Improved Method for Quantitative Measurement of OH Radicals Based on Absorption Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. Equivalent Absorption Path Length
2.2. Integrated Absorption Rate and Temperature Inversion Results
2.3. Calibration Results of Constant C and Applicability Analysis
3. Experimental System and Method
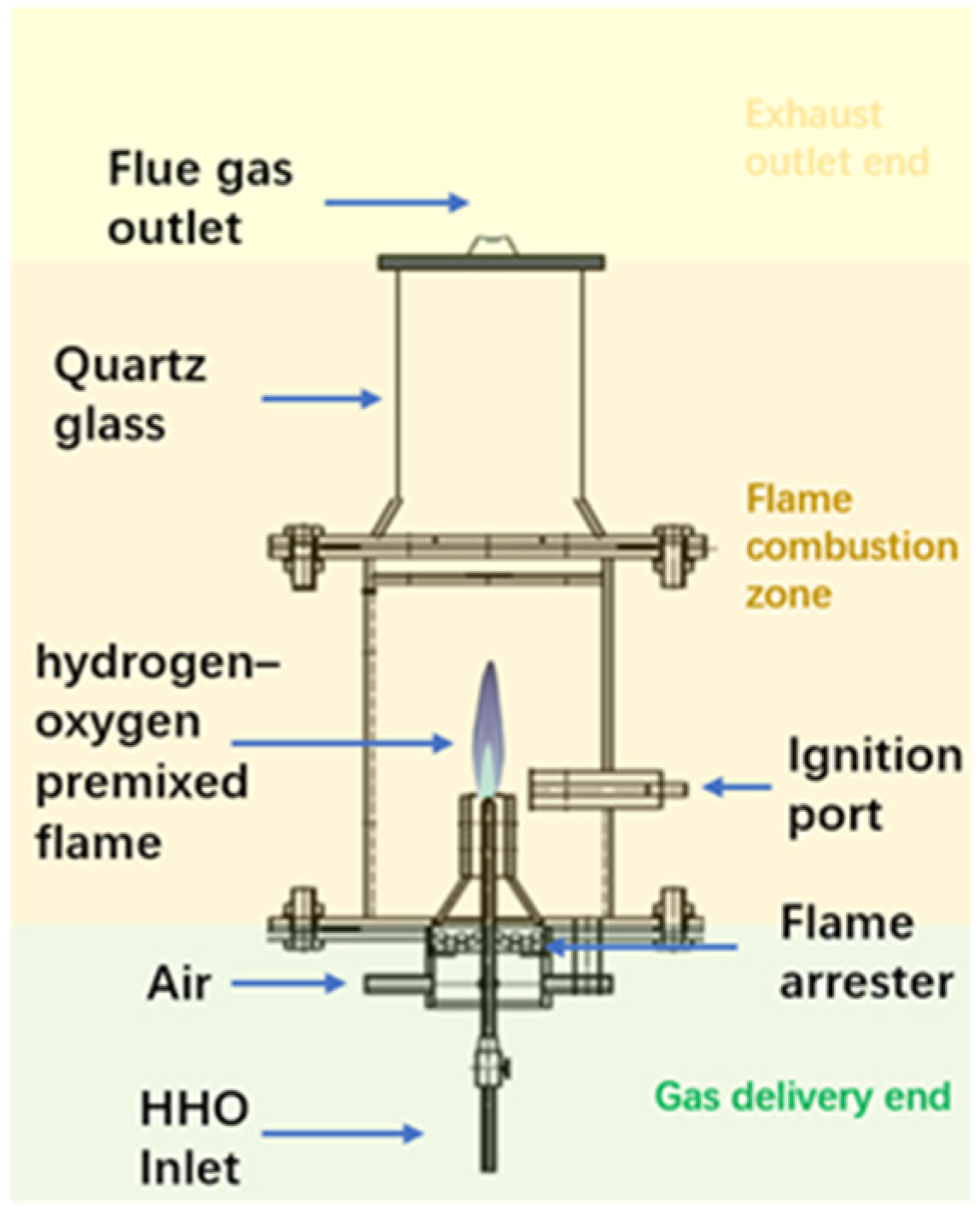
3.1. Experimental System
3.2. Theoretical Basis and Model Construction of Quantitative OH-PLIF Measurement
- (1)
- Relationship Between OH Fluorescence Signal and Radical Concentration
- (2)
- Absorption Spectroscopy and Two-Line Temperature Inversion Theory
- (3)
- Integrated Absorbance and Temperature Inversion Model
4. Experimental Scheme and Operating Conditions
4.1. Experimental Conditions and Operating Procedure
4.2. Laser System Configuration and Dye Wavelength Optimization
4.3. Image Acquisition Procedure and Operating Condition Selection Strategy
5. Conclusions
- (1)
- A quantitative calibration model for OH-PLIF based on absorption spectroscopy and dual-line temperature inversion was established. By introducing an explicit temperature-dependent absorption cross-section, the model achieved dynamic correction of the relationship between integrated absorbance and flame temperature.
