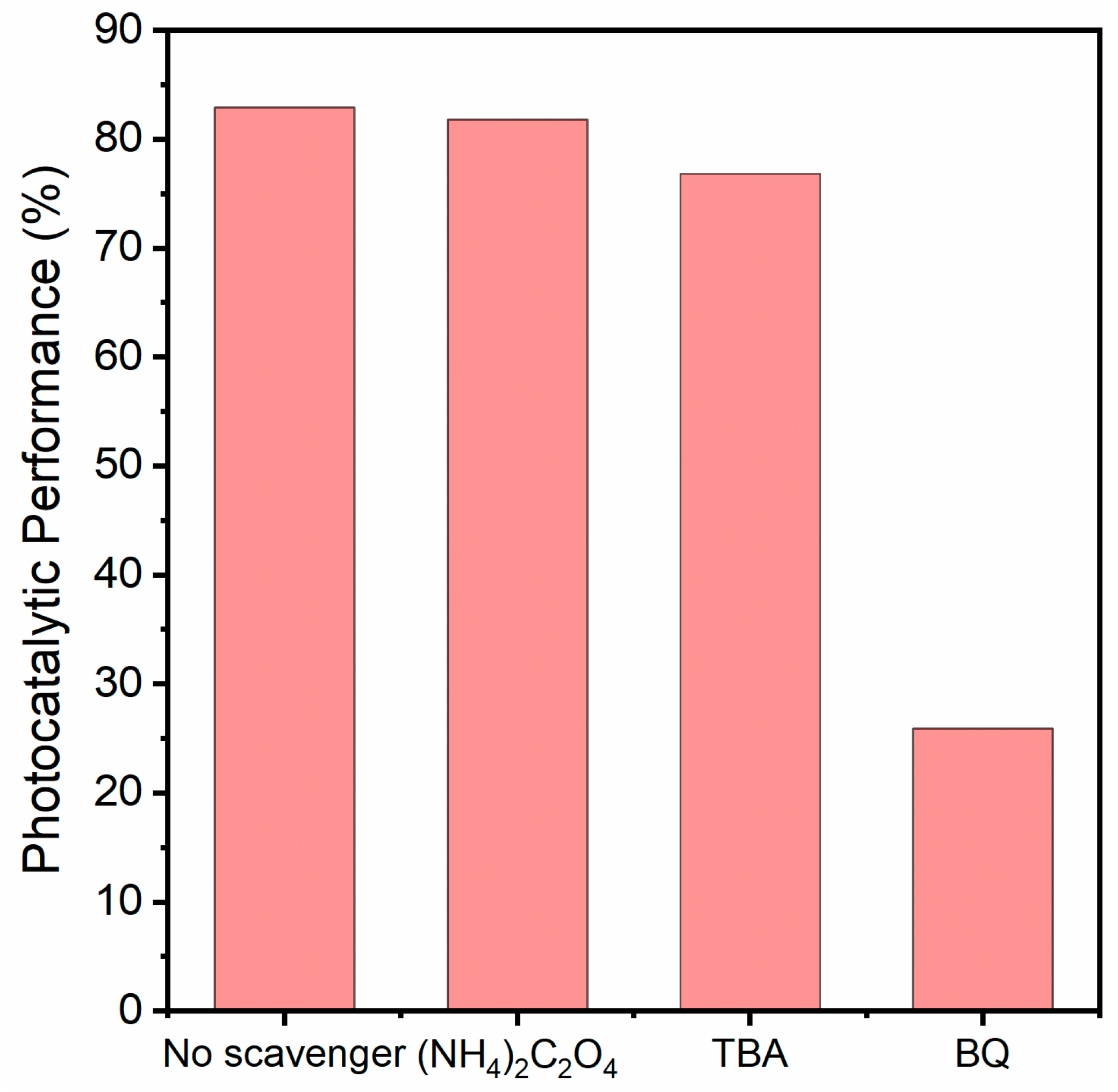
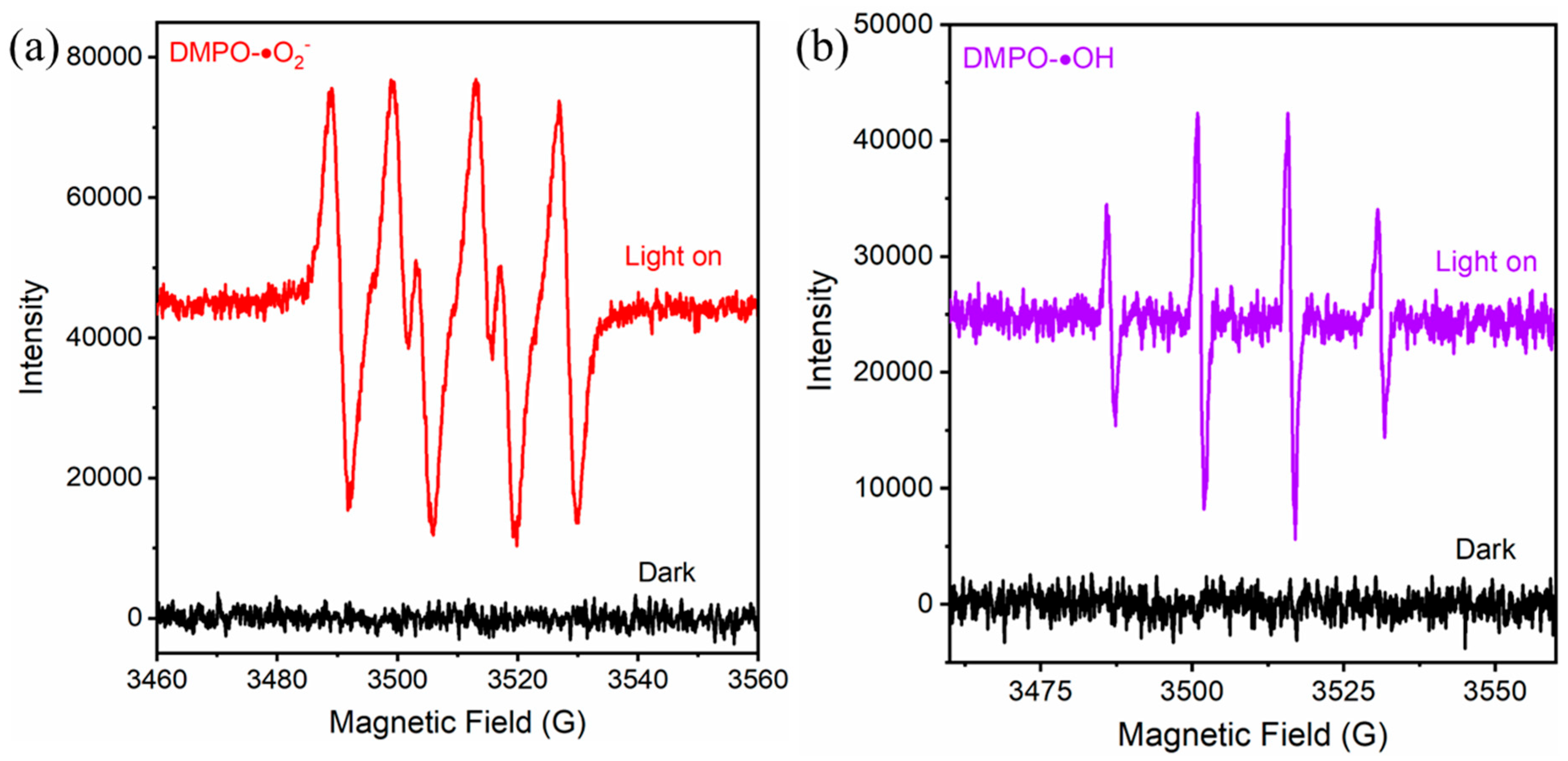
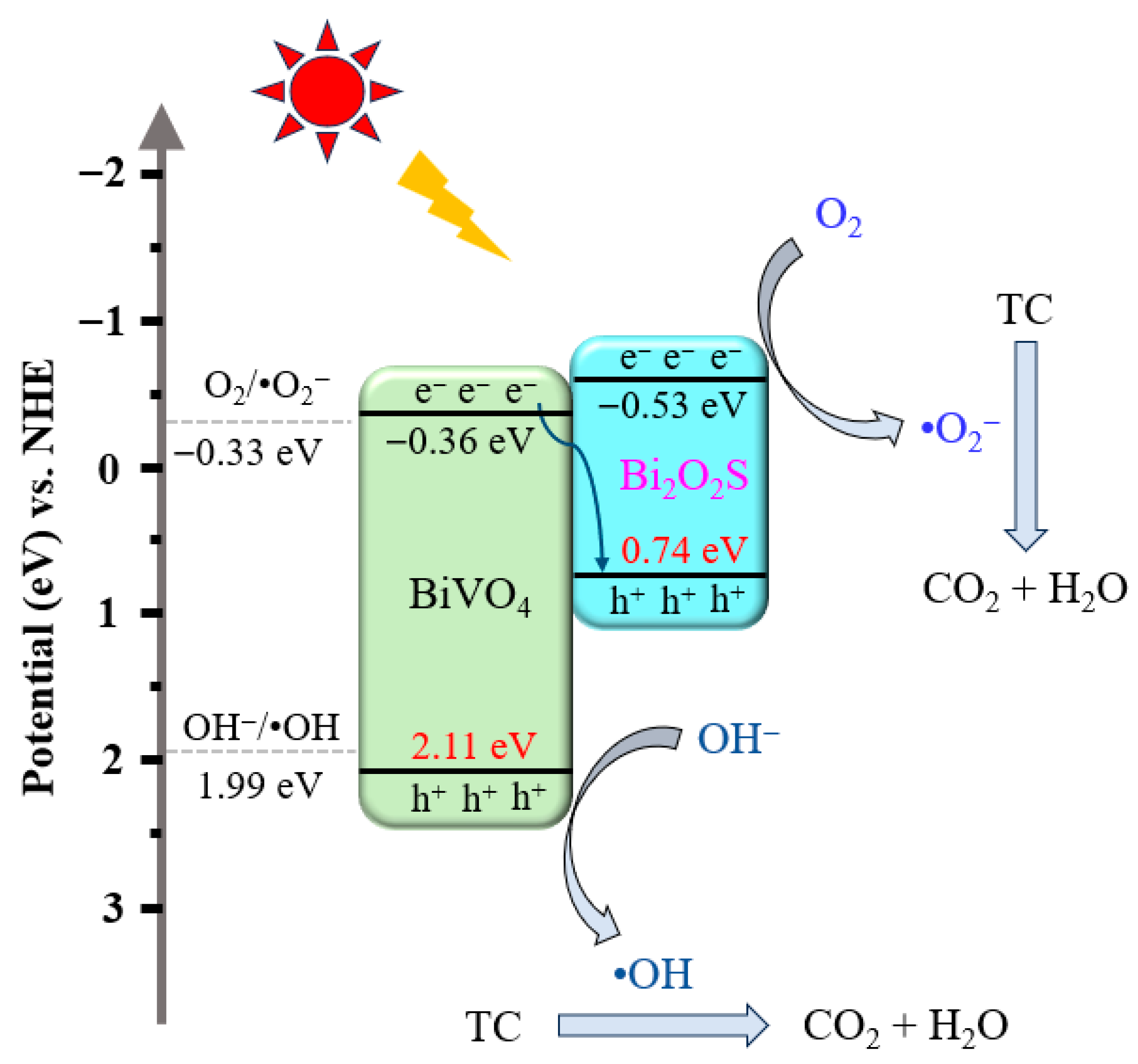
2.3. Photocatalytic Mechanism
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Preparation of Bi2O2S Nanosheets
3.1.2. Preparation of BiVO4/Bi2O2S and BiVO4 Catalysts
3.2. Catalyst Characterization
3.3. Photoelectrochemical Measurements
3.4. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breczko, J.; Basa, A.; Niemirowicz-Laskowska, K.; Skonieczna, B.; López-Martín, R.; De Toro, J.A.; Car, H.; Regulska, E. Magnetic AuNPs@TiO2@NF heterojunction for solar-light degradation of antibiotics and mitigation of bacterial resistance risk. RSC Adv. 2025, 15, 41862–41873. [Google Scholar] [CrossRef] [PubMed]
- Regulska, E.; Breczko, J.; Basa, A.; Niemirowicz-Laskowska, K.; Kiszkiel-Taudul, I. Photocatalytic degradation of oxytetracycline with the REMs (Er, Tm, Yb)-doped nickel and copper aluminates. Mat. Sci. Eng. B-Adv. 2022, 285, 115959. [Google Scholar] [CrossRef]
- Niemirowicz-Laskowska, K.; Basa, A.; Breczko, J.; Kiszkiel-Taudul, I.; Wielgat, P.; Skonieczna, B.; Car, H.; Regulska, E. Sunlight-activated nanocomposites for antibiotic degradation: How NiFe2O4@TiO2@Au tackles tigecycline and its biotoxicity. Surf. Interfaces 2025, 58, 105889. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, T.; Wu, Z.; Razzaque, S.; Zhao, Z.; Cai, J.; Xie, W.; Wang, J.; Zhao, Q.; Wang, K. In Situ construction of thiazole-linked covalent organic frameworks on Cu2O for high-efficiency photocatalytic tetracycline degradation. Molecules 2025, 30, 3233. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, M.; Sun, C.; Jiang, J.; Guo, Y. Highly efficient photocatalytic degradation of tetracycline antibiotics by BiPO4/g-C3N4: A novel heterojunction nanocomposite with nanorod/stacked-like nanosheets structure. Molecules 2025, 30, 2905. [Google Scholar] [CrossRef]
- Du, H.; Zhang, A.; Zhang, Q.; Sun, Y.; Zhu, H.; Wang, H.; Tan, Z.; Zhang, X.; Chen, G. Fabrication of recoverable Bi2O2S/Bi5O7I/ZA hydrogel beads for enhanced photocatalytic Hg0 removal in the presence of H2O2. Sep. Purif. Technol. 2025, 359, 130597. [Google Scholar] [CrossRef]
