Abstract
Given the crucial roles of carbohydrates in energy supply, biochemical processes, signaling events and the pathogenesis of several diseases, the development of carbohydrate analogues, called glycomimetics, is a key research area in Glycobiology, Pharmacology, and Medicinal Chemistry. Among the many structural transformations explored, the replacement of endo- and exocyclic oxygen atoms by carbon (carbasugars) or heteroatoms, such as nitrogen (aza- and iminosugars), phosphorous (phosphasugars), sulfur (thiosugars), selenium (selenosugars) or tellurium (tellurosugars) have garnered significant attention. These isosteric substitutions can modulate the carbohydrate bioavailability, stability, and bioactivity, while introducing new properties, such as redox activity, interactions with pathological lectins and enzymes, or cytotoxic effects. In this manuscript we have focused on three major families of glycomimetics: thio-, seleno-, and tellurosugars. We provide a comprehensive review of the most relevant synthetic pathways leading to substitutions primarily at the endocyclic and glycosidic positions. The scope includes metal-catalyzed reactions, organocatalysis, electro- and photochemical transformations, free-radical processes, and automated syntheses. Additionally, mechanistic insights, stereoselectivity, and biological properties are also discussed. The structural diversity and promising bioactivities of these glycomimetics underscore their significance in this research area.
1. Introduction
Carbohydrates are ubiquitous biomolecules with essential biological functions across all domains of life. Beyond serving as a primary energy source and storage form, they play crucial structural roles (e.g., cellulose, chitin), and act as key building blocks in anabolic pathways, including nucleic acids biosynthesis [1]. Complex carbohydrates (glycans) [1] are also integral components of glycoconjugates such as glycolipids and glycoproteins, which are embedded in cell membranes and participate in critical signaling events, both endogenous and pathogen-mediated.
Moreover, carbohydrates serve as templates for the development of novel therapeutics [1] targeting metabolic disorders [2], cancer (e.g., carbohydrate-based vaccines) [3], infectious diseases [4], and drug delivery systems, including nanocarriers [5].
Given their immense biological significance, carbohydrate analogues, referred to as glycomimetics, have emerged as a promising research area, offering deeper insights into carbohydrate interactions, modulating bioactivity, and even introducing novel properties. Among the vast array of synthetic modifications applicable to carbohydrate structures, bioisosteric replacements of oxygen and carbon atoms deserve attention. The substitution of the endocyclic oxygen, or the anomeric carbon with carbon (carbasugars) [6], nitrogen (iminosugars) [7], phosphorous (phosphasugars) [8], sulfur (thiosugars) [9], selenium (selenosugars) [10] and tellurium (tellurosugars) [11] (Figure 1) has led to the development of an arsenal of compounds that have expanded the frontiers of Glycobiology research, and constitute some of the most relevant glycomimetics.
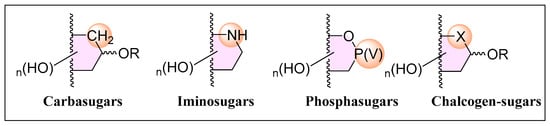
Figure 1.
General structure of relevant glycomimetics.
In this review, we will discuss recent advances in the development of chalcogen-containing glycomimetics and their implication in Glycoscience.
2. S-Containing Carbohydrates
Thiosugars represent a vast family of glycoconjugates in which either a hydroxyl group or the endocyclic oxygen is replaced by sulfur. This isosteric substitution induces significant conformational and physico-chemical changes in the sugar mimic. Due to its bigger atomic radius, sulfur forms longer C–S bonds compared to oxygen, resulting in smaller dihedral angles [12]. Additionally, sulfur has smaller electronegativity and a more hydrophobic character, while not exhibiting hydrogen bonding acceptance capacity, which affects water solubility [13]; the stronger sulfur-π interactions between thiosugars and lectins compared to oxygen can be useful in the design of effective drugs [14]. Many thiosugars exhibit substantially improved hydrolytic or enzymatic stability compared to their oxygen-containing counterparts, which is undoubtedly advantageous for the design of new drugs [9]. There are some examples found in nature, like 5-thio-d-mannose, thioglycosides (e.g., glucosinolates) and 1,4-thioanhydrosugars (e.g., kotalanol, salacinol, Figure 2) [15].
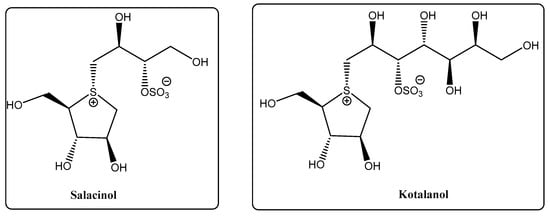
Figure 2.
Structure of salacinol and kotalanol.
2.1. 4′- and 5′-Thiosugars
Classical synthetic approaches for introducing a sulfur atom into a ring to give thiosugars have been recently reviewed [16]; one of the most successful approaches involves the introduction of a leaving group at the appropriate position of the sugar, and reaction with a sulfur-containing nucleophile. Herein, recent syntheses of thiosugars with practical interest will be reviewed.
Ueda and co-workers reported [17] the preparation of the sucrose analogues (+)-5-thiosucrose (5) and (+)-5-thioisosucrose (6) as potential sweeteners and glycosidase inhibitors. The key step of the synthesis was a stereoselective glycosylation reaction between a d-psicose or a d-fructose donor, respectively, and a 5-thio-d-glucose derivative (1 and 3) as acceptors (Scheme 1). Due to the strong anomeric effect induced by 1 and 3, they acted as α-directing glycosyl acceptors. Unfortunately, none of the compounds displayed appreciable activity as sweeteners or inhibitors [17].
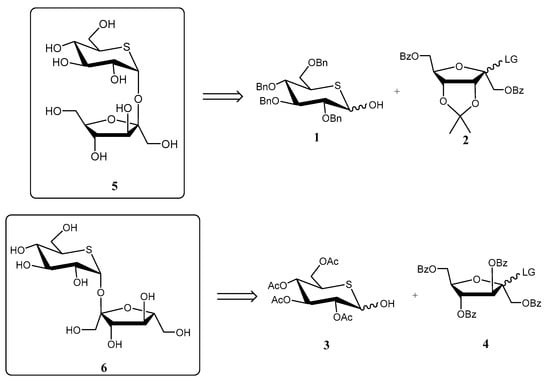
Scheme 1.
Retrosynthetic analysis for the preparation of (+)-5-thiososucrose (5) and (+)-5-thioisosucrose (6).
Díaz-Fernández and Pino-González reported [18] a simple approach for the synthesis of the thiomonosaccharide 10 from d-mannose. The synthesis involved the use of di-O-isopropylidene d-mannose 7, which was transformed into the diastereomeric E/Z mixture of oxime 8 using hydroxylamine hydrochloride under basic conditions. The hydroxyl groups of 8 were subsequently mesylated to produce compound 9. Finally, the mesylate group underwent an SN2 substitution reaction with Na2S, followed by intramolecular cyclization on the nitrile group, yielding the thiosugar 10 (Scheme 2).
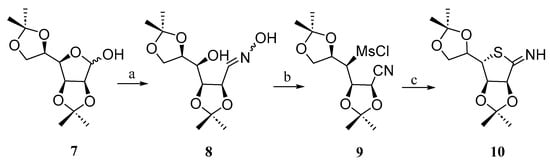
Scheme 2.
Synthesis of thiomonosaccharide 10. Reagents and conditions: (a) NH2OH·HCl, NaHCO3; (b) MsCl, 0 °C→r.t.; (c) Na2S·9H2O, DMF.
Liu and coworkers explored [19] the preparation of S-linked uronates (12–15) using a thiolation reaction (PhSH, BnSH) under alkaline and radical-mediated conditions on Δ4,5-unsaturated methyl uronate 11 (Scheme 3). The reaction demonstrated to be inefficient under radical conditions, which was attributed to the electron-withdrawing effect of the ester moiety located on C-5, although it proceeded with good regio- and stereoselectivity at C-4 and C-5 positions. Under basic conditions, good stereoselectivity was obtained at C-4, but, conversely, poor stereoselectivity at C-5, tentatively due to a retro thiol-Michael reaction. Attempts to obtain a S-linked pseudodisaccharide failed as, unexpectedly, when 13 was treated with a Lewis acid (AlCl3) to remove the benzyl group, the thiofuranoside 16 was obtained serendipitously (Scheme 3).
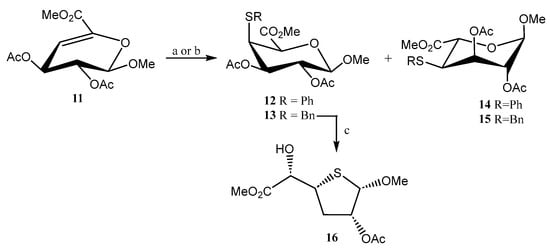
Scheme 3.
Synthesis of S-linked uronate derivatives and formation of the thiofuronoside 16. Reagents and conditions: (a) PhSH, BnSH; (b) BnSH, thermal/photo-inititator; (c) AlCl3, toluene, 48 h.
A family of thiosugars that has gained considerable attention is the sulfonium-containing carbohydrates, as they are analogues of the naturally occurring kotalanol and salacinol (Figure 2), both isolated from Salacia reticulata extracts, and known to be potent inhibitors of intestinal glycosidases [20]. These sulfonium glycomimetics are therefore promising candidates for the treatment of diabetes [21].
In this context, Takashima et al. hypothesized [22] that the incorporation of a hydrophobic moiety into the side chain of salacinol could enhance its inhibitory activity against glycosidases through favorable van der Waals interactions. To test this hypothesis, derivatives 19–24 were prepared (Scheme 4), the alkylation of O-protected thiosugar 17 using cyclic sulfates 18 being the key step. The final compounds were evaluated as inhibitors of rat maltase, sucrose and isomaltase, as well as human maltase. While no clear effects of the hydrocarbon residue were observed on maltase or isomaltase inhibition, sucrose inhibition displayed a length-dependent inhibitory activity, with longer appendages yielding the most potent compounds (IC50 = 0.15–0.73 µM for 19–22, 1.4 and 0.38 µM for 23 and 24). This resulted in an up to ten-fold increase in activity compared to parent salacinol [22]. Additionally, de-O-sulfonated analogues at C-3′ position (23 and 24) exhibited significantly stronger inhibition against isomaltase than 19–22, regardless of the hydrocarbon residue length.
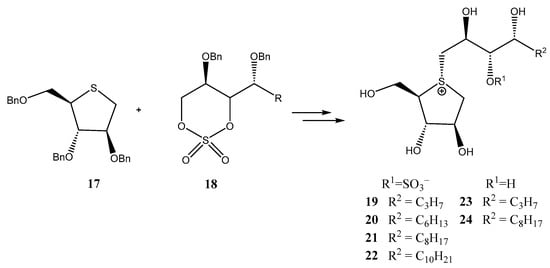
Scheme 4.
Preparation of salacinol derivatives with an elongated side chain.
With the aim of evaluating whether the 5-membered thiosugar is essential for the α-glucosidase inhibitory activity exhibited by sulfonium-based thiosugars, Tanabe et al. recently synthesized [23] ring-cleaved salacinol analogues (25–28) and truncated salacinol analogues (29–31) (Figure 3). This investigation was prompted by a previous report [24] demonstrating that some acyclic analogues derived from 1-deoxynojirimycin, a potent α-glucosidase inhibitor, retained activity, indicating that the five-membered ring may not be crucial for the inhibitory effect. The results for the ring-cleaved salacinol analogues revealed that these compounds were inactive, highlighting the essential role of the 5-membered thiosugar in their potent activity.
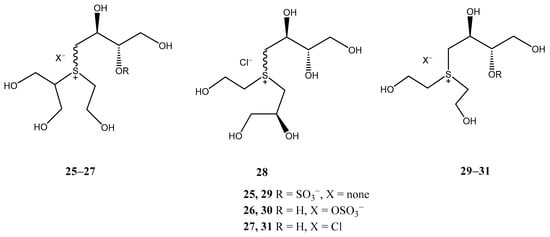
Figure 3.
Ring-cleaved salacinol analogues.
A frequent drawback found in the synthesis of sulfonium thiosugars as potential antidiabetic agents is the limited diastereoselectivity found in the alkylation reaction on the sulfur atom, hampering the scale-up of the process. Tanabe et al. reported [25] the alkylation of O-protected thiosugars with epoxides in hexafluoroisopropanol (HFIP), yielding a roughly 90% diastereomeric ratio (~26:1 α/β), which constitutes a 3-fold improvement compared to conventional methodologies. The excellent diastereoselectivity found was attributed to a cooperative mechanism composed of reversible S-alkylation and thermal isomerization. Using this methodology, derivatives 32 (Figure 4) were accessed and tested against human intestinal maltase. Those isomers with an ortho-substitution pattern exhibited strong inhibitory properties (IC50 = 0.11–0.58 µM). In vivo assays in mice revealed a high capacity for suppression of blood glucose, comparable to the antidiabetic drug voblibose [25].
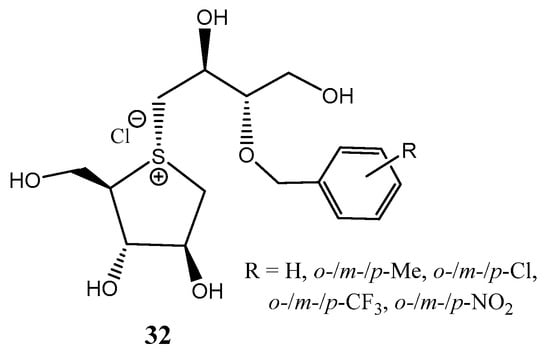
Figure 4.
Salacinol analogues with strong α-glucosidase inhibition.
2.2. Thioglycosides
Thioglycosides are one of the most popular glycosyl donors in glycosylation reactions, widely used in the synthesis of oligosaccharides to furnish 1,2-cis- and trans-linkages [26]. O-glycosides have also been obtained [27] in a stereoselective fashion starting from 1-mercapto-carbohydrates by reaction with an acid and a Cu or Co catalyst (Cu(acac)2, Co(acac)2), Ag2CO3 as the oxidant agent, and under microwave irradiation.
As aforementioned, thioglycosides exhibit a considerably higher stability towards acidic and enzymatic hydrolysis compared to natural glycosidic linkages; this feature is important for increasing their bioavailability when incorporated in bioactive compounds.
For instance, Banisalman et al. prepared [28] glycopeptides 33 by conjugating mono- and disaccharides to the cysteine residues of the peptide through a disulfide linkage, involving the glycosidic position of the carbohydrate residue (Figure 5).
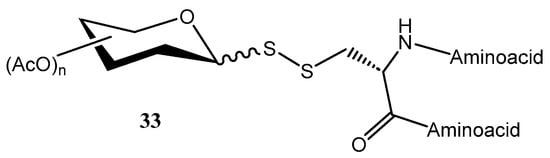
Figure 5.
General structure of disulfide-linked glycopeptides.
Incorporation of S-linked Manα1→2Man termini into oligomannose glycans (Man3, Man4) led to complete stability towards enzymatic hydrolysis mediated by Xanthomonas manihotis mannosidase [29]. 1-S-α-Galp(1 → 3)-β-Galp motif was incorporated [30] into oligosaccharides, leading to the isosteric unit of the epitope recognized by lytic antibodies in T. cruzi, the parasitic agent responsible for the Chagas disease.
An ample variety of thioglycosides have been reported as antiplasmodial [31] and antiviral agents [32], inhibitors of bacterial glycan biosynthesis [33], metabolic decoys of glycosylation [34], or heparanase inhibitors [35], among others; the latter ones are S-linked polysaccharides obtained via chemoenzymatic synthesis (heparosan synthase). Coumarin S-glycosides, obtained by thioglycoligase-mediated connection of 7-mercapto-4-methylcoumarins and p-nitrophenyl-d-glycopyranosides were claimed [36] to be potentially useful in bioimaging, due to their enhanced fluorescence emission properties compared to the parent coumarin.
A relevant example of the improvement of biological activity achieved by isosteric oxygen-sulfur substitution at the glycosidic position was recently reported by Gademann and coworkers [37]. The authors enhanced the acid stability of the natural glycosylated macrolactone Fidaxomicin (Fdx), approved for the Clostridioides difficile (C. diff.) infections; the authors replaced the O-glycosidic bond by the corresponding thioglycoside. Although the natural compound has shown promising activities against other pathogenic agents, its clinical use in other pathogens rather than C. diff. is hampered by its limited acid stability, making it difficult to treat, for example, stomach infections. Treatment of Fdx with Cu(ClO4)·6H2O as catalyst and the corresponding O-protected-1-mercapto carbohydrate, furnished a mixture of three regioisomeric glycosylated products (modest yields) at C-11 (<5%), C-13 (10%) and C-15 (24%) positions, with conserved β-configuration. Figure 6 displays the structure of the thio-isoster of Fdx, obtained as a minor compound. Degradation studies in methanolic HCl revealed that, whereas Fdx underwent degradation (t1/2 = 62.7 min), S-Fdx was stable over a period of 540 min [37]. Additionally, although the substitution slightly reduced the antibacterial activity, S-Fdx retained potent efficacy against C. diff. (Minimum inhibitory concentrations (MIC) ranges of 0.12–4 µg/mL) and Clostridium perfringens (MIC ranges of 0.06–0.5 µg/mL). Regioisomeric thioglycosides at C-13 and C-15 positions demonstrated to be inactive.
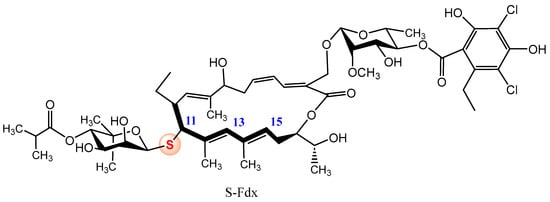
Figure 6.
Structure of thio-Fidaxomicin.
Classical synthetic methodologies reported for the preparation of thioglycosides include reaction of thiols with per-O-acetylated carbohydrates, using Lewis acids as catalysts, reaction of thiolates with acetohalosugars, or 1-thioglycoderivatives with alkyl halides. The building blocks involved in such transformations are depicted in Scheme 5.
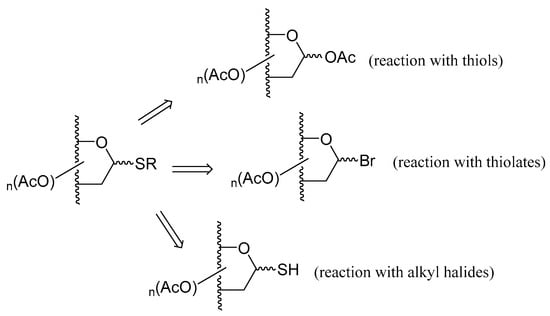
Scheme 5.
Classical procedures for preparing thioglycosides.
In the search for eco-friendly methods to synthesize thioglycosides, Luo and coworkers developed [38] an innovative methodology based on the use of phosphotungstic acid (PTA) as a catalyst for the reaction of per-O-acetylated saccharides 34 (d-galactose, d-xylose, l-fucose) and thiols under microwave-assisted conditions (Scheme 6). Notably, PTA proved to be highly reusable, maintaining yields of 84–88% across multiple reactions with a recovery efficiency exceeding 80%. Furthermore, methanolic PTA was successfully utilized for the one-pot de-O-acetylation of acetoxy compounds to give fully unprotected thioglycosides 35.
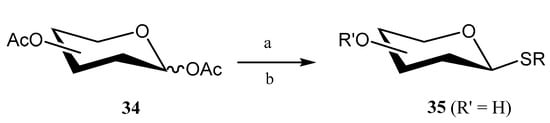
Scheme 6.
One-pot process for the thioglycoside formation and de-O-acetylation. Reagents and conditions: (a) PTA, HSR, DCM, MW, 30 min, 40 °C.; (b) PTA, MeOH, 90 °C, 4 h.
In order to avoid handling alkyl thiols, which are frequently malodorous and toxic, Dong and coworkers developed [39] an efficient and environmentally friendly protocol that consisted of the reaction of sodium alkanethiolates with per-O-acetylated carbohydrates 34 in the presence of BF3·OEt2 under solventless conditions (Scheme 7). Interestingly, 1,2-trans-thioglycosides 35 could be isomerized into challenging 1,2-cis species 36 by treatment with TfOH in non-polar solvents and under mild conditions (Scheme 7).
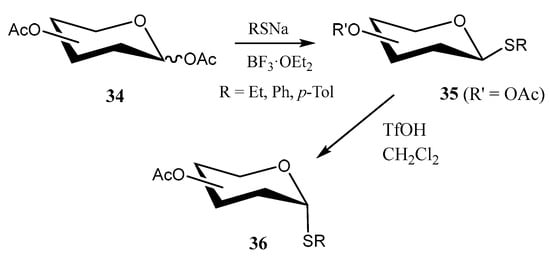
Scheme 7.
Synthesis of thioalkylglycosides.
Pd-catalyzed cross-coupling reactions have also been used for accessing thioglycosides. In this context, Domingues et al. employed [40] the third-generation Buchwald precatalyst (Pd-G3 Xantphos palladacycle) for functionalizing benzo-2,1,3-thiadiazole (BTD) at the C-5 position with 1-mercapto sugars 37 to give 39 (Scheme 8); the interest in such heterocyclic motif lies in the fact that it can be used as a fluorescent marker. The scope of the reaction was analyzed on pyranoses (d-Glc, d-Man, d-Gal, d-Xyl, l-Ara), furanoses (d-Rib) and disaccharides (maltose, lactose), and was found to proceed under mild conditions and to be compatible with an ample variety of functional groups. The yields were from good to almost quantitative, except for per-O-acetylated d-mannopyranose and d-ribofuranose, whose thioglycosides were obtained in modest yields; retention of the β-configuration was observed. The lowest yields could be significantly increased by changing the catalyst to Pd G3-MorDalphos. Products were deprotected using Zemplen-type conditions.
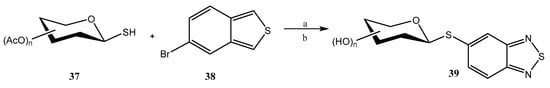
Scheme 8.
Pd-catalyzed C–S cross-coupling reaction between thiosugars and BTD. Reagents and conditions: (a) Pd catalyst 4-5 mol%, Et3N, 1,4-dioxane, 90 °C; (b) K2CO3 (cat.), MeOH.
Pd-catalyzed Migita cross-coupling reaction between a racemic mixture of o-iodo S-trifluoromethyl-S-arylsulfoximines 41 and a great variety of 1-mercapto sugars (mono-, di- and trisaccharides) has been used in the preparation of thioglycosides 42/43 [41]. For that purpose, PdG3-XantPhos was used as the catalyst, and Et3N as base (Scheme 9). A single β-anomer was obtained, in a 1:1 diastereomeric mixture, that was efficiently separated by either crystallization or HPLC.
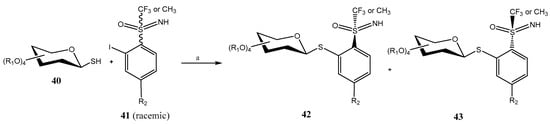
Scheme 9.
