Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review
Abstract
1. Introduction
2. Zeolite A and Its Synthesis
2.1. Synthesis of Low-Silica LTA from Pure Chemical Raw Materials
2.2. Synthesis of Low-Silica LTA from Natural Substances and Solid Waste
2.2.1. Coal Fly Ash (CFA)
2.2.2. Kaolin and Clay
2.2.3. Coal Gangue
2.2.4. Rice Husk Ash and Other Biomass Ash
2.2.5. Diatomite
2.2.6. Mineral Waste Residue
2.2.7. Waste Glass and Sand
2.2.8. Aluminum Waste
2.2.9. Furnace Slag
2.2.10. Natural Zeolites
2.2.11. Spent FCC Catalysts
2.2.12. Waste Ceramics
2.2.13. Electrolytic Manganese Residue
2.2.14. Other Raw Materials
2.3. Synthesis of High/Pure Silica LTA
2.4. Synthesis of AlPO/SAPO LTA
3. Removal of Hazardous Substances from Water by Zeolite A
3.1. Removal of Heavy Metal Cations
3.2. Removal of Radionuclide Cations
3.3. Water Softening
3.4. Removal of Ammonia-Nitrogen
3.5. Removal of Inorganic Anion Pollutants
3.6. Removal of Organic Pollutants
| Raw Material | Adsorbents | Pollutant | Adsorption Capacity | Removal Efficiency | Kinetics | Adsorption Isotherm | Ref. |
|---|---|---|---|---|---|---|---|
| Lithium slag | Zeolite A | Sr(II) | 246.9 mg·g−1 | 99.9% | pseudo-second-order | Langmuir | [32] |
| PCR * | Zeolite A | Sr(II) | 294.1 mg·g−1 | >99% | pseudo-second-order | Langmuir | [235] |
| PCR | Zeolite A | Sr(II) | 204.3 mg·g-1 | > 80% | pseudo-second-order | - | [265] |
| PCR | Zeolite A | Sr(II) | 5.4 meq·g−1 | 83% | - | - | [266] |
| Kaolinite and red mud | Magnetic zeolite A | Sr(II) | 172 mg·g−1 | 96.4% | pseudo-second-order | Langmuir | [267] |
| PCR | magnetic zeolite A | Cs(I) Sr(II) | 229.3 mg·g−1 89.0 mg·g−1 | 81.4% 95.2% | pseudo-second-order | Langmuir | [268] |
| Metakaolin | Zeolite A | Sr(II) Co(II) | 167.5 mg·g−1 118.5 mg·g−1 | - | pseudo-second-order | Langmuir | [269] |
| Halloysite | Zeolite A | Pb(II) Ag(I) | 227.7 mg·g−1 123.0 mg·g−1 | 100% | - | - | [189] |
| PCR | Zeolite A | Cu(II) Pb(II) | 230 mg·g−1 600 mg·g−1 | - | pseudo-second-order | Langmuir | [224] |
| Bagasse ash | Zeolite A | Cu(II) Pb(II) | 63.2 mg·g−1 187.2 mg·g−1 | 40.2% 37.5% | - | - | [136] |
| Metakaolin | Magnetic zeolite NaA | Cu(II) Pb(II) | 2.3mmol·g−1 2.3mmol·g−1 | >95% >95% | - | - | [270] |
| Wheat husk | NaCl-modified LTA | Pb(II) | 321.8 mg·g−1 | - | - | - | [138] |
| BFA and CFA | ZBG ZCF | Pb(II) | 625 mg·g−1 556 mg·g−1 | 100% | pseudo-second-order | Langmuir | [271] |
| CFA | Zeolite A | Pb(II) | 714.3 mg·g−1 | - | first-order | Langmuir | [272] |
| Metakaolin | Zeolite A | Pb(II) | 880 mg·g−1 | 74.5% | pseudo-second-order | Langmuir | [273] |
| Metakaolin | Zeolite A | Pb(II) | 529.7 mg·g−1 | >99% | pseudo-second-order | Langmuir | [274] |
| Lithium leach residue | Zeolite A | Pb(II) Cd(II) | 487.8 mg·g−1 193.8 mg·g−1 | 100% 96.9% | pseudo-second-order | Langmuir | [275] |
| Rare earth tailings | Zeolite A | Cd(II) Cu(II) NH4+ P(V) F− | 247.3 mg·g−1 137.1 mg·g−1 35 mg·g−1 13.8 mg·g−1 5.9 mg·g−1 | 99.6% 98.2% 70.0% 38.2% 15.4% | pseudo-second-order | Langmuir | [276] |
| PCR | Zeolite A | Pb(II) Cu(II) Cr(III) Zn(II) Co(II) | 400 mg·g−1 396 mg·g−1 391 mg·g−1 385 mg·g−1 393.5 mg·g−1 | 100% 99% 97% 96% 98% | pseudo-second-order | Langmuir | [277] |
| Perlite | Zeolite A | Eu(III) Ce(III) | 6.0 mg·g−1 5.1 mg·g−1 | 99% 90% | - | Langmuir | [278] |
| Micrometersized LTA | Nano LTA | Cs(I) | 422 mg·g−1 | 45% | pseudo-first-order | Langmuir | [279] |
| Kaolin | Zeolite A lattices | Cs(I) | 106.3 mg·g−1 | - | pseudo-second-order | Freundlich | [280] |
| PCR | PAN–zeolite A | Cs(I) Sr(II) | 214.1 mg·g−1 98.1 mg·g−1 | 90% 90% | pseudo-second-order | Langmuir and D–R | [281] |
| PCR | MWCNT @Zeolite A | Cs(I) Sr(II) | 113 mg·g−1 107 mg·g−1 | - | pseudo-second-order | Langmuir | [282] |
| CFA | Zeolite A | Cs(I) Sr(II) | 2.1 mmol·g−1 1.9 mmol·g−1 | - | pseudo-second-order | Langmuir | [283] |
| CFA | Zeolite A | Cr(III) | 35.8 mg·g−1 | 97% | - | Langmuir | [228] |
| Bauxite tailings | Zeolite A | Cr(III) | 85.1 mg·g−1 | 96.8% | - | - | [152] |
| CFA | Zeolite A | Cr(VI) | 8.7 mg·g−1 | - | - | - | [229] |
| PCR | 4A/HACC | Cr(VI) | 16.9 mg·g−1 | 92% | pseudo-second-order | Langmuir | [230] |
| Kaolin | Zeolite A | Cr(VI) | 9.7 mg·g−1 | 100% | - | - | [284] |
| PCR | Zeolite A/ Fe3O4/biochar | Cr(VI) | 46.9 mg·g−1 | 93.9% | pseudo-second-order | Langmuir | [285] |
| PCR | Zeolite A | Cr(III) | 70 mg·g−1 | - | pseudo-second-order | - | [197] |
| Kaolin | Zeolite A | Cr(III) | ~200 mg·g−1 | 99.8% | pseudo-second-order | - | [286] |
| PCR | m-ZPC | Cu(II) Cr(III) | 3.9 mg·g−1 2.0 mg·g−1 | - | pseudo-second-order | Redlich–Peterson | [287] |
| Waste materials | Zeolite A | Cd(II) Cu(II) Zn(II) | 103 mg·g−1 99.9 mg·g−1 82.1 mg·g−1 | 96% 98~99.9% 80~85% | - | Freundlich and Langmuir | [225] |
| Woody biomass ash | Zeolite A | Cu(II) Cd(II) Pb(II) | 140.1 mg·g−1 223.5 mg·g−1 850.7 mg·g−1 | >99% | pseudo-second-order | Freundlich and Langmuir | [142] |
| CFA | Zeolite A | Ni(II) Cd(II) Pb(II) | 1.1 mmol·g−1 1.4 mmol·g−1 2.6 mmol·g−1 | - | pseudo-second order | Langmuir | [288] |
| PCR | Hierarchical LTA | Pb(II) Cu(II) Ni(II) | 510 mg·g−1 170 mg·g−1 100 mg·g−1 | - | pseudo-second-order | Langmuir | [25] |
| Red mud | Magnetic 4A-zeolite | Zn(II) Cu(II) Cd(II) Ni(II) Pb(II) | 45.4 mg·g−1 35.6 mg·g−1 56.5 mg·g−1 41.2 mg·g−1 100.0 mg·g−1 | - | pseudo-second-order | Langmuir | [194] |
| Red mud and coal gangue | Magnetic zeolite A | Cu(II) Cd(II) Pb(II) | 76.2 mg·g−1 92.2 mg·g−1 178.4 mg·g−1 | - | pseudo-first-order pseudo-second-order pseudo-second-order | Langmuir | [153] |
| Rice husk and waste aluminum cans | Geopolymer/ zeolite A | Co(II) Cu(II) Zn(II) | 127.2 mg·g−1 119.1 mg·g−1 121.8 mg·g−1 | - | pseudo-second order | Langmuir | [289] |
| Red mud and CGS | Magnetic zeolite A | Pb(II) Cu(II) | 330.7 mg·g−1 142.7 mg·g−1 | 85% | pseudo-second-order Elovich | Langmuir Freundlich | [196] |
| Kaolin | O2-plasma treatment Zeolite A | Cd(II) | 247.0 mg·g−1 | 71% | - | - | [290] |
| PCR | hierarchical LTA | Cd(II) | 324.3 mg·g−1 | 90% | pseudo-first-order | Langmuir | [291] |
| CFA | Zeolite A | Pb(II) Cd(II) | 277.8 mg·g−1 87.7 mg·g−1 | - | pseudo-second order | Langmuir | [292] |
| PCR | Zeolite A | Zn(II) | 117.4 mg·g−1 | 79.6% | pseudo-second-order | Langmuir | [293] |
| PCR | Zeolite A | Zn(II) Cd(II) | 4.0 mmol·g−1 2.0 mmol·g−1 | - | pseudo-second-order | Freundlich and D-R | [294] |
| PCR | Zeolite A | Cu(II) | 202.8 mg·g−1 | 70.3% | pseudo-first-order | Langmuir | [295] |
| PCR | Fe3O4@zeolite NaA | Cu(II) | 86.5 mg·g−1 | 86.5% | pseudo-second-order | Langmuir | [296] |
| PCR | magnetic zeolite A | Cu(II) | 170 mg·g−1 | 57% | - | Langmuir | [297] |
| PCR | Zeolite A | Cu(II) | 155.4 mg·g−1 | 93.3% | - | - | [298] |
| Coal gangue and aluminum ash | Zeolite A | Cu(II) | 75.0 mg·g−1 | 99% | pseudo-second-order | - | [121] |
| Coal gangue | ZMC ZTC ZAC | Cu(II) | 118.1 mg·g−1 116.7 mg·g−1 116.1 mg·g−1 | - | pseudo-second-order | Langmuir | [125] |
| PCR | Hierarchical LTA | Cu(II) | 341.5 mg·g−1 | - | pseudo-second-order | Freundlich | [299] |
| Low-grade bauxite | Zeolite A | Cd(II) | 161.3 mg·g−1 | 99.9% | pseudo-second-order | Langmuir and Freundlich | [300] |
| PCR | tGO-Zeo | Cd(II) | 196 mg·g−1 | - | - | Langmuir | [301] |
| Metakaolin | Zeolite A | Cu(II) | 698.1 mg·g−1 | - | pseudo-second-order | Langmuir | [302] |
| SFCC | Zeolite A | Co(II) | 180.5 mg·g−1 | 99.2% | pseudo-second-order | Langmuir | [33] |
| Agricultural waste | AAS AWS | Co(II) | 235.2 mg·g−1 202.9 mg·g−1 | - | - | Freundlich | [303] |
| PCR | Zeolite A | Ni(II) | 94 mg·g−1~(25 °C) 132 mg·g−1~(45 °C) 185 mg·g−1~(60 °C) | 99.9% | pseudo-second-order | Langmuir | [227] |
| PCR | NaA/XG -alginate | Co(II) Ni(II) | 43.9 mg·g−1 81.3 mg·g−1 | - | pseudo-second-order | Langmuir | [304] |
| Rice husk and waste aluminum cans | geopolymer/ zeolite A/ chitosan | Hg(II) Pb(II) | 211.9 mg·g−1 269.5 mg·g−1 | - | pseudo-second-order | Langmuir | [305] |
| CFA | NH3 modified zeolite 4A | Hg(II) | 53.6 mg·g−1 | 99.2% | pseudo-second-order | Langmuir | [306] |
| CFA | ZnS-zeolite NaA | Hg(II) | 553.2 mg·g−1 | >99.9% | pseudo-second-order | Langmuir and Freundlich | [231] |
| PCR | C@zeolite-ZnS | Hg(II) | 795.8 mg·g−1 | 99.9% | pseudo-second-order | Langmuir | [307] |
| Opal | Zeolite A | Hg(II) | 42.0 mg·g−1 | 70% | pseudo-second order | Langmuir | [308] |
| Commercial | Zeolite A | Th(IV) | 2.8 meq·g−1 | 50.4% | - | Langmuir | [309] |
| PCR | Na2SO4@ zeolite A with MnO2 | 226Ra 228Ra | - | 78.7% 66.7% | - | - | [310] |
| PCR | Co-Zn-LTA | Tc(VII) | - | 88.3% | pseudo-second-order | - | [311] |
| PCR | Zeolite A | U(VI) | 0.95 mg·g−1 | 60-67% | pseudo-first-order | Langmuir | [312] |
| PCR | Zeolite A | U(VI) | 1.08 mg·g−1 | >96% | pseudo-first-order | Langmuir | [313] |
| Kaolin | TiO2@ Zeolites-4A | Fe(III) Mn(II) | 150.1 mg·g−1 94.1 mg·g−1 | 94% 100% | pseudo-second-order | Freundlich and Langmuir | [226] |
| PCR | Agarose- Zeolite LTA | Al(III) Mn(II) Fe(III) | 15.8 mg·g−1 3.0 mg·g−1 19.2 mg·g−1 | 99.5% 95.6% 95.3% | - | - | [314] |
| Linz–Donawitz (LD) slag | Zeolite A | Fe(III) | 27.6 mg·g−1 | 99.9% | pseudo-second-order | Langmuir | [167] |
| PCR | Zeolite A | Mn(II) | 30 mg·g−1~(25 °C) 50 mg·g−1~(55 °C) | ~70% | - | Langmuir | [315] |
| Kaolinite | ZC ZF | Mn(II) | 6.8 mg·g−1 7.2 mg·g−1 | 82.2% 99.9% | pseudo-second-order | Langmuir | [316] |
| Electrolytic manganese residue | Zeolite A | Mn(II) Cd(II) | 119.5 mg·g−1 314.2 mg·g−1 | - 85.6% | pseudo-second-order | Langmuir | [184,185] |