- Esmaili, Z.; Sadeghian, Z.; Ashrafizadeh, S.N. Tailoring of BiVO4 morphology for efficient antifouling of visible-light-driven photocatalytic ceramic membranes for oily wastewater treatment. J. Water Process Eng. 2024, 67, 106145. [Google Scholar] [CrossRef]
- Guan, H.; Wang, Q.; Feng, Y.; Sun, H.; Zhang, W.; Hu, Y.; Zhong, Q. Preparation of binary type II α-Bi2O3/Bi12TiO20 cross-shaped heterojunction with enhanced visible light photocatalytic performance. ACS Appl. Electron. Mater. 2022, 4, 1132–1142. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Gao, D.; Wang, X.; Suo, N.; Yu, Y.; Zhang, S. Electrocatalysis degradation of tetracycline in a three-dimensional aeration electrocatalysis reactor (3D-AER) with a flotation-tailings particle electrode (FPE): Physicochemical properties, influencing factors and the degradation mechanism. J. Hazard. Mater. 2021, 407, 124361. [Google Scholar] [CrossRef] [PubMed]
- Koundle, P.; Nirmalkar, N.; Momotko, M.; Makowiec, S.; Boczkaj, G. Tetracycline degradation for wastewater treatment based on ozone nanobubbles advanced oxidation processes (AOPs)—Focus on nanobubbles formation, degradation kinetics, mechanism and effects of water composition. Chem. Eng. J. 2024, 501, 156236. [Google Scholar] [CrossRef]
- Moral-Rodríguez, A.I.; Quintana, M.; Leyva-Ramos, R.; Ojeda-Galván, H.J.; Oros-Ruiz, S.; Peralta-Rodríguez, R.D.; Mendoza-Mendoza, E. Novel and green synthesis of BiVO4 and GO/BiVO4 photocatalysts for efficient dyes degradation under blue LED illumination. Ceram. Int. 2022, 48, 1264–1276. [Google Scholar] [CrossRef]
- Hemmatpour, P.; Nezamzadeh-Ejhieh, A. A Z-scheme CdS/BiVO4 photocatalysis towards Eriochrome black T: An experimental design and mechanism study. Chemosphere 2022, 307, 135925. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, G.; Yin, L.; Liu, W.; Zhang, B.; Feng, S.; Bi, Y.; Liu, Y.; Liu, T.; Wang, Z.; et al. Full-spectrum broad-spectrum degradation of antibiotics by BiVO4@BiOCl crystal plane S-type and Z-type heterojunctions. Chem. Eng. J. 2023, 467, 143450. [Google Scholar] [CrossRef]
