Capillary Electrophoresis Electrospray Ionization Mass Spectrometry Reveals Metabolic Perturbations During Nematode Infection in Drosophila melanogaster
Abstract
1. Introduction
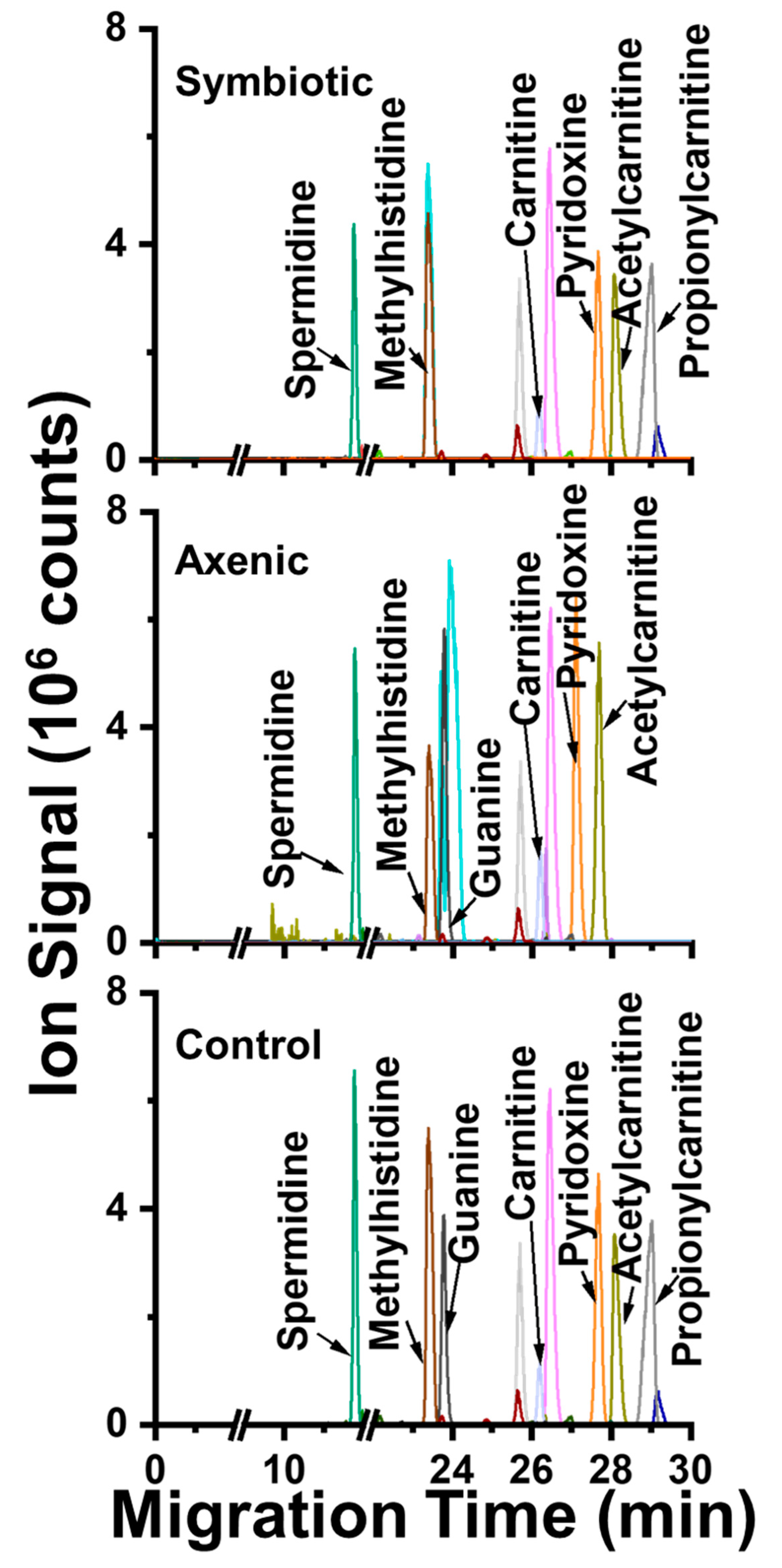
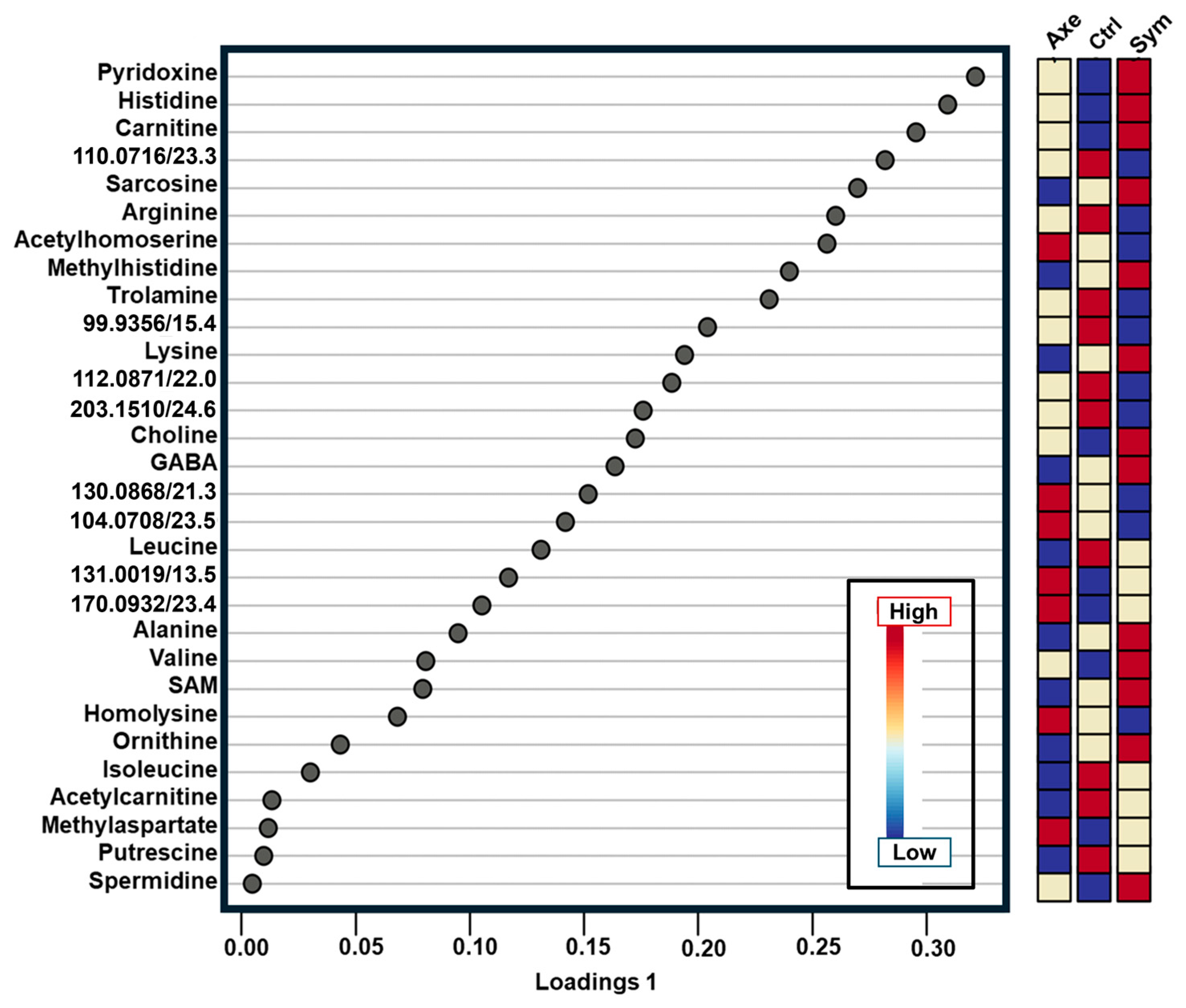
2. Results and Discussion
3. Conclusions
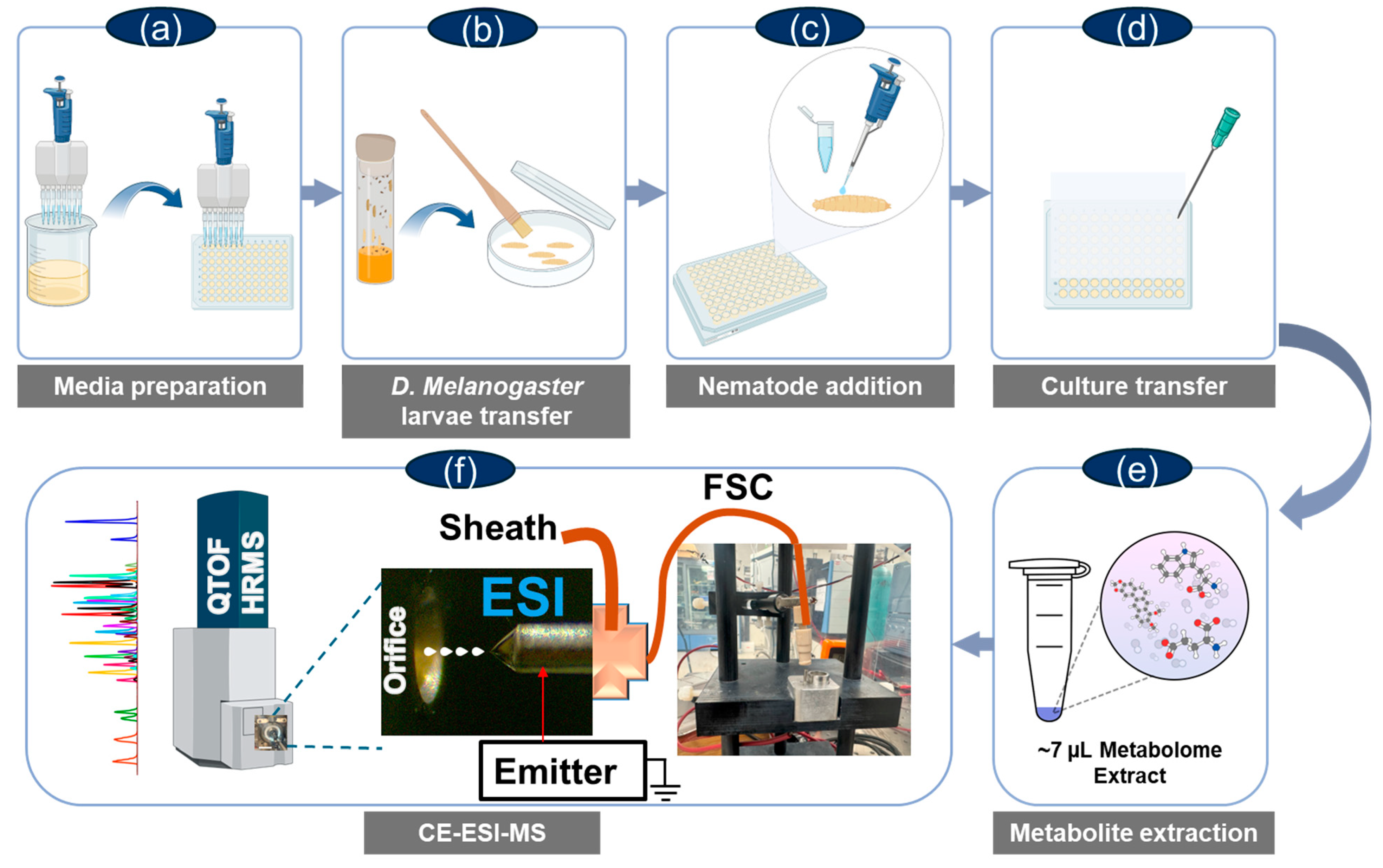
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ctrl | Control |
| Axe | Axenic |
| BGE | Background electrolyte |
| CE-ESI-MS | Capillary electrophoresis electrospray ionization mass spectrometry |
| FA | Formic acid |
| GABA | Gamma–aminobutyrate |
| GC | Gas chromatography |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| MT | Migration time |
| sPLS-DA | Sparse least squares–discriminant analysis |
| Sym | Symbiotic |
| MS/MS | Tandem mass spectrometry |
References
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 2000, 68, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Walker, B.D. Host-pathogen interactions. Curr. Opin. Immunol. 2006, 18, 371–373. [Google Scholar] [CrossRef]
- Megha; Preeti; Prasad, T. Metabolomics: A Promising Tool to Study Disease Biomarkers and Host-Pathogen Interactions. In Integrated Omics Approaches to Infectious Diseases; Hameed, S., Fatima, Z., Eds.; Springer: Singapore, 2021; pp. 403–423. [Google Scholar]
- Raamsdonk, L.M.; Teusink, B.; Broadhurst, D.; Zhang, N.; Hayes, A.; Walsh, M.C.; Berden, J.A.; Brindle, K.M.; Kell, D.B.; Rowland, J.J.; et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotechnol. 2001, 19, 45–50. [Google Scholar] [CrossRef]
- Lewis, E.B. Developmental genetics of Drosophila. Ann. N. Y. Acad. Sci. 2004, 1038, 94–97. [Google Scholar] [CrossRef]
- Cox, J.E.; Thummel, C.S.; Tennessen, J.M. Metabolomic Studies in Drosophila. Genetics 2017, 206, 1169–1185. [Google Scholar] [CrossRef]