- (2)
- Experimental calibration was conducted under different hydrogen-oxygen mixture flow rates (0.4–1.2 L/min). The results show that the calibration constant C fluctuates by less than ±5% across all conditions, with the optimal constant determined as Copt = 0.01844. The average relative error under validation conditions was maintained within 4–6%, demonstrating good applicability and consistency of the calibration results.
- (3)
- Compared with the uncorrected Matynia model, the overall error decreased from approximately 9.1% to 5.2% after applying the temperature correction, indicating that the inclusion of the temperature-dependent absorption cross-section significantly improves the quantitative calibration performance of OH–PLIF measurements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion, 5th ed.; Academic Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 3rd ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Eckbreth, A.C. Laser Diagnostics for Combustion Temperature and Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Lucht, R.P. Applications of laser-induced fluorescence spectroscopy to combustion diagnostics. Appl. Spectrosc. 1985, 39, 153–167. [Google Scholar]
- Zheng, Y.; Weller, L.; Hochgreb, S. Instantaneous flame front identification by Mie scattering vs. OH PLIF in low-turbulence Bunsen flame. Exp. Fluids 2022, 63, 180–191. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Aldén, M. Structure and Laminar Flame Speed of an Ammonia/Methane/Air Premixed Flame under Varying Pressure and Equivalence Ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, Q.; Tang, Y.; Li, S. Stabilization and Emission Characteristics of Gliding Arc-Assisted NH3/CH4/Air Premixed Flames in a Swirl Combustor. Energy Fuels 2022, 36, 8520–8527. [Google Scholar] [CrossRef]
- Allison, P.; Frederickson, K.; Kirik, J.W.; Rockwell, R.D.; Lempert, W.R.; Sutton, J.A. Investigation of flame structure and combustion dynamics using CH2O PLIF and high-speed CH chemiluminescence in premixed dual-mode scramjet combustor. AIAA Aerosp. Sci. Meet. 2016, 1, 441–452. [Google Scholar]