| PCR | Cu-LTA | As(III) As(V) | 1.4 mg·g−1 1.5 mg·g−1 | >98% | pseudo-first-order | Langmuir | [254] |
| CFA | NZVI-5A | As(V) | 72.1 mg·g−1 | 84.0% | pseudo-second-order | Langmuir | [317] |
| PCR | Fe-HZ | As(V) | 5.1 mg·g−1 | - | pseudo-second-order | Langmuir | [318] |
| PCR | Zeolite 5A | As(V) Pb(II) | 36.4 mg·g−1 46.7 mg·g−1 | >95% | Elovich model | Langmuir and Freundlich | [319] |
| CFA and red mud | Zeolite A | Ca(II) Mg(II) | 184.6 mg·g−1 253.9 mg·g−1 | - | pseudo-second-order | - | [240] |
| Waste aluminum and silica gel | Zeolite A | Ca(II) Mg(II) | 111.1 mg·g−1 for Ca(II) | 90% | pseudo-second-order intraparticle diffusion | Freundlich and Temkin | [241] |
| PCR | Mesoporous LTA | Ca(II) Mg(II) | 3.1 mmol·g−1 2.8 mmol·g−1 | - | pseudo-second-order | Dual-site Langmuir | [242] |
| Metakaolin | Zeolite A | Ca(II) Mg(II) | 935 mg·g−1 | >94% | pseudo-second-order | Langmuir | [320] |
| Kaolin | Acid-activated zeolite A | Ca(II) Mg(II) | - | 95.9% 94.9% | - | - | [239] |
| Perlite | Zeolite A | Ca(II) Mg(II) | 2.7 mmol·g−1 | 99.8% 93.4% | - | - | [243] |
| PCR | Zeolite A | Cs(I) Sr(II) Ca(II) Mg(II) | 1.6 mmol·g−1 5.5 mmol·g−1 4.8 mmol·g−1 4.2 mmol·g−1 | - | - | Dubinin–Radushkevich | [321] |
| CFA | Zeolite A | Ca(II) | 184 mg·g−1 | - | - | - | [322] |
| PCR | CTAB modified Zeolite A | Ca(II) | 129.3 mg·g−1 | 95.0% | pseudo-second-order | D–A and Langmuir | [323] |
| Bauxite tailings | Zeolite A | Ca(II) | 296 mg·g−1 | 38.4% | - | - | [324] |
| Coal gangue | Zeolite A | Ca(II) | 296.0 mg CaCO3·g−1 | - | - | - | [122] |
| Coal gangue | Zeolite A | Ca(II) | 358 mg·g−1 | - | - | - | [44] |
| PCR | Polyamidoxime-modified zeolite A | U(VI) | 4.9 mg·g−1 | 98% | pseudo-second-order | Langmuir | [236] |
| PCR | Chitosan/ zeolite A | Cd(II) As(V) | 170 mg·g−1 125 mg·g−1 | 100% | pseudo-first-order pseudo-second-order | Freundlich | [255] |
| PCR | Nanomagnetite/LTA | Dy(III) | 35 mg·g−1 | 100% | pseudo-second-order | Langmuir and Temkin | [325] |
| PCR | NaA membrane | MoO42- | - | 99.8% | - | - | [326] |
| PCR | Pt/LTA | Ba(II) La(III) | 30.0 mg·g−1 14.8 mg·g−1 | 99.9% 99.9% | pseudo-second-order | Freundlich Langmuir | [327] |
| PCR | Tetraethylenepentamine- modified zeolite 4A | P(V) | 23 mg·g−1 | - | pseudo-second-order | Langmuir | [253] |
| PCR | Zeolite A– goethite | P(V) | 0.5 mmol·g−1 | - | - | Langmuir | [252] |
| PCR | Fe-LTA | P(V) | 5.8 mg·g−1 | 80% | pseudo-second-order | Langmuir | [328] |
| Coal gangue | LMZ | P(V) | 44.6 mg·g−1 | 99.6% | - | - | [126] |
| Kaolin | Co3O4@ zeolite@ nano SiO2 | P(V) | 344.8 mg·g−1 | - | pseudo-first-order | Langmuir | [329] |
| PCR | CF/ZA | Se(VI) Se(IV) Se(Mt) | 163 mg·g−1 212.4 mg·g−1 109.3 mg·g−1 | 100% 100% 79.7% | pseudo-first-order | Langmuir | [256] |
| Opal waste rock | Zeolite A | NH4+ | 53.1 mg·g−1 | - | - | Freundlich | [151] |
| PCR | Zeolite A | NH4+ | 42.6 mg·g−1 | - | pseudo-second-order | Langmuir | [330] |
| PCR | Zeolite A | NH4+ | 31.9 mg·g−1 | - | - | Langmuir | [331] |
| PCR | Fe3O4/LTA | NH4+ | 10.5 mg·g−1 | 84.0% | pseudo-second-order | Freundlich | [332] |
| Halloysite | Zeolite A | NH4+ | 44.3 mg·g−1 | - | - | Langmuir and Freundlich | [190] |
| CFA | Zeolite A | NH4+ | 41.2 mg·g−1 | >40% | pseudo-second-order | Langmuir | [248] |
| CFA | Zeolite A | NH4+ | 60.6 mg·g−1 | 59.6% | - | Freundlich | [333] |
| CFA | Zeolite A | NH4+ | 2.3 mmol·g−1 | - | - | - | [247] |
| Foundry dust | Zeolite A | NH4+ | 37.8 mg·g−1 | 96.2% | pseudo-second-order | Langmuir | [198] |
| Halloysite | Chitosan/ zeolite A | NH4+ | 47.6 mg·g−1 | - | - | Langmuir | [334] |
| Kaolin | Zeolite A | NH4+ | 122.0 mg·g−1 | - | pseudo-second-order | Langmuir | [335] |
| PCR | Zeolite A | NH4+ | 73.0 mg·g−1 | 95.8% | pseudo-second-order | Langmuir and Freundlich | [336] |
| PCR | Zeolite A | NH4+ | 94.2 mg N·g−1 | 70.2% | pseudo-second-order | Freundlich | [337] |
| PCR | Zeolite A | NH4+ | 2.6 mmol·g−1 | - | - | Langmuir | [338] |
| PCR | Zeolite A-MgO | F− | 107.6 mg·g−1 | 72% | pseudo-second-order | Langmuir | [251] |
| Coal gangue | Zeolite A | F− | 4.7 mg·g−1 | 94.8% | pseudo-second-order | - | [249] |
| Rice husk ash | Zeolite A | F− | 104.2 mg·g−1 | 99% | pseudo-second-order | Langmuir | [250] |
| PCR | Zeolite A/Fe3O4 | Methylene blue | 2.6 mg·g−1 | 97.5% | pseudo-second-order | Langmuir | [262] |
| Linz–Donawitz (LD) slag | Zeolite A | Methylene blue | 25.3 mg·g−1 | 98.1% | pseudo-second-order | Langmuir | [168] |
| PCR | Fe3O4/ZA | Methylene blue | 40.4 mg·g−1 | ∼96.8% | pseudo-second-order | - | [339] |
| Kaolin | Zeolite A | Methylene blue | 44.4 mg·g−1 | 99.4% | pseudo-second-order | Langmuir | [340] |
| CFA | Zeolite A | Methylene blue | 23.2 mg·g−1 (25 °C) 43.8 mg·g−1 (40 °C) | 82.9% | pseudo-second-order | Langmuir | [341] |
| PCR | NaAmw | Methylene blue | 64.8 mg·g−1 | - | pseudo-first-order | Langmuir | [342] |
| PCR | MZ-A/RGO | Methylene blue Pb(II) | 666.7 mg·g−1 416.7 mg·g−1 | 98.5% 93.9% | pseudo-second-order | Langmuir | [343] |
| PCR | 3A zeolite | Methyl violet | 136 mg·g−1 | 60% | pseudo-first-order | Langmuir | [260] |
| PCR | 3A zeolite | Malachite green | 186 mg·g−1 | 96% | pseudo-first-order | Langmuir | [260] |
| Waste aluminum cans | Zeolite A | Malachite green | 29.7 mg·g−1 | - | pseudo-second-order | Langmuir | [160] |
| PCR | HDTMABr modified LTA | Congo red | 21.1 mg·g−1 | 99.2% | pseudo-second-order | Temkin | [264] |
| CFA | Zeolite A | Acid fuchsin | 40.6 mg·g−1 | - | pseudo-first-order | Langmuir | [261] |
| CFA | Surfactant-modified-LTA | Glyphosate | 769.2 mg·g−1 | 98.9% | pseudo-second-order | Freundlich | [257] |
| PCR | Cu-LTA | Glyphosate | 112.7 mg·g−1 | - | pseudo-first-order and pseudo-second-order | Langmuir | [339] |
| Kaolin | Zeolite A | Ciprofloxacin | 87.7 mg·g−1 | 91.8% | pseudo-second-order | Langmuir | [258] |
| PCR | CS/ZA | AC OM MP | 650.7 mg·g−1 506.5 mg·g−1 560.8 mg·g−1 | 78% 57.6% 74.3% | pseudo-first-order | Langmuir | [259] |
| Clay | Zeolite A | Methylene blue 8-HQ | 77.1 mg·g−1 33.5 mg·g−1 | - | - | Freundlich | [344] |
| PCR | CS/ZA | Bezactive Orange 16 | 305.8 mg·g−1 | - | pseudo-second-order | Langmuir | [345] |
| Coal gangue | ZMC ZTC ZAC | Rh-B | 5.4 mg·g−1 13.1 mg·g−1 32.8 mg·g−1 | - | pseudo-second-order | Langmuir | [125] |
| PCR | GO/4A | Rh-B | 62.8 mg·g−1 | - | pseudo-second-order | Langmuir | [346] |
| CFA | Zeolite A | AR 66 | 416.7 mg·g−1 | 100% | pseudo-second-order | Freundlich | [347] |
| PCR | CTAB modified Zeolite A | Methyl orange | 22.4 mg·g−1 | 87.2% | - | Langmuir | [348] |
4. Ion-Exchange Selectivity Order on Zeolite A
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opoku-Kwanowaa, Y.; Furaha, R.K.; Yan, L.; Wei, D. Effects of planting field on groundwater and surface water pollution in China. Clean Soil Air Water 2020, 48, 1900452. [Google Scholar] [CrossRef]
- Meng, W. System engineering for water pollution control at the watershed level in China. Front. Environ. Sci. Eng. China 2009, 3, 443–452. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef]
- Bellver-Domingo, Á.; Castellet-Viciano, L.; Hernández-Chover, V.; Hernández-Sancho, F. The quantification of non-action costs as an incentive to address water pollution problems. Water 2023, 15, 582. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human health risks due to exposure to water pollution: A review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Duan, H.B.; Shi, P.X. Heavy metal contamination of surface soil in electronic waste dismantling area: Site investigation and source-apportionment analysis. Waste Manage. Res. 2011, 29, 727–738. [Google Scholar]
- Ejidike, I.P.; Onianwa, P.C. Assessment of trace metals concentration in tree barks as indicator of atmospheric pollution within Ibadan City, South-West, Nigeria. J. Anal. Methods Chem. 2015, 2015, 243601. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustainable Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Chang, S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—A review. Environ. Sci. Pollut. Res. 2020, 27, 32371–32388. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Liu, W.; Bu, L.; Qu, L.; Chu, K.; Guo, N.; Zhang, X.; Su, X.; Li, Y.; et al. Synthesis of LSX Using Seed-iteration Approach with High N2 Adsorption Capacity for Air Separation. Chem. Res. Chin. Univ. 2024, 40, 1192–1200. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef] [PubMed]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar]
- Roshanfekr Rad, L.; Anbia, M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Ernst, S. Zeolites and catalysis. Synthesis, reactions and applications. Edited by Jiri Cejka, Avelino Corma and Stacey Zones. Angew. Chem. Int. Ed. 2011, 50, 5425–5426. [Google Scholar] [CrossRef]
- Pérez-Pellitero, J.; Pirngruber, G.D. Industrial zeolite applications for gas adsorption and separation processes. Struct. Bond. 2020, 184, 195–225. [Google Scholar]
- Vermeiren, W.; Gilson, J.-P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Jin, K.; Liu, S.; Li, Q.; Hou, P.; Liu, S.; Song, Q.; Wang, Z.; Tian, P.; et al. Fluoride-free synthesis of high-silica RHO zeolite for the highly selective synthesis of methylamine. Inorg. Chem. Front. 2024, 11, 5473–5483. [Google Scholar] [CrossRef]
- Hao, W.; Yan, X.; Guo, X.; Wang, W.; Yan, T.; Zhang, J.-N.; Yan, W. Synthesis of a low-silica zeolite with exceptional selectivity for radioactive 137Cs+. Inorg. Chem. Front. 2023, 10, 1894–1906. [Google Scholar] [CrossRef]