- Tolwinski, N.S. Introduction: Drosophila-A Model System for Developmental Biology. J. Dev. Biol. 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Daisley, B.A.; Dube, J.S.; Reid, G. Drosophila melanogaster as a High-Throughput Model for Host–Microbiota Interactions. Front. Microbiol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Younes, S.; Al-Sulaiti, A.; Nasser, E.A.A.; Najjar, H.; Kamareddine, L. Drosophila as a Model Organism in Host-Pathogen Interaction Studies. Front. Cell. Infect. Microbiol. 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A. Innate Immunity of Insects. Curr. Opin. Immunol. 1995, 7, 4–10. [Google Scholar] [CrossRef]
- Hultmark, D. Immune-Reactions in Drosophila and Other Insects—A Model for Innate Immunity. Trends Genet. 1993, 9, 178–183. [Google Scholar] [CrossRef]
- Marshall, E.K.P.; Dionne, M.S. Drosophila versus Mycobacteria: A model for mycobacterial host–pathogen interactions. Mol. Microbiol. 2022, 117, 600–609. [Google Scholar] [CrossRef]
- Brandt, S.M.; Dionne, M.S.; Khush, R.S.; Pham, L.N.; Vigdal, T.J.; Schneider, D.S. Secreted bacterial effectors and host-produced eiger/TNF drive death in a Salmonella-infected fruit fly. PLoS Biol. 2004, 2, 2067–2075. [Google Scholar] [CrossRef]
- Dionne, M.S.; Ghori, N.; Schneider, D.S. Drosophila melanogaster is a genetically tractable model host for Mycobacterium marinum. Infect. Immun. 2003, 71, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.T.; Liegeois, S.; Kele, B.; Giammarinaro, P.; Pradel, E.; Hoffmann, J.A.; Ewbank, J.J.; Ferrandon, D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007, 3, 1694–1709. [Google Scholar] [CrossRef]
- Igboin, C.O.; Griffen, A.L.; Leys, E.J. The Drosophila melanogaster host model. J. Oral Microbiol. 2012, 4, 10368. [Google Scholar] [CrossRef]
- Fisch, D.; Yakimovich, A.; Clough, B.; Wright, J.; Bunyan, M.; Howell, M.; Mercer, J.; Frickel, E. Defining host-pathogen interactions employing an artificial intelligence workflow. eLife 2019, 8, 21. [Google Scholar] [CrossRef]
- Ayres, J.S.; Freitag, N.; Schneider, D.S. Identification of drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics 2008, 178, 1807–1815. [Google Scholar] [CrossRef]