Migita cross-coupling reaction for the synthesis of thioglycosides. Reagents and conditions: (a) PdG3-XantPhos, Et3N (1.5 equiv), dioxane, 15–30 min.
O-Protected 1-thio-mono- and disaccharides 37 were efficiently coupled [42] with aryl-naphthoquinones 44 (Scheme 10) in the presence of 45, a chiral squaramide as the organocatalyst. The corresponding axially chiral thioglycosides 46 were obtained, in general, with high diastereoselectivity (14:1 to >19:1 dr). It was claimed that the hydrogen bonding established between the bifunctional organocatalyst and the quinone had a pivotal role in the activation of the substrate and the stereocontrol, locking a major conformation of the biphenyl residue at the transition state with the lowest sterical hindrance.
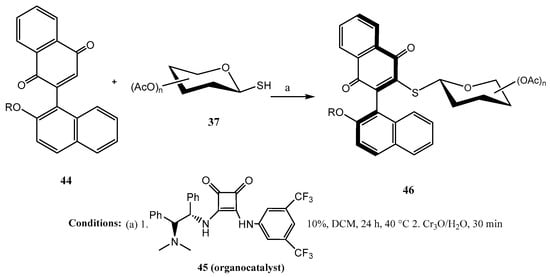
Scheme 10.
Stereoselective synthesis of naphthoquinone thioglycosides using a squaramide-based organocatalyst.
Isothiouronium salts are interesting synthetic intermediates, and can be obtained from fully O-acetylated carbohydrates 34 by treatment with BF3·OEt2 to give tentatively a 1,2-acyloxonium ion [43]. Reaction of the latter with thiourea as a nucleophile, affords the corresponding isothiouronium derivative 47, exclusively with the 1,2-trans-arrangement (Scheme 11). Hydrolysis of 47 under weakly basic conditions (Et3N) gives access to a transient thiolate (48), which exerts a 1,6 nucleophilic addition on p-quinone methide, with the subsequent formation of diarylmethyl thioglycosides 49.
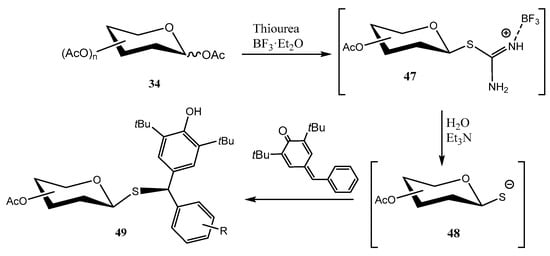
Scheme 11.
Synthesis of diarylmethyl thioglycosides.
Aryldiazonium salts are known for their inherent electrophilicity, and have been used in the formation of C–S bonds. Building on this property, Venkatesh et al. developed [44] a reaction between thiosugars 40 and activated aryldiazonium salts 50 (Scheme 12) to efficiently form C–S bonds (S-arylation, compounds 51). The optimized conditions involved the use of CuCl as the catalyst, DBU as the base, low temperatures (0–5 °C) and short reaction times (<5 min). Under these conditions, diazosulfide (R-S-N=N-Ar) is not observed, and only small amounts of the competing dimer (R-S-S-R) was obtained. The reaction was compatible with an ample number of protecting groups (NHAc, Ac, Bn, Bz), and even with unprotected carbohydrates; however, in this case, the ratio of the undesired dimeric disulfides increased [44]. It was postulated that the key intermediate is a free radical, obtained upon reaction of the aryldiazonium salt with the Cu(I) catalyst. This mechanism was demonstrated by the addition of a free radical scavenger (TEMPO), furnishing only traces of 51 [44].
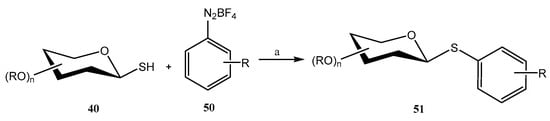
Scheme 12.
Synthesis S-aryl thioglycosides. Reagents and conditions: (a) CuCl (5%), DBU (1 eq), MeCN, 0–5 °C.
This approach enabled the successful synthesis of thio-analogues of bioactive compounds, including the antidiabetic agent dapagliflozin (52) [45,46], and the tyrosinase inhibitor arbutin (53) (Figure 7).
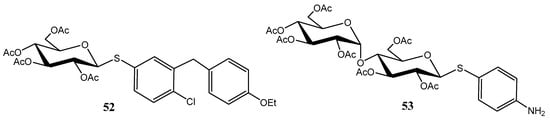
Figure 7.
Thio-analogues of dapagliflozin and arbutin.
A stereospecific metal-free synthesis of aryl thioglycosides 51 was achieved by using a boron catalyst for promoting a reductive deoxygenation coupling reaction between O-protected α-acetobromohexoses 54 (also di- and trisaccharides) and sulfonyl chlorides 55 (Scheme 13) [47]; B2pin2 (Bis(pinacolato)diboron) and PPh3 were used as additives. This afforded exclusive β-stereoselectivity, even with challenging β-mannosides and β-rhamnosides. This methodology also enabled the preparation of thioglycoconjugates of bioactive and natural compounds including naproxen, ibuprofen, indomethacin and lithocholic acid.
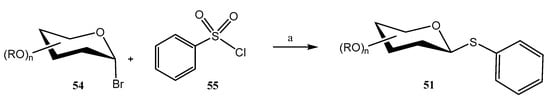
Scheme 13.
Synthesis of boron-mediated arylthioglycosides. Reagents and conditions: (a) B2pin2 (2.5 equiv), PPh3 (3.0 equiv), KOH (4.0 equiv), DME, 25 °C.
Synthetic organic electrosynthesis involving cross-coupling reactions has emerged as a valuable strategy [48], particularly useful for forming new C–C, C–O, C–N, and C–S bonds. In connection to the preparation of thioglycosides, O-protected 1-thiosugars 40 were coupled to preactivated phenols and ketones (aryl/alkenyl triflates) (Scheme 14A) [49], aryl/alkenyl/alkynyl bromides (Scheme 14B) [50], or xanthenes/heteroxanthenes (Scheme 14C) [51] to give the corresponding β-configured thioglycosides 51, 56, 57, 58. Reactions A and B from Scheme 14 were catalyzed by Ni (NiBr2·diglyme), requiring a ligand (di-tBubpy) and an additive (LiBr), and were performed in undivided cells. In a plausible mechanism, some of the key steps would be the cathodic reduction of the Ni salt to Ni(0), the oxidative addition into the triflates/bromides, and the single electron oxidation of 40 to give a thiyl free radical [49].
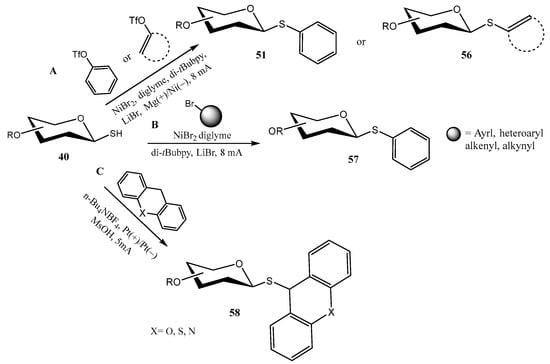
Scheme 14.
Electrochemical synthesis of thioglycosides.
On the contrary, reaction C did not require a metallic catalyst; n-Bu4NBF4 was used as the electrolyte, and MsOH as an additive. Control experiments and cyclic voltammetry assays suggest that both the thiol group and the xanthene underwent an anodic oxidation, to give free radicals (the latter at the benzylic position); cross-coupling reaction between both free radical species would furnish thioglycosides 58 [51]. Reduced current intensity compared to pathways A and B (5 mA vs. 8 mA, Scheme 14) proved to be essential, due to the instability of 58 at higher currents.
Photoredox processes have also emerged as a valuable alternative to classical S-glycosilation reactions, as they can be conducted under mild conditions, and at late synthetic stages, due to their compatibility with an ample variety of functional groups. In this context, the contributions of Messaoudi’s [52] and Ji’s [53] groups are remarkable. O-Protected sugar-derived thiols 40 could be transformed into aryl thioglycosides 51 (Scheme 15A) using reactive aryl radicals obtained from in situ generated diazonium salts (anilines + tBuONO) upon irradiation with visible light (blue LEDs) in the presence of a photoredox catalyst ([Ru(bpy)3](PF6]2) [52], through a Stadler-Ziegler reaction. Diazonium salt is hypothesized to react with sugar-derived thiol to give a photolabile diazosulfide (Sugar-S-N=N-Ar). Next, a single electron transfer (SET) process takes place from the excited state of the photocatalyst to the diazosulfide, which releases N2, a thiolate and a reactive aryl free radical. The combination of these reactive species furnishes a thioether radical anion, which through a second SET process gives the thioglycoside and restores the Ru-based catalytic cycle [52].
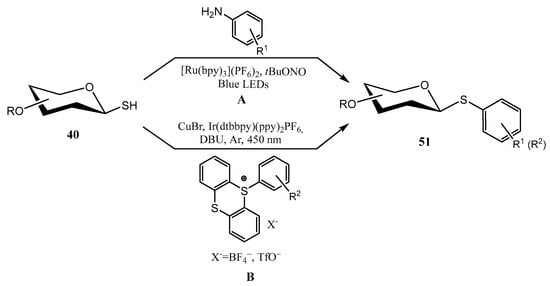
Scheme 15.
Photoredox reactions for the synthesis of thioglycosides.
The photoredox formation of thioglycosides using thianthrenium salts (TT) as aryl donors and 1-thiosugars 40 has also been explored [53] (Scheme 15B). Optimization of the reaction conditions indicated that the use of Ir(dtbbpy)(ppy)2PF6 as the photoredox catalyst, CuBr as co-catalyst, DBU as base and irradiation under blue light (450 nm) achieved the best results. A wide scope was also found, as it was compatible with numerous electron-donating/withdrawing substituents, mono-and disaccharides, and proved to be efficient also for the derivatization of natural products/drugs. These include derivatives of estrone, gemfibrozil, pyriproxyfen, or flurbiprofen [53].
The proposed mechanism for the photoredox formation of thioglycosides 51 from TT is depicted in Scheme 16 [53]. Control experiments revealed that, analogously to what was found to the preparation of thiolglycosides from aryldiazonium salts (Scheme 15, pathway A), aryl and thiyl free radical species (E and C) were involved. Thiolate anion was postulated to quench the excited state of the photocatalyst, to give IrII and the thiyl radical. Subsequently, upon a SET process between the quenched catalyst and the thianthrenium salt, the aryl radical intermediate E was generated. Additionally, the CuI-thiol complex A was oxidized by C to give the corresponding CuII complex D. Thioglycoside 51 was then obtained after oxidative ligation between the aryl radical E and D, followed by reductive elimination (Scheme 17) [53].
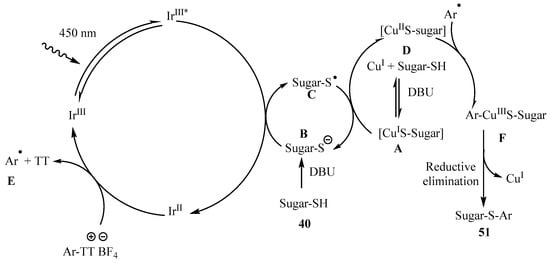
Scheme 16.
Plausible mechanism for the photoredox preparation of thioglycosides 56.
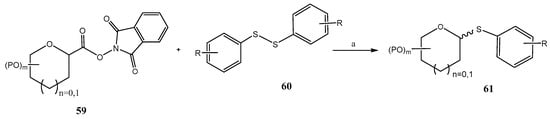
Scheme 17.
Synthesis of thioglycosides from glycosyl redox-active species. Reagents and conditions: (a) DIPEA, DMSO, 12 W LEDs, r.t.
Another recent application of photoredox processes in the preparation of thioglycosides was recently reported by Zhang et al. (Scheme 17) [54]. In this approach, that does not require metal photocatalysts, glycosyl redox-active esters 59 and disulfides 60 were irradiated with a blue LED (455 nm) in the presence of DIPEA to give thioglycosides 61 with acceptable stereoselectivity. Instead of a SET process, commonly proposed when metal photocatalysts are present (e.g., Scheme 16), a photoactive electron-donor-acceptor (EDA) complex between 59 and DIPEA was proposed as the key transient species.
Figure 8, Figure 9 and Figure 10 depict three families of thioglycosides endowed with relevant bioactivities: naphthalene diimide (NDI) conjugates (62–69) [55], N-heterocyclic carbenes (NHCs) [56,57] (70–75), and multivalent β-thio-glycoclusters (76–78) [58].
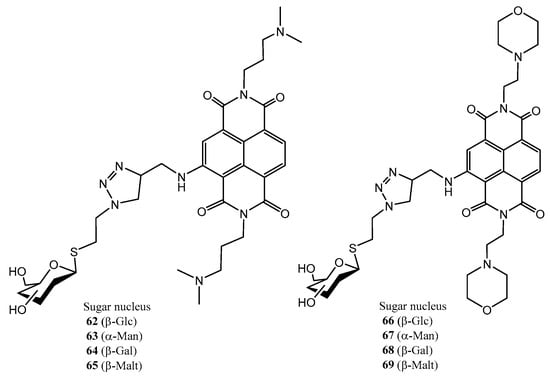
Figure 8.
Thiosugar-NDI conjugates as G-quadruplex ligands.
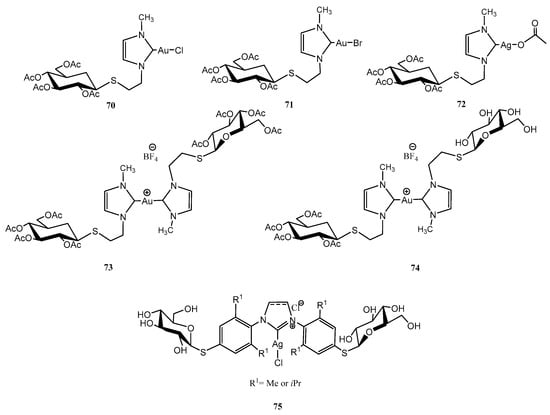
Figure 9.
Bioactive NHCs containing thioglycosides.
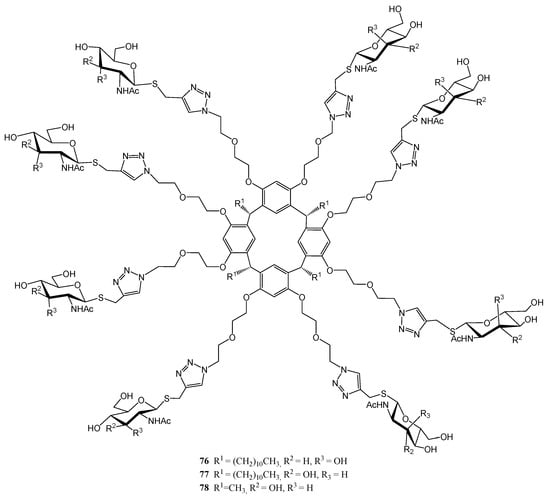
Figure 10.
Structure of multivalent β-thio-glycoclusters.
Naphthalene diimide derivatives (NDI) were designed as ligands of the G-quadruplex, a guanine tetrad located in key regions of the genome; binding to G-quadruplex can confer anticancer and antiparasitic properties. The carbohydrate residue may be recognized by the glucose transporters (GLUT), overexpressed in cancer cells, whereas the thioglycosides-type linkage can provide enhanced hydrolytic and enzymatic stability [55]. Notably, dimethylamino derivatives 62–65 exhibited improved cytotoxicity against both, colon cancer cells and parasites (T. brucei and L. major), compared to morpholine-containing counterparts 66–69 (Figure 8); this was attributed to a less efficient binding to G-quadruplexes of the latter compounds. Thioglycosides, although exhibiting a similar potency against the cancer cell compared to their bioisosteric O-glycosides, showed a better selectivity against non-tumour cells. Thiomaltosyl derivative 65 was found to be the best compound (submicromolar activity against colon cancer cell, selectivity index = 9.8). This compound also had nanomolar activity against T. brucei [55].
NHCs, monodentate ligands that act as two-electron donors, are analogous to phosphines, although with stronger donation capacity; structural modifications can tune their stereoelectronic properties [59]. Numerous Ag/Au-NHC complexes have shown anticancer properties, like those depicted in Figure 9. Ag-NHC complexes 70–74, although less stable in aqueous solutions than Au-counterparts, exhibited superior cytotoxicity against ovarian A2780 cancer cells (IC50 = 1.1–1.3 μM, similar to the chemotherapeutic agent cisplatin, IC50 = 1.5 μM) [56]. Au-NHC complexes demonstrated strong binding properties to thiol-rich biomolecules, acting as potent inhibitors of thioredoxin reductase (TrxR).
A Pd-catalysed Migita cross-coupling reaction and a mechano-chemistry approach were used for the preparation of Ag-NHC thioglycosides 75 (imidazolium and imidazolinium) [57]. Imidazolinium derivative with R1 = Me exhibited the best antiproliferative activity against HCT116 tumour cells (colon, 41% viability at 10 µM concentration).
Cristófalo et al. reported [58] the preparation of calix[4]resorcinarene-based octavalent ligands 76–78 (Figure 10), bearing β-S-GlcNAc (N-acetylglucosamine) and, for the first time, β-S-AllNAc (N-acetylallosamine) residues as recognition elements for the lectin Wheat Germ Agglutinin (WGA). These compounds were obtained via copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction between the corresponding alkynyl thioglycosides and the octa-azido scaffold derived from resorcinarene. Turbidimetry and isothermal titration calorimetry assays revealed that the highest affinity was found for allosamidine-derivative 76 [58], a hitherto unknown property for such monosaccharide. This behavior was explained through in silico calculations.
Thioglycosides in Pseudo-Disaccharides and Pseudo-Oligosaccharides
The thioglycosidic linkage has also been employed to connect a second sugar unit through different positions, and thus, to obtain pseudo-disaccharides and pseudo-oligosaccharides.
In this context, Misra’s group developed [60] a simple two-step methodology for preparing pseudo-disaccharides connected through a thioglycosidic linkage. The approach involves treating per-O-acetyl-α-d-mannopyranosyl bromide (79) or per-O-acetyl-β-l-rhamnopyranosyl bromide (80) with a mixture of Na2S·9H2O and CS2 for a short time (5 min); 1-mercapto derivatives 81 and 82 were obtained, respectively. Subsequent addition of an electrophilic carbohydrate, like 6-iodo-glucopyranoside 83, and Et3N gave β(1→6) pseudo-disaccharides in good yield (Scheme 18).
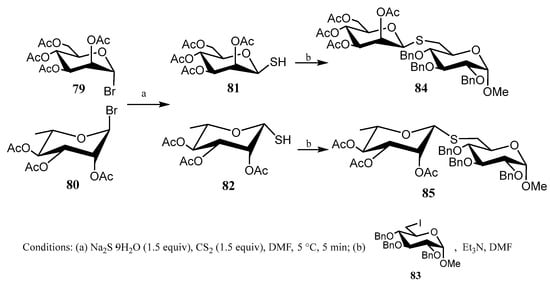
Scheme 18.
Synthesis of pseudo-disaccharides from 1-thio-β-d-mannosides and 1-thio-β-l-rhamnosides.
Photoinitiated thiol-ene coupling reactions were used by Borbás and co-workers for preparing S-linked pseudo-disaccharides [61]. These glycomimetics were obtained by UV-induction of hydrothiolation reactions between unsaturated sugars bearing an exocyclic double bond at C1, C2, C3, C4, C5 and C6 positions and a thiol-containing sugar (Scheme 19). 2,2-Dimethoxy-2-phenylacetophenone (DPAP) was used as the photoinitiator. Reaction proceeds in a two-step pathway: reversible addition of the thiyl free radical, and the irreversible donation of hydrogen atom from a thiol; although the stereoselectivity strongly depends on the carbohydrate involved, a preferential axial H-transfer is frequently observed, with the thiol substituent occupying the equatorial position [61].
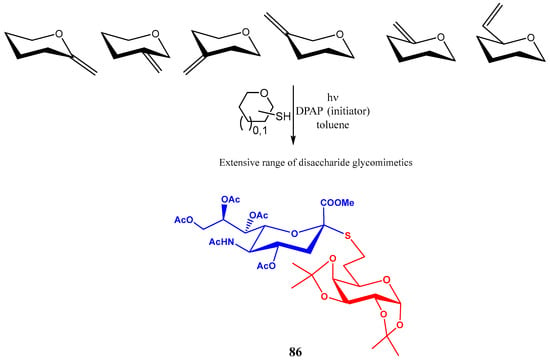
Scheme 19.
Reactions of enopyranoses bearing an exocyclic double bond.
Using this methodology, the sialyl thioglycoside 86 was obtained in good yield (Scheme 20).
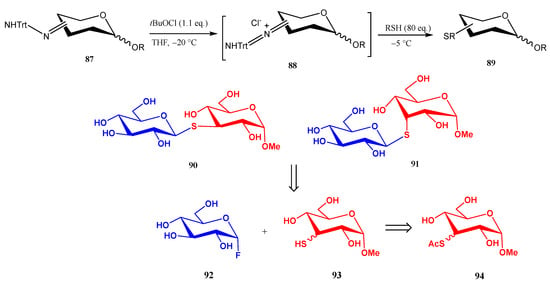
Scheme 20.
Synthesis of thiosugars from sugar-derived trityl hydrazones.
A straightforward alternative for preparing O-unprotected S-linked pseudo-disaccharides consists of the preparation of sugar-derived trityl hydrazones 87, which upon treatment with tBuOCl generate a chloro-azo intermediate on the sugar moiety [62]. Thermolysis of such intermediate in the presence of an excess of simple thiols generates thioethers 89 in up to almost quantitative yield (Scheme 20).
This methodology was applied to the preparation of pseudo-disaccharides 90 and 91, which were obtained by treatment of trityl hydrazone 87 with AcSH to give 94, followed by hydrolysis of the acetyl moiety, and coupling of the corresponding 3-thiols 93 with glucopyranosyl fluoride 92 (Scheme 20) [62].
Automated syntheses have also been applied to the selective incorporation of S-glycosides into oligomannopyranosides [63] using an inverse glycosylation protocol instead of the conventional methodology. The synthesis of trisaccharide 97 was accomplished with good yield (73%) employing reactive glycosyl acceptor 95, glycosyl donor 96, and TMSOTf as the promoter (Scheme 21). The process comprised three sequential cycles of glycosylation coupling, deprotection, and purification. The automated protocol was executed on a platform equipped with a robotic arm and dual syringe pumps, enabling precise delivery of reagents to an array of double-jacketed reaction vessels. The reaction setup was maintained under an inert atmosphere, ensuring controlled and reproducible conditions for each step of the synthesis [63].
