Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization of Target Compounds
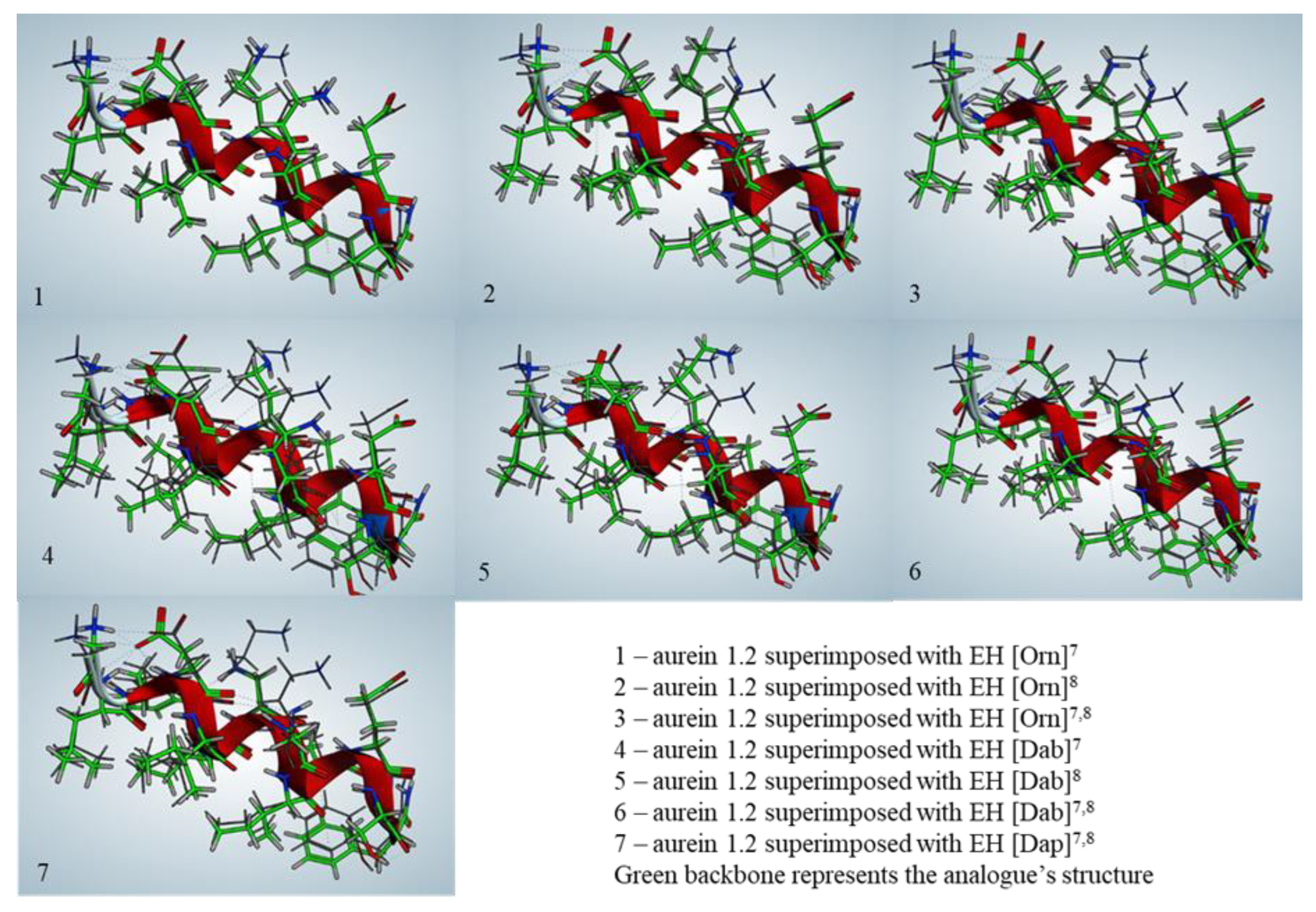
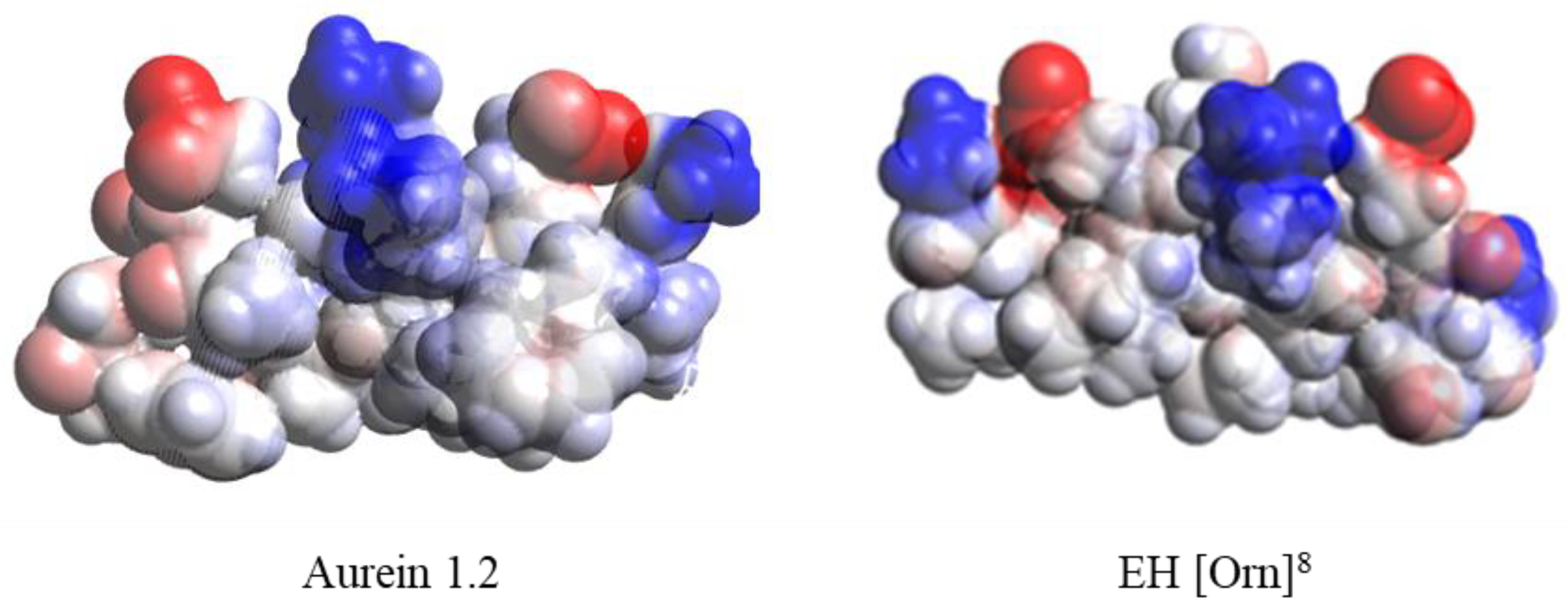
2.2. Secondary Structure Prediction
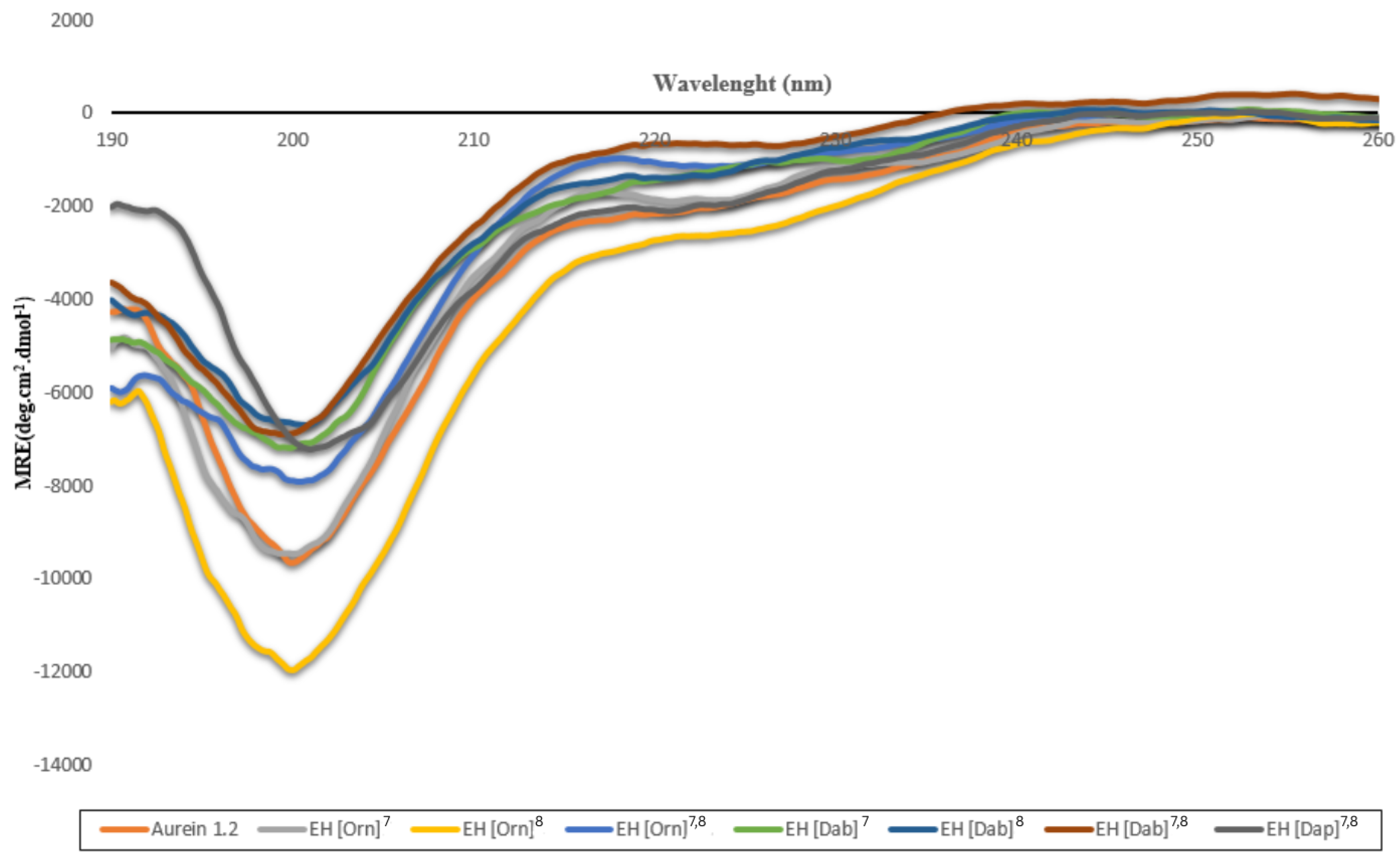
2.3. Secondary Structures of the Peptides Studied by CD
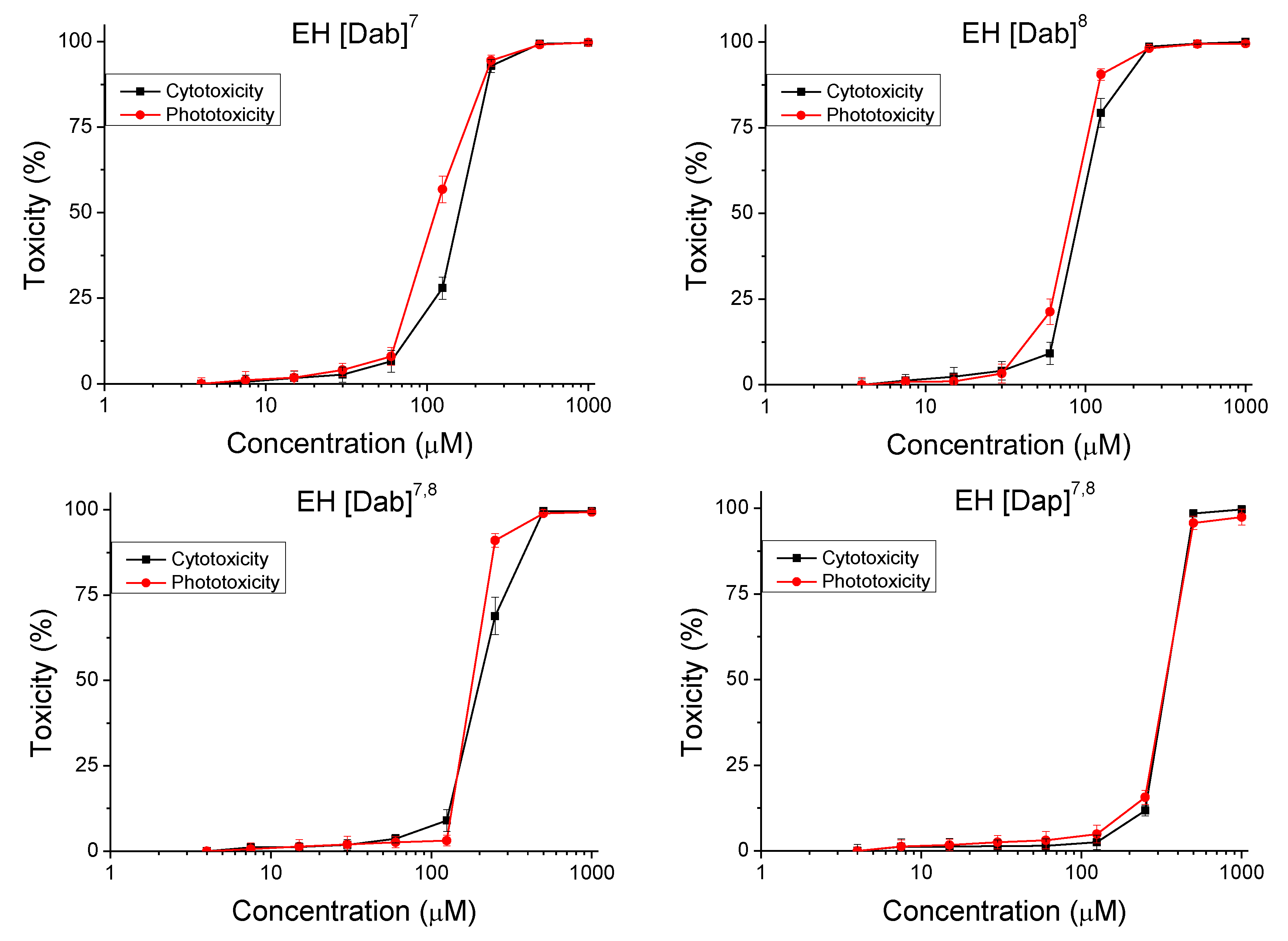
2.4. In Vitro Safety Testing
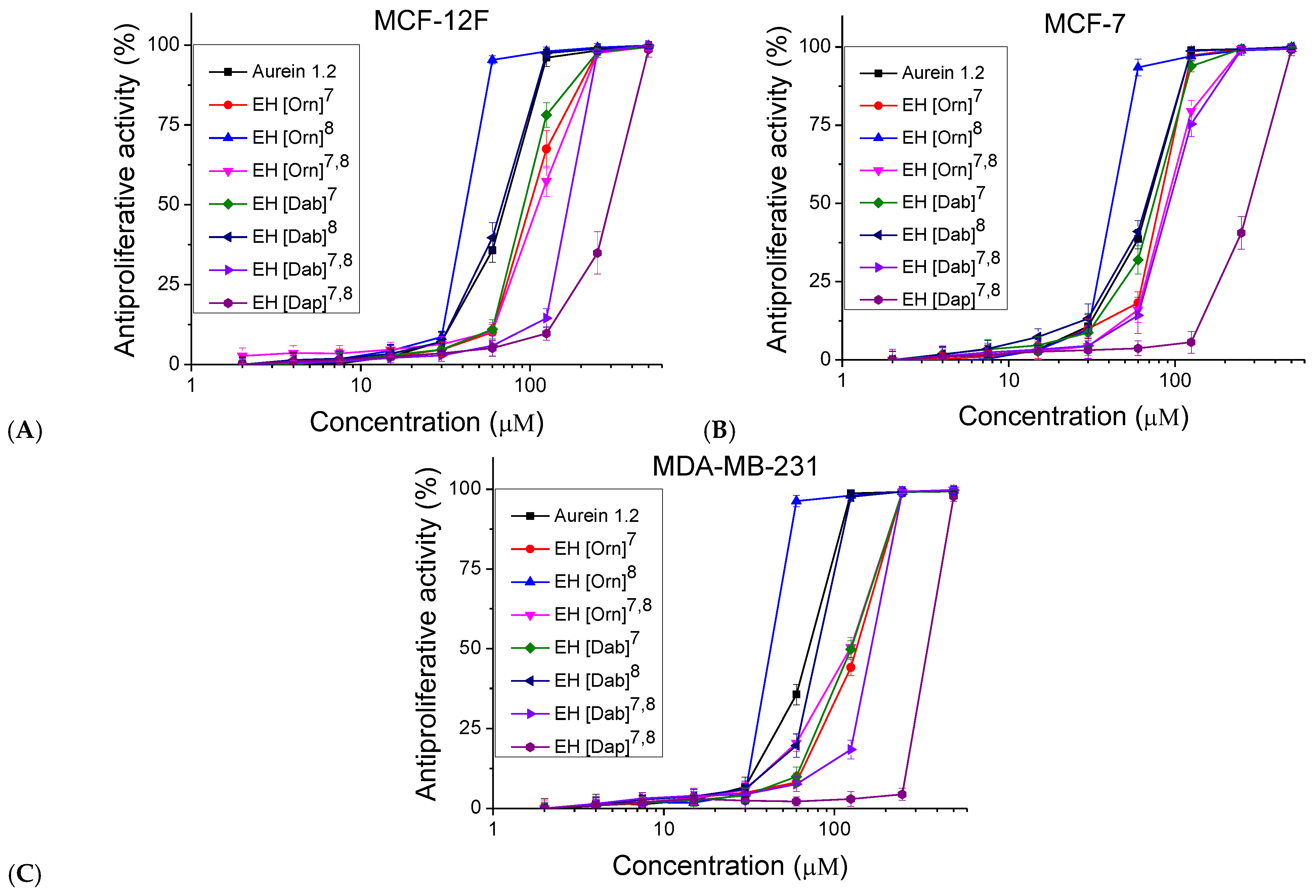
2.5. In Vitro Antiproliferative Activity
2.6. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Chemical Analysis
4.3. Cell Cultures
4.4. Safety Testing
4.5. Antiproliferative Activity
4.6. Antimicrobial Assays
4.6.1. Test Microorganisms and Culture Conditions
4.6.2. Determination of Minimum Inhibitory and Minimum Bactericidal Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deslouches, B.; Peter, Y. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [PubMed]
- Oelkrug, C.; Hartke, M.; Schubert, A. Mode of action of anticancer peptides (ACPs) from amphibian origin. Anticancer Res. 2015, 35, 635–644. [Google Scholar] [PubMed]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Won, H.S.; Jung, S.J.; Kim, H.E.; Seo, M.D.; Lee, B.J. Systematic peptide engineering and structural characterization to search for the shortest antimicrobial peptide analogue of gaegurin 5. J. Biol. Chem. 2004, 279, 14784–14791. [Google Scholar] [CrossRef]
- Zelezetsky, I.; Tossi, A. Alpha-helical antimicrobial peptides—Using a sequence template to guide structure–activity relationship studies. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1436–1449. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Brown, S.; Howard, A.; Kasprzak, A.; Gordon, K.; East, P. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2009, 39, 792–800. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian bell frogs Litoria aurea and Litoria raniformis: The solution structure of aurein 1.2. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef] [PubMed]