- Liu, Y.; Gordesky-Gold, B.; Leney-Greene, M.; Weinbren, N.L.; Tudor, M.; Cherry, S. Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 2018, 24, 57. [Google Scholar] [CrossRef]
- Herbert, E.E.; Goodrich-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.R.; Goodrich-Blair, H. Masters of conquest and pillage: Xenorhabdus nematophila global regulators control transitions from virulence to nutrient acquisition. Cell Microbiol. 2009, 11, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.F.; Akhurst, R.J. The Nature Of The Intestinal Vesicle In Nematodes Of The Family Steinernematidae. Int. J. Parasit. 1983, 13, 599–606. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.; Waterfield, N.; Daborn, P.; Joyce, S.; Bennett, H.; Au, C.; Dowling, A.; Boundy, S.; Reynolds, S.; Clarke, D. Photorhabdus: Towards a functional genomic analysis of a symbiont and pathogen. Fems Microbiol. Rev. 2003, 26, 433–456. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass. Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative Metabolome Analysis Using Capillary Electrophoresis Mass Spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef]
- Lapainis, T.; Rubakhin, S.S.; Sweedler, J.V. Capillary electrophoresis with electrospray ionization mass spectrometric detection for single-cell metabolomics. Anal. Chem. 2009, 81, 5858–5864. [Google Scholar] [CrossRef] [PubMed]
- Nemes, P.; Rubakhin, S.S.; Aerts, J.T.; Sweedler, J.V. Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Protoc. 2013, 8, 783–799. [Google Scholar] [CrossRef]
- Nemes, P.; Knolhoff, A.M.; Rubakhin, S.S.; Sweedler, J.V. Metabolic differentiation of neuronal phenotypes by single-cell capillary electrophoresis-electrospray ionization-mass spectrometry. Anal. Chem. 2011, 83, 6810–6817. [Google Scholar] [CrossRef]
- Onjiko, R.M.; Portero, E.P.; Moody, S.A.; Nemes, P. In Situ Microprobe Single-Cell Capillary Electrophoresis Mass Spectrometry: Metabolic Reorganization in Single Differentiating Cells in the Live Vertebrate (Xenopus laevis) Embryo. Anal. Chem. 2017, 89, 7069–7076. [Google Scholar] [CrossRef]
- Onjiko, R.M.; Moody, S.A.; Nemes, P. Single-cell mass spectrometry reveals small molecules that affect cell fates in the 16-cell embryo. Proc. Natl. Acad. Sci. USA 2015, 112, 6545–6550. [Google Scholar] [CrossRef]
- Lombard-Banek, C.; Portero, E.P.; Onjiko, R.M.; Nemes, P. New-generation mass spectrometry expands the toolbox of cell and developmental biology. Genesis 2017, 55, 14. [Google Scholar] [CrossRef]
- Sun, L.L.; Dubiak, K.M.; Peuchen, E.H.; Zhang, Z.B.; Zhu, G.J.; Huber, P.W.; Dovichi, N.J. Single Cell Proteomics Using Frog (Xenopus laevis) Blastomeres Isolated from Early Stage Embryos, Which Form a Geometric Progression in Protein Content. Anal. Chem. 2016, 88, 6653–6657. [Google Scholar] [CrossRef] [PubMed]
- Onjiko, R.M.; Nemes, P.; Moody, S.A. Altering metabolite distribution at Xenopus cleavage stages affects left-right gene expression asymmetries. Genesis 2021, 59, e23418. [Google Scholar] [CrossRef] [PubMed]
- Everts, B. Metabolomics in Immunology Research. In Clinical Metabolomics: Methods and Protocols; Giera, M., Ed.; Springer: New York, NY, USA, 2018; pp. 29–42. [Google Scholar]
- Lee, C.J.; Schnieders, J.H.; Rubakhin, S.S.; Patel, A.V.; Liu, C.Y.; Naji, A.; Sweedler, J.V. D-amino acids and classical neurotransmitters in healthy and type 2 diabetes-affected human pancreatic islets of Langerhans. Metabolites 2022, 12, 13. [Google Scholar] [CrossRef]
- Yadav, S.; Shokal, U.; Forst, S.; Eleftherianos, I. An improved method for generating axenic entomopathogenic nematodes. BMC Res. Notes 2015, 8, 461. [Google Scholar] [CrossRef]
- Lombard-Banek, C.; Li, J.; Portero, E.P.; Onjiko, R.M.; Singer, C.D.; Plotnick, D.O.; Al Shabeeb, R.Q.; Nemes, P. In vivo subcellular mass spectrometry enables proteo-metabolomic single-cell systems biology in a chordate embryo developing to a normally behaving tadpole (X. laevis). Angew. Chem. Int. Ed. 2021, 60, 12852–12858. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Contestabile, R.; di Salvo, M.L.; Bunik, V.; Tramonti, A.; Vernì, F. The multifaceted role of vitamin B6 in cancer: Drosophila as a model system to investigate DNA damage. Open Biol. 2020, 10, 200034. [Google Scholar] [CrossRef] [PubMed]
- Froldi, F.; Pachnis, P.; Szuperák, M.; Costas, O.; Fernando, T.; Gould, A.P.; Cheng, L.Y. Histidine is selectively required for the growth of Myc-dependent dedifferentiation tumours in the Drosophila CNS. EMBO J. 2019, 38, e99895. [Google Scholar] [CrossRef]
- McGarry, J.D.; Woeltje, K.F.; Kuwajima, M.; Foster, D.W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes/Metab. Rev. 1989, 5, 271–284. [Google Scholar] [CrossRef]
- Carillo, M.R.; Bertapelle, C.; Scialò, F.; Siervo, M.; Spagnuolo, G.; Simeone, M.; Peluso, G.; Digilio, F.A. L-Carnitine in Drosophila: A Review. Antioxidants 2020, 9, 1310. [Google Scholar] [CrossRef]
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.E.; Chan, M.T.; Lenz, O.T.; Keenan, B.T.; Maislin, G.; Pack, A.I. Glutamate Is a Wake-Active Neurotransmitter in Drosophila melanogaster. Sleep 2017, 40, zsw046. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Lylyk, M.P.; Maniukh, O.V.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Dietary L-arginine accelerates pupation and promotes high protein levels but induces oxidative stress and reduces fecundity and life span in Drosophila melanogaster. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2018, 188, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Dziedziech, A.; Shivankar, S.; Theopold, U. Drosophila melanogaster Responses against Entomopathogenic Nematodes: Focus on Hemolymph Clots. Insects 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Nemes, P.; Marginean, I.; Vertes, A. Spraying mode effect on droplet formation and ion chemistry in electrosprays. Anal. Chem. 2007, 79, 3105–3116. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef]
- Okuda, S.; Yamada, T.; Hamajima, M.; Itoh, M.; Katayama, T.; Bork, P.; Goto, S.; Kanehisa, M. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008, 36, W423–W426. [Google Scholar] [CrossRef]
| No. | Metabolite | Migration Time | m/z | |||
|---|---|---|---|---|---|---|
| (min) | Δ (%) | Measured | Theoretical | Δ (ppm) | ||
| 1 | Spermidine *,** | 8.5 | 1.6 | 146.1648 | 146.1652 | 0.4 |
| 2 | Putrescine * | 9.1 | −0.9 | 89.1072 | 89.1073 | 0.1 |
| 3 | Choline ** | 15.3 | −1.8 | 104.1068 | 104.1070 | 0.2 |
| 4 | S-adenosylmethionine *,** | 16.3 | −2.7 | 399.1447 | 399.1445 | −0.2 |
| 5 | Ornithine *,** | 16.4 | −1.3 | 133.0973 | 133.0972 | −0.1 |
| 6 | Sarcosine * | 16.6 | −1.6 | 90.0550 | 90.0550 | 0.0 |
| 7 | Lysine *,** | 16.6 | −1.5 | 147.1128 | 147.1128 | 0.0 |
| 8 | Arginine *,** | 17.2 | −1.2 | 175.1190 | 175.1190 | 0.0 |
| 9 | Homolysine *,** | 17.3 | −1.1 | 161.1286 | 161.1285 | −0.1 |
| 10 | γ-aminobutyrate *,** | 17.4 | −0.9 | 104.0703 | 104.0706 | 0.3 |
| 11 | Histidine ** | 17.4 | −0.8 | 156.0769 | 156.0768 | −0.1 |
| 12 | N6,N6,N6-trimethyllysine *,** | 17.8 | −2.6 | 189.1607 | 189.1598 | −0.9 |
| 13 | Methylhistidine *,** | 18.3 | −2.3 | 170.0924 | 170.0924 | 0.0 |
| 14 | Guanidinopropanoate *,** | 18.5 | −1.9 | 132.074 | 132.0768 | 2.8 |
| 15 | Acetylcholine *,** | 18.7 | −1.6 | 146.1175 | 146.1176 | 0.1 |
| 16 | Guanine *,** | 18.7 | 2.1 | 152.0570 | 152.0567 | −0.3 |
| 17 | Trolamine * | 18.8 | −0.9 | 150.1126 | 150.1125 | −0.1 |
| 18 | Carnitine *,** | 20.5 | −3.9 | 162.1127 | 162.1125 | −0.2 |
| 19 | Pyridoxine (Vitamin B6) *,** | 20.9 | −1.3 | 168.0660 | 168.0655 | −2.4 |
| 20 | Acetylcarnitine *,** | 22.3 | −1.8 | 204.1233 | 204.1230 | −0.3 |
| 21 | Methylaspartate *,** | 22.3 | 1.8 | 148.0606 | 148.0604 | −0.2 |
| 22 | Propionylcarnitine *,** | 23.1 | −2.3 | 218.1399 | 218.1387 | −1.2 |
| 23 | Glycine * | 23.2 | −1.7 | 76.0395 | 76.0393 | −0.2 |
| 24 | Creatine *,** | 23.6 | −2.4 | 132.0771 | 132.0768 | −0.3 |