- Hanson, R.K.; Seitzman, J.M.; Paul, P.H. Planar laser-fluorescence imaging of combustion gases. Appl. Phys. B 1990, 50, 441–454. [Google Scholar] [CrossRef]
- Seitzman, J.M.; Hanson, R.K. Quantitative two-dimensional imaging of species concentrations in combustion gases using laser-induced fluorescence. Combust. Flame 1985, 61, 1–19. [Google Scholar]
- Li, B. Quantitative Measurement of OH Radical Concentration in n-Heptane Laminar Flame by PLIF Combined with Absorption Spectroscopy. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Chen, B.; Li, T.; Wang, Z.; Yang, F.; Zhang, C. Calibration of OH planar laser-induced fluorescence in methane–air premixed flames. Combust. Sci. Technol. 2017, 189, 1989–2003. [Google Scholar]
- Cheskis, S.; Goldman, A. Laser diagnostics of trace species in low-pressure flat flame. Prog. Energy Combust. Sci. 2009, 35, 362–382. [Google Scholar] [CrossRef]
- Matynia, A.; Idir, M.; Molet, J.; Roche, C.; de Persis, S.; Pillier, L. Absolute OH concentration profiles measurements in high-pressure counterflow flames by coupling LIF, PLIF, and absorption techniques. Appl. Phys. B 2012, 108, 393–405. [Google Scholar] [CrossRef]
- Jalbert, A.M. A Study of Quantitative Concentrations of Hydroxyl (OH) in Laminar Flat Flames Using Planar Laser Induced Fluorescence (PLIF). Master’s Thesis, Northeastern University, Boston, MA, USA, 2011. [Google Scholar]
- Luque, J.; Crosley, D.R. LIFBASE: Database and Spectral Simulation for Diatomic Molecules; SRI International Report: Menlo Park, CA, USA, 1999. [Google Scholar]
- Yao, Q. Optics: A Textbook, 3rd ed.; Higher Education Press: Beijing, China, 2002. [Google Scholar]
- Gordon, I.; Rothman, L.; Hargreaves, R.; Hashemi, R.; Karlovets, E.; Skinner, F.; Conway, E.; Hill, C.; Kochanov, R.; Tan, Y.; et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Demtröder, W. Laser Spectroscopy: Basic Concepts and Instrumentation, 4th ed.; Springer: Berlin, Germany, 2014. [Google Scholar]



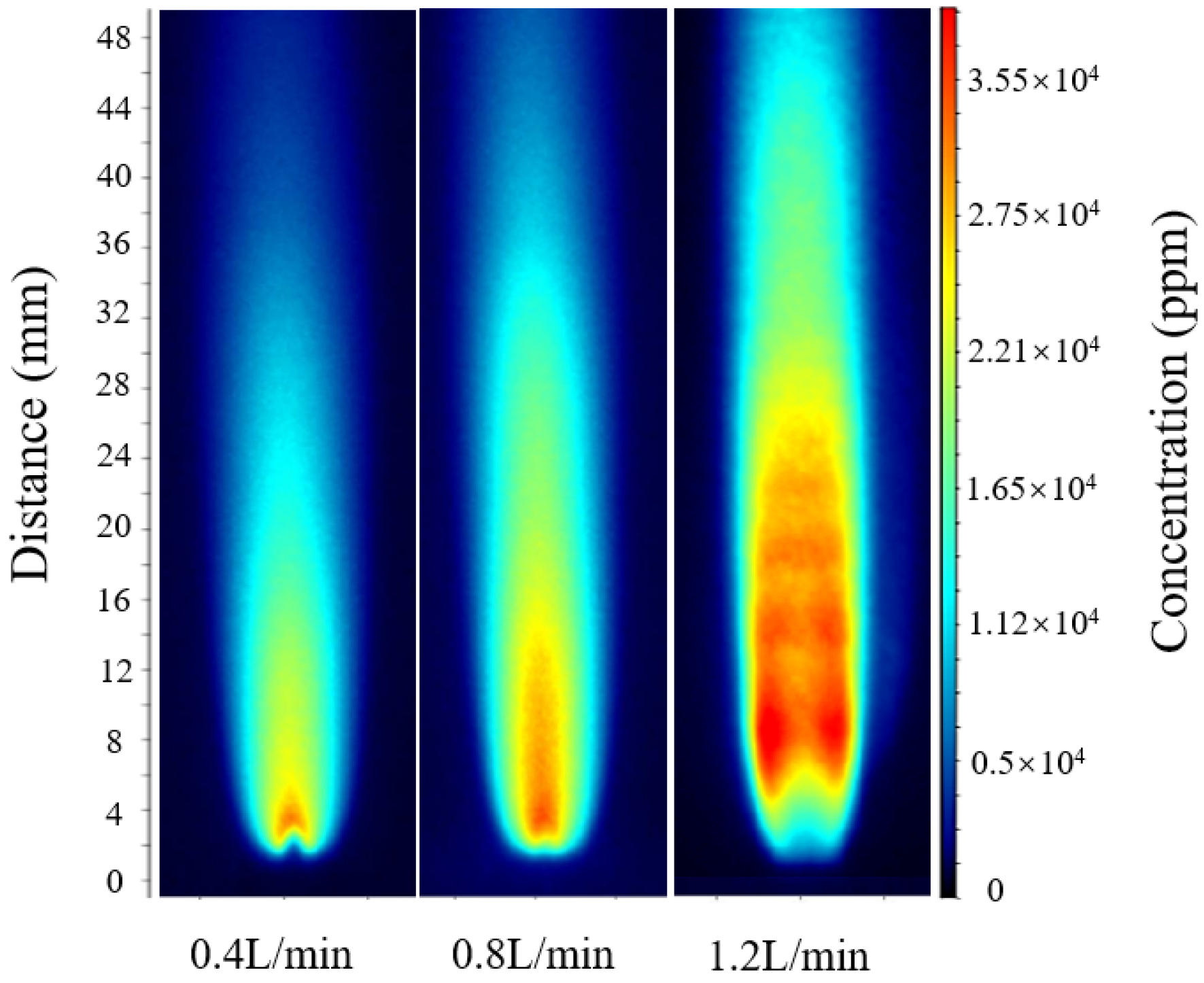




| Height (mm) | Hydrogen-Oxygen Mixture Flow Rate (L/min) | Fluorescence Intensity (283.455 nm) | Fluorescence Intensity (283.62 mm) | Temperature (K) |