- Mulder, K.C.; Lima, L.A.; Miranda, V.J.; Dias, S.C.; Franco, O.L. Current scenario of peptide-based drugs: The key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013, 4, 321. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Rubinchik, E.; Dugourd, D.; Algara, T.; Pasetka, C.; Friedland, H.D. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonization models. Int. J. Antimicrob. Agents 2009, 34, 457–461. [Google Scholar] [CrossRef]
- Rivas, L.; Luque-Ortega, J.R.; Andreu, D. Amphibian antimicrobial peptides and Protozoa: Lessons from parasites. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1570–1581. [Google Scholar] [CrossRef]
- Agier, J.; Efenberger, M.; Brzezinska-Blaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef]
- Chou, H.T.; Kuo, T.Y.; Chiang, J.C.; Pei, M.J.; Yang, W.T.; Yu, H.C.; Chen, W.J. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int. J. Antimicrob. Agents 2008, 32, 130–138. [Google Scholar] [CrossRef]
- De la Fuente-Nunez, C.; Silva, O.N.; Lu, T.K.; Franco, O.L. Antimicrobial peptides: Role in human disease and potential as immunotherapies. Pharmacol. Ther. 2017, 178, 132–140. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Roudi, R.; Syn, N.L.; Roudbary, M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: A comprehensive overview. Front. Immunol. 2017, 8, 1320. [Google Scholar] [CrossRef]
- Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post-feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action, and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Meyer, J.; Beyermann, M.; Maul, B.; Hoischen, C.; Bienert, M. General aspects of peptide selectivity towards lipid bilayers and cell membranes studied by variation of the structural parameters of amphipathic helical model peptides. Biochim. Biophys. Acta Biomembr. 2002, 1558, 171–186. [Google Scholar] [CrossRef]
- Fernández-Vidal, M.; Jayasinghe, S.; Ladokhin, A.S.; White, S.H. Folding amphipathic helices into membranes: Amphiphilicity trumps hydrophobicity. J. Mol. Biol. 2007, 370, 459–470. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical application of AMPs in antimicrobial peptides. In Advances in Experimental Medicine and Biology; Matsuzaki, K., Ed.; Springer: Singapore, 2019; Volume 1117, pp. 307–322. [Google Scholar]
- Huan, Y.; Bhaskar, B.; Zhang, Y.; Hu, Y.; Fang, X.; Jin, Z. Antimicrobial peptides: Classification, design, application, and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Chegini, P.P.; Nikokar, I.; Hosseinabadi, T.; Tabarzad, M. Concerns in the design and development of novel antimicrobial peptides. Trends Pept. Protein Sci. 2017, 1, 135–143. [Google Scholar]
- Barreto-Santamaría, A.; Patarroyo, M.E.; Curtidor, H. Designing and optimizing new antimicrobial peptides: All targets are not the same. Crit. Rev. Clin. Lab. Sci. 2019, 56, 351–373. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.J.; Lee, Y.S.; Song, M.D.; Kim, I.H.; Won, H.S. De novo generation of short antimicrobial peptides with simple amino acid composition. Regul. Pept. 2011, 166, 36–41. [Google Scholar] [CrossRef]
- Ramesh, S.; Govender, T.; Kruger, H.G.; de la Torre, B.G.; Albericio, F. Short antimicrobial peptides (SAMPs) as a class of extraordinary promising therapeutic agents. J. Pept. Sci. 2016, 22, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Joseph, S.; Ghawali, S.; Martis, E.A.; Madan, T.; Venkatesh, K.V.; Idicula-Thomas, S. Designing antibacterial peptides with enhanced killing kinetics. Front. Microbiol. 2018, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.F.; Marques, L.S.; Oliveira, J.T.; Lima, P.G.; Dias, L.P.; Neto, N.A.; Lopes, F.E.; Sousa, J.S.; Silva, A.F.; Caneiro, R.F. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 177, 132–145. [Google Scholar] [CrossRef]
- Saravanan, R.; Li, X.; Lim, K.; Mohanram, H.; Peng, L.; Mishra, B.; Basu, A.; Lee, J.M.; Bhattacharjya, S.; Leong, S.S.J. Design of short membrane-selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnol. Bioeng. 2014, 111, 37–49. [Google Scholar] [CrossRef]
- Liu, Z.; Brady, A.; Young, A.; Rasimick, B.; Chen, K.; Zhou, C.; Kallenbach, N.R. Length effects in antimicrobial peptides of the (RW)n series. Antimicrob. Agents Chemother. 2007, 51, 597–603. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, X.; Zhang, Q.; Tangthianchaichana, J.; Guo, M.; Du, S.; Lu, Y. Anticancer mechanisms and potential anticancer applications of antimicrobial peptides and their nano agents. Int. J. Nanomed. 2024, 19, 1017–1039. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, L.; Yu, L.C.; Huang, J.; Huang, S.; Yao, Y. A Review for antimicrobial peptides with anticancer properties: Re-Purposing of potential anticancer agents. BIO Integr. 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Rozek, T.; Bowie, J.H.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active Aurein peptides from the Australian Bell frogs Litoria aurea and Litoria raniformis. Part 2. Sequence determination using electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Chen, Y.; Shu, A.; Chen, X.; Wang, T.; Jiang, Y.; Wang, L. A novel strategy for the design of Aurein 1.2 analogs with enhanced bioactivities by conjunction of cell-penetrating regions. Antibiotics 2023, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Riva, A.; Kamysz, W.; Silvestri, C.; Nadolski, P.; Della Vittoria, A.; Lukasiak, J.; Scalise, G. In vitro activity of Aurein 1.2 alone and in combination with antibiotics against Gram-positive nosocomial Cocci. Antimicrob. Agents Chemother. 2007, 51, 1494–1496. [Google Scholar] [CrossRef]
- Lorenzón, E.N.; Sanches, P.R.S.; Nogueira, L.G.; Bauab, T.M.; Cilli, E.M. Dimerization of Aurein 1.2: Effects in structure, antimicrobial activity, and aggregation of Candida albicans cells. Amino Acids 2013, 44, 1521–1528. [Google Scholar] [CrossRef]
- Fernandez, D.I.; Le Brun, A.P.; Whitwell, T.C.; Sani, M.A.; James, M.; Separovic, F. The Antimicrobial peptide Aurein 1.2 disrupts model membranes via the carpet mechanism. Phys. Chem. Chem. Phys. 2012, 14, 15739–15751. [Google Scholar] [CrossRef]
- Rai, D.K.; Qian, S. Interaction of the antimicrobial peptide Aurein 1.2 and charged lipid bilayer. Sci. Rep. 2017, 7, 3719. [Google Scholar] [CrossRef]
- Laadhari, M.; Arnold, A.A.; Gravel, A.E.; Separovic, F.; Marcotte, I. Interaction of the antimicrobial peptides Caerin 1.1 and Aurein 1.2 with intact bacteria by 2H Solid-State NMR. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2959–2964. [Google Scholar] [CrossRef]