- Nuić, I.; Trgo, M.; Vukojević Medvidović, N.; Ugrina, M. A mass transfer analysis of competitive binding of Pb, Cd, and Zn from binary systems onto a fixed zeolite bed. Int. J. Environ. Res. Public Health 2019, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Zhou, X.; Hao, W.; Zhang, S.; Lan, C.; Wang, X.; Wang, Z.; Xu, J.; Zhang, J.-N.; et al. Facile activation of lithium slag for the hydrothermal synthesis of zeolite A with commercial quality and high removal efficiency for the isotope of radioactive 90Sr. Inorg. Chem. Front. 2022, 9, 468–477. [Google Scholar] [CrossRef]
- Wang, B.; Yan, Y.; Zhou, X.; Su, H.; Zhang, H.; Zhang, J.-N.; Xu, J.; Pan, Q.; Yan, W. Mild activation of spent fluid catalytic cracking (FCC) catalysts for the pilot synthesis of zeolite a with commercial quality and excellent Co2+ removal ability. Chem. Eng. J. 2024, 490, 151733. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, P.; Jin, Z.; Li, Y.; Li, Y.; Shi, W.; Zhou, X.; Xu, J.; Yan, W.; Xu, R. Stellerite-seeded facile synthesis of zeolite heulandite with exceptional aqueous Cd2+ capture performance. Inorg. Chem. Front. 2019, 6, 1785–1792. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ba Mohammed, B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.P.; Georgin, J.; Lima, E.C.; et al. Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: A critical review. Arabian J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 22 January 2025).
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A critical review of waste resources, synthesis, and applications for zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Ikeda, T.; Kodaira, T.; Oh, T.; Nisawa, A. K+ ion distribution in zeolite ZK-4’s with various Si/Al ratios and the contribution of K+ ions to K cluster formation. Microporous Mesoporous Mater. 2003, 57, 249–261. [Google Scholar] [CrossRef]
- Kodaira, T.; Murakami, Y.; Inoue, S.-i. Magnetism based on the cluster orbital of K metal loaded ZK-4 zeolite. Phys. B 2005, 359, 1445–1447. [Google Scholar] [CrossRef]
- Igarashi, M.; Kodaira, T.; Shimizu, T.; Goto, A.; Hashi, K. NMR property of arrayed K clusters in zeolite LTA with Si/Al=1.5. J. Phys. Chem. Solids 2006, 67, 1063–1066. [Google Scholar] [CrossRef]
- Granato, M.A.; Vlugt, T.J.H.; Rodrigues, A.E. Study on hexane adsorption in zeolite ITQ-29 by molecular simulation. Adsorption 2008, 14, 763–770. [Google Scholar] [CrossRef][Green Version]
- Tiscornia, I.; Valencia, S.; Corma, A.; Téllez, C.; Coronas, J.; Santamaría, J. Preparation of ITQ-29 (Al-free zeolite A) membranes. Microporous Mesoporous Mater. 2008, 110, 303–309. [Google Scholar] [CrossRef]
- Cheung, O.; Bacsik, Z.; Krokidas, P.; Mace, A.; Laaksonen, A.; Hedin, N. K+ exchanged zeolite ZK-4 as a highly selective sorbent for CO2. Langmuir 2014, 30, 9682–9690. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- El Bojaddayni, I.; Emin Küçük, M.; El Ouardi, Y.; Jilal, I.; El Barkany, S.; Moradi, K.; Repo, E.; Laatikainen, K.; Ouammou, A. A review on synthesis of zeolites from natural clay resources and waste ash: Recent approaches and progress. Miner. Eng. 2023, 198, 108086. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Sabbar, H.A.; Waisi, B.I.; Lafta, H.A. Effect of synthesis parameters on the formation 4A zeolite crystals: Characterization analysis and heavy metals uptake performance study for water treatment. Desalin. Water Treat. 2019, 165, 290–300. [Google Scholar] [CrossRef]
- Pham, T.-H.; Lee, B.-K.; Kim, J.; Lee, C.-H. Enhancement of CO2 capture by using synthesized nano-zeolite. J. Taiwan Inst. Chem. Eng. 2016, 64, 220–226. [Google Scholar] [CrossRef]
- Zahmakiran, M. Preparation and characterization of LTA-type zeolite framework dispersed ruthenium nanoparticles and their catalytic application in the hydrolytic dehydrogenation of ammonia–borane for efficient hydrogen generation. Mater. Sci. Eng. B 2012, 177, 606–613. [Google Scholar] [CrossRef]
- Breck, D.W.; Eversole, W.G.; Milton, R.M. New synthetic crystalline zeolites. J. Am. Chem. Soc. 1956, 78, 2338–2339. [Google Scholar] [CrossRef]
- Wakihara, T.; Sasaki, Y.; Kato, H.; Ikuhara, Y.; Okubo, T. Investigation of the surface structure of zeolite A. Phys. Chem. Chem. Phys. 2005, 7, 3416–3418. [Google Scholar] [CrossRef]
- Newsam, J.M. The zeolite cage structure. Science 1986, 231, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cui, J.; Zhang, H.; Zhang, H.; Zhu, J. Kinetic and thermodynamic study of Ag+, Cu2+, and Zn2+ ion adsorption on LTA for high-performance antibacterial coating. Coatings 2024, 14, 1524. [Google Scholar] [CrossRef]
- Belviso, C.; Lettino, A.; Cavalcante, F. Influence of synthesis method on LTA time-dependent stability. Molecules 2018, 23, 2122. [Google Scholar] [CrossRef] [PubMed]
- Madhu, J.; Madurai Ramakrishnan, V.; Santhanam, A.; Natarajan, M.; Palanisamy, B.; Velauthapillai, D.; Lan Chi, N.T.; Pugazhendhi, A. Comparison of three different structures of zeolites prepared by template-free hydrothermal method and its CO2 adsorption properties. Environ. Res. 2022, 214, 113949. [Google Scholar] [CrossRef] [PubMed]
- Tatlier, M.; Atalay-Oral, C.; Bayrak, A.; Maraş, T.; Erdem, A. Impact of ion exchange on zeolite hydrophilicity/hydrophobicity monitored by water capacity using thermal analysis. Thermochim. Acta 2022, 713, 179240. [Google Scholar] [CrossRef]
- Anand, C.; Yamaguchi, Y.; Liu, Z.; Ibe, S.; Elangovan, S.P.; Ishii, T.; Ishikawa, T.; Endo, A.; Okubo, T.; Wakihara, T. Pioneering in situ recrystallization during bead milling: A top-down approach to prepare zeolite A nanocrystals. Sci. Rep. 2016, 6, 29210. [Google Scholar] [CrossRef] [PubMed]
- Yanyan, J.; Bing, Z.; Wu, Z.; Bo, Z.; Hongbo, L.; Dongmei, W.; Ying, L. High-efficient synthesis of zeolite LTA via a wet-gel crystallization route. Chem. Res. Chin. Univ. 2017, 33, 520–524. [Google Scholar]
- Palčić, A.; Sekovanić, L.; Subotić, B.; Bronić, J. Zeolite A synthesis under dynamic conditions, after hydrogel ageing. Croat. Chem. Acta 2012, 85, 297–301. [Google Scholar] [CrossRef]
- Balkus, K.J.; Ly, K.T. The preparation and characterization of an X-type zeolite: An experiment in solid-state chemistry. J. Chem. Educ. 1991, 68, 875. [Google Scholar] [CrossRef]
- Arafat, S.M.Y.; Reza, M.N.; Khan, G.M.A. Linde type-A zeolite synthesis and effect of crystallization on its surface acidity. Indian J. Chem. Technol. 2011, 17, 303–308. [Google Scholar]
- Paprica, N.; Filipović, R.; Perušić, M.; Kostić, D.; Pantić, S.; Damjanović, V. Influence of the SiO2/Al2O3 molar ratio on the specific properties of NaA zeolite. Chem. Pap. 2022, 76, 5421–5428. [Google Scholar] [CrossRef]
- Wang, S.; Vaughan, J.; Xia, F.; Etschmann, B.; Brugger, J.; Brand, H.; Peng, H. Revealing the effect of anions on the formation and transformation of zeolite LTA in caustic solutions: An in situ synchrotron PXRD study. Cryst. Growth Des. 2023, 23, 3660–3670. [Google Scholar] [CrossRef]
- Jafari, M.; Mohammadi, T.; Kazemimoghadam, M. Synthesis and characterization of ultrafine sub-micron Na-LTA zeolite particles prepared via hydrothermal template-free method. Ceram. Int. 2014, 40, 12075–12080. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Shi, C.; Liu, Z.; Ren, B. Synthesis of ultra-small NaA zeolite nanocrystals at near room temperature. J. Porous Mater. 2023, 30, 1143–1147. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, D.; Jia, W.; Tang, D.; Li, X.; Yang, R. Studies on room-temperature synthesis of zeolite NaA. Mater. Res. Bull. 2014, 52, 96–102. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Jiang, Y.; Maturura, E.; Wang, W.; Yang, R.; Xing, C.; Chen, J.; Tsubaki, N. Facile synthesis of zeolites under an atmospheric reflux system. Microporous Mesoporous Mater. 2022, 331, 111646. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N. Microwave assisted synthesis of zeolite A from metakaolin. Microporous Mesoporous Mater. 2008, 108, 152–161. [Google Scholar] [CrossRef]
- Youssef, H.; Ibrahim, D.; Komarneni, S. Microwave-assisted versus conventional synthesis of zeolite A from metakaolinite. Microporous Mesoporous Mater. 2008, 115, 527–534. [Google Scholar] [CrossRef]
- Andaç, Ö.; Tatlıer, M.; Sirkecioğlu, A.; Ece, I.; Erdem-Şenatalar, A. Effects of ultrasound on zeolite A synthesis. Microporous Mesoporous Mater. 2005, 79, 225–233. [Google Scholar] [CrossRef]
- Sharma, P.; Yeo, J.-g.; Kim, D.K. Organic additive free synthesis of mesoporous naoncrystalline NaA zeolite using high concentration inorganic precursors. J. Mater. Chem. 2012, 22, 2838–2843. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, Y.; Liang, X.; Wang, S.; Zuo, S.; Gao, X.; Zhang, Z. Microwave hydrothermal synthesis and performance of NaA zeolite monolithic adsorbent with honeycomb ceramic matrix. Microporous Mesoporous Mater. 2018, 259, 116–122. [Google Scholar] [CrossRef]
- Mintova, S.; Olson, N.; Valtchev, V.; Bein, T. Mechanism of zeolite A nanocrystal growth from colloids at room temperature. Science 1999, 283, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kwon, S.; Na, K. Synthesis of LTA zeolites with controlled crystal sizes by variation of synthetic parameters: Effect of Na+ concentration, aging time, and hydrothermal conditions. J. Sol-Gel Sci. Technol. 2021, 98, 411–421. [Google Scholar] [CrossRef]