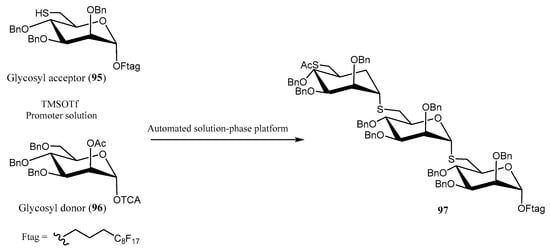
Scheme 21.
Automated approach for the synthesis of oligomannopyranosides.
2.3. 3-Thiosugars
Incorporation of a sulfur atom at C-3 position of a sugar residue has been used for accessing valuable synthetic intermediates, as well as for preparing derivatives with potential biological activities [64]. Despite that, this kind of sulfur-containing carbohydrates is significantly less studied compared to other positions on the sugar, like the endocyclic oxygen, or the glycosidic position. A frequent approach for accessing 3-thiosugars is the reaction of glycals with different thiols and catalysts, as displayed in this section.
Mukherjee et al. reported [65] the biomimetic synthesis of a family of 3-thiosugars (100, Scheme 22) via reaction of 2-ketophenyl-glycals 98, 99 (d-glucal, d-galactal, l-rhamnal) with different thiols. The regio- and stereoselective displacement of the acetoxy group at the C-3 position of the glycal was achieved using aromatic and cyclic aliphatic thiols under mild basic conditions (Et3N), furnishing excellent axial selectivity. The presence of a carbonyl group at C-2 was found to be crucial [65]. It was hypothesized that the thiol attacks the carbonyl group at C-2, and then it undergoes a 1,3-migration through the opposite face of the acetoxy group at C-3. This process was inspired by the thiolation of glucosamine by cytosolic esterases.
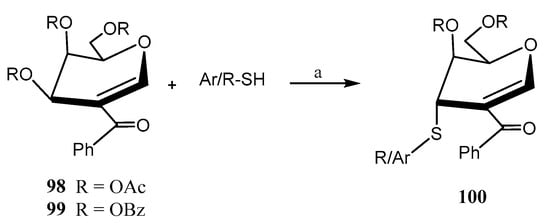
Scheme 22.
Synthesis of a library of 3-aryl/alkyl thiosugars prepared from 2-ketoglycals. Reagents and conditions: (a) Et3N (1 equiv.), MeCN, r.t.
Liu and coworkers reported [66] the stereo- and regioselective synthesis of thiosugars by reaction of 3,4-O-carbonate glycals 101 with different thiols; competitive experiments revealed that the thiol group reacted with total chemoselectivity in the presence of other nucleophilic moieties, like alcohols, phenols, amides, or amines. A regiodivergent approach was developed, as depending on the catalyst employed, either 3-thiosugars (with Co(BF4)2, axial position, compound 102) or 1-thiosugars (with Pd2(dba)3, equatorial position, compound 103) were obtained, with general good yields (Scheme 23). Based on computational calculations, it was hypothesized that under Pd catalysis, the thiol group established hydrogen bonding on the top face with the oxygen atom at C-4 position. Nevertheless, coordination with Co took place through the bottom face.
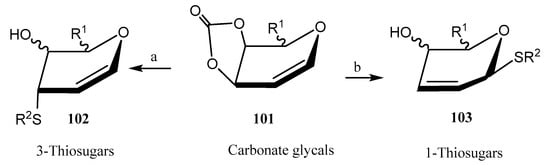
Scheme 23.
Synthesis of 1- and 3-thiosugars using Co and Pd-based catalysts. Reagents and conditions: (a) RSH, Co(BF4)2 catalyst, CH2Cl2, 35 °C; (b) RSH, Pd2(dba)3 catalyst, 100 °C.
Chen and co-workers noticed [67] that per-O-acetylated unnatural monosaccharides and the cysteine residues on proteins undergo an atypical glycosylation process, yielding 3-thiolated sugars in their hemiacetal form. In their study, the authors elucidated an elimination-addition mechanism, which involves a base-promoted β-elimination step followed by a Michael addition of the cysteine residue to the α,β-unsaturated aldehyde. (Scheme 24). This process, called S-glycosylation, might compromise the specificity of the metabolic glycan labelling (MGL) used, for example, in glycan tagging with fluorophores.
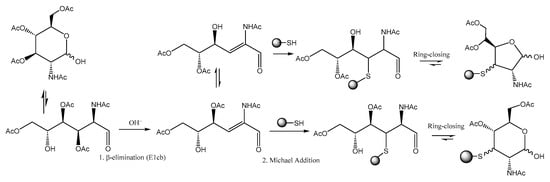
Scheme 24.
Proposed two-step elimination–addition mechanism for S-glyco-modification.
2.4. Other Thiosugars
1,6-Anhydro-1-thio-β-d-hexopyranose derivatives are relevant molecules in Medicinal and Synthetic Chemistry, functioning as precursors of glycomimetics. Misra’s group has developed [68] a fast, effective, and scalable method for their synthesis by treatment of protected 6-O-tosylated glycopyranosyl bromide derivatives 104 with Na2S·9H2O (2 equiv.) and CS2 (2 equiv.) at rt (Scheme 25). The reaction was completed in just 5 min, yielding the corresponding thiolevoglucosan derivatives 105 with yields ranging from 82% to 92%.
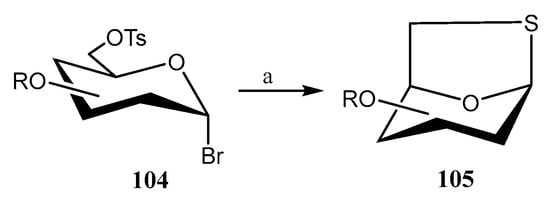
Scheme 25.
Synthesis of 6-anhydro-1-thio-β-d-hexopyranose derivatives. Reagents and conditions: (a) Na2S·9H2O (2 equiv), CS2 (2 equiv), DMF, r.t.
Using the same combination of reactants, the authors extended [69] the methodology to synthesize unsymmetrical glycosyl disulfides directly from glycosyl bromides by incorporating symmetrical disulfides. Under optimized conditions, a series of anomeric glycosyl bromides 54 were treated with symmetrical alkyl, aryl, and glycosyl disulfides, affording unsymmetrical β-glycosyl disulfides 106 in yields ranging from 72% to 90% (Scheme 26).
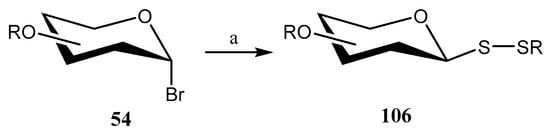
Scheme 26.
Synthesis of unsymmetrical glycosyl disulfides. Reagents and conditions: (a) CS2 (2 equiv.), Na2S·9H2O (2 equiv.), RSSR, DMF, 0 °C, rt, 5–20 min.
3. Se-Containing Carbohydrates
The incorporation of selenium into organic frameworks has led to the development of potent bioactive compounds with antioxidant [70], antiviral [71], antiparasitic [72], anti-Alzheimer’s [73,74,75], or anticancer properties [76,77,78,79,80], among others. Combining the diverse biological activities of selenium with the unique structural features of carbohydrates offers a promising strategy for the design of novel drug candidates with enhanced therapeutic potential.
3.1. 4′- and 5′-Selenosugars
Interest in replacing the endocyclic oxygen atom in carbohydrates by selenium dates back to the 1970’s. However, most early attempts were unsuccessful, either yielding undesired by-products, or producing the desired selenosugars in very low yields (e.g., derivative 107 (Figure 11) [81]. Later, Schiesser and co-workers, in the search for water-soluble antioxidants, pioneered [82] a more practical approach for synthesizing selenosugars (108–110). This was achieved through the thermolysis of per-O-bezylated-5-benzylseleno formates of d-ribo-, xylo- and arabino-configurations. Their method involved an intramolecular attack of the benzylseleno scaffold, and elimination of CO2 and phenylselenoate.
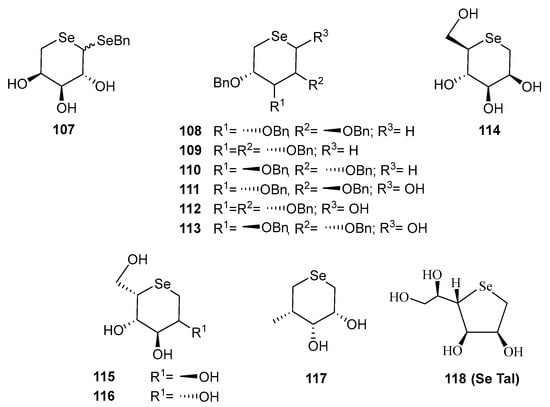
Figure 11.
Structures of some of representative examples of selenosugars.
To prepare reducing selenosugars, the same group reported [82] the SmI2-mediated transformation of O-protected 5-benzylseleno aldoses into derivatives 111–113 via an intramolecular homolytic substitution [83]. Nevertheless, only d-arabino-configured 113 was obtained in pure form and with moderate yield. Unfortunately, none of such compounds could be successfully deprotected.
Liu and Pinto developed [84] a procedure for achieving unprotected selenofuranoses and pyranoses by using acetals as protecting groups in their efforts to synthesize selenonium sulfates as analogues of natural salacinol and kotalanol (Figure 2), potent α-glucosidase inhibitors. Their strategy for accessing the selenosugars was based on four steps: appropriate O-protection of the starting carbohydrate with isopropylidene groups, reduction of the latent aldehyde of the reducing sugar, di-O-mesylation and double nucleophilic displacement with in situ generated Na2Se (treatment of elemental selenium with the appropriate number of equivalents of NaBH4), as depicted in Scheme 27 [84]. This simple synthetic pathway inspired Schiesser’s and other groups to access a wide variety of pyranoses and furanoses with diverse configurations. These included compounds like 114–116, 118 [83,85], which are efficient scavengers of hypohalous acids and 117, a good mimetic of glutathione peroxidase (GPx) [86].
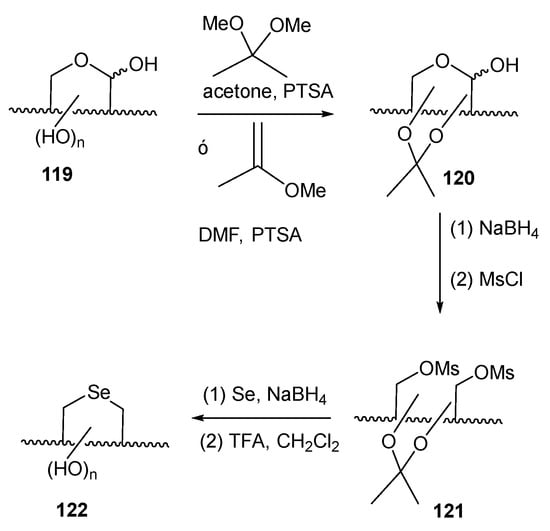
Scheme 27.
General procedure for the preparation of unprotected selenosugars.
Undoubtedly, due to its synthetic accessibility, the most extensively studied compound in this series is 1,4-anhydro-4-seleno-d-talitol (SeTal, 118, Figure 11). It has been considered as a privileged structure because of the diverse biological properties it exhibits. This water-soluble compound has demonstrated [87] potent scavenging activity against oxidizing agents, like HOCl and HOBr, the former being produced by the enzyme myeloperoxidase (MPO) to eliminate pathogens from inflamed tissues. Alongside other analogues with different configurations, SeTal and its derivatives have shown antioxidant potency up to twice that of their sulfur-counterparts. Moreover, 118 has been shown to repair damaged skin tissues in animal models, including diabetic wounds [87]. SeTal exhibits remarkable stability under acidic conditions (as those found when drugs have an oral administration) and also in artificial gastric or intestinal fluids, achieving steady-state intracellular concentrations ranging 2–10 μM. However, the exact internalization mechanism remains undetermined [88]. In vitro experiments have revealed that SeTal protects against oxidative damage in human coronary artery cells and mouse aortic rings [88]. Interestingly, topical application of 118 to induced skin injuries in mice modulated inflammatory markers [89]. Furthermore, its incorporation into gelatin and alginate polymeric films, either alone, or in combination with hydrocortisone or vitamin C, has recently shown promise as a treatment of atopic dermatitis [90]. Studies on its potential hepatotoxicity revealed [91] that SeTal only affects hepatic cells viability at very high concentrations, much higher than its potential therapeutic dose, making it a promising candidate for drug development.
Another significant contribution to the field of water-soluble selenosugars was made by Iwaoka and Tomoda. They synthesized trans-dihydroxy selenonane 124 (DHSred) and diselenane 126 by nucleophilic opening of racemic 1,3-butadiene diepoxide with NaHSe or Na2Se2, respectively (Scheme 28) [92]. DHSred was shown to mimic the catalytic cycle of GPX by reducing H2O2 in the presence of a thiol-containing compound as a cofactor such as dithiothreitol (DTT) [93]. GPx is a metalloenzyme that maintains the homeostasis in Reactive Oxygen Species (ROS) levels by eliminating H2O2 and alkyl peroxides, with glutathione (GSH) as a cofactor. The proposed catalytic cycle in aqueous media involves the slow oxidation of DHSred with H2O2 to produce the selenoxide 128, which is subsequently reduced by DTT to give DSHred [93] (Scheme 29A).
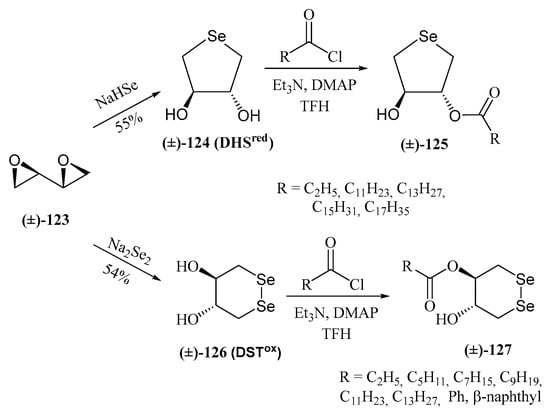
Scheme 28.
Preparation of trans-dihydroxy selenonanes and diselenanes and lipophilic derivatives.
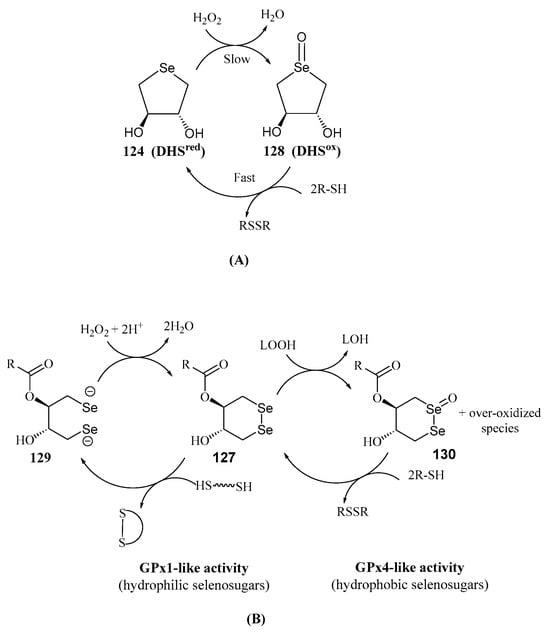
Scheme 29.
Catalytic cycles for the GPx-like activity of DHSred (A) and DSTox (B).
DSHred exhibited superior ROS scavenging capacity compared to its non-cyclic isomer. The enhanced activity was attributed to increased HOMO energy due to the strain caused by the cyclic structure [94], facilitating oxidation. Unexpectedly, when GPx-like activity was tested in MeOH, the reaction became much more complex. Over-oxidized species, like hydroxyselenonium and hydroxy perhydroxyselane were suggested to play an active role in the scavenging process [95].
DHSred also demonstrated [96] radio-protective effects in mice when administrated intraperitoneally before and after the irradiation with 60Co. Reduced DNA damage, decreased lipid peroxidation, and down-regulation of pro-inflammatory genes were observed [96]. These effects were similar to those exhibited by the seleno-amino acid SeMet. The mechanism included GPx-dependent DNA repair enhancement [97].
Monoesterification of DHSred with acid chlorides derived from propionic, lauric, myristic, palmitic, and stearic acids yielded fatty acid conjugates (125), with lipid peroxide (LOOH) scavenging activity in lecithin/cholesterol liposome membranes [98]. The myristate derivative displayed interfacial redox activity. Amphiphilic diselenides (127) were also synthesized [99].
Diselenides such as 127 (Scheme 28) were shown [100] to mimic protein disulfide isomerases (PDIs), enzymes critical for redox homeostasis in the endoplasmic reticulum and in the prevention of amyloid plaque pathogenicity. Furthermore, GPx-like activity was observed for 126 and 127. Depending on their hydrophilic (R = H, C3) or hydrophobic (R = C6–C14) nature, GPx1- or GPx4-like activity predominated [99], respectively. These compounds reduced H2O2 or lipid peroxides (LOOH) to harmless H2O and an alcohol (LOH), respectively (Scheme 29B). Antioxidant properties were also confirmed in cultured cells [99]. A similar behavior was proposed for DHSred (124) and monoesters counterparts (125) [101].
Mugesh and co-workers described [102] the synthesis of enantiomerically pure trans-dihydroxy diselenide (126) and selenyl sulfide (136) as antioxidants to protect erythrocytes (RBCs, Red Blood Cells) from oxidative stress-induced eryptosis (programmed RBC death). The synthesis involved treatment of 1,4-di-O-tosyl-2,3-O-isopropylidene-l-threitol 131 with substoichiometric selenol 133 (obtained by reduction of p-methoxybenzyl diselenide 132) to give benzyl selenide 134. Subsequent nucleophilic displacement of the second tosylate with KSAc, followed by I2-mediated oxidation afforded O-protected selenyl sulfide 135 (Scheme 30). Final deprotection under acidic conditions furnished 136. Alternatively, treatment of ditosylated 131 with an excess of selenol 133 gave the dibenzylselenide 137. Final oxidation and deprotection led to the expected diselenide 126 [102].
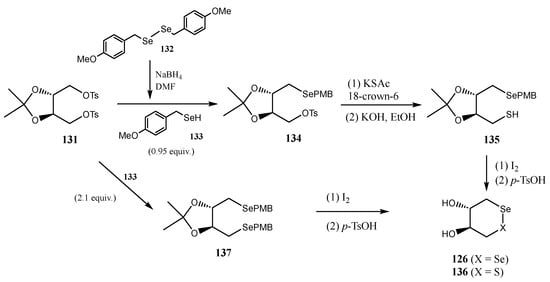
Scheme 30.
Synthesis of trans-dihydroxy diselenide 126 and selenenyl sulfide 136.
When RBCs were exposed to H2O2 to simulate severe oxidative stress, treatment with 126 and 127 reduced ROS levels, demonstrating effective antioxidant activity in cells. Structural aspects proved critical; decreasing conformational flexibility (via isopropilidene protection) or replacing selenium atoms with sulfur impaired activity [102]. O-protected derivatives exhibited high toxicity, even without H2O2. Inhibition of glutathione reductase (GR), leading to reduced glutathione (GSH) levels, significantly diminished the protective effects of 126 and 136. This confirmed that their antioxidant activity primarily relies on GPx-like mechanisms, with GSH as a cofactor [102].
Selenosugars have been conjugated with hydroxycinnamic acids, known for their potent antioxidant agents, via a Mitsunobu reaction to achieve synergic effects [103]. Initially, the authors intended [104] to use l-sugars (the C-4 epimer of 139), obtained from d-ribose through a 6-step synthetic pathway: isopropylidene protection of the C-2 and C-3 positions, TBDPS protection at C-5, reduction of latent aldehyde, di-O-mesylation, nucleophilic displacement with NaHSe and acidic removal of TBDPS protecting group. However, unexpectedly, when the C-4 epimer of 139 was subjected to the Mitsunobu reaction with monoaceytlated hydroquinone, a configurational inversion occurred at C-4, resulting in a d-sugar instead [104]. Alternatively, d-configured selenosugar 139 can be obtained in a 5-step methodology starting from O-protected d-ribonolactone 138 [104]: mesylation of C-5 position, inversion of configuration on C-4 promoted by KOH, TBDPS-protection of C-5, reduction of the lactone moiety, di-O-mesylation, nucleophilic displacement with NaHSe, and C-5 deprotection. Subsequently, p-coumaric, caffeic and feluric acids were attached to the free OH at C-5 through a Mitsunobu reaction (giving compounds 140–142) [103,104], in the presence of diisopropyl azodicarboxilate (DIAD) and PPh3 (Scheme 31).
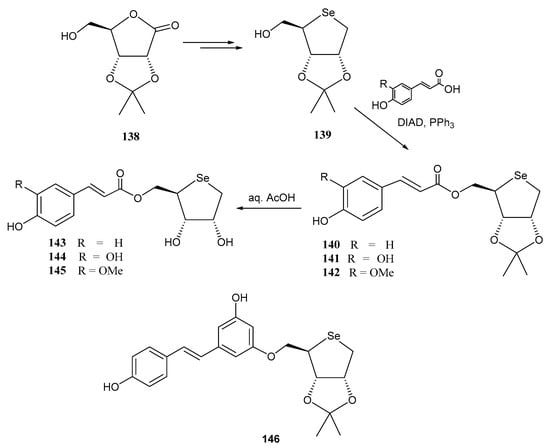
Scheme 31.
Preparation of selenosugar-phenolic acid conjugates through a Mitsunobu reaction.
Final deprotection of the isopropylidene protecting group under acidic conditions afforded conjugates 143–145. These conjugates exhibited notable free-radical scavenging properties, reduced toxicity at concentrations up to 100 µM, and promising wound-healing properties in keratinocytes (in vitro scratch wound model), making them potential cosmeceutical ingredients [103,104]. Unprotected derivatives 144 and 145, derived from p-caffeic and ferulic acids, demonstrated dose-dependent healing activity, at lower doses compared to the corresponding unconjugated cinnamic acids. The authors did not find a correlation between a higher cellular uptake and the wound healing properties, suggesting interaction with outer cell membrane components [103].
Using a similar approach with the C-4-epimer of 139, conjugate 146 was synthesized [105] using diacetylated resveratrol in a Mitsunobu-type reaction (Scheme 31). trans-Resveratrol, a natural phytoalexina found in grapes and red wine, exhibits numerous biological properties like antioxidant, anti-inflammatory, cardio- and neuroprotective, and antidiabetic effects [106]. Caffeic acid and resveratrol conjugates 144 and 146 were loaded on a hydroxyl film to develop a pH-sensitive delivery system for accelerating skin wound healing. For this purpose, a copolymer comprised of poly(ethyleneglycol diacrylate) (PEGDA) and poly(hydroxyethyl methacrylate) (HEMA), in a 1:4.2 molar ratio, was used. Compound 144 was released at pH 7.4, suitable for acute wounds, whereas resveratrol-containing 146 was released at pH 9.6, optimal for chronic wounds [105]. The pH-depending selective release was attributed to weak interactions between the selenoconjugates and the hydroxyl groups of HEMA [105].