- Boualam, O.; El Alami, S.; Miyah, Y.; Belaabed, R.; El Knidri, H.; Kherbeche, A.; Addaou, A.; Laajeb, A. Multifunctional BiVO4@Illite-Chitosan hybrid for visible-light photocatalytic degradation of phenol and diluted olive mill wastewater via synergistic photocatalysis-coagulation. J. Environ. Chem. Eng. 2025, 13, 120389. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Chen, C.; You, T.; Yu, X.; Zhang, Y.; Liu, H.; Zhu, Y. Fabrication of CuO/BiVO4 composites for enhanced visible-light-driven photocatalytic antibacterial activity. RSC Adv. 2025, 15, 45038–45047. [Google Scholar] [CrossRef] [PubMed]
- Popović, M.; Pandey, S.K.; Zjačić, J.P.; Dananić, V.; Roković, M.K.; Kovačić, M.; Kušić, H.; Šuligoj, A.; Štangar, U.L.; Božić, A.L. Elucidating semiconducting properties and photocatalytic performance of surface-decorated BiVO4 for the removal of contaminants of emerging concern. Molecules 2025, 30, 2454. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, Y.; Wu, H.; Xie, R. Recent progress in BiVO4-based heterojunction nanomaterials for photocatalytic applications. Mat. Sci. Eng. B-Adv. 2023, 289, 116278. [Google Scholar] [CrossRef]
- Bai, Y.; Fang, Z.; Fang, Y.; Lin, C.; Bai, H.; Fan, W. Recent advances in BiVO4-based heterojunction photocatalysts for energy and environmental applications. Chem. Commun. 2025, 61, 5264–5280. [Google Scholar] [CrossRef] [PubMed]
- Nalini, P.; Raja, A.; Sweekaran, S.; Thulasika, R.; Poonguzhali, K.; Yuvarani, K.; Sridhar, S.; Saravanakumar, M.; Kang, M.; El-marghany, A. Preparation of Zn-doped BiVO4 nanoparticles by hydrothermal process for solar photocatalytic activity. J. Mater. Sci. Mater. Electron. 2025, 36, 516. [Google Scholar] [CrossRef]
- Qin, C.; Tang, X.; Chen, J.; Liao, H.; Zhong, J.; Li, J. In-situ fabrication of Bi/BiVO4 heterojunctions with N-doping for efficient elimination of contaminants. Colloids Surf. A 2021, 617, 126224. [Google Scholar] [CrossRef]