- Bayati, B.; Babaluo, A.A.; Karimi, R. Hydrothermal synthesis of nanostructure NaA zeolite: The effect of synthesis parameters on zeolite seed size and crystallinity. J. Eur. Ceram. Soc. 2008, 28, 2653–2657. [Google Scholar] [CrossRef]
- Anbia, M.; Koohsaryan, E.; Borhani, A. Novel hydrothermal synthesis of hierarchically-structured zeolite LTA microspheres. Mater. Chem. Phys. 2017, 193, 380–390. [Google Scholar] [CrossRef]
- Feng, Y.C.; Meng, Y.; Li, F.X.; Lv, Z.P.; Xue, J.W. Synthesis of mesoporous LTA zeolites with large BET areas. J. Porous Mater. 2013, 20, 465–471. [Google Scholar] [CrossRef]
- Nagase, T.; Miyakawa, M.; Nishioka, M. Template-free mesoporous LTA zeolite synthesized using microwave heating in the flow system. Microporous Mesoporous Mater. 2020, 306, 110375. [Google Scholar] [CrossRef]
- Song, G.; Li, F.; Lv, Z.; Xue, J. Synthesis and characterization of a novel single-crystalline core–shell zeolite A. Mater. Lett. 2015, 161, 57–59. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.-f.; Pan, D.-h.; Ma, J.-h. A hierarchically micro-meso-macroporous zeolite CaA for methanol conversion to dimethyl ether. Crystals 2016, 6, 155. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.-S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef]
- Cho, K.; Cho, H.S.; de Ménorval, L.-C.; Ryoo, R. Generation of mesoporosity in LTA zeolites by organosilane surfactant for rapid molecular transport in catalytic application. Chem. Mater. 2009, 21, 5664–5673. [Google Scholar] [CrossRef]
- Hasan, F.; Singh, R.; Li, G.; Zhao, D.; Webley, P.A. Direct synthesis of hierarchical LTA zeolite via a low crystallization and growth rate technique in presence of cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2012, 382, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Singh, R.; Webley, P.A. Formation of LTA zeolite crystals with multi-hollow polycrystalline core–shell structure via aggregation–recrystallization route in presence of emulsion droplets. Microporous Mesoporous Mater. 2012, 160, 75–84. [Google Scholar] [CrossRef]
- Bingre, R.; Louis, B.; Nguyen, P. An overview on zeolite shaping technology and solutions to overcome diffusion limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Müller, P.; Russell, A.; Tomas, J. Influence of binder and moisture content on the strength of zeolite 4A granules. Chem. Eng. Sci. 2015, 126, 204–215. [Google Scholar] [CrossRef]
- Yu, L.; Gong, J.; Zeng, C.; Zhang, L. Synthesis of monodisperse zeolite A/chitosan hybrid microspheres and binderless zeolite A microspheres. Ind. Eng. Chem. Res. 2012, 51, 2299–2308. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.; Chen, Z.; Xiao, F.-S. Recent Advances in the Synthesis of Zeolites from Solid Wastes. Chem. Res. Chin. Univ. 2024, 40, 646–656. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Q.; Yan, N.; Zhang, X.; Yang, M.; Tian, P.; Liu, Z. Highly Selective CO2 Separation on Na-exchanged DNL-6 Synthesized by Utilization of Spent Industrial Catalyst. Chem. Res. Chin. Univ. 2024, 40, 1171–1178. [Google Scholar] [CrossRef]
- Han, X.; Xia, H.; Tu, W.; Wei, Y.; Xue, D.; Li, M.; Yan, W.; Zhang, J.-N.; Han, Y.-F. Zeolite-confined Fe-site Catalysts for the Hydrogenation of CO2 to Produce High-value Chemicals. Chem. Res. Chin. Univ. 2024, 40, 78–95. [Google Scholar] [CrossRef]
- Bo, S.; Wang, T.; Lv, T.; Wu, H.; Feng, Z.; Miao, L.; Feng, Z.; Ren, L.; Meng, C. Rapid Synthesis of Boron-MWW Zeolite Through a Solvent-free Strategy. Chem. Res. Chin. Univ. 2024, 40, 1245–1255. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hosseini Asl, S.M.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Cleaner Prod. 2019, 208, 1131–1147. [Google Scholar] [CrossRef]
- Kazemian, H.; Naghdali, Z.; Ghaffari Kashani, T.; Farhadi, F. Conversion of high silicon fly ash to Na-P1 zeolite: Alkaline fusion followed by hydrothermal crystallization. Adv. Powder Technol. 2010, 21, 279–283. [Google Scholar] [CrossRef]
- Freitas, J.V.; Farinas, C.S. Sugarcane bagasse fly ash as a no-cost adsorbent for removal of phenolic inhibitors and improvement of biomass saccharification. ACS Sustainable Chem. Eng. 2017, 5, 11727–11736. [Google Scholar] [CrossRef]
- Kumar, S.; Patil, C.B. Estimation of resource savings due to fly ash utilization in road construction. Resour. Conserv. Recycl. 2006, 48, 125–140. [Google Scholar] [CrossRef]
- Guozhi, L.; Zhang, T.; Cheng, C.; Zhang, W.; Wang, L.; Wang, Y.; Zhang, Z. Zeolite a preparation from high alumina fly ash of china using alkali fusion and hydrothermal synthesis method. Mater. Res. Express 2019, 6, 065049. [Google Scholar] [CrossRef]
- Liu, L.; Singh, R.; Xiao, P.; Webley, P.A.; Zhai, Y. Zeolite synthesis from waste fly ash and its application in CO2 capture from flue gas streams. Adsorption 2011, 17, 795–800. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Sun, Q. Green synthesis of magnetic zeolite LTA using NaOH activated fly ash. Z. Anorg. Allg. Chem. 2020, 646, 1666–1670. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, R.; Liu, Q.; Cao, Q.; Guo, R. Synthesis of zeolite A from fly ash and its application in the slow release of urea. Waste Manage. 2023, 158, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qian, X.; Yuan, P.; Bai, H.; Miki, T.; Men, F.; Li, H.; Nagasaka, T. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J. Cleaner Prod. 2019, 212, 250–260. [Google Scholar] [CrossRef]
- Nowak, P.; Muir, B.; Solińska, A.; Franus, M.; Bajda, T. Synthesis and characterization of zeolites produced from low-quality coal fly ash and wet flue gas desulphurization wastewater. Materials 2021, 14, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kunecki, P.; Panek, R.; Wdowin, M.; Franus, W. Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash. Int. J. Miner. Process. 2017, 166, 69–78. [Google Scholar] [CrossRef]
- Chareonpanich, M.; Jullaphan, O.; Tang, C. Bench-scale synthesis of zeolite A from subbituminous coal ashes with high crystalline silica content. J. Cleaner Prod. 2011, 19, 58–63. [Google Scholar] [CrossRef]
- Hadi, A.A.; Malek, N.A.N.N.; Williams, C.D. Structural characterization and antibacterial activity of antibiotic streptomycin immobilized on zeolite synthesized from natural kaolinite. Biointerface Res. Appl. Chem. 2021, 11, 13573–13586. [Google Scholar]
- Mgbemere, H.E.; Ekpe, I.C.; Lawal, G.; Ovri, H.; Chaudhary, A.-L. Preparation and characterization of zeolite type 4A using kaolin from ajebo, nigeria. Pertanika J. Sci. Technol. 2019, 27, 2427–2438. [Google Scholar]
- Wang, P.; Sun, Q.; Zhang, Y.; Cao, J.; Li, X. Hydrothermal synthesis of Zeolite NaA from kaolin. Funct. Mater. Lett. 2019, 12, 1950075. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Influence of metakaolinization temperature on the formation of zeolite 4A from kaolin. Clay Miner. 1996, 31, 253–261. [Google Scholar] [CrossRef]
- Holmes, S.M.; Alomair, A.A.; Kovo, A.S. The direct synthesis of pure zeolite-A using ‘virgin’ Kaolin. RSC Adv. 2012, 2, 11491–11494. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Silveira, D.R.; Saorin Puton, B.M.; Cansian, R.L.; Bernardo-Gusmão, K. Sustainable conversion of Brazilian Amazon kaolin mining waste to zinc-based Linde Type A zeolites with antibacterial activity. J. Cleaner Prod. 2022, 338, 130659. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Q. Synthesis and characterisation of zeolite LTA with sheet structure. Micro Nano Lett. 2020, 15, 433–436. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Huang, Y.-X.; Pan, Y.; Mi, J.-X. Hydrothermal synthesis of high purity zeolite A from natural kaolin without calcination. Microporous Mesoporous Mater. 2014, 199, 50–56. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-assisted synthesis of zeolite A from metakaolinite for CO2 adsorption. Int. J. Mol. Sci. 2023, 24, 14040. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Eigenvalues, inequalities and ergodic theory. Chin. Sci. Bull. 2000, 45, 769–774. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Chen, G. Solvent-free preparation of hierarchical 4A zeolite monoliths: Role of experimental conditions. J. Cryst. Growth 2019, 528, 125286. [Google Scholar] [CrossRef]
- Si, J.; Guo, R.; Zhang, Y.; Ning, W.; Sun, Y.; Li, W.; Miao, S. Synthesis of Linde A-type zeolite from ball clay with incorporated ruthenium and application in hydrogenation catalysis. Appl. Clay Sci. 2023, 239, 106897. [Google Scholar] [CrossRef]
- García, G.; Aguilar-Mamani, W.; Carabante, I.; Cabrera, S.; Hedlund, J.; Mouzon, J. Preparation of zeolite A with excellent optical properties from clay. J. Alloys Compd. 2015, 619, 771–777. [Google Scholar] [CrossRef]
- Foroughi, M.; Salem, A.; Salem, S. Potential of fusion technique in production of mesoporous zeolite A powder from poor kaolin through modification by boehmite: Effect of clay mineralogy on particle morphology. Adv. Powder Technol. 2021, 32, 2423–2432. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhao, B.; Zhang, C.-S.; Zhang, Q.-L. Paste-like self-flowing transportation backfilling technology based on coal gangue. Min. Sci. Technol. 2009, 19, 137–143. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Cai, J.; Yu, Q.; Min, J. Study on the synthesis of LTA-Type molecular sieves from coal gangue and aluminum ash and its adsorption properties towards Cu2+. Crystals 2024, 14, 379. [Google Scholar] [CrossRef]
- Liu, S.; Yan, S.; Li, Y.; Cao, J. Synthesis of zeolite A with high whiteness from coal gangue by two-step pretreatment method. Asia-Pac. J. Chem. Eng. 2022, 17, e2760. [Google Scholar] [CrossRef]