|---|---|---|---|---|
| 4 | 0.4 | 385 | 534 | 2048.9 |
| 0.8 | 453 | 611 | 2269.3 | |
| 1.2 | 523 | 700 | 2340.3 | |
| 10 | 0.4 | 354 | 492 | 2027.4 |
| 0.8 | 424 | 585 | 2098.1 | |
| 1.2 | 476 | 651 | 2145.6 | |
| 16 | 0.4 | 335 | 467 | 2013.6 |
| 0.8 | 357 | 494 | 2058.7 | |
| 1.2 | 425 | 587 | 2077.5 | |
| 22 | 0.4 | 312 | 441 | 1997.8 |
| 0.8 | 346 | 478 | 2035.6 | |
| 1.2 | 350 | 481 | 2042.6 |
| Hydrogen-Oxygen Flow Rate (L/min) | Incident Energy (mJ) | Transmitted Energy (mJ) | ln(I0/I) | OH Intensity at 4 mm | OH Number Density (mol·m−3) | Volume Fraction (ppm) | C |
|---|---|---|---|---|---|---|---|
| 0.4 | 15.0 | 7.1 | 0.739 | 520 | 1.67 × 10−1 | 28,062 | 0.01853 |
| 0.8 | 15.0 | 6.8 | 0.793 | 580 | 1.77 × 10−1 | 33,320 | 0.017407 |
| 1.2 | 15.0 | 6.4 | 0.853 | 700 | 1.90 × 10−1 | 36,501 | 0.019178 |
| Experimental Condition | Hydrogen-Oxygen Mixture Flow Rate (L/min) |
|---|---|
| 1 | 0.2 |
| 2 | 0.4 |
| 3 | 0.6 |
| 4 | 0.8 |
| 5 | 1.0 |
| 6 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yang, X.; Cui, J.; Ma, R.; Yue, L.; Yin, Y.; Qi, J.; Xu, Y.; Xu, B.; Zhu, L. Improved Method for Quantitative Measurement of OH Radicals Based on Absorption Spectroscopy. Molecules 2026, 31, 118. https://doi.org/10.3390/molecules31010118
Yang X, Cui J, Ma R, Yue L, Yin Y, Qi J, Xu Y, Xu B, Zhu L. Improved Method for Quantitative Measurement of OH Radicals Based on Absorption Spectroscopy. Molecules. 2026; 31(1):118. https://doi.org/10.3390/molecules31010118
Chicago/Turabian StyleYang, Xiu, Jie Cui, Rui Ma, Lindan Yue, Yongzhuo Yin, Janhua Qi, Youning Xu, Benchuan Xu, and Liang Zhu. 2026. "Improved Method for Quantitative Measurement of OH Radicals Based on Absorption Spectroscopy" Molecules 31, no. 1: 118. https://doi.org/10.3390/molecules31010118
APA StyleYang, X., Cui, J., Ma, R., Yue, L., Yin, Y., Qi, J., Xu, Y., Xu, B., & Zhu, L. (2026). Improved Method for Quantitative Measurement of OH Radicals Based on Absorption Spectroscopy. Molecules, 31(1), 118. https://doi.org/10.3390/molecules31010118