- Li, X.; Fang, G.; Tian, Q.; Wu, T. Crystal regulation of BiVO4 for efficient photocatalytic degradation in g-C3N4/BiVO4 heterojunction. Appl. Surf. Sci. 2022, 584, 152642. [Google Scholar] [CrossRef]
- Peng, Y.; Kong, F.; Ren, J.; Cai, J.; Xu, L.; Jia, M. Oxygen vacancy-rich CuOx/BiVO4 heterojunction engineering with optimized charge separation for high-efficiency solar-driven photocatalysis. J. Phys. Chem. C 2025, 129, 18993–19001. [Google Scholar] [CrossRef]
- Li, C.; Feng, F.; Jian, J.; Xu, Y.; Li, F.; Wang, H.; Jia, L. Boosting carrier dynamics of BiVO4 photoanode via heterostructuring with ultrathin BiOI nanosheets for enhanced solar water splitting. J. Mater. Sci. Technol. 2021, 79, 21–28. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Fang, Y.; Li, R.; Huang, Y. Hydrothermal synthesis of m-BiVO4/t-BiVO4 heterostructure for organic pollutants degradation: Insight into the photocatalytic mechanism of exposed facets from crystalline phase controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Gan, L.; Ji, X.; Wu, Z.; Zhang, R. All-solid-state Z-scheme BiVO4−Bi6O6(OH)3(NO3)3 heterostructure with prolonging electron-hole lifetime for enhanced photocatalytic hydrogen and oxygen evolution. J. Mater. Sci. Technol. 2021, 77, 117–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xie, W.; Gong, X.; Wang, Z.; Liu, Y.; Wang, P.; Cheng, H.; Dai, Y.; Huang, B.; et al. In-situ topotactic synthesis of decahedron BiVO4/2D networks Bi2S3 heterojunctions for efficient Cr(VI) reduction: Improved charge separation and imaged at the single-particle level. Chem. Eng. J. 2024, 485, 149794. [Google Scholar] [CrossRef]
- Jiamprasertboon, A.; Waehayee, A.; Eknapakul, T.; Li, S.; Carmalt, C.J.; Phonsuksawang, P.; Siritanon, T. Efficient synthesis of Bi2O2S nanoparticles for photocatalytic tetracycline degradation. Mater. Lett. 2024, 369, 136776. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Li, Y.; Wu, X.; Zhang, G. Promoted charge separation from nickel intervening in [Bi2O2]2+ layers of Bi2O2S crystals for enhanced photocatalytic CO2 conversion. Appl. Catal. B-Environ. 