- Kong, D.; Jiang, R. Preparation of NaA zeolite from high iron and quartz contents coal gangue by acid leaching—Alkali melting activation and hydrothermal synthesis. Crystals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Jin, Y.; Li, L.; Liu, Z.; Zhu, S.; Wang, D. Synthesis and characterization of low-cost zeolite NaA from coal gangue by hydrothermal method. Adv. Powder Technol. 2021, 32, 791–801. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.-m.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Song, L.; Li, J.; Zhang, Z.; Li, Z.; Hu, T.; Li, F.; Zhao, Y.; Peng, X. La-containing magnetic zeolite synthesized from gangue by ball-milling method. Mater. Lett. 2021, 303, 130542. [Google Scholar] [CrossRef]
- Bohra, S.; Kundu, D.; Naskar, M.K. One-pot synthesis of NaA and NaP zeolite powders using agro-waste material and other low cost organic-free precursors. Ceram. Int. 2014, 40, 1229–1234. [Google Scholar] [CrossRef]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Schmitz, T.; Schneider, H.; Schwanke, A.J.; Tessaro, I.C.; Marcilio, N.R. LTA zeolite from rice husk ash: Influence of the silicon source. Ind. Biotechnol. 2022, 18, 191–196. [Google Scholar] [CrossRef]
- Simanjuntak, W.; Pandiangan, K.D.; Sembiring, Z.; Simanjuntak, A.; Hadi, S. The effect of crystallization time on structure, microstructure, and catalytic activity of zeolite-A synthesized from rice husk silica and food-grade aluminum foil. Biomass Bioenergy 2021, 148, 106050. [Google Scholar] [CrossRef]
- Yusof, A.M.; Nizam, N.A.; Rashid, N.A.A. Hydrothermal conversion of rice husk ash to faujasite-types and NaA-type of zeolites. J. Porous Mater. 2010, 17, 39–47. [Google Scholar] [CrossRef]
- Madhu, J.; Santhanam, A.; Natarajan, M.; Velauthapillai, D. CO2 adsorption performance of template free zeolite A and X synthesized from rice husk ash as silicon source. RSC Adv. 2022, 12, 23221–23239. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Z.; Younesi, H. Preparation of free-template nanometer-sized Na–A and –X zeolites from rice husk ash. Waste Biomass Valorization 2012, 3, 61–74. [Google Scholar] [CrossRef]
- Tan, W.-C.; Yap, S.-Y.; Matsumoto, A.; Othman, R.; Yeoh, F.-Y. Synthesis and characterization of zeolites NaA and NaY from rice husk ash. Adsorption 2011, 17, 863–868. [Google Scholar] [CrossRef]
- Nawog, M.A.; Muhid, M.N.M.; Malek, N.A.N.N.; Hamdan, H. Eco-friendly synthesis of nanozeolite NaA from rice husk ash and its efficiency in removing ammonium ions. Key Eng. Mater. 2014, 594–595, 168–172. [Google Scholar] [CrossRef]
- Hameed, Y.N.-u.-A.; Alam, S.; Sultana, S.; Gul, S.; Naveed, A. Synthesis of zeolite-A from bagasse ash and removal of heavy metals from industrial effluents. Adv. Cem. Res. 2021, 33, 398–402. [Google Scholar]
- Esmaeili, A.; Saremnia, B. Synthesis and characterization of NaA zeolite nanoparticles from Hordeum vulgare L. husk for the separation of total petroleum hydrocarbon by an adsorption process. J. Taiwan Inst. Chem. Eng. 2016, 61, 276–286. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Yücel, S.; Öztürk, M. Application of Box–Behnken design for modeling of lead adsorption onto unmodified and NaCl-modified zeolite NaA obtained from biosilica. Water Sci. Technol. 2016, 75, 358–365. [Google Scholar] [CrossRef]
- Tuaimah, S.K.; Al-Nasri, S.K.; Al-Rahmani, A.A.; abbas, T.K. Using dates leaves midribs to prepare hierarchical structures incorporating porous carbon and zeolite A composites for cesium137Cs ion exchange. Baghdad Sci. J. 2020, 17, 818–825. [Google Scholar] [CrossRef]
- Esmaeili, A.; Movahedi Far, F. Synthesis of granular nanozeolite NaA from Phragmites australis for removal of total petroleum hydrocarbon. Water Qual. Res. J. 2016, 51, 307–320. [Google Scholar] [CrossRef]
- Ng, E.-P.; Chow, J.-H.; Mukti, R.R.; Muraza, O.; Ling, T.C.; Wong, K.-L. Hydrothermal synthesis of zeolite a from bamboo leaf biomass and its catalytic activity in cyanoethylation of methanol under autogenic pressure and air conditions. Mater. Chem. Phys. 2017, 201, 78–85. [Google Scholar] [CrossRef]
- Küçük, M.E.; Makarava, I.; Kinnarinen, T.; Häkkinen, A. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.N.; Dehnavi, A.R.; Joorabdoozha, A. Synthesis and characterization of LTA nanozeolite using barley husk silica: Mercury removal from standard and real solutions. Mater. Res. Bull. 2013, 48, 1753–1759. [Google Scholar] [CrossRef]
- Moisés, M.P.; da Silva, C.T.P.; Meneguin, J.G.; Girotto, E.M.; Radovanovic, E. Synthesis of zeolite NaA from sugarcane bagasse ash. Mater. Lett. 2013, 108, 243–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental applications of diatomite minerals in removing heavy metals from water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Frangie, S.; Martinez, V.; Valiente, L.; Webb, O.A. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J. Hazard. Mater. 2012, 241–242, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Veselý, D.; Kalendova, A.; Kalenda, P. A study of diatomite and calcined kaoline properties in anticorrosion protective coatings. Prog. Org. Coat. 2010, 68, 173–179. [Google Scholar] [CrossRef]
- Nascimento, C.R.; Sobrinho, E.M.O.; Assis, R.B.; Fagundes, R.F.; Bieseki, L.; Pergher, S.B.C. Synthesis of zeolite A using diatomite as silicon and aluminum source. Cerâmica 2014, 60, 63–68. [Google Scholar] [CrossRef]
- El-Kordy, A.; Elgamouz, A.; Abdelhamid, A.; Kawde, A.-N.; Tijani, N.; Lemdek, E.M. Manufacturing of novel zeolite-clay composite membrane from natural clay and diatomite, an electrochemical study of the surface and application towards heavy metals removal. J. Environ. Chem. Eng. 2024, 12, 112143. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Liu, Z.; Wang, D. Insight into the growth mechanism of low-temperature synthesis of high-purity lithium slag-based zeolite A. Materials 2024, 17, 568. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, J.; Liu, H.; Chen, T.; Cheng, P.; Wang, C.; Kong, D. Preparation, characterization, and performance of 4A zeolite based on opal waste rock for removal of ammonium ion. Adsorpt. Sci. Technol. 2018, 36, 1700–1715. [Google Scholar] [CrossRef]
- Lei, P.-c.; Shen, X.-j.; Li, Y.; Guo, M.; Zhang, M. An improved implementable process for the synthesis of zeolite 4A from bauxite tailings and its Cr3+ removal capacity. Int. J. Miner. Metall. Mater. 2016, 23, 850–857. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, J.; Liu, D.; Meng, X.; Guo, Y.; Cheng, F. Feasible synthesis of magnetic zeolite from red mud and coal gangue: Preparation, transformation and application. Powder Technol. 2023, 423, 118495. [Google Scholar] [CrossRef]
- Kuroki, S.; Hashishin, T.; Morikawa, T.; Yamashita, K.; Matsuda, M. Selective synthesis of zeolites A and X from two industrial wastes: Crushed stone powder and aluminum ash. J. Environ. Manage. 2019, 231, 749–756. [Google Scholar] [CrossRef]
- Terzano, R.; D’Alessandro, C.; Spagnuolo, M.; Romagnoli, M.; Medici, L. Facile zeolite synthesis from municipal glass and aluminum solid wastes. Clean Soil Air Water 2015, 43, 133–140. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, D.; Liu, J.; Wu, W.; Zhao, H.; Tang, J. Recycling of typical difficult-to-treat e-waste: Synthesize zeolites from waste cathode-ray-tube funnel glass. J. Hazard. Mater. 2017, 324, 673–680. [Google Scholar] [CrossRef]
- Lee, W.-H.; Lin, Y.-W.; Lin, K.-L. Parameter optimization, characterization, and crystallization mechanisms underlying the synthesis of zeolite A using liquid crystal display waste glass and sandblasting waste as alternative raw materials. J. Environ. Chem. Eng. 2022, 10, 108506. [Google Scholar] [CrossRef]
- Prasertsab, A.; Leangsiri, W.; Salakhum, S.; Yomthong, K.; Ittisanronnachai, S.; Watcharasing, S.; Kiattikomol, P.; Wattanakit, C. Transformation of production sand waste to FAU and LTA zeolites for selective moisture adsorption and ethanol conversion. Top. Catal. 2023, 66, 1631–1648. [Google Scholar] [CrossRef]
- Tounsi, H.; Mseddi, S.; Djemel, S. Preparation and characterization of Na-LTA zeolite from Tunisian sand and aluminum scrap. Phys. Procedia 2009, 2, 1065–1074. [Google Scholar] [CrossRef]
- Abdelrahman, E.A. Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq. 2018, 253, 72–82. [Google Scholar] [CrossRef]
- Ladomerský, J.; Janotka, I.; Hroncová, E.; Najdená, I. One-year properties of concrete with partial substitution of natural aggregate by cupola foundry slag. J. Cleaner Prod. 2016, 131, 739–746. [Google Scholar] [CrossRef]
- Murakami, T.; Sugano, Y.; Kinami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Alkali hydrothermal synthesis of zeolite A using oxide by-products. ISIJ Int. 2011, 51, 158–165. [Google Scholar] [CrossRef]
- Sugano, Y.; Sahara, R.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Hydrothermal synthesis of zeolite A using blast furnace slag. ISIJ Int. 2005, 45, 937–945. [Google Scholar] [CrossRef]
- Anuwattana, R.; Khummongkol, P. Conventional hydrothermal synthesis of Na-A zeolite from cupola slag and aluminum sludge. J. Hazard. Mater. 2009, 166, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Li, C.; Li, L. Synthesis, characterization of NaA zeolite from blast furnace slag (BFS) via alkaline fusion and hydrothermal treatment. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2023, 38, 401–407. [Google Scholar] [CrossRef]