- Aghamiri, S.; Zandsalimi, F.; Raee, P.; Abdollahifar, M.-A.; Tan, S.C.; Low, T.Y.; Najafi, S.; Ashrafizadeh, M.; Zarrabi, A.; Ghanbarian, H.; et al. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 2021, 171, 105777. [Google Scholar] [CrossRef]
- Enbäck, J.; Laakkonen, P. Tumour-homing peptides: Tools for targeting, imaging, and destruction. Biochem. Soc. Trans. 2007, 35, 780–783. [Google Scholar] [CrossRef]
- Jaber, S.; Iliev, I.; Angelova, T.; Nemska, V.; Sulikovska, I.; Naydenova, E.; Georgieva, N.; Givechev, I.; Grabchev, I.; Danalev, D.S. Synthesis, antitumor, and antibacterial studies of new shortened analogues of (KLAKLAK)2-NH2 and their conjugates containing unnatural amino acids. Molecules 2021, 26, 898. [Google Scholar] [CrossRef]
- Jaber, S.; Nemska, V.; Iliev, I.; Ivanova, E.; Foteva, T.; Georgieva, N.; Danalev, D. Synthesis and Biological Studies on (KLAKLAK)2-NH2 analog containing unnatural amino acid β-Ala and conjugates with second pharmacophore. Molecules 2021, 26, 7321. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, V.; Antonova, K.; Petkov, O.; Stoyanova-Ivanova, A.; Jaber, S.; Ivanova, V.; Danalev, D. Interaction of KLAKLAK-NH2 and snalogs with biomimetic membrane models. Pharmaceutics 2024, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Evstatieva, Y.; Nemska, V.; Nikolova, D.; Naydenova, E.; Georgieva, N.; Danalev, D. Antimicrobial activity of (KLAKLAK)–NH2 analogs against pathogenic microbial strains. Curr. Res. Biotechnol. 2024, 8, 100236. [Google Scholar] [CrossRef]
- Jaber, S.; Nemska, V.; Iliev, I.; Ivanova, E.; Foteva, T.; Georgieva, N.; Danalev, D. Synthesis, antiproliferative and antimicrobial activities of (KLAKLAK) 2-NH2 analogue containing nor-Leu and its conjugates with a second pharmacophore. Biotechnol. Biotechnol. Equip. 2023, 37, 151–158. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), Chemical Computing Group, Montreal, Canada. 2015. Available online: http://www.chemcomp.com (accessed on 24 February 2024).
- Wang, G.; Li, Y.; Li, X. Correlation of three-dimensional structures with the antibacterial activity of a group of peptides designed based on a nontoxic bacterial membrane Anchor. J. Biol. Chem. 2005, 280, 5803–5811. [Google Scholar] [CrossRef]
- Afacan, J.N.; Yeung, A.T.T.; Pena, O.M.; Hancock, R.E.W. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Naydenova, E.D.; Zhivkova, V.I.; Zamfirova, R.N.; Vezenkov, L.T.; Dobrinova, Y.G.; Mateeva, P.I. Synthesis and biological activity of Nociceptin/Orphanin FQ (1–13) NH2 analogues modified in 9 and/or 13 position. Bioorg. Med. Chem. Lett. 2006, 16, 4071–4074. [Google Scholar] [CrossRef]
- Vollmer, W.; Holtje, J.-V. The Architecture of the Murein (Peptidoglycan) in Gram-negative bacteria: Vertical scaffold or horizontal layers. J. Bacteriol. 2004, 186, 5978–5987. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Institute CaLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; Institute CaLS: Wayne, PA, USA, 2012; Available online: https://clsi.org/ (accessed on 24 February 2024).

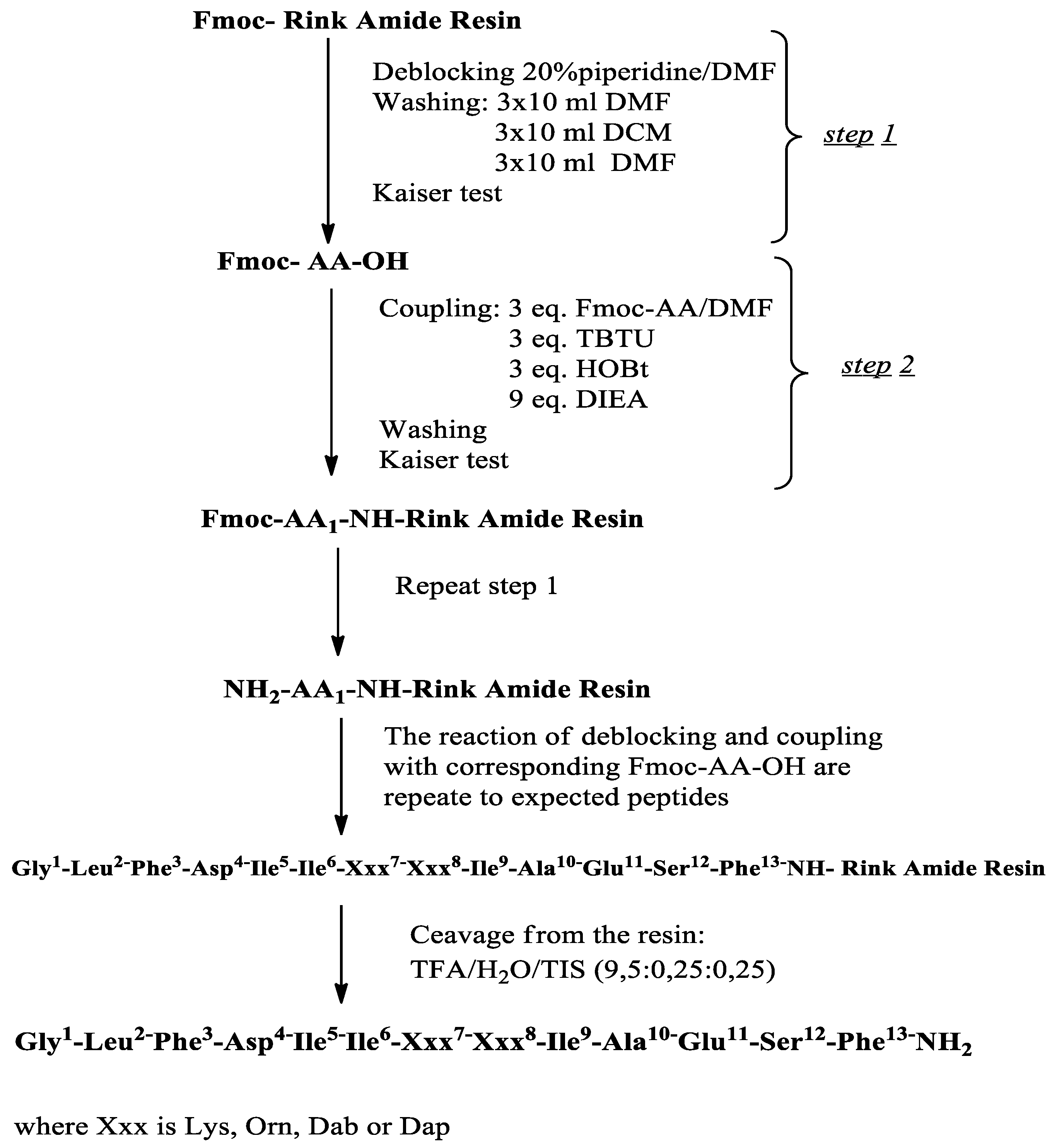
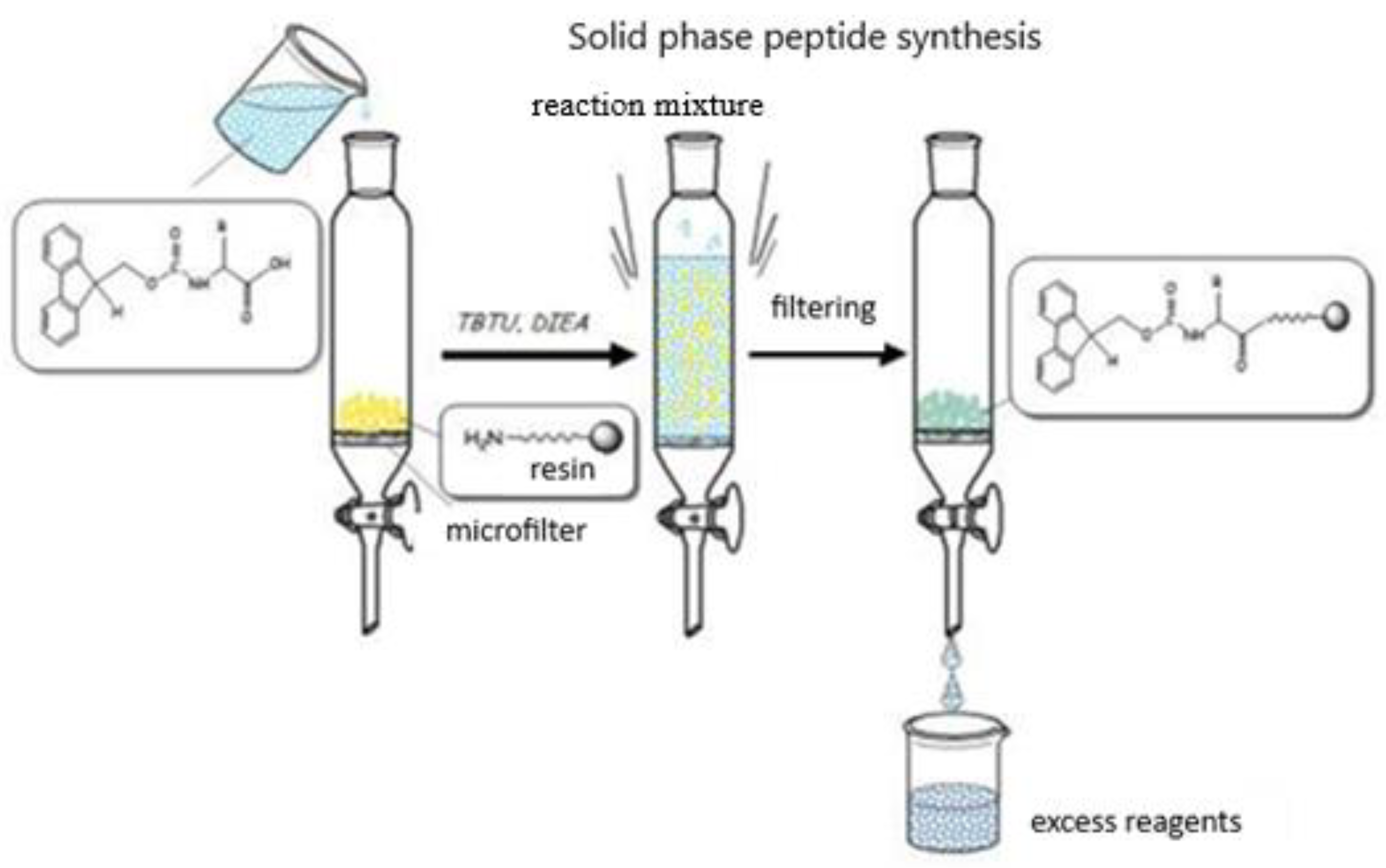

| Code | Structure | Molecular Formula | MM Exact | [M+H] + Found | RT (min) | M.P (°C) | α 20546 (°) * |
|---|---|---|---|---|---|---|---|
| Aurein 1.2 | Gly1-Leu2-Phe3-Asp4-Ile5-Ile6-Lys7-Lys8-Ile9-Ala10-Glu11-Ser12-Phe13-NH2 | C71H114N16O18 | 1479.76 | 1480.10 | 2.013 | 242.0 | −28 |
| EH [Orn]7 | Gly-Leu-Phe-Asp-Ile-Ile-Orn7-Lys-Ile-Ala-Glu-Ser-Phe-NH2 | C70H112N16O18 | 1465.73 | 1466.47 | 7.09 | 233.4 | −10 |
| EH [Orn]8 | Gly-Leu-Phe-Asp-Ile-Ile-Lys-Orn8-Ile-Ala-Glu-Ser-Phe-NH2 | C70H112N16O18 | 1465.73 | 1466.84 | 8.45 | 234.1 | −8 |
| EH [Orn]7,8 | Gly-Leu-Phe-Asp-Ile-Ile-Orn7-Orn8-Ile-Ala-Glu-Ser-Phe-NH2 | C69H110N16O18 | 1451.71 | 1452.86 | 7.21 | 212.3 | −16 |
| EH [Dab]7 | Gly-Leu-Phe-Asp-Ile-Ile-Dab7-Lys-Ile-Ala-Glu-Ser-Phe-NH2 | C69H110N16O18 | 1451.70 | 1452.86 | 7.07 | 238.5 | 24 |
| EH [Dab]8 | Gly-Leu-Phe-Asp-Ile-Ile-Lys-Dab8-Ile-Ala-Glu-Ser-Phe-NH2 | C69H110N16O18 | 1451.70 | 1452.86 | 7.22 | 250.8 | −50 |
| EH [Dab]7,8 | Gly-Leu-Phe-Asp-Ile-Ile-Dab7-Dab8-Ile-Ala-Glu-Ser-Phe-NH2 | C67H106N16O18 | 1423.65 | 1424.83 | 7.20 | 243.9 | 14 |
| EH [Dap]7,8 | Gly-Leu-Phe-Asp-Ile-Ile-Dap7-Dap8-Ile-Ala-Glu-Ser-Phe-NH2 | C65H102N16O18 | 1394.71 | 1395.76 | 7.86 | 240.0 | −26 |
| Code | Calculated Energy of the Generated Structure, kJ/mol | RMSD, Å |
|---|---|---|
| Aurein 1.2 | 293.48 | - |
| EH [Orn]7 | 38.25 | 0.152 |
| EH [Orn]8 | 311.40 | 0.268 |
| EH [Orn]7,8 | 294.03 | 0.381 |
| EH [Dab]7 | 249.32 | 0.371 |
| EH [Dab]8 | 120.72 | 0.586 |
| EH [Dab]7,8 | 276.15 | 0.528 |
| EH [Dap]7,8 | 466.28 | 0.365 |
| Compounds | Mean CC50 ± SD (µM) | PIF * | |
|---|---|---|---|
| −Irr | +Irr ** | ||
| Aurein 1.2 | 87.59 ± 2.45 | 87.22 ± 1.88 | 1 |
| EH [Orn]7 | 133.65 ± 7.4 | 102.50 ± 7.40 | 1.30 |
| EH [Orn]8 | 75.62 ± 3.33 | 59.45 ± 5.21 | 1.27 |
| EH [Orn]7,8 | 157.25 ± 6.38 | 101.25 ± 3.70 | 1.55 |
| EH [Dab]7 | 158.29 ± 4.31 | 113.30 ± 5.57 | 1.40 |
| EH [Dab]8 | 92.08 ± 1.97 | 81.38 ± 2.34 | 1.13 |
| EH [Dab]7,8 | 201.81 ± 10.11 | 181.11 ± 1.81 | 1.11 |
| EH [Dap]7,8 | 360.00 ± 2.52 | 357.46 ± 3.03 | 1.01 |
| Chlorpromazine *** | 12.37 ± 0.93 | 2.04 ± 0.07 | 6.06 |
| Compounds | Mean IC50 ± SD (µM) | SI * | |||
|---|---|---|---|---|---|
| MCF-12F | MCF-7 | MDA-MB-231 | MCF-7 | MDA-MB-231 | |
| Aurein 1.2 | 71.37 ± 2.88 | 68.87 ± 2.12 | 70.93 ± 2.06 | 1.04 | 1.01 |
| EH [Orn]7 | 100.70 ± 5.25 | 80.51 ± 1.55 | 134.56 ± 3.94 | 1.25 | 0.75 |
| EH [Orn]8 | 44.33 ± 0.52 | 44.38 ± 1.08 | 44.85 ± 0.71 | 1.00 | 0.99 |
| EH [Orn]7,8 | 112.12 ± 7.14 | 88.93 ± 3.14 | 122.70 ± 6.47 | 1.26 | 0.91 |
| EH [Dab]7 | 92.07 ± 2.12 | 74.39 ± 3.29 | 124.91 ± 4.97 | 1.24 | 0.74 |
| EH [Dab]8 | 68.31 ± 3.51 | 67.21 ± 2.57 | 79.74 ± 1.78 | 1.02 | 0.86 |
| EH [Dab]7,8 | 167.93 ± 2.54 | 92.23 ± 1.80 | 163.82 ± 2.51 | 1.82 | 1.03 |
| EH [Dap]7,8 | 307.96 ± 21.71 | 288.65 ± 19.83 | 372.21 ± 3.17 | 1.07 | 0.83 |
| Doxorubicin *** | 0.601 ± 0.059 | 0.68 ± 0.031 | 2.528 ± 0.11 | 0.88 | 0.237 |
| Peptides | Bacillus subtilis NBIMCC 3562 | Escherichia coli NBIMCC K12 407 | ||
|---|---|---|---|---|
| MIC, [µg/mL] | MBC, [µg/mL] | MIC, [µg/mL] | MBC, [µg/mL] | |
| Aurein 1.2 | 160 | NI | 40 | NI |
| EH [Orn]7 | 80 | 320 | 80 | NI |
| EH [Orn]8 | 40 | NI | 40 | NI |
| EH [Orn]7,8 | 80 | 320 | 80 | NI |
| EH [Dab]7 | 80 | 320 | 80 | NI |
| EH [Dab]8 | 80 | 320 | 80 | NI |
| EH [Dab]7,8 | 160 | NI | 160 | NI |
| EH [Dap]7,8 | 320 | NI | NI | NI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelova, N.; Iliev, I.; Nemska, V.; Dzimbova, T.; Georgieva, N.; Danalev, D.; Naydenova, E. Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids. Molecules 2025, 30, 2050. https://doi.org/10.3390/molecules30092050
Angelova N, Iliev I, Nemska V, Dzimbova T, Georgieva N, Danalev D, Naydenova E. Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids. Molecules. 2025; 30(9):2050. https://doi.org/10.3390/molecules30092050
Chicago/Turabian StyleAngelova, Nora, Ivan Iliev, Veronica Nemska, Tatyana Dzimbova, Nelly Georgieva, Dancho Danalev, and Emilia Naydenova. 2025. "Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids" Molecules 30, no. 9: 2050. https://doi.org/10.3390/molecules30092050
APA StyleAngelova, N., Iliev, I., Nemska, V., Dzimbova, T., Georgieva, N., Danalev, D., & Naydenova, E. (2025). Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids. Molecules, 30(9), 2050. https://doi.org/10.3390/molecules30092050