The same group recently developed [107] a second generation of selenosugar-cinnamic acid conjugates as cosmeceutical agents, introducing an acetoxy group at the pseudo-anomeric position to enhance cellular uptake. This structural feature was incorporated via a seleno-Pummerer rearrangement of selenoxide 148 upon heating in Ac2O (Scheme 32). This reaction has been used extensively by Iwaoka’s group for accessing selenonucleosides [108] (see Section 3.2). Subsequent silyl-O-deprotection, followed by Mitsunobu reaction with the same phenolic acids as described in Scheme 32, and final O-deprotection furnished conjugates 151–153.
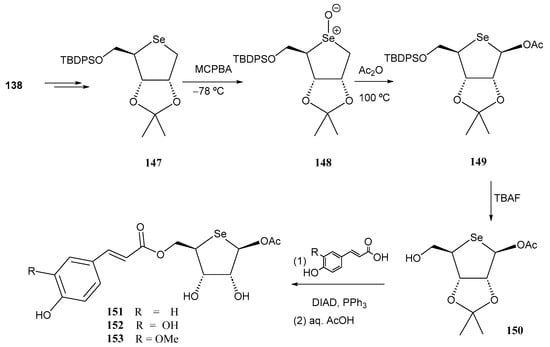
Scheme 32.
Preparation of the second-generation selenosugar-phenolic acid conjugates.
Compared to the first generation counterparts, the acetoxy derivatives 151–153 displayed a dose-dependent cytotoxicity, potentially affecting mitochondria redox activity at concentrations above 25 µM [107]. Among these, 153 exhibited a 7.5-fold increase in cell membrane permeability compared to 145, which lacks the acetoxy group (HaCaT cells). The caffeoyl derivative 152 showed the strongest protective effect against H2O2, while selenosugars derived from p-coumaric and ferulic acids (151 and 153) demonstrated pro-oxidant properties.
Using a percutaneous absorption assay, first generation compounds, without the acetoxy group, generally exhibited higher skin penetration [107]. Compound 144 displayed the fastest penetration rate. However, 152 (derived from caffeic acid) was the only second-generation compound found in the receptor fluid layer.
3.2. Selenonucleosides
The incorporation of selenium into nucleosides has been proposed as a strategy to modulate or enhance their anticancer or antiviral properties [10]. Herein we focused specifically on replacing the endocyclic oxygen atom in the carbohydrate moiety with selenium. Previous studies exploring selenium substitution within the nitrogen base have been comprehensively reviewed [10].
Most reported examples of selenonucleosides describe the synthesis of the 4′-selenosugar moiety 154 via a double nucleophilic displacement reaction using in situ generated NaHSe on a di-O-mesylated derivative, as illustrated in Scheme 27. Subsequently, the nitrogen base is usually introduced into the selenosugar framework through two main strategies (Scheme 33). Pathway A: treatment of a selenoxide (155) directly with a silylated nitrogen base in the presence of TMSOTf and Et3N. Pathway B: via seleno-Pummerer rearrangement with Ac2O followed by N-glycosylation with a nitrogen base (Vorbrüggen glycosylation). In this approach, the selenoxide undergoes heating in the presence of Ac2O to produce the acetoxy derivative 157, that acts as the glycosyl donor. The acetoxy intermediates then react with the appropriate nitrogen base in the presence of N,O-bis(trimethylsilyl)acetamide (BSA) and TMSOTf (Scheme 33).
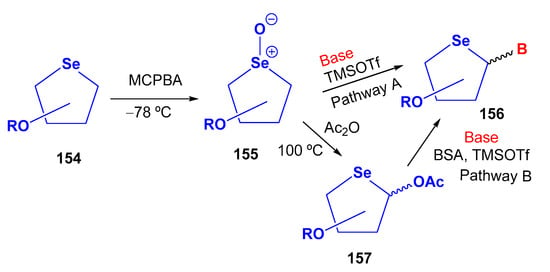
Scheme 33.
General procedure for the preparation of selenonucleosides.
In the search for novel anti hepatitis C virus agents (HCV), Jeong and co-workers prepared Se-analogues of Sofosbuvir, an oral drug that is prescribed for the treatment of chronic HCV [109]. It was claimed that the presence of selenium might increase the lipophilicity compared to the 4′-oxo nucleosides, thus enabling these compounds to be transported across cell membranes; furthermore, the bulkier character of the selenium atom can also affect the nucleoside conformation [109]. Commercially available 2-C-methyl-d-ribono-γ-lactone 158 was transformed into the key 4′-selenofuranose 159 in a 5-step procedure, which included basic-promoted epimerization at C-4′ and nucleophilic displacement of a transient di-O-mesylate by selenide as the key steps. Then, two families of selenonucleosides were obtained [109] from 159 (Scheme 34): 4′-selenopyrimidine and purine nucleosides. The former family (161–163) was obtained following the pathway A of Scheme 33; MCPBA-promoted oxidation of 159 to give the glycosyl donor 160 followed by treatment with the appropriate pyrimidine base, TMSOTf and Et3N furnished the corresponding O-protected nucleosides in a roughly 1:2.5 α:β ratio [109]. Deprotection afforded derivatives 161–163 (Scheme 34).
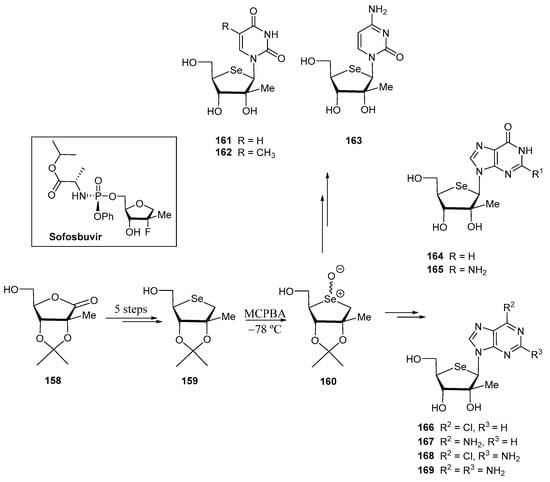
Scheme 34.
Preparation of 2′-C-methyl-4′-seleno pyrimidines and purines.
For purine nucleosides, incorporation of the nitrogen base proceeded more efficiently using the Vorbrüggen glycosylation (pathway B of Scheme 33). Following this methodology, nucleosides 164–169 were obtained.
2D-NOESY experiments and X-ray crystallographic studies demonstrated that the selenofurane backbone of 2′-C-methyl selenonucleosides adopts a North conformation (C2′-exo/C-3′-endo), the same as the 4′-oxo derivatives, and opposed to the unusual conformation exhibited by 4′-selenoribofuranosyl nucleosides (South conformation, C2′-endo/C3′-exo) [110]. The authors claimed that probably, the sterical hindrance of the C2′-Me counteracted the effect of the selenium atom [109].
With the aim of mimicking the structure of Sofosbuvir, the authors also incorporated [109] a phosphoramidate residue at C-5′ position, affording compounds 170 and 171 (Figure 12), considered as prodrugs. The phosphoramidate moiety is incorporated to both increase the cell uptake, and to avoid the rate-determining 5′-monophosphorylation step by cellular kinases. Unfortunately, none of the 2′-C-methyl selenonucleosides showed relevant anti-HCV activity at the highest concentration tested (100 µM).
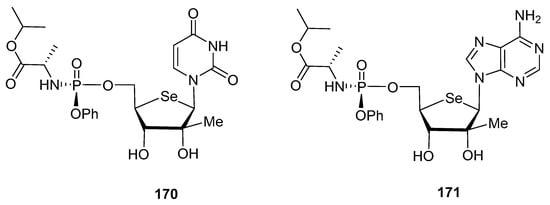
Figure 12.
Structure of 5′-phosphoramidate-selenonucleosides 170 and 171.
Other nucleosides designed as antiviral agents are depicted in Scheme 35. Minakawa and co-workers envisioned [111] the synthesis of imidazole-derived thio- and selenonucleosides 174–177, with potential anti-dengue virus (DENV) activity. The primary goal pursued by using an endocyclic chalcogen atom (S, Se) was to reduce the intrinsic cytotoxicity of their 4′-oxo analogues. Starting from 6-chloropurine nucleosides 172 and 173, the purine moiety was degraded into the imidazole scaffold by first, conversion into the corresponding inosine derivatives with 2-ethanethiol in basic medium, followed by subsequent treatment with 1-chloro-2,4-dinitrobenzene and ethylenediamine [111].
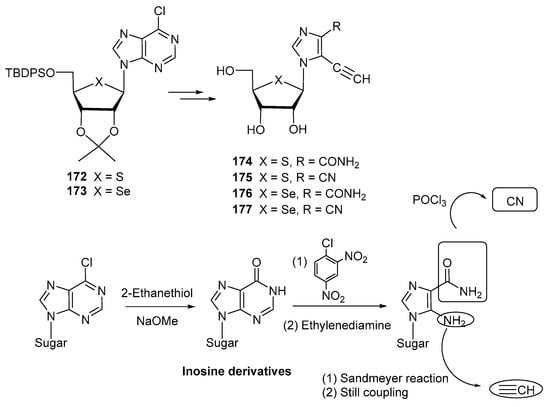
Scheme 35.
Preparation of imidazole thio- and selenonucleosides 174–177.
The amido moiety was dehydrated with POCl3 to give a cyano motif (175, 177), whereas the ethynyl functionality was obtained by subjecting the amino group on the imidazole to a Sandmeyer reaction, followed by a Stille-type coupling with the appropriate stannane derivative (Scheme 35). The compound with the highest capacity of inhibiting DENV replication was the thionucleoside 175; although sensibly less potent than the analogous 4′-oxonucleosides (up-to-24 fold-less potent), the undesired cytotoxicity was considerably reduced (up to 40-fold) [111]. This compound exhibited virtually the same potency as ribavirin, a 1,2,4-triazolyl nucleoside used as a positive control.
It has been hypothesized that the lack of antiviral activity exerted by some 4′-selenonucleosides could be attributed to either a change of the sugar reside conformation or to the bulky character of the selenium atom; this might hinder the kinase approach, thus preventing the phosphorylation at C-5′ position [112]. In order to prove this issue, Jeong and co-workers designed [112] 5′-homo-4′selenonucleosides, where the elongation of the exocyclic residue in one carbon might alleviate the sterical hindrance (181, 183, Figure 13).
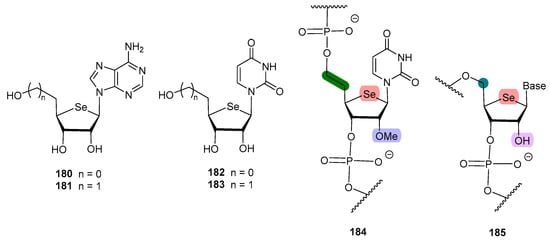
Figure 13.
Selenonucleosides and their homologous isomers.
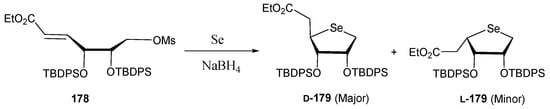
For this purpose, they used an innovative approach for accessing the selenosugar scaffold; instead of using the typical nucleophilic substitution on di-O-mesylated intermediates, a Se-Michael reaction was applied for the first time for constructing a carbohydrate backbone [112]. The use of bulky protecting groups allowed a good facial selectivity to achieve the d-sugar over the l-counterpart (Scheme 36).
Scheme 36.
Se-Michael reaction for accessing 4′-selenofuranoses.
The results align with the initial hypothesis, as derivatives 181 and 183 exhibited potent anti-herpes simplex virus (HSV-1) activity (EC50 = 2.9 and 2.3 µM, respectively) [112], whereas 4′-selenonucleosides 180 and 182, previously synthesized by the same authors [110,113], turned out to be inactive (EC50 > 100 µM).
In order to develop oligonucleotides with therapeutic interest, their stability towards ribonucleases must be improved, by hindering the hydrolysis of the phosphodiester bonds. The most widely used procedure is to incorporate a methoxy group at the C-2′ position of the sugar residue. Jeong and co-workers combined the homologation approach [112] to maintain the biological activity of the selenonucleoside with a methoxy group at C-2′, and prepared oligonucleotides containing the 5′-homo-4′-selenouridine fragment (184, Figure 13) [114]. This modified selenonucleoside was proved to reduce the capacity of the oligonucleotide to form duplexes, probably because of the free rotation enabled by the extra carbon, and had reduced thermal stability. However, a remarkable increase in its stability towards nuclease-mediated digestion was found; tentatively, the homologated structure caused a reduced affinity with the enzyme [114].
Interestingly, Minakawa and co-workers prepared 4′-selenoRNA 185 (uridine, cytidine, adenosine, guanosine), with no homologation, and without a masked hydroxyl group at C-2′ [115]. The key synthetic intermediates were the corresponding phosphoramidites, which afforded the 4′-selenoRNA using a DNA/RNA synthesizer. These compounds enhanced the formation or RNA duplexes, and exhibited a high stability towards endonucleases, despite the presence of the free 2′-OH moiety [115].
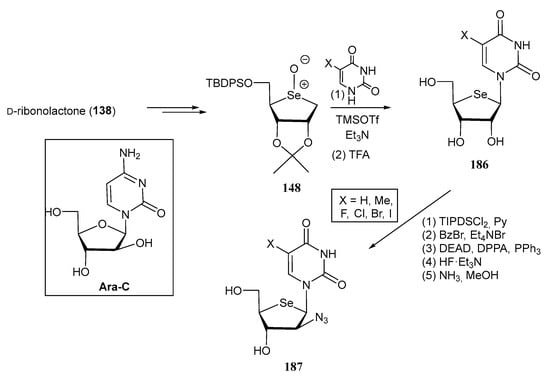
Many selenonucleosides also exhibit notable anticancer properties. In this context, d-arabino-configured Arabine-C (cytarabine), is a well-known nucleoside widely used as a chemotherapeutic agent for the treatment of leukaemia and lymphomas. Isosteric derivatives bearing F and N3 motifs at the C-2′ position have demonstrated noteworthy antiviral and anticancer properties [116]. These structural templates inspired Jeong and co-workers [116] to develop an extensive library of 2′-substituted-4′-selenoarabinofuranosyl pyrimidines. Thus, starting from d-ribonolactone 138, and following the pathway A illustrated in Scheme 33, the pyrimidine moiety was installed on the glycosyl donor 148 to give selenonucleosides 186. Selenonucleosides 187, with d-arabino configuration and bearing an azido motif on C-2′ were accessed from 186 in 5 steps: TIPDS protection at C-3 and C-5 positions of the sugar residue, benzoylation of the nitrogen base, Mitsunobu reaction with dipheynylphosphoryl azide (DPPA), and subsequent removal of silyl and benzoate protecting groups (Scheme 37).
Scheme 37.
Synthetic pathway for the preparation of selenonucleosides 187.
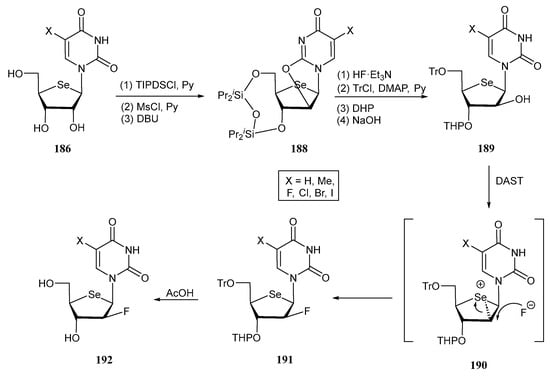
Fluorinated selenonucleosides 192 were prepared from the fully unprotected derivative 186 as outlined in Scheme 38 [116]. After TIPDS protection, mesylation at the C-2′ position and DBU-treatment afforded anhydro nucleoside 188, with configurational inversion at C-2′. Subsequent O-deprotection, followed by chemoselective tritylation of the primary alcohol, regioselective protection at C-3′ and hydrolysis of the anhydro moiety gave 189. Finally, fluorination on C-2′ with retention of the configuration was accomplished with diethylaminosulfur trifluoride (DAST) through a double SN2 reaction involving the selenium atom, followed by acidic deprotection to afford 192.
Scheme 38.
Synthetic pathway for the preparation of selenonucleosides 192.
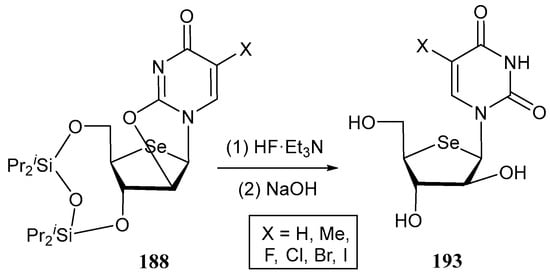
The third family of 2′-substituted-4′-selenoarabinofuranosyl pyrimidines prepared by Jeong and co-workers consisted of derivatives 193, as shown in Scheme 39 [116]. Deprotection of C-3′ and C-5, followed by hydrolysis of the anhydro moiety in 188 afforded d-arabino-configured selenonucleosides 193 with an OH at the C-2′ position.
Scheme 39.
Synthetic pathway for the preparation of selenonucleosides 193.
Selenonucleosides of the cytidine type were obtained using the approach illustrated in Scheme 40. Derivatives 187, 194 and 195 (uracyl selenonucleosides, X = H) were acetylated at C-3′ and C-5′ positions, and treated with 1,2,4-triazole to furnish unstable triazole derivatives 196–198. Final treatment with NH4OH in dioxane and methanolic NH3 gave cytidines 199–201.
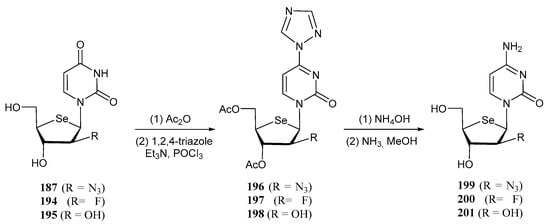
Scheme 40.
Synthetic pathway for the preparation of selenonucleosides of cytidine-type.
Compounds 187, 194, 195 and 199–201 were tested in vitro as potential antiproliferative agents against a panel of 6 tumour cell lines. Although most of them were either inactive at the highest concentration tested (100 µM), or moderate, compound 200 turned out to be a potent antiproliferative agent, with IC50 ranging from 0.14–1.1 µM, with a better profile than Ara-C, used as a positive control [116].
A very remarkable example of anticancer activities exhibited by selenonucleosides is 4′-selenofuranosyl-2,6-dichloropurine (202, LJ-2618, Figure 14). This compound was tested [117] in vitro and in vivo (xenograft mouse model) against drug-sensitive and paclitaxel-resistant prostate cancer cells (PC-3 and PC-3-Pa, respectively). Although less potent than paclitaxel, compound LJ-2618 maintained the same activity in both drug-sensitive and drug-resistant cells (IC50 = 11.6 and 11.7 µM, respectively); paclitaxel exhibited a 108-fold impairment of activity in PC-3-Pa. Moreover, mice implanted with PC-3-Pa cells showed a significant diminishment of the tumour volume after 29 days of treatment with LJ-2618 (3 or 10 mg/Kg, 3 times/week) of 50.0 and 66.1%. These results strongly contrast with those exhibited by paclitaxel (26.6% reduction of the tumour, 5 mg/Kg treatment) [117]. This compound was shown to induce a G2/M cell cycle arrest via down-regulation of the Skp2 (S-Phase Kinase Associated Protein 2) expression in PC-3-Pa cells, a hitherto unknown target for selenonucleosides [117].
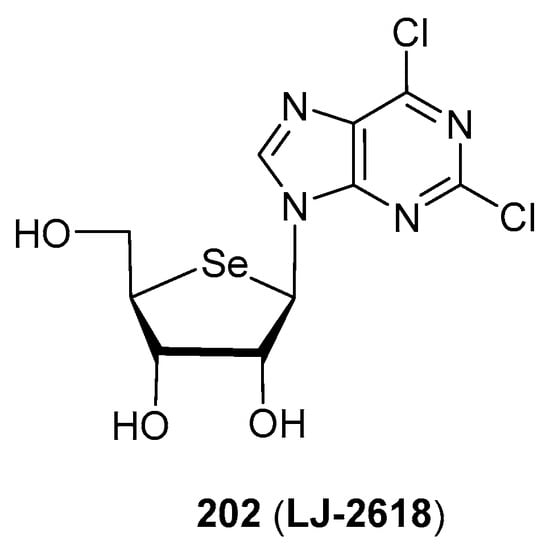
Figure 14.
Structure of the anticancer selenonucleoside LJ-2618.
A template that has been extensively studied is 4′-selenoadenosine in connection with peroxisome proliferator-activated and A3 adenosine receptors (PPAR and A3AR, respectively). PPARs (classified as PPARα, γ and δ, depending on the tissue distribution and specific function), have a key role in maintaining the metabolic homeostasis, with a direct involvement in the metabolism of lipids and glucose, adipogenesis, and also in inflammatory responses [118]. Accordingly, PPARs are interesting therapeutic targets for metabolic disorders and inflammatory diseases, like inflammatory bowel disease. Jeon, Noh and co-workers developed a series of selenoadenosine nucleosides (203, Figure 15) by modification of the substituents at C-2 (H, Cl) and C-6 positions N6-(cycloalkyl, aryl, halobenzyl) of the base [119]. Interestingly, derivative 203, decorated with a Cl atom at C-2 and a 3-iodobenzyl moiety at N6 exhibited an enhanced antagonist activity against PPARδ compared to its 4′-oxo and 4′-thio counterparts. This behaviour was attributed to the unusual South conformation; docking simulations confirmed additional hydrogen bonding within the hydrophobic pocket of the enzyme, what was lacking in its other chalcogen isosters [119].
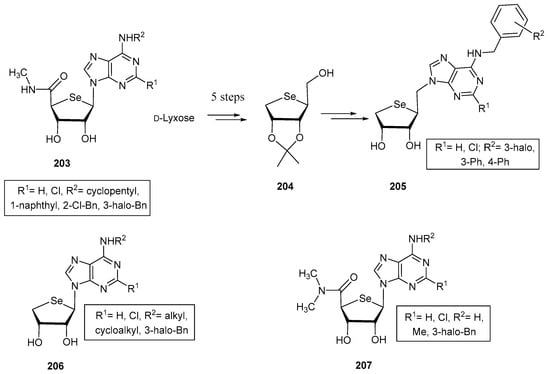
Figure 15.
Structure of 5′-N-methylcarbamoyl-4′-selenoadenosines and truncated/homologated analogues.
In subsequent investigations, structures 205 were obtained (Figure 15). These compounds are truncated homologated analogues of 203, where the carboxamido motif has been eliminated, and an extra carbon has been introduced at C-1′ position [120]. These selenoucleosides were obtained using the selenofuranose 204 as the key synthetic intermediate (Figure 15), which in turn was obtained starting from d-lyxose in 6 synthetic steps: acetal protection of C-2 and C-3 positions, chemoselective protection of the primary alcohol, reduction of the latent aldehyde, di-O-mesylation, nucleophilic displacement with in situ generated selenide and primary O-deprotection. The nucleobase was introduced in this case via an SN2 reaction prior mesylation of 204 [120]. Again, the best compound in the series within 205 incorporated a Cl atom at C-2 and a 3-iodobenzyl moiety at N6. This compound behaved as a potent PPARγ partial agonist (Ki = 2.8 μM) and a PPARδ antagonist (Ki = 43 nM). Additionally, it improved the production of adiponectin, a hormone secreted for controlling the metabolism of fatty acids and glucose, increasing sensitivity to insulin. This effect demonstrates that the synergic modulation of both receptors can lead to promising agents for the treatment of metabolic disorders, specifically those associated with hypoadiponectinemia [120].