- Singh, S.K.; Rekha, P.; Surya, M. Utilization of Linz–Donawitz slag from steel industry for waste minimization. J. Mater. Cycles Waste Manage. 2020, 22, 611–627. [Google Scholar] [CrossRef]
- Samanta, N.S.; Banerjee, S.; Mondal, P.; Anweshan; Bora, U.; Purkait, M.K. Preparation and characterization of zeolite from waste Linz-Donawitz (LD) process slag of steel industry for removal of Fe3+ from drinking water. Adv. Powder Technol. 2021, 32, 3372–3387. [Google Scholar] [CrossRef]
- Samanta, N.S.; Das, P.P.; Mondal, P.; Bora, U.; Purkait, M.K. Physico-chemical and adsorption study of hydrothermally treated zeolite A and FAU-type zeolite X prepared from LD (Linz–Donawitz) slag of the steel industry. Int. J. Environ. Anal. Chem. 2024, 104, 3219–3241. [Google Scholar] [CrossRef]
- Cheng, X.-w.; Zhong, Y.; Wang, J.; Guo, J.; Huang, Q.; Long, Y.-c. Studies on modification and structural ultra-stabilization of natural STI zeolite. Microporous Mesoporous Mater. 2005, 83, 233–243. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Faghihian, H.; Maragheh, M.G.; Amini, M.K.; Nezamzadeh, A.R. Thorium ion uptake by zeolite a synthesized from natural clinoptilolite tuffs. Adsorpt. Sci. Technol. 2004, 22, 707–717. [Google Scholar] [CrossRef]
- Faghihian, H.; Amini, M.K.; Nezamzadeh, A.R. Cerium uptake by zeolite A synthesized from natural clinoptilolite tuffs. J. Radioanal. Nucl. Chem. 2005, 264, 577–582. [Google Scholar] [CrossRef]
- Kazemian, H.; Modarress, H.; Kazemi, M.; Farhadi, F. Synthesis of submicron zeolite LTA particles from natural clinoptilolite and industrial grade chemicals using one stage procedure. Powder Technol. 2009, 196, 22–25. [Google Scholar] [CrossRef]
- Yue, M.B.; Huang, L.; Dong, X.; Xu, J.H.; Zhu, J.H. Attempts to synthesize zeolitic composites from stellerite. Mater. Manuf. Processes 2007, 22, 700–704. [Google Scholar] [CrossRef]
- Pathak, A.; Rana, M.S.; Marafi, M.; Kothari, R.; Gupta, P.; Tyagi, V.V. Waste petroleum fluid catalytic cracking catalysts as a raw material for synthesizing valuable zeolites: A critical overview on potential, applications, and challenges. Sustainable Mater. Technol. 2023, 38, e00733. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Qin, Y.; Liu, H.; Zhao, X.; Gao, X.; Song, L.; Sun, Z. Optimized zeolite distribution of FCC catalysts for promoting heavy-oil catalytic cracking. Ind. Eng. Chem. Res. 2022, 61, 11628–11635. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Pereyra, A.M.; Zerbino, R.; Basaldella, E.I. Removal and cementitious immobilization of heavy metals: Chromium capture by zeolite-hybridized materials obtained from spent fluid cracking catalysts. J. Cleaner Prod. 2015, 91, 187–190. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Pereyra, A.M.; Bosch, P.; Fetter, G.; Lara, V.H.; Basaldella, E.I. Structural and morphological evolutions of spent FCC catalyst pellets toward NaA zeolite. J. Mater. Sci. 2016, 51, 5061–5072. [Google Scholar] [CrossRef]
- Monzón, J.D.; Gonzalez, M.R.; Mardones, L.E.; Conconi, M.S.; Pereyra, A.M.; Basaldella, E.I. The role of alkaline activation in the structural transformations of aluminosiliceous industrial wastes towards zeolite production. Mater. Today Commun. 2019, 21, 100624. [Google Scholar] [CrossRef]
- Mallapur, V.P.; Oubagaranadin, J.U.K. A brief review on the synthesis of zeolites from hazardous wastes. Trans. Indian Ceram. Soc. 2017, 76, 1–13. [Google Scholar] [CrossRef]
- Wajima, T.; Ikegami, Y. Synthesis of zeolitic materials from waste porcelain at low temperature via a two-step alkali conversion. Ceram. Int. 2007, 33, 1269–1274. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Banan, Z. A comparison between the efficiency of CdS nanoparticles/zeolite A and CdO/zeolite A as catalysts in photodecolorization of crystal violet. Desalination 2011, 279, 146–151. [Google Scholar] [CrossRef]
- Li, W.; Jin, H.; Xie, H.; Wang, D. Progress in comprehensive utilization of electrolytic manganese residue: A review. Environ. Sci. Pollut. Res. 2023, 30, 48837–48853. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, H.; Xie, H.; Ma, L. Synthesis of zeolite A and zeolite X from electrolytic manganese residue, its characterization and performance for the removal of Cd2+ from wastewater. Chin. J. Chem. Eng. 2023, 62, 31–45. [Google Scholar] [CrossRef]
- Li, W.; Jin, H.; Xie, H.; Wang, D.; Lei, E. Utilization of electrolytic manganese residue to synthesize zeolite A and zeolite X for Mn ions adsorption. J. Ind. Eng. Chem. 2023, 120, 147–158. [Google Scholar] [CrossRef]
- Li, W.; Jin, H.; Xie, H.; Wang, M.; Han, Y. Utilization of electrolytic manganese residue and bauxite to synthesize zeolite a for pickle liquor adsorption: Characterization, mechanisms and performance. J. Cleaner Prod. 2023, 429, 139537. [Google Scholar] [CrossRef]
- Kasai, M.; Kobayashi, Y.; Yoshida, K.; Sasaki, Y.; Togo, M.; Nakahira, A. Synthesis and evaluation of zeolite surface-modified perlite. J. Ceram. Soc. Jpn. 2018, 126, 115–121. [Google Scholar] [CrossRef]
- Monzón, J.D.; Gonzalez, M.R.; Muñoz, M.; Pereyra, A.M.; Basaldella, E.I. Phase-transition process in the hydrothermal zeolitization of volcanic ash into LTA and FAU structures. Clays Clay Miner. 2021, 69, 735–745. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, H.; Lin, J.; Lin, Z.; Sun, J. Zeolite A synthesized from alkaline assisted pre-activated halloysite for efficient heavy metal removal in polluted river water and industrial wastewater. J. Environ. Sci. 2017, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef]
- Fernandes-Machado, N.R.C.; Miotto-Bigatão, D.M.M. Utilização de zeólitas sintetizadas a partir de xisto retortado na remoção de arsênio em águas contaminadas. Quim. Nova 2007, 30, 1108–1114. [Google Scholar] [CrossRef]
- Miyake, M.; Tamura, C.; Matsuda, M. Resource recovery of waste incineration fly ash: Synthesis of zeolites A and P. J. Am. Ceram. Soc. 2002, 85, 1873–1875. [Google Scholar] [CrossRef]
- Tamura, C.; Matsuda, M.; Miyake, M. Conversion of waste incineration fly ash into zeolite A and zeolite P by hydrothermal treatment. J. Ceram. Soc. Jpn. 2007, 114, 205–209. [Google Scholar] [CrossRef]
- Xie, W.-M.; Zhou, F.-P.; Bi, X.-L.; Chen, D.-D.; Li, J.; Sun, S.-Y.; Liu, J.-Y.; Chen, X.-Q. Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Joseph, I.V.; Roncaglia, G.; Tosheva, L.; Doyle, A.M. Waste peat ash mineralogy and transformation to microporous zeolites. Fuel Process. Technol. 2019, 194, 106124. [Google Scholar] [CrossRef]
- Cui, K.-B.; Lyu, J.-W.; Liu, H.-Z.; Yang, J.-L.; Yan, Z.-Q.; Yang, W.; Liu, X.; Qiu, J. Eco-friendly synthesis of magnetic zeolite A from red mud and coal gasification slag for the removal of Pb2+ and Cu2+. J. Environ. Chem. Eng. 2024, 12, 113739. [Google Scholar] [CrossRef]
- Belaabed, R.; El Knidri, H.; Addaou, A.; Laajeb, A.; Lahsini, A. Optimization of zeolite LTA synthesis parameters for chromium removal from tannery wastewater. Chem. Afr. 2024, 7, 3329–3341. [Google Scholar] [CrossRef]
- Wang, M.; Xu, D.; Ma, H.; Li, B.; Howard, A. Synthesis of NaA zeolite from foundry dust and its adsorption capacity of ammonia. J. Environ. Manag. 2023, 331, 117297. [Google Scholar] [CrossRef]
- Corma, A.; Rey, F.; Rius, J.; Sabater, M.J.; Valencia, S. Supramolecular self-assembled molecules as organic directing agent for synthesis of zeolites. Nature 2004, 431, 287–290. [Google Scholar] [CrossRef]
- Boal, B.W.; Schmidt, J.E.; Deimund, M.A.; Deem, M.W.; Henling, L.M.; Brand, S.K.; Zones, S.I.; Davis, M.E. Facile synthesis and catalysis of pure-silica and heteroatom LTA. Chem. Mater. 2015, 27, 7774–7779. [Google Scholar] [CrossRef]
- Cheung, O.; Bacsik, Z.; Fil, N.; Krokidas, P.; Wardecki, D.; Hedin, N. Selective adsorption of CO2 on zeolites NaK-ZK-4 with Si/Al of 1.8-2.8. ACS Omega 2020, 5, 25371–25380. [Google Scholar] [CrossRef] [PubMed]
- Rigo, R.T.; Prigol, C.; Antunes, A. Synthesis of ZK4 zeolite: An LTA-structured zeolite with a Si/Al ratio greater than 1. Mater. Lett. 2013, 102, 87–90. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Ou, S.Y.; Gu, Q.; Shang, J. Direct air capture of CO2 by metal cation-exchanged LTA zeolites: Effect of the charge-to-size ratio of cations. AIChE J. 2023, 69, 10. [Google Scholar] [CrossRef]
- Ahn, N.H.; Ryu, T.; Kang, Y.; Kim, H.; Shin, J.; Nam, I.-S.; Hong, S.B. The origin of an unexpected increase in NH3–SCR activity of aged Cu-LTA catalysts. ACS Catal. 2017, 7, 6781–6785. [Google Scholar] [CrossRef]
- Jo, D.; Park, G.T.; Ryu, T.; Hong, S.B. Economical synthesis of high-silica LTA zeolites: A step forward in developing a new commercial NH3-SCR catalyst. Appl. Catal., B 2019, 243, 212–219. [Google Scholar] [CrossRef]
- Park, M.B.; Lee, Y.; Zheng, A.; Xiao, F.-S.; Nicholas, C.P.; Lewis, G.J.; Hong, S.B. Formation pathway for LTA zeolite crystals synthesized via a charge density mismatch approach. J. Am. Chem. Soc. 2013, 135, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Ahn, N.H.; Seo, S.; Cho, J.; Kim, H.; Jo, D.; Park, G.T.; Kim, P.S.; Kim, C.H.; Bruce, E.L.; et al. Fully copper-exchanged high-silica LTA zeolites as unrivaled hydrothermally stable NH3-SCR catalysts. Angew. Chem. Int. Ed. 2017, 56, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal., B 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Liu, X.; Song, X.; Zeng, S.; Wang, B.; Li, X.; Tao, S. Construction of the mosaic aluminophosphate zeotypes based on nonclassical crystallization and in situ selective etching strategies. Microporous Mesoporous Mater. 2024, 367, 112999. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, K.; Wang, M.; Tao, S.; Zhang, L.; Wei, Y. Tetraalkylammonium hydroxide-assisted ionothermal synthesis and characterization of LTA-type aluminophosphate zeotypes with high structural stability after detemplation and hydration. New J. Chem. 2018, 42, 15453–15459. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wang, B.; Zi, W.; Ji, Q.; Li, Y.; Zhang, X.; Wang, Y.; Ding, Y.; Liu, J.; et al. Ultrafast synthesis of discrete submicron AlPO4-LTA molecular sieve crystals and their application in molecular sieve membrane. Microporous Mesoporous Mater. 2022, 334, 111771. [Google Scholar] [CrossRef]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Huang, A.; Caro, J. Preparation of large and well-shaped LTA-type AlPO4 crystals by using crown ether Kryptofix 222 as structure directing agent. Microporous Mesoporous Mater. 2010, 129, 90–99. [Google Scholar] [CrossRef]
- Azim, M.M.; Mohsin, U. An efficient method for the ionothermal synthesis of aluminophospahte with the LTA framework type. Microporous Mesoporous Mater. 2020, 295, 109957. [Google Scholar] [CrossRef]
- Xu, X.T.; Zhai, J.P.; Chen, Y.P.; Li, I.L.; Chen, H.Y.; Ruan, S.C.; Tang, Z.K. Synthesis of large single crystals of AlPO–LTA by using n-Propylamine as structure directing agent. J. Cryst. Growth 2014, 407, 1–5. [Google Scholar] [CrossRef]
- Pinilla-Herrero, I.; Márquez-Álvarez, C.; Sastre, E. Complex relationship between SAPO framework topology, content and distribution of Si and catalytic behaviour in the MTO reaction. Catal. Sci. Technol. 2017, 7, 3892–3901. [Google Scholar] [CrossRef]
- Pinilla-Herrero, I.; Olsbye, U.; Márquez-Álvarez, C.; Sastre, E. Effect of framework topology of SAPO catalysts on selectivity and deactivation profile in the methanol-to-olefins reaction. J. Catal. 2017, 352, 191–207. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, Y.; Zhang, L.; Guo, K.; Wang, M.; Huang, P.; Meng, X.; Zhang, R. Facile ionothermal synthesis of SAPO-LTA zeotypes with high structural stability and their catalytic performance in MTO reaction. Microporous Mesoporous Mater. 2019, 288, 109611. [Google Scholar] [CrossRef]
- Vinaches, P.; Pergher, S.B.C. Organic structure-directing agents in SAPO synthesis: The case of 2-Ethyl-1,3,4-trimethylimidazolium. Eur. J. Inorg. Chem. 2018, 2018, 122. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Cantín, Á.; Vidal-Moya, A.; Moliner, M.; Corma, A. Self-assembled aromatic molecules as efficient organic structure directing agents to synthesize the silicoaluminophosphate SAPO-42 with isolated Si species. Chem. Mater. 2015, 27, 2981–2989. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.; Xi, B.; Zhang, J.; He, Z.; Ma, C.; Wu, F. Accumulation of arsenic, mercury and heavy metals in lacustrine sediment in relation to eutrophication: Impacts of sources and climate change. Ecol. Indic. 2018, 93, 771–780. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Gong, W.; Xu, Y.; Ding, Q.; Cui, L. Distribution, risk assessment of heavy metals in sediments, and their potential risk on water supply safety of a drinking water reservoir, middle China. Environ. Sci. Pollut. Res. Int. 2023, 30, 73702–73713. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, D.; Wang, B.-B.; Feng, C.-L.; Su, H.-L.; Wang, Y.; Qin, N. Ecological risk assessment and water quality standard evaluation of 10 typical metals in eight basins in China. China Environ. Sci. 2019, 39, 2970–2982. [Google Scholar]
- Cui, W.; Tang, K.; Chen, Y.; Chen, Z.; Lan, Y.; Hong, Y.; Lan, W. Regulating the particle sizes of NaA molecular sieves toward enhanced heavy metal ion adsorption. New J. Chem. 2024, 48, 7863–7874. [Google Scholar] [CrossRef]
- Nseke, J.M.; Baloyi, N.P. Ecofriendly synthesized Zeolite 4A for the treatment of a multi-cationic contaminant-based effluent: Central composite design (CCD) statistical approach. Heliyon 2024, 10, e35176. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, M.F.; Mohamed, A.M.G.; Keshawy, M.; elMoghny, T.A.; Shehata, N. Adsorption of heavy metals and hardness ions from groundwater onto modified zeolite: Batch and column studies. Alexandria Eng. J. 2022, 61, 4189–4207. [Google Scholar] [CrossRef]
- Ghasemi, M.; Javadian, H.; Ghasemi, N.; Agarwal, S.; Gupta, V.K. Microporous nanocrystalline NaA zeolite prepared by microwave assisted hydrothermal method and determination of kinetic, isotherm and thermodynamic parameters of the batch sorption of Ni (II). J. Mol. Liq. 2016, 215, 161–169. [Google Scholar] [CrossRef]
- Rentsennorov, U.; Davaabal, B.; Dovchin, B.; Temuujin, J. Adsorption of Cr(III) from aqueous media on zeolite a prepared from fused fly ash by hydrothermal synthesis. J. Ceram. Process. Res. 2021, 22, 232–239. [Google Scholar]
- Xiao, M.; Hu, X.; Gong, Y.; Gao, D.; Zhang, P.; Liu, Q.; Liu, Y.; Wang, M. Solid transformation synthesis of zeolites from fly ash. RSC Adv. 2015, 5, 100743–100749. [Google Scholar] [CrossRef]
- Guan, Q.; Gao, K.; Ning, P.; Miao, R.; He, L. Efficient removal of low-concentration Cr(VI) from aqueous solution by 4A/HACC particles. New J. Chem. 2019, 43, 17220–17230. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Li, X.; Miki, T.; Nagasaka, T. A composite adsorbent of ZnS nanoclusters grown in zeolite NaA synthesized from fly ash with a high mercury ion removal efficiency in solution. J. Hazard. Mater. 2021, 411, 125044. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xu, Z.; Luo, Y.; Ren, L.; Hua, W. Removal of radionuclides from laundry wastewater containing organics and suspended solids using inorganic ion exchanger. Procedia Environ. Sci. 2016, 31, 375–381. [Google Scholar] [CrossRef]
- Murukutti, M.K.; Jena, H. Synthesis of nano-crystalline zeolite-A and zeolite-X from Indian coal fly ash, its characterization and performance evaluation for the removal of Cs+ and Sr2+ from simulated nuclear waste. J. Hazard. Mater. 2022, 423, 127085. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, H.S.; Jeong, H.-K.; Park, M.; Chung, D.-Y.; Lee, K.-Y.; Lee, E.-H.; Lim, W.T. Selective removal of radioactive cesium from nuclear waste by zeolites: On the origin of cesium selectivity revealed by systematic crystallographic studies. J. Phys. Chem. C 2017, 121, 10594–10608. [Google Scholar] [CrossRef]
- Hao, W.; Yan, N.; Xie, M.; Yan, X.; Guo, X.; Bai, P.; Guo, P.; Cheng, T.; Yan, W. Origin of the exceptional selectivity of NaA zeolite for the radioactive isotope 90Sr2+. Inorg. Chem. Front. 2022, 9, 6258–6270. [Google Scholar] [CrossRef]
- Dahake, R.; Tiwari, P.; Bansiwal, A. Multicycle adsorption and desorption for recovery of U(VI) from aqueous solution using oxime modified zeolite-A. J. Radioanal. Nucl. Chem. 2021, 327, 133–142. [Google Scholar] [CrossRef]
- Park, J.-S.; Song, J.-H.; Yeon, K.-H.; Moon, S.-H. Removal of hardness ions from tap water using electromembrane processes. Desalination 2007, 202, 1–8. [Google Scholar] [CrossRef]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Kusrini, E.; Mualim, N.M.; Rahman, A.; Usman, A.; Nugraha, I.G.D. Application of activated Na-zeolite a as a water softening agent to remove Ca2+ and Mg2+ ions from water. AIP Conf. Proc. 2020, 2255, 1–5. [Google Scholar]
- Manna, M.; Sen, S. Tuning crystallization for controlled morphology of zeolite A by an eco-friendly sonochemical precursor-less method. Mater. Chem. Phys. 2023, 309, 128378. [Google Scholar] [CrossRef]
- El-Nahas, S.; Osman, A.I.; Arafat, A.S.; Al-Muhtaseb, A.a.H.; Salman, H.M. Facile and affordable synthetic route of nano powder zeolite and its application in fast softening of water hardness. J. Water Process Eng. 2020, 33, 101104. [Google Scholar] [CrossRef]
- Xue, Z.; Li, Z.; Ma, J.; Bai, X.; Kang, Y.; Hao, W.; Li, R. Effective removal of Mg2+ and Ca2+ ions by mesoporous LTA zeolite. Desalination 2014, 341, 10–18. [Google Scholar] [CrossRef]
- Painer, F.; Baldermann, A.; Gallien, F.; Eichinger, S.; Steindl, F.; Dohrmann, R.; Dietzel, M. Synthesis of zeolites from fine-grained perlite and their application as sorbents. Materials 2022, 15, 4474. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Q.; Zhang, Y.; Cao, J. Alkali-dissolving hydrothermal synthesis of zeolite P from fly ash. Micro Nano Lett. 2019, 14, 572–576. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Qin, W.; Wei, S.P.; Zheng, Y.; Choi, E.; Li, X.; Johnston, J.; Wan, X.; Abrahamson, B.; Flinkstrom, Z.; Wang, B.; et al. Ammonia-oxidizing bacteria and archaea exhibit differential nitrogen source preferences. Nat. Microbiol. 2024, 9, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xiao, L.; Qu, R.; Liu, S.; Ye, D.; Song, H.; Wu, W.; Zheng, C.; Wu, X.; Gao, X. Synthesis and characterization of a single phase zeolite A using coal fly ash. RSC Adv. 2018, 8, 42200–42209. [Google Scholar] [CrossRef]
- Jiang, Q.; He, J.; Wang, Y.; Chen, B.; Tian, K.; Yang, K.; Wei, H.; Xu, X. Efficient removal of ammonia–nitrogen in wastewater by zeolite molecular sieves prepared from coal fly ash. Sci. Rep. 2024, 14, 21064. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y. Synthesis and characterization of zeolite A obtained from coal gangue for the adsorption of F− in wastewater. Sci. Adv. Mater. 2019, 11, 277–282. [Google Scholar] [CrossRef]
- Naskar, M.K. Preparation of colloidal hydrated alumina modified NaA zeolite derived from rice husk ash for effective removal of fluoride ions from water medium. J. Asian Ceram. Soc. 2020, 8, 437–447. [Google Scholar] [CrossRef]