2021, 294, 120249. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Jin, W.; Zhang, D.; Sun, T.; Liu, E.; Hu, X.; Miao, H. In-situ construction of 2D/1D Bi2O2S/Bi2S3 heterojunction from the topological transformation of Bi2O2S with significantly enhanced photoelectrochemical performance. Int. J. Hydrog. Energy 2024, 51, 1545–1557. [Google Scholar] [CrossRef]
- Rong, P.; Gao, S.; Zhang, M.; Ren, S.; Lu, H.; Jia, J.; Jiao, S.; Zhang, Y.; Wang, J. Large-area hierarchical Bi2O2S flowers composed of 2D ultrathin nanosheets for high performance self-powered IR photodetector. J. Alloys Compd. 2022, 928, 167128. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Madhusudan, P.; Zhang, J. Half reaction over a Ni2P/Bi2O2S composite for photocatalytic overall nitrogen fixation. New J. Chem. 2025, 49, 1262–1267. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Wu, Y.; Wang, T.; Wang, W.; Su, X.; Shi, D.; Zhan, H.; Wang, Y. Interfacial engineering of [Bi2O2]2+-layer structures at Bi2O2S/Bi12TiO20 heterojunctions for enhanced photocatalytic degradation of 2,4-dichlorophenol. J. Environ. Chem. Eng. 2024, 12, 111778. [Google Scholar] [CrossRef]
- Pacquette, A.L.; Hagiwara, H.; Ishihara, T.; Gewirth, A.A. Fabrication of an oxysulfide of bismuth Bi2O2S and its photocatalytic activity in a Bi2O2S/In2O3 composite. J. Photochem. 2014, 277, 27–36. [Google Scholar] [CrossRef]
- Liaqat, M.; Younas, A.; Iqbal, T.; Afsheen, S.; Zubair, M.; Kamran, S.K.S.; Syed, A.; Bahkali, A.H.; Wong, L.S. Synthesis and characterization of ZnO/BiVO4 nanocomposites as heterogeneous photocatalysts for antimicrobial activities and waste water treatment. Mater. Chem. Phys. 2024, 315, 128923. [Google Scholar] [CrossRef]
- Seling, T.R.; Kumar Shringi, A.; Wang, K.; Riaz, U.; Yan, F. Bi2O2S nanosheets for effective visible light photocatalysis of anionic dye degradation. Mater. Lett. 2024, 361, 136136. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, K.; Zhang, C.; Han, S.; Shi, D. One-step construction of Bi2O2S-Bi2O3 S-scheme heterojunction for enhanced photocatalytic degradation of ciprofloxacin. J. Alloys Compd. 2025, 1032, 181042. [Google Scholar] [CrossRef]
- Alomayri, T. Enhanced interfacial charge transfer in BiVO4/rGO/FeVO4 heterojunction composite for improved photocatalysis water purification. Ceram. Int. 2025, 51, 10193–10199. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Wannapop, S.; Sakhon, T.; Kuntalue, B.; Thongtem, T.; Thongtem, S. Characterization and photocatalytic properties of BiVO4 synthesized by combustion method. J. Mol. Struct. 2023, 1274, 134420. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Shim, J.; Byon, C. Synthesis, characterization, and optical properties of visible light-driven Bi2S3 nanorod photocatalysts. J. Mater. Sci. Mater. Electron. 2017, 28, 14282–14292. [Google Scholar] [CrossRef]
- Sasikala, S.; Balakrishnan, M.; Kumar, M.; Chang, J.-H.; Manivannan, M.; Thangabalu, S. Effect of sulfur source and temperature on the morphological characteristics and photocatalytic activity of Bi2S3 nanostructure synthesized by microwave irradiation technique. J. Mater. Sci. Mater. Electron. 2023, 34, 997. [Google Scholar] [CrossRef]
- Liu, Y.; Wygant, B.R.; Kawashima, K.; Mabayoje, O.; Hong, T.E.; Lee, S.-G.; Lin, J.; Kim, J.-H.; Yubuta, K.; Li, W.; et al. Facet effect on the photoelectrochemical performance of a WO3/BiVO4 heterojunction photoanode. Appl. Catal. B-Environ. 2019, 245, 227–239. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, T.; Chen, Y.; Chen, J.; Zhong, J.; Li, J.; Huang, S.; Dou, L.; Li, M.; Jiang, Z. In-situ preparation of N-doped Bi0/OVs-BiVO4 photocatalysts with enhanced photocatalytic properties. J. Alloys Compd. 2024, 972, 172852. [Google Scholar] [CrossRef]
- Lamonier, C.; Bennani, A.; D’Huysser, A.; Aboukaïs, A.; Wrobel, G. Evidence for different copper species in precursors of copper–cerium oxide catalysts for hydrogenation reactions. An X-ray diffraction, EPR and X-ray photoelectron spectroscopy study. J. Chem. Soc. Faraday Trans. 1996, 92, 131–136. [Google Scholar] [CrossRef]
- Posada-Borbón, A.; Bosio, N.; Grönbeck, H. On the signatures of oxygen vacancies in O1s core level shifts. Surf. Sci. 2021, 705, 121761. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of oxygen 1s X-ray photoelectron spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, L.; Zhang, M.; Li, Y.; Liu, S.; Lin, J.; Song, F.; Zhang, W.; Zhu, S.; Pan, J. Construction of a bioinspired hierarchical BiVO4/BiOCl heterojunction and its enhanced photocatalytic activity for phenol degradation. ACS Appl. Mater. Interfaces 2021, 13, 32906–32915. [Google Scholar] [CrossRef]
- Cooper, J.K.; Gul, S.; Toma, F.M.; Chen, L.; Liu, Y.-S.; Guo, J.; Ager, J.W.; Yano, J.; Sharp, I.D. Indirect bandgap and optical properties of monoclinic bismuth vanadate. J. Phys. Chem. C 2015, 119, 2969–2974. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, Y.; Hu, J.; Zhou, C.; Shi, X.; Li, D.; Jiang, D. Synergistic effects of surface Lewis Base/Acid and nitrogen defect in MgAl layered double Oxides/Carbon nitride heterojunction for efficient photoreduction of carbon dioxide. Appl. Surf. Sci. 2021, 563, 150369. [Google Scholar] [CrossRef]
- Li, B.; Cao, Z.; Wang, S.; Wei, Q.; Shen, Z. BiVO4 quantum dot-decorated BiPO4 nanorods 0D/1D heterojunction for enhanced visible-light-driven photocatalysis. Dalton Trans. 2018, 47, 10288–10298. [Google Scholar] [CrossRef]
- Pandey, A.; Shrestha, S.; Kandel, R.; Gyawali, N.; Acharya, S.; Nepal, P.; Gaire, B.; Fualo, V.; Hahn, J.R. Visible-light-driven Co3O4/Nb2O5 heterojunction nanocomposites for efficient photocatalytic and antimicrobial performance in wastewater treatment. Molecules 2025, 30, 2561. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Chen, G.; Yao, S.; Cong, B.; Liu, P. Construction photothermal/pyroelectric property of hollow FeS2/Bi2S3 nanostructure with enhanced full spectrum photocatalytic activity. Appl. Catal. B-Environ. 2021, 298, 120573. [Google Scholar] [CrossRef]
- Li, Z.; Jin, C.; Wang, M.; Kang, J.; Wu, Z.; Yang, D.; Zhu, T. Novel rugby-like g-C3N4/BiVO4 core/shell Z-scheme composites prepared via low-temperature hydrothermal method for enhanced photocatalytic performance. Sep. Purif. Technol. 2020, 232, 115937. [Google Scholar] [CrossRef]
- Luo, X.-L.; Yang, S.-Y.; Wang, Z.-L.; Xu, Y.-H. Synthesis of Z–scheme Bi2S3/RGO/BiVO4 photocatalysts with superior visible light photocatalytic effectiveness for pollutant degradation. Sep. Purif. Technol. 2023, 318, 123966. [Google Scholar] [CrossRef]
- Yang, S.; Li, K.; Huang, P.; Liu, K.; Li, W.; Zhuo, Y.; Yang, Z.; Han, D. Dual-functional Cu2O/g-C3N4 heterojunctions: A high-performance SERS sensor and photocatalytic self-cleaning system for water pollution detection and remediation. Microsyst. Nanoeng. 2024, 10, 198. [Google Scholar] [CrossRef]
- Yu, W.; Hu, C.; Bai, L.; Tian, N.; Zhang, Y.; Huang, H. Photocatalytic hydrogen peroxide evolution: What is the most effective strategy? Nano Energy 2022, 104, 107906. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chen, D.; Hu, S.; Jia, Z.; Zhang, Y.; Zhang, B.; Liu, S.; Li, X. Construction of S-Scheme BiVO4/Bi2O2S Heterojunction for Highly Effective Photocatalysis of Antibiotic Pollutants. Molecules 2026, 31, 136. https://doi.org/10.3390/molecules31010136
Chen D, Hu S, Jia Z, Zhang Y, Zhang B, Liu S, Li X. Construction of S-Scheme BiVO4/Bi2O2S Heterojunction for Highly Effective Photocatalysis of Antibiotic Pollutants. Molecules. 2026; 31(1):136. https://doi.org/10.3390/molecules31010136
Chicago/Turabian StyleChen, Dongdong, Siting Hu, Zhenzhen Jia, Yang Zhang, Bo Zhang, Shasha Liu, and Xiang Li. 2026. "Construction of S-Scheme BiVO4/Bi2O2S Heterojunction for Highly Effective Photocatalysis of Antibiotic Pollutants" Molecules 31, no. 1: 136. https://doi.org/10.3390/molecules31010136
APA StyleChen, D., Hu, S., Jia, Z., Zhang, Y., Zhang, B., Liu, S., & Li, X. (2026). Construction of S-Scheme BiVO4/Bi2O2S Heterojunction for Highly Effective Photocatalysis of Antibiotic Pollutants. Molecules, 31(1), 136. https://doi.org/10.3390/molecules31010136