Bioisosteric replacement of oxygen and sulfur in previous nucleosides by selenium led to A3AR agonists, a feature that can allow the development of new drugs for the treatment of inflammatory and autoimmune diseases. In this context, derivative 205 bearing a H at H-2 and a N6-3-iodobenzyl moiety (Figure 15), behaved as a subnanomolar agonist of such receptor (Ki = 0.57 nM) [121]. This represents an outstanding selectivity compared to A1AR and A3AR (selectivity index > 800 and 1900, respectively). Structural analysis revealed that these selenonucleosides exhibit again a South furanose puckering, and a syn orientation of the nucleobase; surprisingly, previous A3AR agonist exhibit the complete opposite conformational preference. The lead compound also showed an improved inhibition of the MCP-1 induced microglial chemotaxis, suggesting potential anti-stroke properties [121].
Further Structure-Activity studies conducted on adenosine receptors led to the preparation of selenonucleosides 206, with a truncated structure [122], and 207 [123], with a disubstituted nitrogen atom on the carboxamido motif (Figure 15). Compounds 206 exhibited strong binding to A3AR, within the nanomolar range. Surprisingly, the two most potent compounds (R1 = Cl) incorporated alkyl groups on R2 positions (Me and cycloalkyl, Ki = 5.2 and 5.7 nM). Computational calculations demonstrated that the South puckering of the carbohydrate residue allowed a closer location within the binding site of OH-3 to Thr94, compared to 4′-oxo nucleosides [122]. Furthermore, an agonist effect was observed, unlike their truncated oxygen and sulfur counterparts, which behaved as antagonist of this receptor.
Remarkably, the addition of a second methyl to the carboxamido moiety (207) shifted the activity of the 4-‘selenonucleosides to antagonism, with medium to high binding affinity [123]. The best compounds lacked a chlorine atom at the 2-position of the nucleobase, and the lead one was decorated with a 3-iodobenzyl scaffold at N6-position (Ki = 22.7 nM). The same behaviour, and similar binding affinities were found for isosteric 4′-oxo and 4′-thionucleosides. Theoretical calculations revealed the essential role of the NH moiety for exhibiting agonist activity [123].
3.3. Selenoglycosides
The substitution of the oxygen atom in the glycosidic bond with selenium has emerged as a prominent strategy in glycomimetic synthesis. This isosteric modification enables the development of biomimetics with enhanced metabolic stability compared to their natural counterparts, while maintaining dynamic and conformational properties similar to those of thioglycosides [124]. Selenoglycosides, through 77Se-NMR, X-ray crystallography or calorimetry titrations, are widely used in structural studies [124,125,126,127], particularly for gaining deeper insights into carbohydrate recognition by protein receptors, such as lectins. Notable examples of these glycomimetics include the seleno- and diselenodiglycosides 208–210 [124,126,127], which have been studied for their binding to human galectins 1- and 3, as well as Se-sialoside 211, a mimetic of sialyl α(2,6) and α(2,3)-galactose epitopes, relevant for investigating glycan-pathogen interactions (Figure 16). Numerous selenoglycosides also exhibit relevant biological properties; for instance, Comini and co-workers evaluated [128] an ample panel of selenoglycosides as potential antiparasitic agents against trypanosomiasis; the lead compounds exhibited activity within the submicromolar range, and good selectivity.
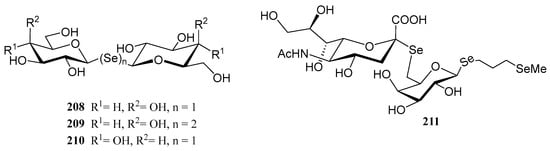
Figure 16.
Representative selenoglycosides for structural studies.
The growing interest in selenoglycosides has driven the need for practical and stereoselective synthetic methods. Most of these approaches are based on nucleophilic displacements carried out by Se-based nucleophiles (Scheme 41) on glycosyl halides (212), triflates, acetates (215) and glycals (216). For instance, the treatment of glycosyl halides with p-methylselenobenzoic anhydride in the presence of Cs2CO3 and piperidine, generates p-methylselenobenzoate in situ, which then undergoes a SN2 reaction at the anomeric position, with inversion of configuration, yielding p-methylbenzoylselenoglycoside 213 (Scheme 41, pathway A). Further reaction with alkyl halides, including those containing sugar residues, under basic conditions enables the preparation of the corresponding selenoglycosides [125,129]. Using this methodology as one of the key steps, Murphy and coworkers accomplished [129] the preparation of 219 (Figure 17), the Se-isoster of the immunostimulant α-GalCer. In their synthetic pathway, TiCl4 was used to isomerize an equatorial β-selenoglycoside into its axial α-counterpart.
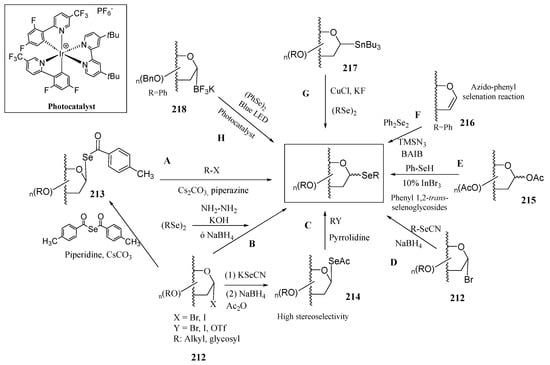
Scheme 41.
Main synthetic procedures for the preparation of selenoglycosides.
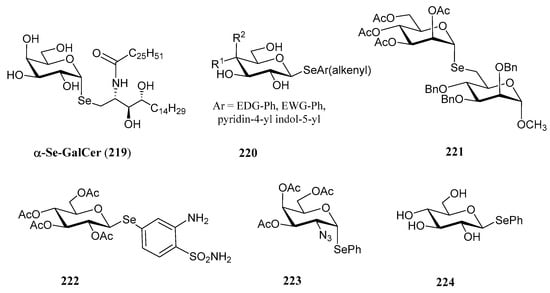
Figure 17.
Relevant selenoglycosides.
A transient p-methylselenobenzoate intermediate was also used [130] in cross-coupling reactions by treatment with heteroaryl and alkenyl halides using a Pd-based catalyst (PdG3 XantPhos) under mild conditions. This process furnished the unprecedented formation of a C(sp2)-Se bond, like in 220 (Figure 17). This methodology proved to be compatible with a variety of functional groups, including aldehydes, ketones, or nitriles, among others. However, it was claimed [131] that, despite being an elegant approach, it lacks atom economy, as the acyl moiety in 213 is not incorporated into the final structure. This issue was overcome by Liang and coworkers by using modified conditions of the Castellani reaction (Scheme 42), originally envisioned for the synthesis of arenes, and based on Pd/norbornene (NBE) cooperative catalysis [132]. They reported the unprecedented use of two-component Castallani-type reaction for coupling p-methylbenzoylselenoglycosides 213 with (hetero)aryl iodides to furnish selenoglycosides upon formation of a new C(sp2)-Se bond, and concomitant Se–C(=O) breaking. A similar procedure was also employed for accessing the corresponding thioisosters. This strategy proved to have a broad scope, being effective in O-protected pyranoses and furanoses (ribose, glucose, mannose, galactose, xylose, arabinose), including disaccharides like cellobiose and maltose, and tolerates a variety of functional groups (Ac, Piv, Bn, TBDPS, Me). A tentative catalytic cycle (adapted from the one reported for thioglycosides) is illustrated in Scheme 42. (Pd(MeCN)2Cl2 is used as the Pd(II) source, and P(p-Cl-C6H4)3 as the ligand; the formation of the selenoglycoside is postulated to take place through seven steps [131]: oxidative addition of Pd(0) into aryl iodides, migratory insertion of 5-norbornene-2-carbonitrile, ortho C-H activation to give the five-membered palladacycle 227, oxidative addition of p-methylbenzoylselenoglycoside 213, reductive elimination, norbornene extrusion, and a second reductive elimination to give selenoglycoside 231, where no loss of atoms has taken place. The main concerns of this approach are the high temperatures (95–100 °C) and the long reaction times (16 h).
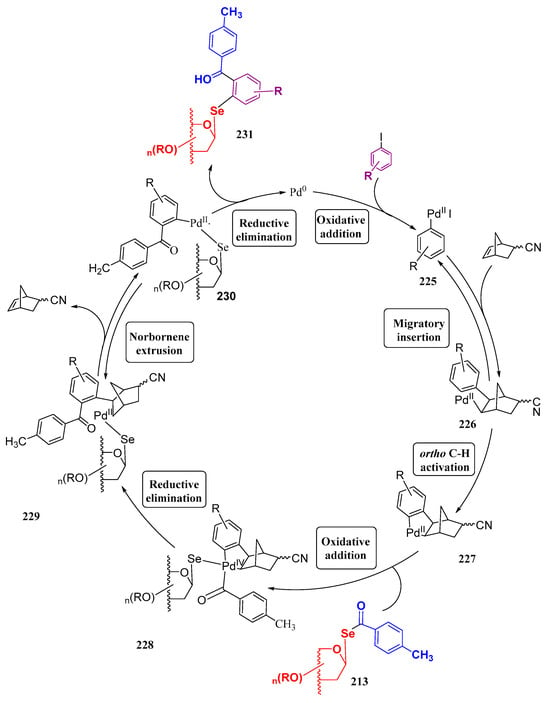
Scheme 42.
Catalytic cycle of the Castellani-type reaction for the synthesis of (hetero)aryl selenoglycosides.
Azeem and Mandal described [133] the atom-economic and gram-scale synthesis of unsymmetrical gem-diarylmethyl(thio)seleno glycosides. For this purpose, glycosyl thio(seleno) acetates and other acylated derivatives 232 (analogues to 213) were treated with Cs2CO3 and p-quinone methides (p-QMs). It was hypothesized that Cs2CO3 promotes the cleavage of the X-C(=O) bond, generating a transient thiolate/selenolate that undergoes a 1,6-addition on the p-QMs and an acyl transfer via a concerted mechanism (Scheme 43). Reactions proceeded smoothly (rt), with short reaction times (30 min-2 h), and the selenoglycosides 233 were isolated in a roughly 1:1 diasteromeric ratio.
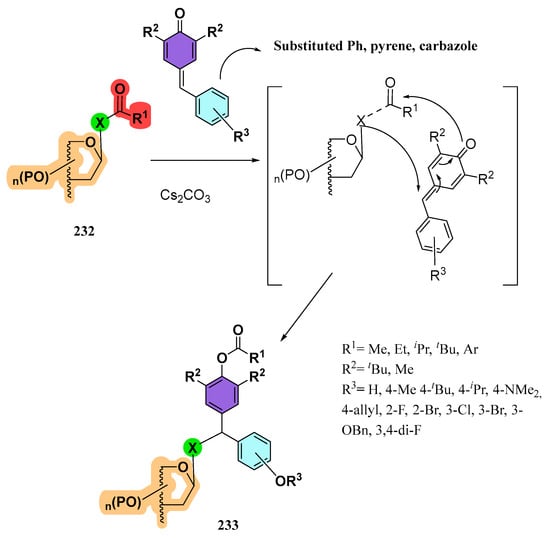
Scheme 43.
Preparation of unsymmetrical gem-diarylmethylthio(seleno)glycosides.
1,2-trans-Selenoglycosides (alkyl, aryl, glycosyl) can be obtained [134] through the nucleophilic attack of selenides (Scheme 41, Pathway B), which are generated via the reductive cleavage of symmetrical diselenides. These selenides then react with per-O-acetylated glycosyl bromides under phase transfer catalysis. Using a similar approach, Oscarson and coworkers reported [135] the preparation of selenoglycosides bearing a fluorine atom as useful tools for studying protein-carbohydrate interactions with 19F and 77Se-NMR spectroscopy.
Another approach to prepare selenoglycosides from glycosyl halides 212 is illustrated in Scheme 41, Pathway C. Treatment with KSeCN affords glycosyl selenocyanates, typically with inversion of configuration. Reduction of the selenocyanato motif with NaBH4 generates a configurationally stable selenide, which is subsequently trapped through acetylation (214). Further reaction with alkyl halides under basic conditions leads to the formation of the corresponding selenoglycoside [136] in good yields, and short reaction times (10–20 min). Disaccharides, like 221, were also obtained with this procedure.
Glycosyl bromides 212 were also treated with aryl selenides, generated via the NaBH4-mediated reduction of the corresponding aryl selenocyanates (Scheme 41, Pathway D). This strategy was used by Angeli and coworkers [137] to develop novel sulfonamides bearing a selenoglycoside linkage, like 222, designed for targeting seizures associated with glucose transporter type 1 deficiency syndrome (GLUT1-DS).
Reactions involving Se-based nucleophiles can also take place in per-O-acetylated aldoses, using benzene selenol and InBr3 as a moisture-stable Lewis acid (Scheme 41, Pathway E) [138]. This strategy affords 1,2-trans selenoglycosides, due to neighbouring group participation, in moderate to almost quantitative yields. The methodology is also applicable to the preparation of phenyl thioglycosides.
Nifantiev and coworkers reported [139] the heterogeneous and reproducible 2-azido-phenylselenylation of 3,5,6-tri-O-acetyl-d-galactal with Ph2Se2 and TMSN3 (Scheme 41, Pathway F) in the presence of the hypervalent iodine compound BAIB (PhI(OAc)2, [bis(acetoxy)iodo]benzene). This reaction afforded crystalline phenyl 3,4,6-tri-O-acetyl-2-azido-2-deoxy-1-seleno-α-d-galactopyranoside (223), used for the preparation of galactosamine building blocks. Conversely, the use of d-glucal did not proceed with stereoselectivity, resulting in a mixture of d-gluco and d-manno selenoglycosides. The use of flow chemistry allowed a reduction of side-products, and the reaction time, compared to the batch process [140]; optimised conditions led to the production of the above 2-azidoselenoglycoside in 1.2 mmol/h rate, affording the processing of 5 mmol of galactal over a 3 h-period.
Alternatively, Walczak and coworkers reported the stereoretentive synthesis of selenoglycosides starting from glycosyl tributyl stannanes and symmetrical diselenides in the presence of CuCl and KF (Scheme 41, Pathway G) [141]. No directing groups are required, and the reaction proceeds successfully with free hydroxyl groups (e.g., 224), affording a broad scope that enabled the preparation of a vast library of compounds. It was hypothesized that the tributyl stannane undergoes a stereoretentive transmetallation reaction with CuCl, enabled by the presence of F−. Then, the glycosyl organocopper intermediate reacts with the diselenide, collapsing to the corresponding selenoglycoside [141]. The main limitations are the temperatures (110 °C) and hazardous organotin reagents.
The same group recently developed a procedure for the stereoretentive cross-coupling reaction of 2-deoxysugars bearing anomeric trifluoroborates. The main targets were C-arylation and etherification reactions under photoredox conditions (via a single electron transfer mechanism) [142]. As the only example of a selenoglycoside, they described the preparation of phenyl 2-deoxy-3,4,6-tri-O-benzyl-α-d-glucopyranoside with total retention of configuration (Scheme 41, Pathway H).
Corzana and coworkers reported [143] the preparation of S- and Se-mimetics of a GalNAc glycopeptide (compound 238, Scheme 44) derived from mucin MUC1, an O-glycoprotein overexpressed in a series of tumours. This glycopeptide acts as a tumour-associated antigen, and thus, is potentially useful in the development of cancer vaccines. The substitution of oxygen by bulkier S and Se atoms increases the distance between the saccharide residue and the oligopeptide, and also alters the orientation of the glycosidic bond. These structural modifications allowed the glycopeptide 238 and its sulfur isoster to adopt a pre-organized conformation that improved binding to the MUC1 antibody [143]. The preparation of selenoglycoside 237, a key synthetic intermediate latter subjected to solid-phase peptide synthesis, involved a nucleophilic displacement exerted by a selenium nucleophile (Scheme 44). Unlike previous examples, in this case the diselenide motif was located at the anomeric position. This was achieved starting from per-O-acetylated GalNAc, which upon treatment with Woolin’s reagent afforded a transient selenoamide that evolved to bicyclic 2-methylselenazoline 235 through a spontaneous intramolecular nucleophilic cyclization. Subsequent treatment with TFA furnished diselenide 236 which was then reduced and coupled to an iodinated derivative of l-threonine [143].
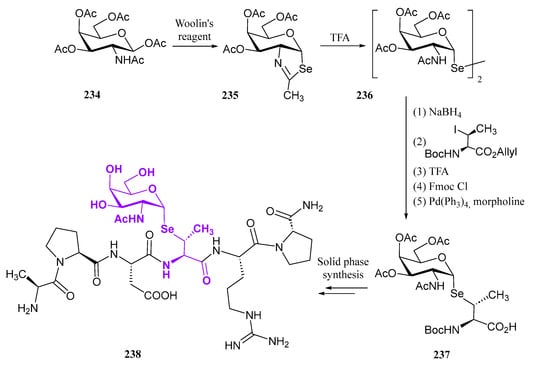
Scheme 44.
Synthesis of Se-GalNAc-glycopeptide as a tumour-associated antigen.
Selenoglycosides are not only relevant for structural studies or for the development of bioactive compounds, but they are also valuable intermediates in organic synthesis, as they can be used as glycosyl donors with different alcohols (including partially protected saccharides) and promoters. This strategy has also been used in the preparation of complex oligosaccharides [144]. Phenyl α-selenoglycosides, obtained via an azido-phenyl selenation reaction from glycals as depicted in Scheme 41 (Pathway F) were activated using classical glycosylation conditions with NIS and TMSOTf, and treated with either primary or secondary (sugar-derived) alcohols (Scheme 45); in the latter case, disaccharides were obtained [145]. 2-Azido motifs are the precursors of amino and acetamido scaffolds, present in numerous bioactive glycans. When d-gluco-configured selenoglycosides were used (e.g., 240), a good α:β selectivity was observed (3–5:1); interestingly, mannopyranosides afforded α-linked glycosides exclusively.
Scheme 45.
Use of selenoglycosides in glycosylation reactions.
Alternatively, Li and co-workers converted the 2-azido functionality in 239 (O-benzyl protected) into 2-deoxy-2-(2,4-dinitrobenzenesulfonyl)amino (DNsNH) (compound 241, Scheme 45) [146], which in turn can be treated with thioacetic acid and DMAP to give an acetamido motif. Activation of 2-deoxy-2-DNsNH phenyl selenoglycoside 241 with a combination of PhSeCl and AgOTf, followed by the addition of the glycosyl acceptor at low temperatures afforded disaccharide 242 with good β-stereoselectivity. This protocol proved to be efficient even with sterically hindered glycosyl acceptors, with reduced nucleophilic properties. The combination of 241 with sequential thioglycoside-based donors enabled the preparation of β-(1→6)-linked tri- and hexasaccharides with potential interest as antigens in the search for vaccines against microbial infections [146].
3.4. Miscellaneous Selenosugars
Other sugar mimetics containing selenium have been reported, besides selenosugars, selenonucleosides and selenoglycosides. Figure 18 shows some recent examples of such structures. Thus, López and co-workers reported [74] the preparation of sugar-derived bicyclic 1,3-selenazolines bearing either an alkylamino (243) or alkoxy (244) residue at the C-2 position of the heterocyclic motif. Such structures were designed as dual inhibitors of cholinesterases and O-GlcNAcase (OGA), two enzymes that are considered as validated targets against Alzheimer’s disease; it was postulated that the presence of a Se atom might contribute to maintain the redox homeostasis. Derivatives 243, and particular the derivative with R = Pr exhibited the best bioactivities. This compound acted as a submicromolar selective inhibitor of butyrylcholinesterase (predominant in advanced stages of the disease; IC50 = 0.46 µM) and a nanomolar selective inhibitor of human OGA (IC50 = 53 nM), while showing no significant activity against glycosidases or cytotoxicity [74].
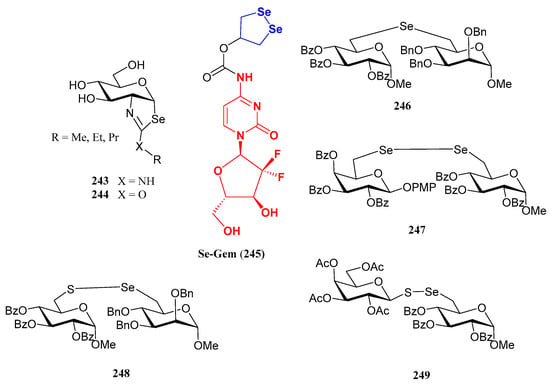
Figure 18.
Miscellaneous selenosugars.
Fan’s group designed prodrugs derived from the antitumour drug gemcitabine and a 1,2-diselenolane moiety connected through a carbamate linker (Se-Gem, 245, Figure 18) [147]. This compound, which acted as a suicide prodrug, improved the antiproliferative activity of gemcitabine (up to 6-fold, IC50 = 0.11–0.88 µM). It was activated by glutathione (GSH), releasing gemcitabine and the diselenide, depleting GSH and increasing the oxidative stress, what in turn induced cell-death by apoptosis [147].
Using O-protected monosaccharides decorated with a selenocyanato motif either at C-4 or C-6 position, Misra’s group accomplished the preparation of non-symmetrical and non-glycosidic pseudodisaccharides connected through a selenide (246) [148] or a diselenide (247) [149] tether (Figure 18). In both cases, the key step is the reduction of the selenocyanato group with hydrazine hydrate to give a transient and reactive sugar selenide, which traps a sugar iodide or triflate to 246, or a symmetrical sugar diselenide to give 247. Additionally, treatment of a 6-iodo sugar derivative with a symmetrical sugar diselenide, CS2 and Na2S·9H2O afforded a pseudo disaccharide with a S-Se linkage (e.g., 248) [150]. When the leaving group is located on the anomeric position (acetobromo aldoses), reaction with sugar diselenides afforded compounds like 249, with exclusive formation of the β-anomer. This methodology proved to be scalable, odourless and high-yielding [150].
4. Te-Containing Carbohydrates
Tellurium was first identified by von Reichstein in 1782, while the synthesis of the first organotellurium compounds (dialkyl tellurides) was achieved in 1840 by Wöher [151]. However, significant advances in the field of organotellurium chemistry did not occur until the 1980s, roughly a decade after organoselenium chemistry did [152]. This delay can be attributed to the fact that low-molecular weight organotellurium derivatives are typically volatile, malodorous and perceived as toxic substances [152]. Initially considered as an extension of Se-containing isomers, organotellurium chemistry has since unlocked new synthetic possibilities [153]. The synthesis and applications of organotellurium derivatives are currently being explored in a wide range of areas, including organo- and metal-based catalysis [154,155], biocatalysts [156], Material Science [157,158,159], and Medicinal Chemistry [160,161], among others.
4.1. Tellurosugars
When it comes to carbohydrate mimetics, the number of tellurium-containing saccharides is significantly smaller compared to their thio- and seleno counterparts. In these derivatives, tellurium has been incorporated in different positions of the carbohydrate skeleton, such as the endocyclic position, the aglycon residue, or other specific sites, like the C-5 position of xylofuranoses (protecting agents against oxidative stress in in vivo experiments) [162], or C-2 and C-6 positions of cyclodextrins (as GPx mimics) [163].
Inspired by the promising antioxidant properties of selenosugars, Schiesser and co-workers reported [11,164] the preparation of isosteric 4-tellurofuranoses (252, 255, 257–259), 5-telluropyranoses 260–262 and 6-tellurepane 256 as water soluble carbohydrate mimics. These compounds were accessed using naturally occurring carbohydrates with different configuration as starting materials. They key step of their syntheses involved a double nucleophilic substitution reaction on dimesylated alditols (e.g., 250, 253, 254) exerted by freshly prepared NaHTe (Scheme 46). NaHTe was in turn obtained by reducing elemental Te with NaBH4. The nucleophilic displacement reaction was found to proceed more efficiently when polytethylene glycol (PEG-400) was used as solvent. Additionally, the choice of the protecting group played a crucial role in the outcome of the reaction. Whereas O-benzylated tellurosugars 260–262 could not be deprotected [164] to yield the corresponding unprotected carbohydrate mimics, acetals were successfully removed upon treatment with TFA. 5-Telluropyranose 260 was found to be highly unstable, even when preserved in the freezer, and spontaneously underwent decomposition, with release of elemental Te; in order to demonstrate the formation of 260, it was transformed into dibromotellurium derivative 261, upon reaction with Br2.
Scheme 46.
Preparation of tellurosugars by nucleophilic substitution on di-O-mesylalditols.
Additionally, racemic trans-3,4-dihydroxy-1-tellurolane 263 was obtained [11] in almost quantitative yield by nucleophilic reaction of buta-1,3-diene bisepoxide 123 with aq. NaHTe (Scheme 47). Alternatively, Capperucci and coworkers reported [165] the synthesis of 263 under on water conditions and using rongalite (sodium hydroxymethanesulfinate) to reduce in situ elemental Te to Na2Te as the nucleophile (Scheme 47). Compound 263 is the Te-isoster of DHSred 124, a selenosugar mimetics endowed with strong antioxidant properties, as considered in the precedent section.
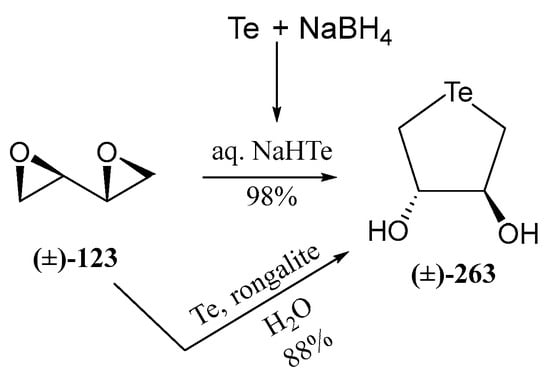
Scheme 47.
Preparation of trans-3,4-dihydroxy-1-tellurolane 263.
Tellurosugars 252, 256, 257, 259 and 263 were assayed [11] for their scavenging properties against common oxidant agents like hypochlorous, hypobromous and peroxynitrous acids (HOCl, HOBr and ONOOH, respectively), generated by the inflammatory enzyme myeloperoxidase. Antioxidant properties against HOCl and HOBr were determined using a competition kinetics assay, while stopped-flow spectroscopy was used for monitoring reaction with ONOOH. 3,4-Dihydroxy-1-tellurolane 263 exhibited the best antioxidant profile against HOCl and ONOOH, whereas 4-tellurofuranose 259 was the best scavenger against HOBr [11]. A wider range of activities towards ONOOH was found among the set of compounds compared to hypohalous acids. The authors postulated the mechanism depicted in Scheme 48 for explaining the antioxidant properties of tellurosugars tested; it was suggested that such glycomimetics underwent oxidation to furnish telluroxide 266 through intermediates 264 (for hypohalous acids) and 265 (for hyponitrous acid).
Scheme 48.
Proposed mechanism for the scavenging properties of tellurosugars against hypohalous and hyponitrous acids.
In silico studies conducted on an ample number of seleno- and tellurosugars demonstrated that the incorporation of heavier chalcogen atoms (Se, Te) improves the antiradical properties of these glycomimetics compared to their sulfur isosters [166].
4.2. Telluroglycosides
Incorporation of Te on the aglycon residue of a carbohydrate can be accomplished by reaction of O-protected (Bz, Ac, Bn) glycosyl bromides with diaryl ditellurides in the presence of NaBH4 [167]. Reactions proceeded smoothy, with good yields, and total stereospecificity; this outcome is compatible with an SN2-type reaction between the glycosyl bromide and the in situ generated aryl telluride, leading to the inversion of the configuration of the anomeric carbon (Scheme 49).
Scheme 49.
Preparation of telluroglycosides.
A relevant application of aryl telluro glycosides is their transformation into glycosyl fluorides, which are important tools in glycobiology, acting as probes in mechanistic studies of glycosidases and glycosyl transferases, and are also key intermediates for synthetic and enzymatic glycosylation reactions. Williams and coworkers reported [168] the straightforward transformation of thio-, seleno- and telluroglycosides into the corresponding glycosyl fluorides 268 using Xtalfluor reagents (E® and M®) as an alternative to DAST and DAST/NBS as fluorine sources (Scheme 50). DAST is moisture sensitive and thermally unstable.
Scheme 50.
Preparation of glycosyl fluorides from telluroglycosides.
4.3. Sugar-Derived α-Alkoxyacyl Tellurides as Synthetic Intermediates
Inoue and coworkers developed [169] a robust convergent methodology for accessing complex polyoxygenated backbones featured with a high density of stereocenters. Such structure is frequently present in natural products, not only in saccharides, but also in secondary metabolites with great pharmaceutical interest. This methodology is based on a decarbonylative radical coupling reaction between sugar-derived α-alkoxyacyl tellurides and double bonds from aldehydes [170,171], oximes [169,172], enones [169,173], and N-heteroarenes [174]; homocoupling reactions between α-alkoxy radicals derived from carbohydrates have also been described [175,176].
α-Alkoxyacyl tellurides derived from carbohydrates (271) can be accessed [170] following the synthetic methodology depicted in Scheme 51. Thus, O-protected uronic acids 269 are converted into transient, non-isolated isobutyl esters 270 by treatment with isobutyl chloroformate in the presence of N-methylmorpholine (NMM). Subsequent reaction with in situ generated phenyl telluride (reduction of diphenyl ditelluride with NaBH4) afforded the corresponding α-alkoxyacyl telluride 271.
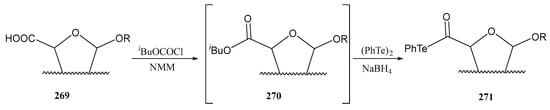
Scheme 51.
General synthetic pathway for the preparation of α-alkoxyacyl tellurides derived from carbohydrates.
Then, treatment of 271 with Et3B and O2 promotes the formation of an ethyl radical which in turn reacts with the organotellurium derivative to furnish an acyl radical (Scheme 52). This intermediate spontaneously undergoes a decarbonylation reaction, with the loss of CO to give an α-alkoxy radical, which is trapped by the acceptor (aldehyde, oxime, enone, or an N-heteroarene) to give derivatives 276–279 [171]; the complete mechanism is depicted, as a model process for coupling with aldehydes. Reaction with simple benzaldehyde failed, presumably due to the reduced electrophilicity of the carbonyl group because of conjugation with the aromatic residue [171]. Replacement of benzaldehyde with o-hydroxybenzaldedyde, and addition of a Lewis acid (Et2AlCl) successfully allowed the radical coupling reaction. It was claimed that complexation with the Lewis acid enhanced its reactivity. Radical-radical homocoupling reaction (without the addition of a free radical trapping reagent) [175] yielded dimers 280 as a mixture of diastereoisomers (Scheme 52). Coupling reactions between two different carbohydrate-derived radicals have also been reported [176].
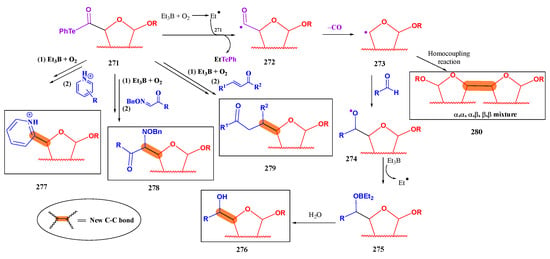
Scheme 52.
Proposed mechanism for the reaction of α-alkoxyacyl tellurides with radical acceptors.
Figure 19 depicts the α-alkoxyacyl tellurides derived from carbohydrates that have been employed as key synthetic intermediates in the preparation of complex compounds, including natural products: 281–283 [169,171,174], 284, 285 [172], 286 [171], 287, 288 [175,176], 289 [170], 290 [173], 291–293 [175].
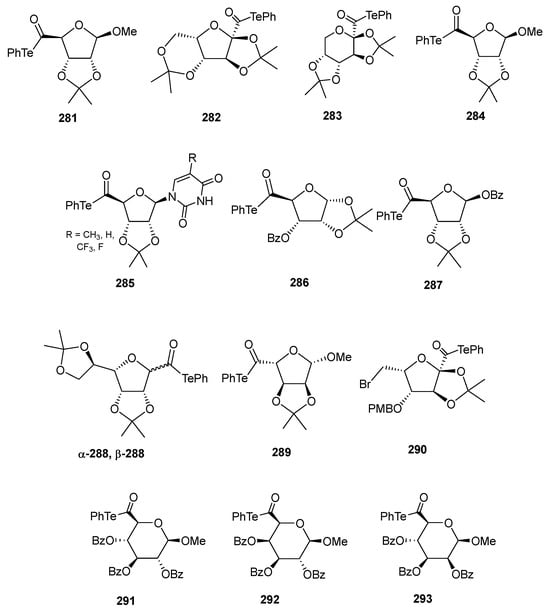
Figure 19.
Sugar-derived α-alkoxyacyl tellurides as intermediates in free-radical reactions.
Figure 20 shows a summary of the most relevant complex derivatives obtained from α-alkoxyacyl tellurides as one of the key intermediates: carbohydrate-N-heteroarene adducts through a hindered C(sp2)-C(sp3) junction (294) [174]; polyoxins J and L and fluoranalogues (295) [172]; the antitumour agent LLY-283 (296) [171]; the C2-symmetric bis-tetrahydrofuran 297 [176], a template that is present in the skeleton of the natural anticancer agent asimicin, as well as dimeric pyranose scaffolds 298 [175], which may be suitable for the construction of polyoxygenated structures commonly found in natural bioactive compounds; the antibacterial agent diospyrodin (299) [170]; or the tricyclic derivative 300 [173], present in the skeleton of bioactive cladiellin diterpenoids.
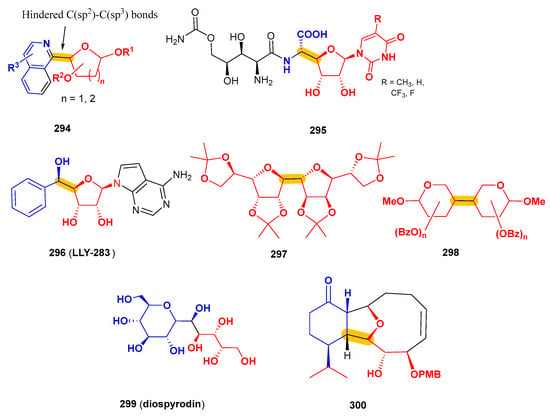
Figure 20.
Examples of structures obtained from α-alkoxyacyl tellurides via free-radical reactions as one of the key steps.
5. Conclusions
In conclusion, the enormous importance of natural carbohydrates in a countless number of biochemical pathways has driven the development of glycomimetics to modulate or enhance their chemo-biological properties. Among the vast number of families of glycomimetics, those derived from the isosteric replacement of oxygen atoms at endocyclic or glycosidic positions by chalcogens (S, Se, Te) has received special attention. This transformation has paved the way for the synthesis of derivatives with unique conformational properties, increased hydrolytic stability, notable redox behavior, improved enzyme or receptor binding, and promising pharmacological effects. The growing interest in these compounds has also led to significant advances in the development of highly chemo- and regioselective reactions, environmentally friendly processes, chemoenzymatic synthesis, and even automated methodologies.
Author Contributions
Conceptualization, Ó.L.; writing—original draft preparation, R.M.-P., M.V.-Z. and Ó.L.; writing—review and editing, R.M.-P., M.V.-Z., J.G.F.-B. and Ó.L. All authors have read and agreed to the published version of the manuscript.
Funding
J.G.F.-B. and O.L. thank the Spanish MICIU (project PID2023-147401OB-I00, funded by MICIU/AEI/10.13039/501100011033, and FEDER, UE) and Junta de Andalucía (FQM134) for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A3AR | A3 Adenosine Receptor |
| AllNAc | N-Acetylallosamine |
| B2pin2 | Bis(pinacolato)diboron |
| BSA | N,O-bis(trimethylsilyl)acetamide |
| BTD | Benzo-2,1,3-thiadiazole |
| C. diff. | Clostridioides difficile |
| Cer | Ceramide |
| CuAAC | Cu(I)-Catalyzed-Azide-Alkyne Cycloaddition |
| DBU | 1,8-Diazabicyclo(5.4.0)undec-7-ene |
| Di-tBubpy | 4,4-Di-tert-butyl-2,2–dipyridyl |
| DAST | Diethylaminosulfur trifluoride |
| DCM | Dichloromethane |
| DENV | Dengue Virus |
| DHS | Dihydroxy selenolane |
| DIAD | Diisopropyl azodicarboxylate |
| DMAP | 4-(N,N-Dimethylamino)pyridine |
| DNsNH | 2,4-(Dinitrobenzenesulfonyl)amino |
| DPAP | 2,2-Dimethoxy-2-phenylacetophenone |
| DPPA | Diphenylphosphoryl azide |
| DTT | Dithiothreitol |
| EDA | Electron-Donor-Aceptor |
| Fdx | Fidaxomicin |
| GHS | Glutahione |
| GLUT | Glucose Transporters |
| GPx | Glutathione peroxidase |
| HCV | Hepatitis C Virus |
| HEMA | Poly(hydroxyethyl methacrylate) |
| HFIP | Hexafluoroisopropanol |
| HOMO | Highest Occupied Molecular Orbital |
| LED | Light Emitting Diode |
| LOOH | Lipid peroxide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MGL | Metabolic Glycan Labelling |
| MIC | Minimum Inhibitory Concentration |
| MPO | Myeloperoxidase |
| MsOH | Methanesulfonic acid |
| MUC1 | Mucin 1 |
| NBS | N-Bromosuccinimide |
| NDI | Naphthalene diimide |
| NHC | N-Heterocyclic carbene |
| NIS | N-Iodosuccinimide |
| NMM | N-Methylmorpholine |
| OGA | O-GlcNAcase |
| PDI | Protein disulfide isomerases |
| PEGDA | Poly(ethyleneglycol diacrylate) |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PTA | Phosphotungstic acid |
| p-QMS | p-Quinone methides |
| RBCs | Red Blood Cells |
| ROS | Reactive Oxygen Species |
| SET | Single Electron Transfer |
| Skp2 | S-Phase Kinase Associated Protein 2 |
| TBDPS | Terc-Butyldiphenylsilyl |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl, free radical |
| TIPDS | Tetraisopropyldisiloxanyl |
| TMSOTf | Trimethylsilyl triflate |
| TT | Thianthrenium |
| WGA | Wheat Germ Agglutin |
| XantPhos | 9,9-Dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane |
References
- Cao, X.; Du, X.; Jiao, H.; An, Q.; Chen, R.; Fang, P.; Wang, J.; Yu, B. Carbohydrate-based drugs launched during 2000−2021. Acta Pharm Sin B 2022, 12, 3783–3821. [Google Scholar] [CrossRef]
- Matassini, C.; Clemente, F.; Cardona, F. Carbohydrate-based therapeutics for lysosomal storage disorders. In Carbohydrate-Based Therapeutics; Adamo, R., Lay, L., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2023; pp. 245–292. [Google Scholar] [CrossRef]
- Sorieul, C.; Papi, F.; Carboni, F.; Pecetta, S.; Phogat, S.; Adamo, R. Recent advances and future perspectives on carbohydrate-based cancer vaccines and therapeutics. Pharmacol. Ther. 2022, 235, 108158. [Google Scholar] [CrossRef]
- De Martos, A.M. Recent advances in the development and synthesis of carbohydrate-based molecules with promising antibacterial activity. Eur. J. Org. Chem. 2023, 26, e202200919. [Google Scholar] [CrossRef]
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-based nanocarriers and their application to target macrophages and deliver antimicrobial agents. Adv. Drug Deliv. Rev. 2019, 151–152, 94–129. [Google Scholar] [CrossRef]
- Zorin, A.; Klenk, L.; Mack, T.; Deigner, H.-P.; Schmidt, M.S. Current synthetic approaches to the synthesis of carbasugars from non-carbohydrate sources. Top. Curr. Chem. 2022, 380, 12. [Google Scholar] [CrossRef]
- Clemente, F.; Matassini, C.; Cardona, F. Reductive amination routes in the synthesis of piperidine iminosugars. Eur. J. Org. Chem. 2020, 29, 4447–4462. [Google Scholar] [CrossRef]
- Hernández-Guerra, D.; Kennedy, A.R.; León, E.I.; Martín, Á.; Pérez-Martín, I.; Rodríguez, M.S.; Suárez, E. Synthetic approaches to phosphasugars (2-oxo-1,2-oxaphosphacyclanes) using the anomeric alkoxyl radical β-fragmentation reaction as the key step. J. Org. Chem. 2020, 85, 4861–4880. [Google Scholar] [CrossRef]
- Gupta, S.; Gauthier, C. 1-Thiosugars: From synthesis to applications. Curr. Org. Chem. 2025, 29, 359–401. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Dias, I.F.C.; Di Lorenzo, I.; Grzes, P.; Palomba, M.; Rosati, O.; Bagnoli, L.; Marini, F.; Santi, C.; Lenardao, E.J.; et al. Sweet selenium: Synthesis and properties of selenium-containing sugars and derivatives. Pharmaceuticals 2020, 13, 211. [Google Scholar] [CrossRef]
- Borges, E.L.; Ignasiak, M.T.; Velichenko, Y.; Perin, G.; Hutton, C.A.; Davies, M.J.; Schiesser, C.H. Synthesis and antioxidant capacity of novel stable 5-tellurofuranose derivatives. Chem. Commun. 2018, 54, 2990–2993. [Google Scholar] [CrossRef]
- Czubatka-Bieńkowska, A.; Sarnik, J.; Poplawski, T. Biological properties of (1–4)-thiodisaccharides. Carbohydr. Res. 2023, 533, 108934. [Google Scholar] [CrossRef]
- Bielski, R.; Mencer, D. New syntheses of thiosaccharides utilizing substitution reactions. Carbohydr. Res. 2023, 532, 108915. [Google Scholar] [CrossRef]
- Ahiadorme, D.; Crich, D. Entropy-enthalpy compensation in the methyl 5-thio-α-D-galactopyranoside–Jacalin interaction. Carbohydr. Res. 2025, 547, 109305. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Culhane, J.M. Thiosugars: New perspectives regarding availability and potential biochemical and medicinal applications. Appl. Microbiol. Biotechnol. 2005, 69, 237–244. [Google Scholar] [CrossRef]
- Al Bujuq, N. Strategies for introducing sulfur atom in a sugar ring: Synthesis of 5-thioaldopyranoses and their NMR data. J. Sulfur Chem. 2019, 40, 664–702. [Google Scholar] [CrossRef]
- Ueda, A.; Pi, J.; Makura, Y.; Tanaka, M.; Uenishi, J. Stereoselective synthesis of (+)-5-thiosucrose and (+)-5-thioisosucrose. RSC Adv. 2020, 10, 9730–9735. [Google Scholar] [CrossRef]
- Díaz-Fernández, M.; Pino-González, S. Thiomonosaccharide derivatives from d-mannose. Chem. Proc. 2021, 3, 28. [Google Scholar] [CrossRef]
- Ma, S.-T.; Lee, C.-W.; Liu, W.-M. Synthesis of 4-thiol-furanosidic uronate via hydrothiolation reaction. RSC Adv. 2021, 11, 18409–18416. [Google Scholar] [CrossRef]
- Johnston, B.D.; Jensen, H.H.; Pinto, B.M. Synthesis of sulfonium sulfate analogues of disaccharides and their conversion to chain-extended homologues of salacinol: New glycosidase inhibitors. J. Org. Chem. 2006, 71, 1111–1118. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Tanabe, G.; Matsuda, H.; Yoshikawa, M.; Muraoka, O. A review of antidiabetic active thiosugar sulfoniums, salacinol and neokotalanol, from plants of the genus Salacia. J. Nat. Med. 2021, 75, 449–466. [Google Scholar] [CrossRef]
- Takashima, K.; Sakano, M.; Kinouchi, E.; Nakamura, S.; Marumoto, S.; Ishikawa, F.; Ninomiya, K.; Nakanishi, I.; Morikawa, T.; Tanabe, G. Elongation of the side chain by linear alkyl groups increases the potency of salacinol, a potent α-glucosidase inhibitor from the Ayurvedic traditional medicine “Salacia”, against human intestinal maltase. Bioorg. Med. Chem. Lett. 2021, 33, 127751. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Nakamura, S.; Nagayama, M.; Marumoto, S.; Ishikawa, F.; Xie, W.; Nakanishi, I.; Muraoka, O.; Morikawa, T.; Tanabe, G. Role of the thiosugar ring in the inhibitory activity of salacinol, a potent natural α-glucosidase inhibitor. RSC Adv. 2024, 14, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Haines, A.H.; Taylor, R.J.K.; Chrystal, E.J.T.; Gravestock, M.B. Synthesis and biological activity of acyclic analogues of nojirimycin. J. Chem. Soc. Perkin Trans. 1 1994, 1, 2229–2235. [Google Scholar] [CrossRef]
- Ishikawa, F.; Jinno, K.; Kinouchi, E.; Ninomiya, K.; Marumoto, S.; Xie, W.; Muraoka, O.; Morikawa, T.; Tanabe, G. Diastereoselective synthesis of salacinol-type α-glucosidase inhibitors. J. Org. Chem. 2018, 83, 185–193. [Google Scholar] [CrossRef]
- Crawford, C.J.; Seeberger, P.H. Advances in glycoside and oligosaccharide synthesis. Chem. Soc. Rev. 2023, 52, 7773–7801. [Google Scholar] [CrossRef]