- Chakraborty, A.; Naskar, M.K. Study on the synthesis and structural properties of zeolite A-MgO composite for defluoridation of water. Trans. Indian Ceram. Soc. 2021, 80, 199–207. [Google Scholar] [CrossRef]
- Kugbe, J.; Matsue, N.; Henmi, T. Synthesis of Linde type A zeolite–goethite nanocomposite as an adsorbent for cationic and anionic pollutants. J. Hazard. Mater. 2009, 164, 929–935. [Google Scholar] [CrossRef]
- Guan, Q.; Deng, L.; Zhang, D.; Ning, P.; Kong, Z.; He, L. Preparation of tetraethylenepentamine-functionalized 4A zeolite for effective removal of phosphate in water. Appl. Organomet. Chem. 2020, 34, e5861. [Google Scholar] [CrossRef]
- Pillewan, P.; Mukherjee, S.; Meher, A.K.; Rayalu, S.; Bansiwal, A. Removal of arsenic (III) and arsenic (V) using copper exchange zeolite-A. Environ. Prog. Sustain. Energy 2014, 33, 1274–1282. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; Jumah, M.N.B.; Al-Khalawi, N.; Alruhaimi, R.S.; Salama, Y.F.; Allam, A.A. Insight into the adsorption properties of chitosan/Zeolite-A hybrid structure for effective decontamination of toxic Cd (II) and As (V) ions from the aqueous environments. J. Polym. Environ. 2022, 30, 295–307. [Google Scholar] [CrossRef]
- Ashraf, M.-T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Haghjoo, S.; Lengauer, C.L.; Kazemian, H.; Roushani, M. Facile and innovative application of surfactant-modified-zeolite from Austrian fly ash for glyphosate removal from water solution. J. Environ. Manage. 2023, 346, 118976. [Google Scholar] [CrossRef] [PubMed]
- Kamgang Djioko, F.H.; Fotsop, C.G.; Kamgang Youbi, G.; Nwanonenyi, S.C.; Oguzie, E.E.; Ada Madu, C. Efficient removal of pharmaceutical contaminant in wastewater using low-cost zeolite 4A derived from kaolin: Experimental and theoretical studies. Mater. Chem. Phys. 2024, 315, 128994. [Google Scholar] [CrossRef]
- Mostafa, M.; Bin Jumah, M.N.; Othman, S.I.; Alruhaimi, R.S.; Salama, Y.F.; Allam, A.A.; Abukhadra, M.R. Effective removal of different species of organophosphorus pesticides (acephate, omthosate, and methyl parathion) using chitosan/zeolite-A as multifunctional adsorbent. Environ. Technol. Innov. 2021, 24, 101875. [Google Scholar] [CrossRef]
- Muniandy, S.; Salleh, L.; Zaini, M.A.A. Evaluation of malachite green and methyl violet dyes removal by 3A molecular sieve adsorbents. Desalin. Water Treat. 2020, 203, 440–448. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.; Shi, T.; Liu, W.; Qi, S. Adsorption of acid fuchsin onto LTA-type zeolite derived from fly ash. Sci. China Technol. Sci. 2014, 57, 1127–1134. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Amedalor, R.; Yaya, A.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and evaluation of zeolite A/Fe3O4 nanocomposite as a potential adsorbent for removal of organic molecules from wastewater. J. Chem. 2019, 2019, 8090756. [Google Scholar] [CrossRef]
- Elfeky, A.S.; Youssef, H.F.; Elzaref, A.S. Adsorption of dye from wastewater onto ZnO nanoparticles-loaded zeolite: Kinetic, thermodynamic and isotherm studies. Z. Phys. Chem. 2020, 234, 255–278. [Google Scholar] [CrossRef]
- Khalaf, I.H.; Al-Sudani, F.T.; AbdulRazak, A.A.; Aldahri, T.; Rohani, S. Optimization of Congo red dye adsorption from wastewater by a modified commercial zeolite catalyst using response surface modeling approach. Water Sci. Technol. 2021, 83, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Choi, Y.; Singh, B.K.; Na, K. Selective and rapid capture of Sr2+ with LTA zeolites: Effect of crystal sizes and mesoporosity. Appl. Surf. Sci. 2020, 506, 145029. [Google Scholar] [CrossRef]
- Merceille, A.; Weinzaepfel, E.; Barré, Y.; Grandjean, A. The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes. Sep. Purif. Technol. 2012, 96, 81–88. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Liu, H.; Xu, W.; Yu, J.; Zang, Y.; Hu, G.; Hu, T.; Jiang, J.; Mao, P.; et al. A novel and cost-effective synthesis of magnetic zeolite 4A using kaolinite and red mud for Sr(II) removal. Microporous Mesoporous Mater. 2024, 370, 113069. [Google Scholar] [CrossRef]
- Faghihian, H.; Moayed, M.; Firooz, A.; Iravani, M. Synthesis of a novel magnetic zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Colloid Interface Sci. 2013, 393, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Wu, Y.-C. Highly efficient adsorption of Sr2+ and Co2+ ions by ambient prepared alkali activated metakaolin. Polymers 2022, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Jangkorn, S.; Youngme, S.; Praipipat, P. Comparative lead adsorptions in synthetic wastewater by synthesized zeolite A of recycled industrial wastes from sugar factory and power plant. Heliyon 2022, 8, e09323. [Google Scholar] [CrossRef] [PubMed]
- Rayalu, S.; Dhopte, S.D.; Kumar, P. Flyash based zeolite-A:A suitable sorbent for lead removal. Indian J. Chem. Technol. 2004, 11, 227–233. [Google Scholar]
- Su, Q.; He, Y.; Yang, S.; Wan, H.; Chang, S.; Cui, X. Synthesis of NaA-zeolite microspheres by conversion of geopolymer and their performance of Pb (II) removal. Appl. Clay Sci. 2021, 200, 105914. [Google Scholar] [CrossRef]
- Wei, E.; Wang, K.; Muhammad, Y.; Chen, S.; Dong, D.; Wei, Y.; Fujita, T. Preparation and conversion mechanism of different geopolymer-based zeolite microspheres and their adsorption properties for Pb2+. Sep. Purif. Technol. 2022, 282, 119971. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Chen, Y. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue. Microporous Mesoporous Mater. 2022, 329, 111553. [Google Scholar] [CrossRef]
- Cheng, J.; Hua, X.; Zhang, G.; Yu, M.; Wang, Z.; Zhang, Y.; Liu, W.; Chen, Y.; Wang, H.; Luo, Y.; et al. Synthesis of high-crystallinity zeolite A from rare earth tailings: Investigating adsorption performance on typical pollutants in rare earth mines. J. Hazard. Mater. 2024, 468, 133730. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Salehirad, A.; Nasri, Z. Preparation of zeolite A by inorganic complex precursor route for efficient elimination of heavy metal cations from aqueous solutions. Inorg. Nano-Met. Chem. 2023, 53, 66–77. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Attia, L.A.; Attallah, M.F. Modification of perlite to prepare low cost zeolite as adsorbent material for removal of 144Ce and 152+154Eu from aqueous solution. Radiochim. Acta 2020, 108, 727–735. [Google Scholar] [CrossRef]
- Torad, N.L.; Naito, M.; Tatami, J.; Endo, A.; Leo, S.-Y.; Ishihara, S.; Wu, K.C.W.; Wakihara, T.; Yamauchi, Y. Highly crystallized nanometer-sized zeolite A with large Cs adsorption capability for the decontamination of water. Chem. Asian J. 2014, 9, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, H.; Fu, S.; Hu, Y.; He, P.; Sun, Z.; Duan, X.; Jia, D.; Colombo, P.; Zhou, Y. Additive manufacturing of hierarchical Zeolite-A lattices with exceptionally high Cs+ adsorption capacity and near-unity immobilization efficiency. Chem. Eng. J. 2023, 474, 145909. [Google Scholar] [CrossRef]
- Faghihian, H.; Iravani, M.; Moayed, M.; Ghannadi-Maragheh, M. Preparation of a novel PAN-zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: Kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2013, 222, 41–48. [Google Scholar] [CrossRef]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Removal of Cs+ and Sr2+ from water using MWCNT reinforced zeolite-A beads. Microporous Mesoporous Mater. 2016, 224, 84–88. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Application of fly ash-based materials for stabilization/solidification of cesium and strontium. Environ. Sci. Pollut. Res. 2019, 26, 23542–23554. [Google Scholar] [CrossRef] [PubMed]
- Salimkhani, S.; Siahcheshm, K.; Kadkhodaie, A.; Salimkhani, H. Structural analysis and the effect of the chromium on LTA (Na) zeolite synthesized from kaolin. Mater. Chem. Phys. 2021, 271, 124957. [Google Scholar] [CrossRef]
- Derbe, T.; Zereffa, E.A.; Sani, T. Synthesis of zeolite-A/Fe3O4/biochar composite for removal of Cr(VI) from aqueous solution. Int. J. Environ. Sci. Technol. 2024, 21, 10027–10046. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez, E.; Mayoral, Á.; Chebude, Y.; Díaz, I. Synthesis of zeolite A using raw kaolin from Ethiopia and its application in removal of Cr(III) from tannery wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 146–154. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Lee, C.-G.; Kim, J.-H.; Kang, J.-K.; Park, J.-A.; Kim, S.-B. Characterization of magnetic zeolite-polymer composites for Cu(II) and Cr(III) removal from aqueous solutions. Desalin. Water Treat. 2017, 67, 261–270. [Google Scholar] [CrossRef]
- Miyake, M.; Jha, V.K.; Kimura, Y.; Matsuda, M. Effective utilization of coal fly ash containing unburned carbon. WIT Trans. Ecol. Environ. 2009, 109, 203–212. [Google Scholar]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Elzaref, A.; Youssef, H.; Shehata, H.; Wassel, M.; Friedrich, J.; Poncin-Epaillard, F.; Debarnot, D. Plasma O2 modifies the structure of synthetic zeolite-A to improve the removal of cadmium ions from aqueous solutions. Turk. J. Chem. 2019, 43, 172–184. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Kang, S.; He, S.; Dong, X.; Zhao, Y.; Li, F.; Cen, Q. Study on using crystalline celluloses as templates for preparation of hierarchical LTA Zeolite for fast Cd(II) removal. Chem. Eng. Res. Des. 2023, 200, 32–41. [Google Scholar] [CrossRef]
- Sai Krishna, G.V.; Matli, C.S. