Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Results
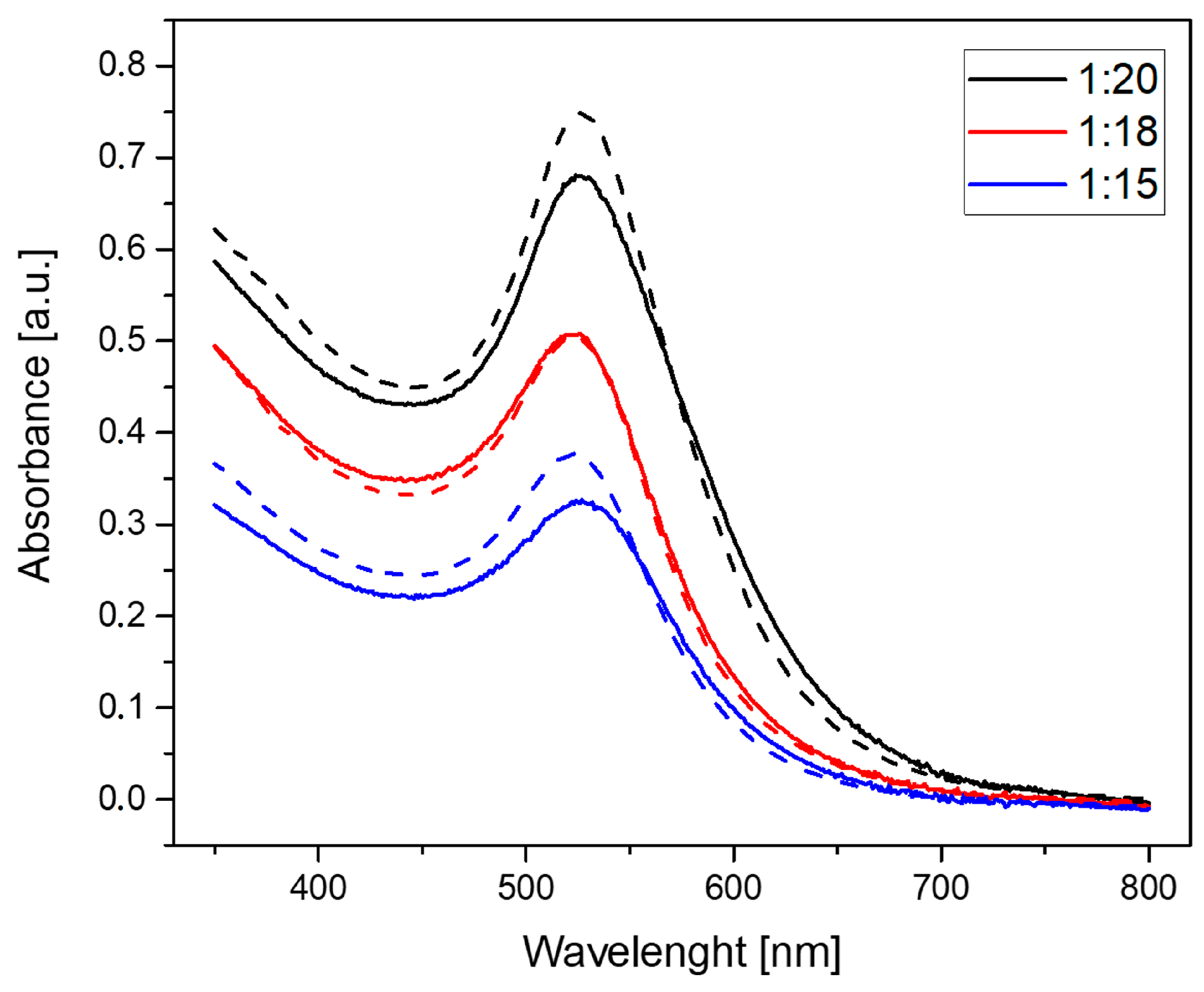
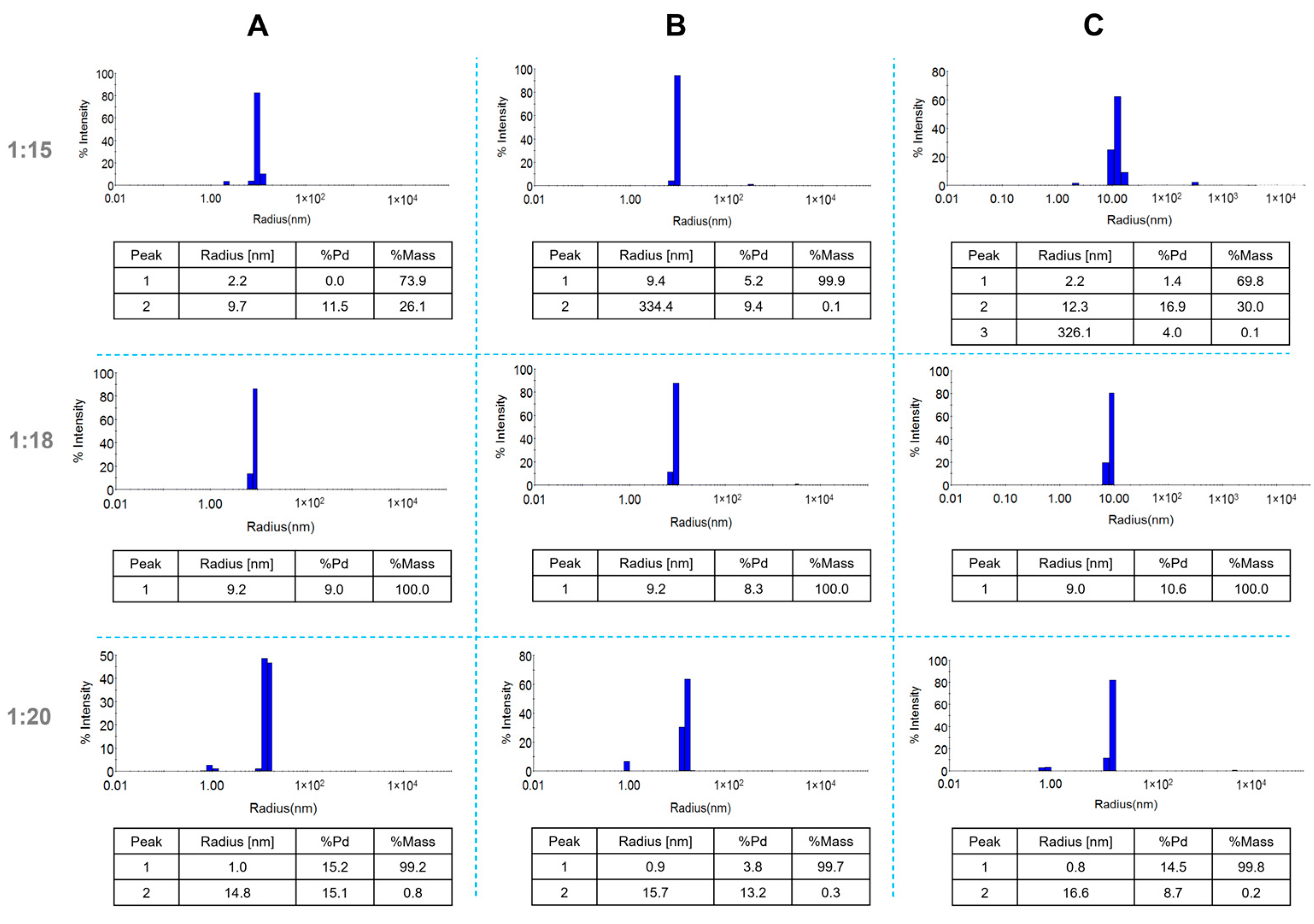
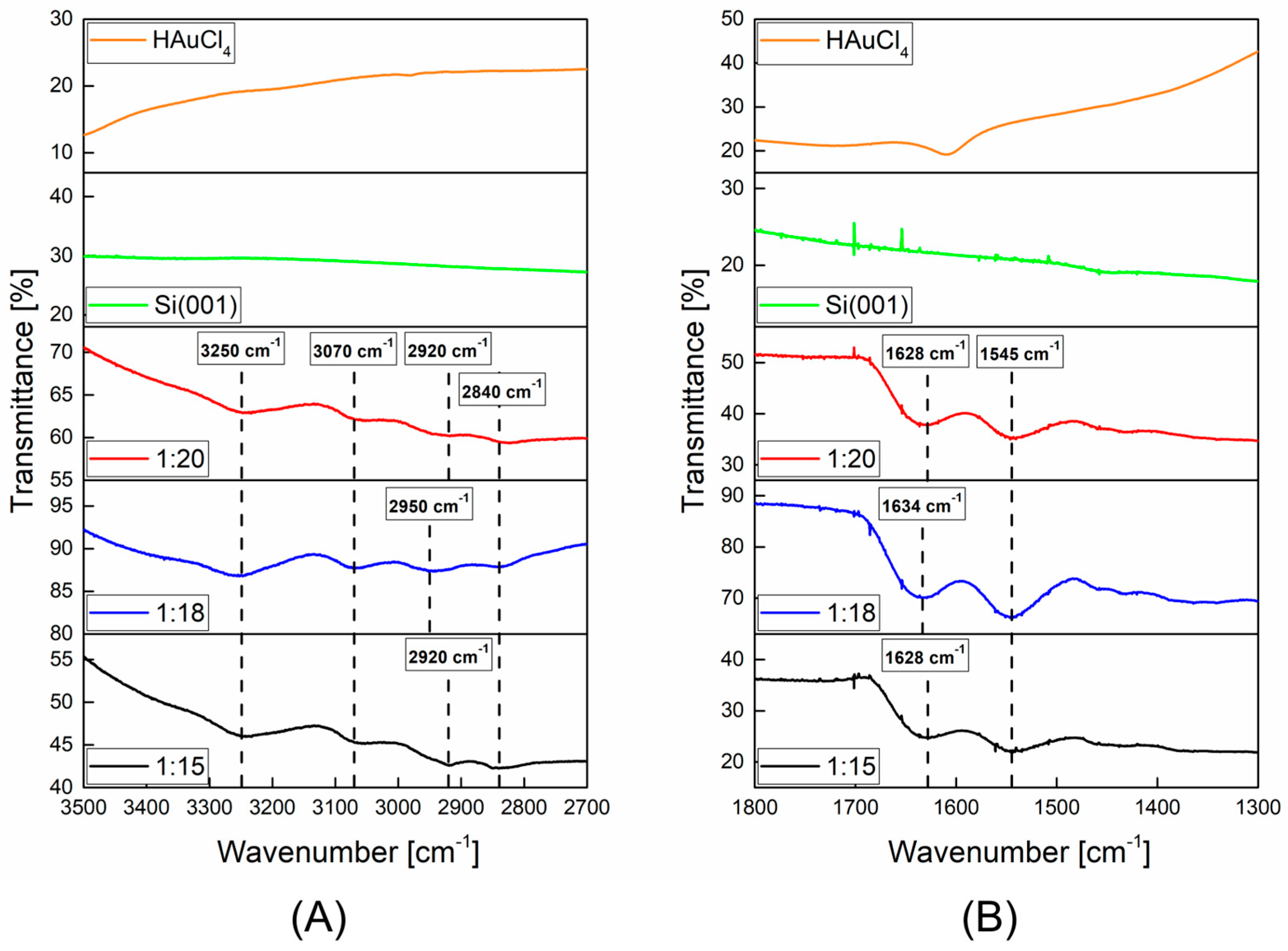
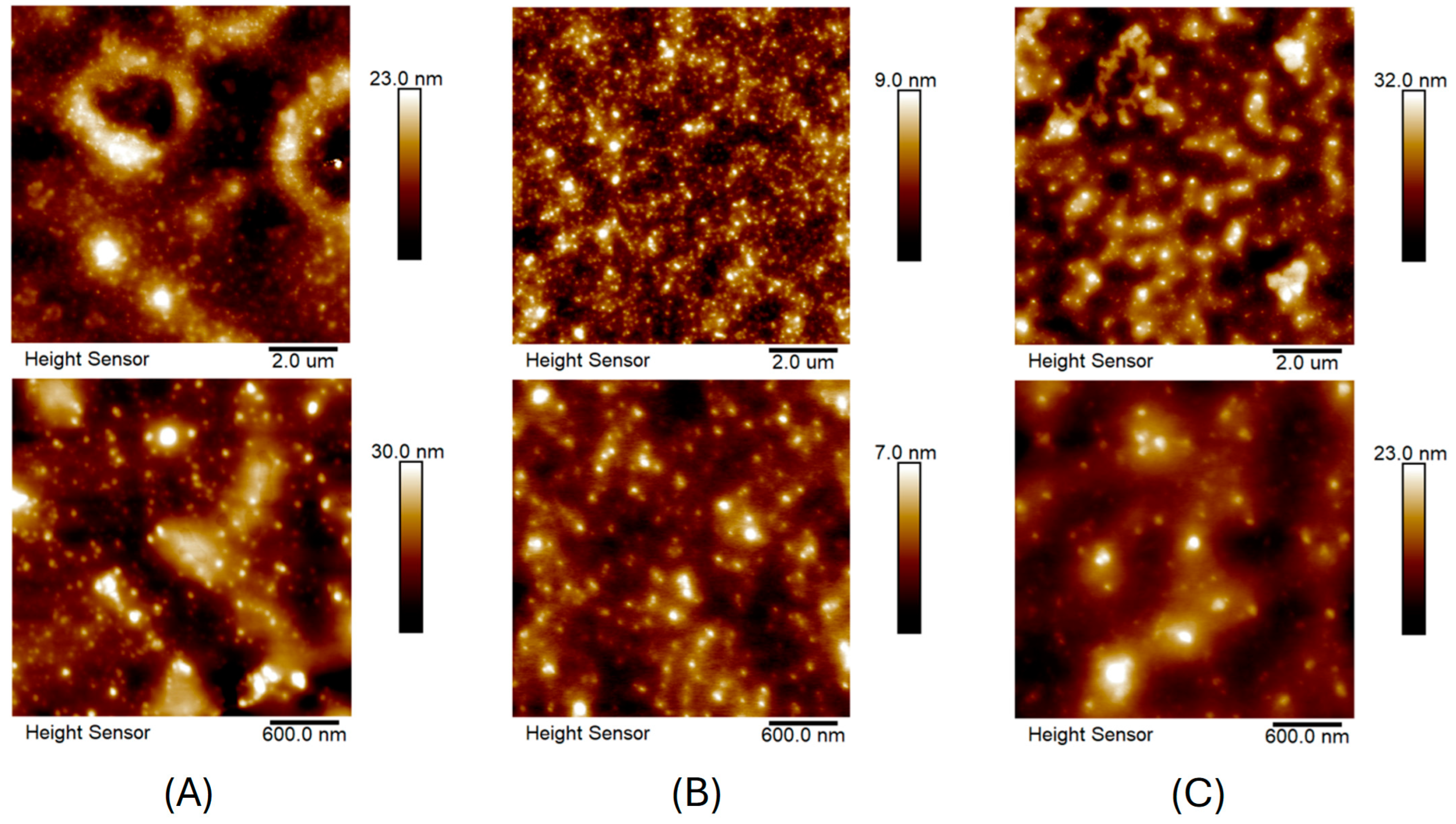
2.1. Characterization of AuNPs/PAMAM
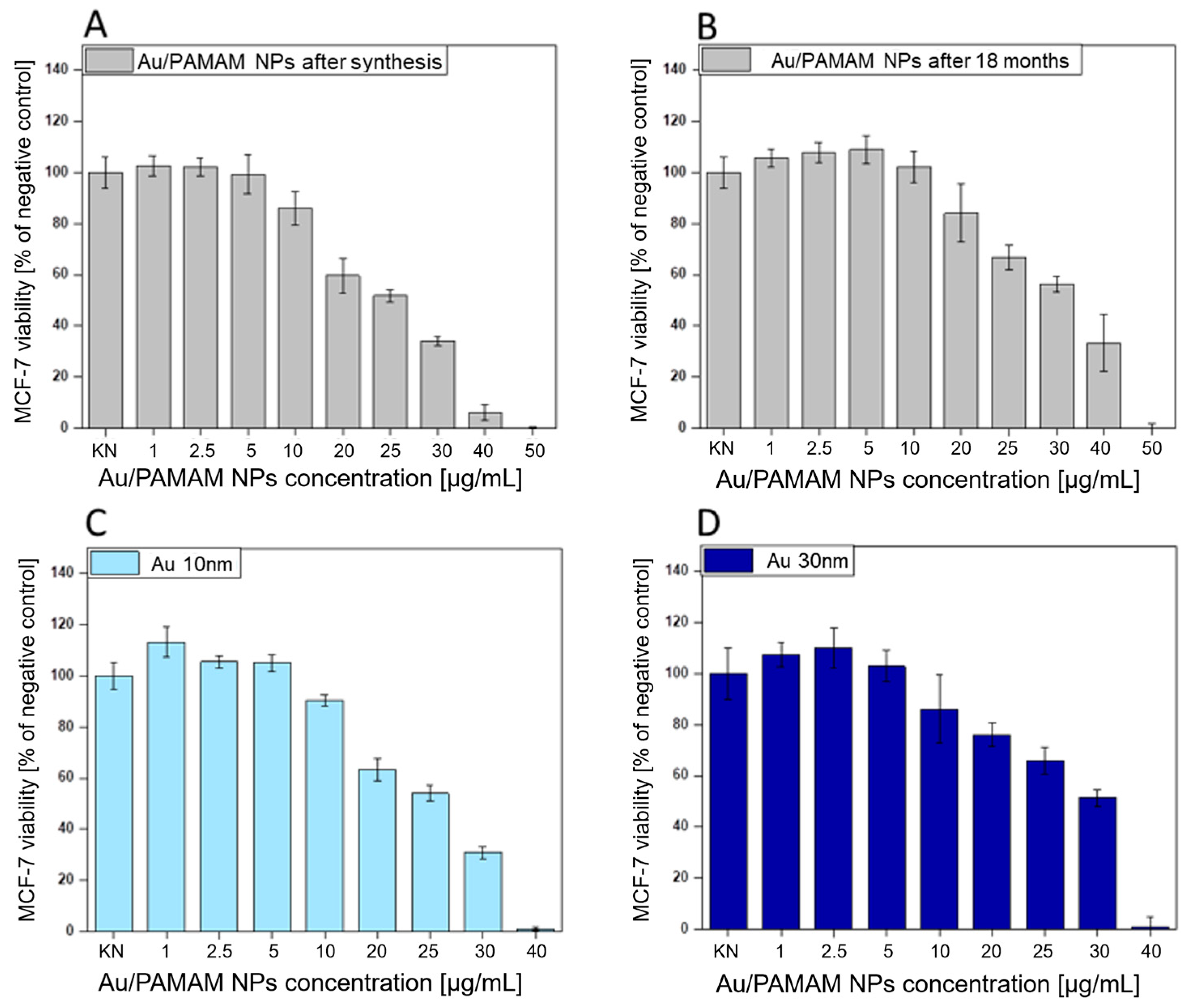
2.2. Investigation of AuNPs/PAMAM Cytotoxicity on MCF-7 Cell Line
3. Discussion
4. Materials and Methods
4.1. Two—Stage Synthesis of AuNPs/PAMAM
4.2. Ultraviolet-Visible Spectroscopy
4.3. Dynamic Light Scattering Technique and Zeta Potential Measurements
4.4. Infra-Red Spectroscopy
4.5. Atomic Force Microscopy
4.6. Transmission Electron Microscopy
4.7. Cell Culture and Cytotoxicity Measurements (XTT Assay)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pippa, N.; Chronopoulos, D.D.; Stellas, D.; Fernández-Pacheco, R.; Arenal, R.; Demetzos, C.; Tagmatarchis, N. Design and Development of Multi-Walled Carbon Nanotube-Liposome Drug Delivery Platforms. Int. J. Pharm. 2017, 528, 429–439. [Google Scholar] [CrossRef]
- Madani, S.Z.M.; Safaee, M.M.; Gravely, M.; Silva, C.; Kennedy, S.; Bothun, G.D.; Roxbury, D. Carbon Nanotube-Liposome Complexes in Hydrogels for Controlled Drug Delivery via Near-Infrared Laser Stimulation. ACS Appl. Nano Mater. 2021, 4, 331–342. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Toderas, F.; Stephan, O.; Baldeck, P.L.; Astilean, S. Uptake and Biological Effects of Chitosan-Capped Gold Nanoparticles on Chinese Hamster Ovary Cells. Mater. Sci. Eng. C 2011, 31, 184–189. [Google Scholar] [CrossRef]
- Khan, J.A.; Pillai, B.; Das, T.K.; Singh, Y.; Maiti, S. Molecular Effects of Uptake of Gold Nanoparticles in HeLa Cells. ChemBioChem 2007, 8, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green Synthesis of Gold Nanoparticles Using Brown Algae Cystoseira Baccata: Its Activity in Colon Cancer Cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Kristl, T.; Andosch, A.; Zimmermann, M.; Tran, N.; Casals, E.; Himly, M.; Puntes, V.; Huber, C.G.; Lütz-Meindl, U.; et al. Chitosan Functionalisation of Gold Nanoparticles Encourages Particle Uptake and Induces Cytotoxicity and Pro-Inflammatory Conditions in Phagocytic Cells, as Well as Enhancing Particle Interactions with Serum Components. J. Nanobiotechnol. 2015, 13, 84. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan-Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Shi, F.; Peng, C.; Yang, Y.; Sha, Y.; Shi, X.; Wu, H. Enhanced CT Imaging of Human Laryngeal Squamous Carcinoma and Indirect CT Lymphography Imaging Using PEGylated PAMAM G5·NH2-Entrapped Gold Nanoparticles as Contrast Agent. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 194–204. [Google Scholar] [CrossRef]
- Thambiraj, S.; Hema, S.; Ravi Shankaran, D. Functionalized Gold Nanoparticles for Drug Delivery Applications. Mater. Today Proc. 2018, 5, 16763–16773. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, J.; Zhang, Z.; Cao, Y.; Li, J.; Cao, S. PEGylated Graphene Oxide-Capped Gold Nanorods/Silica Nanoparticles as Multifunctional Drug Delivery Platform with Enhanced near-Infrared Responsiveness. Mater. Sci. Eng. C 2019, 104, 109889. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Lubin, B.C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold Nanoparticles Stabilize Peptide-Drug-Conjugates for Sustained Targeted Drug Delivery to Cancer Cells. J. Nanobiotechnol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Kim, Y.G.; Oh, S.K.; Crooks, R.M. Preparation and Characterization of 1-2 Nm Dendrimer-Encapsulated Gold Nanoparticles Having Very Narrow Size Distributions. Chem. Mater. 2004, 16, 167–172. [Google Scholar] [CrossRef]
- Mandal, T.; Dasgupta, C.; Maiti, P.K. Engineering Gold Nanoparticle Interaction by PAMAM Dendrimer. J. Phys. Chem. C 2013, 117, 13627–13636. [Google Scholar] [CrossRef]
- Pérignon, N.; Mingotaud, A.F.; Marty, J.D.; Rico-Lattes, I.; Mingotaud, C. Formation and Stabilization in Water of Metal Nanoparticles by a Hyperbranched Polymer Chemically Analogous to PAMAM Dendrimers. Chem. Mater. 2004, 16, 4856–4858. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Liu, R.; Zhang, A.; Yuan, Z. Size Effect of Au/PAMAM Contrast Agent on CT Imaging of Reticuloendothelial System and Tumor Tissue. Nanoscale Res. Lett. 2016, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zhang, H.T.; Xin, L. Hyaluronic Acid-Modified Polyamidoamine Dendrimer G5-Entrapped Gold Nanoparticles Delivering METase Gene Inhibits Gastric Tumor Growth via Targeting CD44+ Gastric Cancer Cells. J. Cancer Res. Clin. Oncol. 2018, 144, 1463–1473. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various Methods of Gold Nanoparticles (GNPs) Conjugation to Antibodies. Sens. Biosensing Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Mashinchian, O.; Alkilany, A.M.; Hajipour, M.J.; Tavakol, S. Gold Nanoparticles for Biomedical Imaging and Their Biological Response. In New Developments in Gold Nanomaterials Research; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Xiao, T.; Wen, S.; Wang, H.; Liu, H.; Shen, M.; Zhao, J.; Zhang, G.; Shi, X. Facile Synthesis of Acetylated Dendrimer-Entrapped Gold Nanoparticles with Enhanced Gold Loading for CT Imaging Applications. J. Mater. Chem. B 2013, 1, 2773–2780. [Google Scholar] [CrossRef]
- Sivanesan, S.; Rajeshkumar, S. Gold Nanoparticles in Diagnosis and Treatment of Alzheimer’s Disease. In Nanobiotechnology in Neurodegenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-Entrapped MUC-1 Aptamer Targeted Dendrimer-Gold Hybrid Nanostructure as a Theranostic System for Colon Adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75. [Google Scholar] [CrossRef]
- Dockery, L.T.; Daniel, M.C. Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles. Pharmaceutics 2023, 15, 2103. [Google Scholar] [CrossRef]
- Reznickova, A.; Slepicka, P.; Slavikova, N.; Staszek, M.; Svorcik, V. Preparation, Aging and Temperature Stability of PEGylated Gold Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 91–97. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Zamiri, R.; Zak, A.K.; Abdullah, A.H.; Ibrahim, N.A. Time-Dependent Effect in Green Synthesis of Silver Nanoparticles. Int. J. Nanomed. 2011, 6, 677–681. [Google Scholar] [CrossRef]
- Paino, I.M.M.; Marangoni, V.S.; de Oliveira, R.d.C.S.; Antunes, L.M.G.; Zucolotto, V. Cyto and Genotoxicity of Gold Nanoparticles in Human Hepatocellular Carcinoma and Peripheral Blood Mononuclear Cells. Toxicol. Lett. 2012, 215, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pericet-Camara, R.; Papastavrou, G.; Borkovec, M. Atomic Force Microscopy Study of the Adsorption and Electrostatic Self-Organization of Poly(Amidoamine) Dendrimers on Mica. Langmuir 2004, 20, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, F.; Bauer, B.J.; Akpalu, Y.A.; Jackson, C.L.; Amis, E.J. Dendrimer Templates for the Formation of Gold Nanoclusters. Macromolecules 2000, 33, 6042–6050. [Google Scholar] [CrossRef]
- Grala, M.; Kołodziejczyk, A.M.; Białkowska, K.; Walkowiak, B.; Komorowski, P. Assessment of the Influence of Gold Nanoparticles Stabilized with PAMAM Dendrimers on HUVEC Barrier Cells. Micron 2023, 168, 103430. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 Polyamidoamine Dendrimers Is a Novel Candidate of Nano-Carrier for Gene Delivery Agents in Breast Cancer Treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Garcia, M.E.; Baker, L.A.; Crooks, R.M. Preparation and Characterization of Dendrimer-Gold Colloid Nanocomposites. Anal. Chem. 1999, 71, 256–258. [Google Scholar] [CrossRef]
- Boyoglu, C.; He, Q.; Willing, G.; Boyoglu-Barnum, S.; Dennis, V.A.; Pillai, S.; Singh, S.R. Microscopic Studies of Various Sizes of Gold Nanoparticles and Their Cellular Localizations. ISRN Nanotechnol. 2013, 2013, 123838. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.; Jakubowska, A.; Kucinska, M.; Wasiak, T.; Komorowski, P.; Makowski, K.; Walkowiak, B. Sensing of Silver Nanoparticles on/in Endothelial Cells Using Atomic Force Spectroscopy. J. Mol. Recognit. 2018, 31, e2723. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.M.; Kucinska, M.; Jakubowska, A.; Sokolowska, P.; Rosowski, M.; Tkacz-Szczesna, B.; Komorowski, P.; Makowski, K.; Walkowiak, B. Endothelial Cell Aging Detection by Means of Atomic Force Spectroscopy. J. Mol. Recognit. 2020, 33, e2853. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.M.; Sokolowska, P.; Zimon, A.; Grala, M.; Rosowski, M.; Siatkowska, M.; Komorowski, P.; Walkowiak, B. Dysfunction of Endothelial Cells Exposed to Nanomaterials Assessed by Atomic Force Spectroscopy. Micron 2021, 145, 103062. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary Biological Evaluation of Polyamidoamine (PAMAM) StarburstTM Dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between Structure and Biocompatibility In Vitro, and Preliminary Studies on the Biodistribution of 125I-Labelled Polyamidoamine Dendrimers In Vivo. J. Control Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. The Influence of Surface Modification on the Cytotoxicity of PAMAM Dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Avila-Salas, F.; González, R.I.; Riós, P.L.; Araya-Durán, I.; Camarada, M.B. Effect of the Generation of PAMAM Dendrimers on the Stabilization of Gold Nanoparticles. J. Chem. Inf. Model. 2020, 60, 2966–2976. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]

| Samples | Zeta Potential As-Synthesized [mV] | Zeta Potential 18 Months Post-Synthesis [mV] |
|---|---|---|
| 1:15 | 43.0 (±2.9) | 13.7 (±0.7) |
| 1:18 | 35.9 (±3.4) | 15.7 (±0.7) |
| 1:20 | 30.3 (±0.9) | 18.6 (±0.6) |
| Designation | AuNPs/PAMAM After Synthesis [µg/mL] | AuNPs/PAMAM 18 Months After Synthesis [µg/mL] | AuNPs 10 nm [µg/mL] | AuNPs 30 nm [µg/mL] |
|---|---|---|---|---|
| EC10 | 10 ± 2.3 | 15 ± 1.5 | 10.5 ± 1.9 | 12 ± 2.8 |
| EC25 | 15 ± 2.3 | 22 ± 1.7 | 15.5 ± 2.2 | 19 ± 3.4 |
| EC50 | 24.5 ± 2.8 | 33 ± 2.0 | 24 ± 2.7 | 31.5 ± 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejczyk, A.M.; Grala, M.; Kołodziejczyk, Ł. Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells. Molecules 2025, 30, 2024. https://doi.org/10.3390/molecules30092024
Kołodziejczyk AM, Grala M, Kołodziejczyk Ł. Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells. Molecules. 2025; 30(9):2024. https://doi.org/10.3390/molecules30092024
Chicago/Turabian StyleKołodziejczyk, Agnieszka Maria, Magdalena Grala, and Łukasz Kołodziejczyk. 2025. "Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells" Molecules 30, no. 9: 2024. https://doi.org/10.3390/molecules30092024
APA StyleKołodziejczyk, A. M., Grala, M., & Kołodziejczyk, Ł. (2025). Evaluation of PAMAM Dendrimer-Stabilized Gold Nanoparticles: Two-Stage Procedure Synthesis and Toxicity Assessment in MCF-7 Breast Cancer Cells. Molecules, 30(9), 2024. https://doi.org/10.3390/molecules30092024