- Bennai, N.; Chabrier, A.; Fatthalla, M.I.; Tran, C.; Yen-Pon, E.; Belkadi, M.; Alami, M.; Grimaud, L.; Messaoudi, S. Reversing reactivity: Stereoselective desulfurative 1,2-trans-O-glycosylation of anomeric thiosugars with carboxylic acids under copper or cobalt catalysis. J. Org. Chem. 2020, 85, 8893–8909. [Google Scholar] [CrossRef]
- Banisalman, K.A.F.; Polykandritou, A.; Barnieh, F.M.; Morais, G.R.; Falconer, R.A. Chemoselective solution- and solid-phase synthesis of disulfide linked glycopeptides. J. Org. Chem. 2022, 87, 14026–14036. [Google Scholar] [CrossRef]
- Neralkar, M.; Tian, L.; Redman, R.L.; Krauss, I.J. Synthesis of mannosidase-stable Man3 and Man4 glycans containing S-linked Manα1→2Man termini. Org. Lett. 2021, 23, 3053–3057. [Google Scholar] [CrossRef]
- Morrone-Pozzuto, P.; Uhrig, M.L.; Agusti, R. Synthesis of oligosaccharides containing the S-Galp(α1 → 3)Galp unit, glycomimetic of the epitope recognized by lytic antibodies. J. Org. Chem. 2022, 87, 13455–13468. [Google Scholar] [CrossRef]
- Bege, M.; Singh, V.; Sharma, N.; Debreczeni, N.; Bereczki, I.; Poonam; Herczegh, P.; Rathi, B.; Singh, S.; Borbás, A. In vitro and in vivo antiplasmodial evaluation of sugar-modified nucleoside analogues. Sci. Rep. 2023, 13, 12228. [Google Scholar] [CrossRef]
- Azzam, R.A.; Gad, N.M.; Elgemeie, G.H. Novel thiophene thioglycosides substituted with the benzothiazole moiety: Synthesis, characterization, antiviral and anticancer evaluations, and NS3/4A and USP7 enzyme inhibitions. ACS Omega 2022, 7, 35656–35667. [Google Scholar] [CrossRef]
- de la Luz Quintana, I.; Paul, A.; Chowdhury, A.; Moulton, K.D.; Kulkarni, S.S.; Dube, D.H. Thioglycosides act as metabolic inhibitors of bacterial glycan biosynthesis. ACS Infect. Dis. 2023, 9, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Gao, X.; del Solar, V.; Yu, X.; Antonopoulos, A.; Friedman, A.E.; Matich, E.K.; Atilla-Gokcumen, G.E.; Nasirikenari, M.; Lau, J.T.; et al. Thioglycosides are efficient metabolic decoys of glycosylation that reduce selectin dependent leukocyte adhesion. Cell Chem. Biol. 2018, 25, 1519–1532. [Google Scholar] [CrossRef]
- He, P.; Zhang, X.; Xia, K.; Green, D.E.; Baytas, S.; Xu, Y.; Pham, T.; Liu, J.; Zhang, F.; Almond, A.; et al. Chemoenzymatic synthesis of sulfur-linked sugar polymers as heparanase inhibitors. Nat. Commun. 2022, 13, 7438. [Google Scholar] [CrossRef]
- Burrini, N.; Pâris, A.; Collet, G.; Lafite, P.; Daniellou, R. Biocatalytic synthesis of coumarin S-glycosides: Towards non-cytotoxic probes for biomedical imaging and sensing. Molecules 2024, 29, 1322. [Google Scholar] [CrossRef]
- Ferrara, I.; Chesnokov, G.A.; Dittmann, S.; Blacque, O.; Sievers, S.; Gademann, K. Formal single atom editing of the glycosylated natural product fidaxomicin improves acid stability and retains antibiotic activity. JACS Au 2024, 4, 2267–2280. [Google Scholar] [CrossRef]
- Chen, J.-S.; Lo, T.-C.; Hsieh, Y.-C.; Chen, C.-H.; Lin, M.; Lin, H.-Y.; Hung, M.-W.; Wu, H.-R.; Luo, S.-Y. Utilizing reusable catalyst phosphotungstic acid for the synthesis of thioglycoside from per-O-acetyl saccharides with microwave assisted and de-O-acetylation with methanol. ACS Omega 2023, 8, 8885–8893. [Google Scholar] [CrossRef]
- Feng, G.-J.; Luo, T.; Guo, Y.-F.; Liu, C.-Y.; Dong, H. Concise synthesis of 1-thioalkyl glycoside donors by reaction of per-O-acetylated sugars with sodium alkanethiolates under solvent-free conditions. J. Org. Chem. 2022, 87, 3638–3646. [Google Scholar] [CrossRef]
- Santos, B.F.; Silva, B.A.L.; Domingues, N.L.C. Pd-catalyzed functionalization of benzo-2,1,3-thiadiazole at the C-5-position using 1-thiosugars. New, J. Chem. 2022, 46, 19785–19789. [Google Scholar] [CrossRef]
- Bennai, N.; Ibrahim, N.; Marrot, J.; Belkadi, M.; Alami, M.; Magnier, E.; Anselmi, E.; Messaoudi, S. Synthesis of S-trifluoromethyl S-arylsulfoximine thioglycosides through Pd-catalyzed Migita cross-coupling. Eur. J. Org. Chem. 2020, 2020, 4972–4981. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, W.-J.; Yao, L.; Niu, Y.; Zhao, H.; Peng, C.; Han, B.; Huang, W.; Zhan, G. Organocatalytic atroposelective synthesis of naphthoquinone thioglycosides from aryl-naphthoquinones and thiosugars. Chem. Commun. 2023, 59, 7279–7282. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Mandal, P.K. An Efficient one-pot protocol for direct access to diarylmethyl thioglycosides with para-quinone methides via S-glycosyl isothiouronium salts. Synlett 2020, 31, 1713–1719. [Google Scholar] [CrossRef]
- Venkatesh, R.; Tiwari, V.; Kandasamy, J. Copper(I)-catalyzed Sandmeyer-type S-arylation of 1-thiosugars with aryldiazonium salts under mild conditions. J. Org. Chem. 2022, 87, 11414–11432. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.-J.; Guo, Y.-F.; Tang, Y.; Li, M.; Jia, Y.; Li, Z.; Wang, S.; Liu, H.; Wu, Y.; Dong, H. Design, synthesis, and biological evaluation of thioglucoside analogues of gliflozin as potent new gliflozin drugs. J. Med. Chem. 2023, 66, 12536–12543. [Google Scholar] [CrossRef]
- Feng, G.-J.; Wang, S.-S.; Lv, J.; Luo, T.; Wu, Y.; Dong, H. Improved synthesis of 1-glycosyl thioacetates and its application in the synthesis of thioglucoside gliflozin analogues. Eur. J. Org. Chem. 2021, 2021, 2940–2949. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Zhong, L.; Wang, S.; Liu, Z.; Dai, Y.; He, Y.; Feng, Z. Boron-promoted umpolung reaction of sulfonyl chlorides for the stereospecific synthesis of thioglycosides via reductive deoxygenation coupling reactions. Org. Lett. 2022, 24, 2463–2468. [Google Scholar] [CrossRef]
- Malapit, C.A.; Prater, M.B.; Cabrera-Pardo, J.R.; Li, M.; Pham, T.D.; McFadden, T.P.; Blank, S.; Minteer, S.D. Advances on the merger of electrochemistry and transition metal catalysis for organic synthesis. Chem. Rev. 2022, 122, 3180–3218. [Google Scholar] [CrossRef]
- Li, F.; Liu, H.; Xing, W.; Zhang, Q.; Wang, L. Electrochemical nickel-catalyzed cross-coupling of glycosyl thiols with preactivated phenols and ketones. Org. Biomol. Chem. 2024, 22, 3597–3601. [Google Scholar] [CrossRef]
- Zhu, M.; Alami, M.; Messaoudi, S. Electrochemical nickel-catalyzed Migita cross-coupling of 1-thiosugars with aryl, alkenyl and alkynyl bromides. Chem. Commun. 2020, 56, 4464–4467. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Jiang, Q.-H.; Wang, H.-X.; Zhang, X.-W.; Yan, N. Electrochemically mediated S-glycosylation of 1-thiosugars with xanthene derivatives. Org. Lett. 2023, 25, 4252–4257. [Google Scholar] [CrossRef]
- Zhu, M.; Ghouilem, J.; Messaoudi, S. Visible-light-mediated Stadler−Ziegler arylation of thiosugars with anilines. ACS Org. Inorg. Au. 2022, 2, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liang, Q.; Shi, L.; Wen, J.; Liu, X.; Hong, X.; Zha, X.; Ji, F. Site-selective S-arylation of 1-thiosugars with aryl thianthrenium salts through copper(I)-mediated, photoredox catalyzed reactions. Adv. Synth. Catal. 2024, 36, 2344–2351. [Google Scholar] [CrossRef]
- Zhang, L.; He, S.; Hou, J.; Ye, M.; Chen, J.; Lv, G.; Huang, T.; Yang, Z.; Wu, Y. Visible-light-mediated synthesis of non-anomeric S-aryl glycosides via a photoactive electron-donor–acceptor complex. Chem. Commun. 2023, 59, 13759–13762. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Benassi, A.; Peñalver, P.; Cucchiarini, A.; Guédin, A.; Mergny, J.L.; Rosu, F.; Gabelica, V.; Freccero, M.; Doria, F.; et al. Thiosugar naphthalene diimide conjugates: G-quadruplex ligands with antiparasitic and anticancer activity. Eur. J. Med. Chem. 2022, 232, 114183. [Google Scholar] [CrossRef]
- Ceccherini, V.; Giorgi, E.; Mannelli, M.; Cirri, D.; Gamberi, T.; Gabbiani, C.; Pratesi, A. Synthesis, chemical characterization, and biological evaluation of hydrophilic gold(I) and silver(I) N-heterocyclic carbenes as potential anticancer agents. Inorg. Chem. 2024, 63, 16949–16963. [Google Scholar] [CrossRef]
- Ryzhakov, D.; Beillard, A.; Le Bideau, F.; Al-Shuaeeb, R.A.A.; Alami, M.; Bantreil, X.; Bonnemoy, A.; Gautier, A.; Lamaty, F.; Messaoudi, S. Azoliums and Ag(I)-N-heterocyclic carbene thioglycosides: Synthesis, reactivity and bioactivity. Eur. J. Org. Chem. 2022, 2022, e202101499. [Google Scholar] [CrossRef]
- Cristófalo, A.E.; Cagnoni, A.J.; Uhrig, M.L. Synthesis of N-acetylglucosamine and N-acetylallosamine resorcinarene-based multivalent β-thio-glycoclusters: Unexpected affinity of N-acetylallosamine ligands towards wheat germ agglutinin. Org. Biomol. Chem. 2020, 18, 6853–6865. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Shit, P.; Kundu, M.; Misra, A.K. Straightforward stereoselective synthesis of 1-thio-β-d-mannosides and 1-thio-β -l-rhamnosides. Tetrahedron Lett. 2023, 118, 154391. [Google Scholar] [CrossRef]
- Csávás, M.; Eszenyi, D.; Mező, E.; Lázár, L.; Debreczeni, N.; Tóth, M.; Somsák, L.; Borbás, A. Stereoselective synthesis of carbon-sulfur-bridged glycomimetics by photoinitiated thiol-ene coupling reactions. Int. J. Mol. Sci. 2020, 21, 573. [Google Scholar] [CrossRef]
- Reintjens, N.R.M.; Witte, M.D.; Minnaard, A.J. Site-selective introduction of thiols in unprotected glycosides. Org. Biomol. Chem. 2023, 21, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.K.; Pohl, N.L.B. Automated solution-phase synthesis of S-glycosides for the production of oligomannopyranoside derivatives. Org. Lett. 2020, 22, 4156–4159. [Google Scholar] [CrossRef] [PubMed]
- Meena; Sam, M.; Pierce, K.; Szostak, J.W.; McLaughlin, L.W. 2′,3′-Dideoxy-3′-thionucleoside triphosphates: Syntheses and polymerase substrate activities. Org. Lett. 2007, 9, 1161–1163. [Google Scholar] [CrossRef]
- Zargar, I.A.; Sakander, N.; Mukherjee, D. 2-Ketophenyl assisted biomimetic synthesis of 3-thio substituted glycals at room temperature. Eur. J. Org. Chem. 2023, 26, e202300780. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, Y.; Luo, H.; Huang, N.; Lai, M.; Zou, K.; Yao, H. Catalyst-controlled regiodivergent synthesis of 1- and 3-thiosugars with high stereoselectivity and chemoselectivity. ACS Catal. 2021, 11, 5287–5293. [Google Scholar] [CrossRef]
- Qin, K.; Zhang, H.; Zhao, Z.; Chen, X. Protein S-glyco-modification through an elimination–addition mechanism. J. Am. Chem. Soc. 2020, 142, 9382–9388. [Google Scholar] [CrossRef]
- Shit, P.; Kundu, M.; Misra, A.K. Expeditious preparation of 1,6-anhydro-1-thio-β-d-hexopyranose derivatives. Carbohydr. Res. 2023, 525, 108765. [Google Scholar] [CrossRef]
- Kundu, M.; Misra, A.K. Direct synthesis of unsymmetrical glycosyl disulfides from glycosyl bromides. Eur. J. Org. Chem. 2021, 2021, 3759–3767. [Google Scholar] [CrossRef]
- Anghinoni, J.M.; Birmann, P.T.; da Rocha, M.J.; Gomes, C.S.; Davies, M.J.; Brüning, C.A.; Savegnago, L.; Lenardão, E.J. Recent advances in the synthesis and antioxidant activity of low molecular mass organoselenium molecules. Molecules 2023, 28, 7349. [Google Scholar] [CrossRef]
- Jain, V.K.; Priyadarsini, K.I. Selenium compounds as promising antiviral agents. New J. Chem. 2024, 48, 6534–6552. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Fonseca-Berzal, C.; Martínez-Montiel, M.; Álvarez-Márquez, M.; Gómez-Núñez, M.; Lacueva-Arnedo, M.; Espinosa-Buitrago, T.; Martín-Pérez, T.; Escario, J.A.; Merino-Montiel, P.; et al. Thio- and selenosemicarbazones as antiprotozoal agents against Trypanosoma cruzi and Trichomonas vaginalis. J. Enzyme Inhib. Med. Chem. 2022, 37, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Pyka, P.; Garbo, S.; Fioravanti, R.; Jacob, C.; Hittinger, M.; Handzlik, J.; Zwergel, C.; Battistelli, C. Selenium-containing compounds: A new hope for innovative treatments in Alzheimer’s disease and Parkinson’s disease. Drug Discov. Today 2024, 29, 104062. [Google Scholar] [CrossRef] [PubMed]
- Velueta-Viveros, M.; Martínez-Bailén, M.; Puerta, A.; Romero-Hernández, L.L.; Křen, V.; Merino-Montiel, P.; Montiel-Smith, S.; Fernandes, M.X.; Moreno-Vargas, A.J.; Padrón, J.M.; et al. Carbohydrate-derived bicyclic selenazolines as new dual inhibitors (cholinesterases/OGA) against Alzheimer’s disease. Bioorg. Chem. 2022, 127, 105983. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Peña, J.M.; Alejandre-Ramos, D.; López, Ó.; Maya, I.; Lagunes, I.; Padrón, J.M.; Peña-Altamira, L.E.; Bartolini, M.; Monti, B.; Bolognesi, M.L.; et al. New tacrine dimers with antioxidant linkers as dual drugs: Anti-Alzheimer’s and antiproliferative agents. Eur. J. Med. Chem. 2017, 138, 761–773. [Google Scholar] [CrossRef]
- Roldán-Peña, J.M.; Puerta, A.; Dinić, J.; Stojanov, S.J.; González-Bakker, A.; Hicke, F.J.; Mishra, A.; Piyasaengthong, A.; Maya, I.; Walton, J.W.; et al. Biotinylated selenocyanates: Potent and selective cytostatic agents. Bioorg. Chem. 2023, 133, 106410. [Google Scholar] [CrossRef]
- Begines, P.; Martos, S.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Chemoselective preparation of new families of phenolic-organoselenium hybrids-a biological assessment. Molecules 2022, 27, 1315. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Plano, D.; Sanmartín, C. Metal-based compounds containing selenium: An appealing approach towards novel therapeutic drugs with anticancer and antimicrobial effects. Eur. J. Med. Chem. 2022, 244, 114834. [Google Scholar] [CrossRef]
- Begines, P.; Sevilla-Horrillo, L.; Puerta, A.; Puckett, R.; Bayort, S.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Masked phenolic-selenium conjugates: Potent and selective antiproliferative agents overcoming P-gp resistance. Pharmaceuticals 2020, 13, 358. [Google Scholar] [CrossRef]
- Lagunes, I.; Begines, P.; Silva, A.; Galán, A.R.; Puerta, A.; Fernandes, M.X.; Maya, I.; Fernández-Bolaños, J.G.; López, Ó.; Padrón, J.M. Selenocoumarins as new multitarget antiproliferative agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2019, 179, 493–501. [Google Scholar] [CrossRef]
- Blumberg, K.; Fuccello, A.; van Es, T. Selenium derivatives of l-arabinose, d-ribose, and d-xylose. Carbohydr. Res. 1977, 59, 351–362. [Google Scholar] [CrossRef]
- Lucas, M.A.; Nguyen, O.T.K.; Schiesser, C.H.; Zheng, S.-L. Preparation of 5-selenopentopyranose sugars from pentose starting materials by samarium(II) iodide or (phenylseleno)formate mediated ring closures. Tetrahedron 2000, 56, 3995–4000. [Google Scholar] [CrossRef]
- Schiesser, C.H. The quest for selenocycles: From an ESR spectrum to a commercial product. J. Chem. Res. 2022, 46. [Google Scholar] [CrossRef]
- Liu, H.; Pinto, M.B. Synthesis of zwitterionic selenonium and sulfonium sulfates from d-mannose as potential glycosidase inhibitors. Can. J. Chem. 2006, 84, 497–505. [Google Scholar] [CrossRef]
- Schiesser, C.H.; Storkey, C.; Davies, M.J. Seleno-compounds and therapeutic uses thereof. U.S. Patent 2015/0191446 A1, 24 July 2014. [Google Scholar]
- Merino-Montiel, P.; López, Ó.; Fernández-Bolaños, J.G. l-Isofucoselenofagomine and derivatives: Dual activities as antioxidants and as glycosidase inhibitors. Tetrahedron 2012, 68, 3591–3595. [Google Scholar] [CrossRef]
- Davies, M.J.; Schiesser, C.H. 1,4-Anhydro-4-seleno-d-talitol (SeTal): A remarkable selenium-containing therapeutic molecule. New J. Chem. 2019, 43, 9759–9765. [Google Scholar] [CrossRef]
- Zacharias, T.; Flouda, K.; Jepps, T.A.; Gammelgaard, B.; Schiesser, C.H.; Davies, M.J. Effects of a novel selenium substituted-sugar (1,4-anhydro-4-seleno-d-talitol, SeTal) on human coronary artery cell lines and mouse aortic rings. Biochem. Pharmacol. 2020, 173, 113631. [Google Scholar] [CrossRef]
- Voss, G.T.; de Oliveira, R.L.; Davies, M.J.; Domingues, W.B.; Campos, V.F.; Soares, M.P.; Luchese, C.; Schiesser, C.H.; Wilhelm, E.A. Suppressive effect of 1,4-anhydro-4-seleno-d-talitol (SeTal) on atopic dermatitis-like skin lesions in mice through regulation of inflammatory mediators. J. Trace Elem. Med. Biol. 2021, 67, 126795. [Google Scholar] [CrossRef]
- Voss, G.T.; Davies, M.J.; Schiesser, C.H.; de Oliveira, R.L.; Nornberg, A.B.; Soares, V.R.; Barcellos, A.M.; Luchese, C.; Fajardo, A.R.; Wilhelm, E.A. Treating atopic-dermatitis-like skin lesions in mice with gelatin-alginate films containing 1,4-anhydro-4-seleno-d-talitol (SeTal). Int. J. Pharm. 2023, 642, 123174. [Google Scholar] [CrossRef]
- di Vito, R.; Acito, M.; Fatigoni, C.; Schiesser, C.H.; Davies, M.J.; Mangiavacchi, F.; Villarini, M.; Santi, C.; Moretti, M. Genotoxicity assessment of 1,4-anhydro-4-seleno-d-talitol (SeTal) in human liver HepG2 and HepaRG cells. Toxicology 2023, 499, 153663. [Google Scholar] [CrossRef]
- Iwaoka, M.; Takahashi, T.; Tomoda, S. Syntheses and structural characterization of water-soluble selenium reagents for the redox control of protein disulfide bonds. Heteroat. Chem. 2001, 12, 293–299. [Google Scholar] [CrossRef]
- Arai, K.; Kumakura, F.; Takahira, M.; Sekiyama, N.; Kuroda, N.; Suzuki, T.; Iwaoka, M. Effects of ring size and polar functional groups on the glutathione peroxidase-like antioxidant activity of water-soluble cyclic selenides. J. Org. Chem. 2015, 80, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.G.; Kumar, P.; Phadnis, P.; Iwaoka, M.; Priyadarsini, K.I. Free radical induced selenoxide formation in isomeric organoselenium compounds: The effect of chemical structures on antioxidant activity. New J. Chem. 2019, 43, 13357–13362. [Google Scholar] [CrossRef]
- Arai, K.; Tashiro, A.; Osaka, Y.; Iwaoka, M. Glutathione peroxidase-like activity of amino-substituted water-soluble cyclic selenides: A shift of the major catalytic cycle in methanol. Molecules 2017, 22, 354. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Verma, P.; Bhilwade, H.N.; Iwaoka, M.; Priyadarsini, K.I. Dihydroxyselenolane (DHS) supplementation improves survivalfollowing whole-body irradiation (WBI) by suppressing tissue-specificinflammatory responses. Mutat. Res. 2016, 807, 33–46. [Google Scholar] [CrossRef]
- Verma, P.; Kunwar, A.; Arai, K.; Iwaoka, M.; Priyadarsini, K.I. Mechanism of radioprotection by dihydroxy-1-selenolane (DHS): Effect of fatty acid conjugation and role of glutathione peroxidase (GPx). Biochimie 2018, 144, 122–133. [Google Scholar] [CrossRef]
- Iwaoka, M.; Sano, N.; Lin, Y.-Y.; Katakura, A.; Noguchi, M.; Takahashi, K.; Kumakura, F.; Arai, K.; Singh, B.G.; Kunwar, A.; et al. Fatty acid conjugates of water-soluble (±)-trans-selenolane-3,4-diol: Effects of alkyl chain length on the antioxidant capacity. ChemBioChem 2015, 16, 1226–1234. [Google Scholar] [CrossRef]
- Arai, K.; Sato, Y.; Nakajima, I.; Saito, M.; Sasaki, M.; Kanamori, A.; Iwaoka, M. Glutathione peroxidase-like functions of 1,2-diselenane-4,5-diol and its amphiphilic derivatives: Switchable catalytic cycles depending on peroxide substrates. Bioorg. Med. Chem. 2021, 29, 115866. [Google Scholar] [CrossRef]
- Arai, K.; Ueno, H.; Asano, Y.; Chakrabarty, G.; Shimodaira, S.; Mugesh, G.; Iwaoka, M. Protein folding in the presence of water-soluble cyclic diselenides with novel oxidoreductase and isomerase activities. ChemBioChem 2018, 19, 207–211. [Google Scholar] [CrossRef]
- Iwaoka, M.; Katakura, A.; Mishima, J.; Ishihara, Y.; Kunwar, A.; Priyadarsini, K.I. Mimicking the lipid peroxidation inhibitory activity of phospholipid hydroperoxide glutathione peroxidase (GPx4) by using fatty acid conjugates of a water-soluble selenolane. Molecules 2015, 20, 12364–12375. [Google Scholar] [CrossRef]
- Chakrabarty, G.; NaveenKumar, S.K.; Kumar, S.; Mugesh, G. Modulation of redox signaling and thiol homeostasis in red blood cells by peroxiredoxin mimetics. ACS Chem. Biol. 2020, 15, 2673–2682. [Google Scholar] [CrossRef]
- Cimmino, G.; De Nisco, M.; Piccolella, S.; Gravina, C.; Pedatella, S.; Pacifico, S. Innovative cosmeceutical ingredients: Harnessing selenosugar-linked hydroxycinnamic acids for antioxidant and wound-healing properties. Antioxidants 2024, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Serpico, L.; De Nisco, M.; Cermola, F.; Manfra, M.; Pedatella, S. Stereoselective synthesis of selenium-containing glycoconjugates via the Mitsunobu reaction. Molecules 2021, 26, 2541. [Google Scholar] [CrossRef] [PubMed]
- Serpico, L.; Iacono, S.D.; De Stefano, L.; De Martino, S.; Battisti, M.; Dardano, P.; Pedatella, S.; De Nisco, M. pH-sensitive release of antioxidant Se-glycoconjugates through a flexible polymeric patch. Eur. Polym. J. 2022, 178, 111486. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Cimmino, G.; De Nisco, M.; Alonso, C.; Gravina, C.; Piscopo, V.; Lemos, R.; Coderch, L.; Piccolella, S.; Pacifico, S.; Pedatella, S. Novel synthesized seleno-glycoconjugates as cosmeceutical ingredients: Antioxidant activity and in vitro skin permeation. Eur. J. Med. Chem. Rep. 2024, 12, 100240. [Google Scholar] [CrossRef]
- Iwaoka, M.; Hiyoshi, Y.; Arai, S.; Ito, T. Synthesis of 4-selenothreofuranose derivatives via Pummerer-type reactions of trans-3,4-dioxygenated tetrahydroselenophenes mediated by a selenonium intermediate. ACS Omega 2021, 6, 17621–17634. [Google Scholar] [CrossRef]
- Lee, H.; Jarhad, D.B.; Yu, J.; Lee, C.; Jeong, L.S. Asymmetric synthesis of 2-C-methyl-4-selenonucleosides as anti-hepatitis C virus agents. J. Org. Chem. 2019, 84, 14414–14426. [Google Scholar] [CrossRef]
- Jeong, L.S.; Tosh, D.K.; Kim, H.O.; Wang, T.; Hou, X.; Yun, H.S.; Kwon, Y.; Lee, S.K.; Choi, J.; Zhao, L.X. First synthesis of 4-selenonucleosides showing unusual southern conformation. Org. Lett. 2008, 10, 209–212. [Google Scholar] [CrossRef]
- Okano, Y.; Saito-Tarashima, N.; Kurosawa, M.; Iwabu, A.; Ota, M.; Watanabe, T.; Kato, F.; Hishiki, T.; Fujimuro, M.; Minakawa, N. Synthesis and biological evaluation of novel imidazole nucleosides as potential anti-dengue virus agents. Bioorg. Med. Chem. 2019, 27, 2181–2186. [Google Scholar] [CrossRef]
- Sahu, P.K.; Kim, G.; Yu, J.; Ahn, J.Y.; Song, J.; Choi, Y.; Jin, X.; Kim, J.-H.; Lee, S.K.; Park, S.; et al. Stereoselective synthesis of 4-selenonucleosides via seleno-Michael reaction as potent antiviral agents. Org. Lett. 2014, 16, 5796–5799. [Google Scholar] [CrossRef]
- Yu, J.; Kim, J.-H.; Lee, H.W.; Alexander, V.; Ahn, H.-C.; Choi, W.J.; Choi, J.; Jeong, L.S. New RNA purine building blocks, 4′-selenopurine nucleosides: First synthesis and unusual mixture of sugar puckerings. Chem. Eur. J. 2013, 19, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, J.W.; Chandra, G.; Saito–Tarashima, N.; Nogi, Y.; Ota, M.; Minakawa, N.; Jeong, L.S. Synthesis of oligonucleotides containing 5-homo-4-selenouridine derivative and its increased resistance against nuclease. Bioorg. Med. Chem. Lett. 2023, 83, 129172. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Takahashi, H.; Nogi, Y.; Kagotani, Y.; Saito-Tarashima, N.; Kondo, J.; Minakawa, N. Synthesis and properties of fully-modified 4-selenoRNA, an endonuclease-resistant RNA analog. Bioorg. Med. Chem. 2022, 76, 117093. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, J.; Alexander, V.; Choi, J.H.; Song, J.; Lee, H.W.; Kim, H.O.; Choi, J.; Lee, S.K.; Jeong, L.S. Structure-activity relationships of 2′-modified-4′-selenoarabinofuranosyl-pyrimidines as anticancer agents. Eur. J. Med. Chem. 2014, 83, 208–225. [Google Scholar] [CrossRef]
- Byun, W.S.; Jin, M.; Yu, J.; Kim, W.K.; Song, J.; Chung, H.-J.; Jeong, L.S.; Lee, S.K. A novel selenonucleoside suppresses tumor growth by targeting Skp2 degradation in paclitaxel-resistant prostate cancer. Biochem. Pharmacol. 2018, 158, 84–94. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.-C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- An, S.; Yu, J.; Choi, H.; Ko, H.; Ahn, S.; Shin, J.C.; Pyo, J.J.; Jeong, L.S.; Noh, M. Selenium bioisosteric replacement of adenosine derivatives promoting adiponectin secretion increases the binding affinity to peroxisome proliferator-activated receptor δ. Bioorg. Med. Chem. 2020, 28, 115226. [Google Scholar] [CrossRef]
- Choi, H.; An, S.; Hyun, Y.E.; Noh, M.; Jeong, L.S. Design, synthesis and biological evaluation of truncated 1′-homologated 4′-selenonucleosides as PPAR γ/δ dual modulators. Bioorg. Chem. 2025, 154, 108042. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, L.X.; Park, J.; Lee, H.W.; Sahu, P.K.; Cui, M.; Moss, S.M.; Hammes, E.; Warnick, E.; Gao, Z.-G.; et al. N6-Substituted 5-N-methylcarbamoyl-4-selenoadenosines as potent and selective A3 adenosine receptor agonists with unusual sugar puckering and nucleobase orientation. J. Med. Chem. 2017, 60, 3422–3437. [Google Scholar] [CrossRef]
- Kim, M.; Choi, H.; Nayak, A.; Tripathi, S.K.; Aswar, V.R.; Gaikwad, V.B.; Jacobson, K.A.; Jeong, L.S. Structure−activity relationship of truncated 4-selenonucleosides: A3 adenosine receptor activity and binding selectivity. ACS Med. Chem. Lett. 2024, 15, 1620–1626. [Google Scholar] [CrossRef]
- Choi, H.; Jacobson, K.A.; Yu, J.; Jeong, L.S. Design and synthesis of 2,6-disubstituted-4′-selenoadenosine-5′-N,N-dimethyluronamide derivatives as human A3 adenosine receptor antagonists. Pharmaceuticals 2021, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Raics, M.; Timári, I.; Diercks, T.; Szilágyi, L.; Gabius, H.-J.; Kövér, K.E. Selenoglycosides as lectin ligands: 77Se-edited CPMG-HSQMBC NMR spectroscopy to monitor biomedically relevant interactions. ChemBioChem 2019, 20, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hayashi, C.; Komura, N.; Tamai, R.; Uzawa, J.; Ogawa, J.; Tanaka, H.-N.; Imamura, A.; Ishida, H.; Kiso, M.; et al. Synthesis and glycan−protein interaction studies of Se-sialosides by 77Se NMR. Org. Lett. 2019, 21, 6393–6396. [Google Scholar] [CrossRef] [PubMed]
- Diercks, T.; Medrano, F.J.; FitzGerald, F.G.; Beckwith, D.; Pedersen, M.J.; Reihill, M.; Ludwig, A.-K.; Romero, A.; Oscarson, S.; Cudic, M.; et al. Galectin–glycan interactions: Guidelines for monitoring by 77Se NMR spectroscopy, and solvent (H2O/D2O) impact on binding. Chem. Eur. J. 2021, 27, 316–325. [Google Scholar] [CrossRef]
- Raics, M.; Balogh, A.K.; Kishor, C.; Timári, I.; Medrano, F.J.; Romero, A.; Go, R.M.; Blanchard, H.; Szilágyi, L.; Kövér, K.E.; et al. Investigation of the molecular details of the interactions of selenoglycosides and human galectin-3. Int. J. Mol. Sci. 2022, 23, 2494. [Google Scholar] [CrossRef]
- Dibello, E.; Oddone, N.; Franco, J.; Illyés, T.-Z.; Medeiros, A.; Kiss, A.; Hőgye, F.; Kövér, K.E.; Szilágyi, L.; Comini, M.A. Selenosugars targeting the infective stage of Trypanosoma brucei with high selectivity. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100529. [Google Scholar] [CrossRef]
- McDonagh, A.W.; Mahon, M.F.; Murphy, P.V. Lewis acid induced anomerization of Se-glycosides. Application to synthesis of α-Se-GalCer. Org. Lett. 2016, 18, 552–555. [Google Scholar] [CrossRef]
- Zhu, M.; Alami, M.; Messaoudi, S. Room-temperature Pd-catalyzed synthesis of 1-(hetero)aryl selenoglycosides. Org. Lett. 2020, 22, 6584–6589. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Huang, Y.-C.; Shi, W.-Y.; Zheng, N.; Wang, C.-T.; Chen, X.; An, Y.; Zhang, Z.; Liang, Y.-M. Modular synthesis of aryl thio/selenoglycosides via the Catellani strategy. Org. Lett. 2021, 23, 5641–5646. [Google Scholar] [CrossRef]
- Catellani, M.; Frignani, F.; Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 119–122. [Google Scholar] [CrossRef]
- Azeem, Z.; Mandal, P.K. Atom-economic synthesis of unsymmetrical gem-diarylmethylthio/seleno glycosides via base mediated C(O)−S/Se bond cleavage and acyl transfer approach of glycosylthio/selenoacetates. J. Org. Chem. 2023, 88, 1695–1712. [Google Scholar] [CrossRef] [PubMed]
- Shit, P.; Sahaji, S.; Misra, A.K. Synthesis of selenoglycosides and selenium linked disaccharides using reductive cleavage of diselenides. Carbohydr. Res. 2022, 516, 108554. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.; Bengtsson, D.; Infantino, A.S.; Oscarson, S. Synthesis of fluoro- and seleno-containing d-lactose and d-galactose analogues. Org. Biomol. Chem. 2023, 21, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Manna, T.; Misra, A.K. Glycosyl selenoacetates: Versatile building blocks for the preparation of stereoselective selenoglycosides and selenium linked disaccharides. Org. Biomol. Chem. 2019, 17, 8902–8912. [Google Scholar] [CrossRef]
- Angeli, A.; Ferraroni, M.; Granchi, C.; Minutolo, F.; Chen, X.; Shriwas, P.; Russo, E.; Leo, A.; Selleri, S.; Carta, F.; et al. First-in-class dual targeting compounds for the management of seizures in glucose transporter type 1 deficiency syndrome. J. Med. Chem. 2023, 66, 10010–10026. [Google Scholar] [CrossRef]
- Ma, T.; Li, C.; Liang, H.; Wang, Z.; Yu, L.; Xue, W. InBr3-catalyzed synthesis of aryl 1,2-trans-thio(seleno)glycosides. Synlett 2017, 28, 2311–2314. [Google Scholar] [CrossRef]
- Fomitskaya, P.A.; Argunov, D.A.; Tsvetkov, Y.E.; Lalov, A.V.; Ustyuzhanina, N.E.; Nifantiev, N.E. Further investigation of the 2-azido-phenylselenylation of glycals. Eur. J. Org. Chem. 2021, 2021, 5897–5904. [Google Scholar] [CrossRef]
- Guberman, M.; Pieber, B.; Seeberger, P.H. Safe and scalable continuous flow azidophenylselenylation of galactal to prepare galactosamine building blocks. Org. Process Res. Dev. 2019, 23, 2764–2770. [Google Scholar] [CrossRef]
- Zhu, F.; O’Neill, S.; Rodriguez, J.; Walczak, M.A. Stereoretentive reactions at the anomeric position: Synthesis of selenoglycosides. Angew. Chem. Int. Ed. 2018, 57, 7091–7095. [Google Scholar] [CrossRef]
- Miller, E.M.; Walczak, M.A. Light-mediated cross-coupling of anomeric trifluoroborates. Org. Lett. 2021, 23, 4289–4293. [Google Scholar] [CrossRef]
- Compañón, I.; Guerreiro, A.; Mangini, V.; Castro-López, J.; Escudero-Casao, M.; Avenoza, A.; Busto, J.H.; Castillón, S.; Jiménez-Barbero, J.; Asensio, J.L.; et al. Structure-based design of potent tumor-associated antigens: Modulation of peptide presentation by single-atom O/S or O/Se substitutions at the glycosidic linkage. J. Am. Chem. Soc. 2019, 141, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, E.D.; Yashunsky, D.V.; Krylov, V.B.; Bouchara, J.-P.; Cornet, M.; Valsecchi, I.; Fontaine, T.; Latgé, J.-P.; Nifantiev, N.E. Biotinylated oligo-α-(1→4)-d-galactosamines and their N-acetylated derivatives: α-stereoselective synthesis and immunology application. J. Am. Chem. Soc. 2020, 142, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Tokatly, A.I.; Vinnitsky, D.Z.; Kamneva, A.A.; Yashunsky, D.V.; Tsvetkov, Y.E.; Nifantiev, N.E. Glycosylation with derivatives of phenyl 2-azido-2-deoxy-1-seleno-α-d-gluco- and -α-d-mannopyranosides. Russ. Chem. Bull. 2023, 72, 785–792. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Wang, X.; Qiao, Z.; Wang, L.; Wang, P.; Song, N.; Li, M. β-Glycosylations with 2-deoxy-2-(2,4-dinitrobenzenesulfonyl)-amino-glucosyl/galactosyl selenoglycosides: Assembly of partially N-acetylated β-(1→ 6)-oligoglucosaminosides. J. Org. Chem. 2023, 88, 9004–9025. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Zhao, J.; Li, J.; Wang, S.; Fang, J. Combination of chemotherapy and oxidative stress to enhance cancer cell apoptosis. Chem. Sci. 2020, 11, 3215–3222. [Google Scholar] [CrossRef]
- Manna, T.; Misra, A.K. On-water synthesis of glycosyl selenocyanate derivatives and their application in the metal free organocatalytic preparation of nonglycosidic selenium linked pseudodisaccharide derivatives. RSC Adv. 2021, 11, 10902–10911. [Google Scholar] [CrossRef]
- Manna, T.; Rana, A.; Misra, A.K. Synthesis of unsymmetrical glycosyl diselenides by the treatment of symmetrical diselenides with glycosyl selenocyanates. Tetrahedron 2021, 95, 132358. [Google Scholar] [CrossRef]
- Rana, A.; Kundu, M.; Misra, A.K. Convenient synthesis of mixed S-Se-linked pseudodisaccharides by sulfur and selenium exchange. Tetrahedron 2022, 115, 132804. [Google Scholar] [CrossRef]
- Wang, L.; Cao, W.; Xu, H. Tellurium-containing polymers: Towards biomaterials and optoelectronic materials. ChemNanoMat 2016, 2, 479–488. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Barrientos-Astigarraga, R.E. Add a little tellurium to your synthetic plans! Aldrichimica Acta 2000, 33, 66–78. [Google Scholar]
- Irfan, M.; Rehman, R.; Razali, M.R.; Ur-Rehman, S.; Ur-Rehman, A.; Iqbal, M.A. Organotellurium compounds: An overview of synthetic methodologies. Rev. Inorg. Chem. 2020, 40, 193–232. [Google Scholar] [CrossRef]
- Jain, S.; Satpute, S.S.; Jha, R.K.; Patel, M.S.; Kumar, S. Bidentate ligand driven intramolecularly Te…O bonded organotellurium cations from synthesis, stability to catalysis. Chem. Eur. J. 2024, 30, e202303089. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.; Oswal, P.; Bahuguna, A.; Tyagi, A.; Bhatt, N.; Kumar, A. Catalytic system having an organotellurium ligand on graphene oxide: Immobilization of Pd(0) nanoparticles and application in heterogeneous catalysis of cross-coupling reactions. RSC Adv. 2024, 14, 27092–27109. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Gariani, R.A. Biotransformations on organic selenides and tellurides: Synthetic applications. Tetrahedron 2009, 65, 8447–8459. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Zhang, S.; Sun, J.; He, W.; Zhang, L.; Cheng, Z. NIR Photocontrolled organotellurium-mediated reversible-deactivation radical polymerization by activation of C–Te bonds with organotellurium radicals. Macromolecules 2024, 57, 1182–1194. [Google Scholar] [CrossRef]
- Takagi, K.; Hayashi, S.; Sakakibara, N. Controlled cationic polymerization with organotellurium catalysts utilizing redox-mediated chalcogen bonding interaction. Macromolecules 2024, 57, 3358–13367. [Google Scholar] [CrossRef]
- Jiang, Y.; Kibune, M.; Tosaka, M.; Yamago, S. Practical synthesis of dendritic hyperbranched polyacrylates and their topological block polymers by organotellurium-mediated emulsion polymerization in water. Angew. Chem. Int. Ed. 2023, 62, e202306916. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Dubey, A.; Tufail, A.; Tufail, N.; Jangra, K.; Garg, S. Experimental and computational insights into organotellurium (IV) complexes as potent PfNDH2 inhibitors: Synthesis, characterization, and antimalarial evaluation. Appl. Organometal. Chem. 2025, 39, e7778. [Google Scholar] [CrossRef]
- Angeli, A.; Pinteala, M.; Maier, S.S.; Toti, A.; Mannelli, L.D.C.; Ghelardini, C.; Selleri, S.; Carta, F.; Supuran, C.T. Tellurides bearing benzensulfonamide as carbonic anhydrase inhibitors with potent antitumor activity. Bioorg. Med. Chem. Lett. 2021, 45, 128147. [Google Scholar] [CrossRef]
- Wollenhaupt, S.G.N.; Soares, A.T.; Salgueiro, W.G.; Noremberg, S.; Reis, G.; Viana, C.; Gubert, P.; Soares, F.A.; Affeldt, R.F.; Lüdtke, D.S.; et al. Seleno- and telluro-xylofuranosides attenuate Mn-induced toxicity in C. elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2014, 64, 192–199. [Google Scholar] [CrossRef]
- Jiao, A.; Yang, N.; Wang, J.; Xu, X.; Jin, Z. Cyclodextrin-derived chalcogenides as glutathione peroxidase mimics and their protection of mitochondria against oxidative damage. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 155–163. [Google Scholar] [CrossRef]
- Nguyen, O.T.K.; Schiesser, C.H. Preparation of 5-telluropentopyranose sugars from common pentose starting materials. Tetrahedron Lett. 2002, 43, 3799–3800. [Google Scholar] [CrossRef]
- Tanini, D.; Ricci, L.; Capperucci, A. Rongalite-promoted on water synthesis of functionalised tellurides and ditellurides. Adv. Synth. Catal. 2020, 362, 1323–1332. [Google Scholar] [CrossRef]
- Kashyap, C.; Mazumder, L.J.; Rohman, S.S.; Ullah, S.S.; Guha, A.K. Re-visiting the antioxidant activity of Se- and Te-carbohydrates: A theoretical study. ChemistrySelect 2019, 4, 1470–1475. [Google Scholar] [CrossRef]
- Yamago, S.; Kokubo, K.; Masuda, S.; Yoshida, J.-i. Practical synthesis of telluroglycosides. Synlett 1996, 1996, 929–930. [Google Scholar] [CrossRef]
- Tsegay, S.; Williams, R.J.; Williams, S.J. Synthesis of glycosyl fluorides from thio-, seleno-, and telluroglycosides and glycosyl sulfoxides using aminodifluorosulfinium tetrafluoroborates. Carbohydr. Res. 2012, 357, 16–22. [Google Scholar] [CrossRef]
- Nagatomo, M.; Kamimura, D.; Matsui, Y.; Masuda, K.; Inoue, M. Et3B-mediated two- and three-component coupling reactions via radical decarbonylation of α-alkoxyacyl tellurides: Single-step construction of densely oxygenated carboskeletons. Chem. Sci. 2015, 6, 2765–2769. [Google Scholar] [CrossRef]
- Fukuda, T.; Nagatomo, M.; Inoue, M. Total synthesis of diospyrodin and its three diastereomers. Org. Lett. 2020, 22, 6468–6472. [Google Scholar] [CrossRef]
- Nagatomo, M.; Zhang, K.; Fujino, H.; Inoue, M. Et3B/Et2AlCl/O2-mediated radical coupling reaction between α-alkoxyacyl tellurides and 2-hydroxybenzaldehyde derivatives. Chem. Asian J. 2020, 15, 3820–3824. [Google Scholar] [CrossRef]
- Fujino, H.; Nagatomo, M.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Unified total synthesis of polyoxins J, L, and fluorinated analogues on the basis of decarbonylative radical coupling reactions. Angew. Chem. Int. Ed. 2017, 56, 11865–11869. [Google Scholar] [CrossRef]
- Nagatomo, M.; Fujimoto, Y.; Masuda, K.; Inoue, M. Construction of a 6/5/9-membered tricyclic structure of cladiellins via radical-polar crossover reaction. J. Antibiot. 2019, 72, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, D.; Ovadia, B.; Kamimura, D.; Nagatomo, M.; Inoue, M. Installation of O-heterocycles to N-heteroarenes via an Et3B/O2-mediated radical reaction of α-alkoxy and α-alkoxyacyl tellurides. Asian J. Org. Chem. 2019, 8, 1088–1091. [Google Scholar] [CrossRef]
- Masuda, K.; Nagatomo, M.; Inoue, M. Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions. Nat. Chem. 2017, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, T.; Yamaguchi, A.; Nagatomo, M.; Inoue, M. Convergent total synthesis of asimicin via decarbonylative radical dimerization. Chem. Eur. J. 2018, 24, 18907–18912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).