| 25 | Adenosine * | 24.7 | −2.4 | 268.1047 | 268.1040 | −0.7 |
| 26 | Alanine * | 25.5 | 0.6 | 90.0553 | 90.0550 | −0.3 |
| 27 | Argininosuccinate *,** | 27.4 | −0.6 | 291.1305 | 291.1299 | −0.6 |
| 28 | Valine *,** | 29.9 | 1.1 | 118.0863 | 118.0863 | 0.0 |
| 29 | Serine *,** | 30.4 | −0.2 | 106.0498 | 106.0499 | 0.1 |
| 30 | Isoleucine * | 30.2 | 1.2 | 132.1020 | 132.1019 | −0.1 |
| 31 | Leucine * | 30.5 | 1.2 | 132.1020 | 132.1019 | −0.1 |
| 32 | Threonine *,** | 32.5 | 0.6 | 120.0654 | 120.0655 | 0.1 |
| 33 | Asparagine *,** | 32.6 | 0.1 | 133.0610 | 133.0608 | −0.2 |
| 34 | Tryptophan *,** | 33.2 | 0.5 | 205.0975 | 205.0972 | −0.3 |
| 35 | Methionine *,** | 33.4 | 0.7 | 150.0586 | 150.0583 | −0.3 |
| 36 | Glutamine *,** | 33.5 | 0.6 | 147.0767 | 147.0764 | −0.3 |
| 37 | Acetylhomoserine * | 34.1 | 0.4 | 162.0751 | 162.0761 | 1.0 |
| 38 | Citrulline *,** | 34.1 | 0.2 | 176.1032 | 176.1030 | −0.2 |
| 39 | Homocitrulline *,** | 34.1 | 0.5 | 190.1191 | 190.1186 | −0.5 |
| 40 | Glutamate *,** | 34.2 | 0.5 | 148.0607 | 148.0604 | −0.3 |
| 41 | Phenylalanine *,** | 34.9 | 1.1 | 166.0867 | 166.0863 | −0.4 |
| 42 | Acetyllysine * | 35.3 | 1.2 | 189.1237 | 189.1234 | −0.3 |
| 43 | Tyrosine *,** | 35.4 | 1.3 | 182.0817 | 182.0812 | −0.5 |
| 44 | Proline *,** | 36.6 | 0.6 | 116.0706 | 116.0706 | 0.0 |
| 45 | Hypoxanthine *,** | 36.7 | −0.8 | 137.0458 | 137.0458 | 0.0 |
| 46 | Cysteine *,** | 37.1 | 5.0 | 122.0270 | 122.027 | 0.0 |
| 47 | Aspartate *,** | 39.2 | 1.5 | 134.0447 | 134.0448 | 0.1 |
| 48 | Glycine betaine * | 40.0 | 1.4 | 118.0862 | 118.0863 | 0.1 |
| 49 | Hydroxyproline *,** | 43.3 | 1.0 | 132.0657 | 132.0655 | −0.2 |
| 50 | Glutathione *,** | 46.7 | 0.1 | 308.0924 | 308.0911 | −1.3 |
| Pathway | Total | Hits | p-Value | Impact |
|---|---|---|---|---|
| Symbiotic group | ||||
| Arginine and proline metabolism | 29 | 4 | 1.91 × 10−4 | 0.333 |
| Alanine, aspartate, and glutamate metabolism | 23 | 2 | 0.0260 | 0.122 |
| One carbon pool via folate | 23 | 2 | 0.0260 | 0.168 |
| Glutathione metabolism | 26 | 2 | 0.0327 | 0.0179 |
| Vitamin B6 metabolism | 8 | 1 | 0.0872 | 0.0833 |
| Histidine metabolism | 9 | 1 | 0.0976 | 0.400 |
| Arginine biosynthesis | 13 | 1 | 0.138 | 0.146 |
| Butanoate metabolism | 14 | 1 | 0.148 | 0.200 |
| Glycerophospholipid metabolism | 32 | 1 | 0.309 | 0.0542 |
| Cysteine and methionine metabolism | 32 | 1 | 0.309 | 0.0546 |
| Control group | ||||
| Arginine and proline metabolism | 29 | 2 | 0.00418 | 0.271 |
| Arginine biosynthesis | 13 | 1 | 0.0481 | 0.341 |
| Glutathione metabolism | 26 | 1 | 0.0945 | 0.0179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuani, Y.T.; Ayon, N.J.; Onjiko, R.M.; Choi, S.B.; Yadav, S.; Eleftherianos, I.; Nemes, P. Capillary Electrophoresis Electrospray Ionization Mass Spectrometry Reveals Metabolic Perturbations During Nematode Infection in Drosophila melanogaster. Molecules 2025, 30, 2023. https://doi.org/10.3390/molecules30092023
Tuani YT, Ayon NJ, Onjiko RM, Choi SB, Yadav S, Eleftherianos I, Nemes P. Capillary Electrophoresis Electrospray Ionization Mass Spectrometry Reveals Metabolic Perturbations During Nematode Infection in Drosophila melanogaster. Molecules. 2025; 30(9):2023. https://doi.org/10.3390/molecules30092023
Chicago/Turabian StyleTuani, Yayra T., Navid J. Ayon, Rosemary M. Onjiko, Sam B. Choi, Shruti Yadav, Ioannis Eleftherianos, and Peter Nemes. 2025. "Capillary Electrophoresis Electrospray Ionization Mass Spectrometry Reveals Metabolic Perturbations During Nematode Infection in Drosophila melanogaster" Molecules 30, no. 9: 2023. https://doi.org/10.3390/molecules30092023
APA StyleTuani, Y. T., Ayon, N. J., Onjiko, R. M., Choi, S. B., Yadav, S., Eleftherianos, I., & Nemes, P. (2025). Capillary Electrophoresis Electrospray Ionization Mass Spectrometry Reveals Metabolic Perturbations During Nematode Infection in Drosophila melanogaster. Molecules, 30(9), 2023. https://doi.org/10.3390/molecules30092023







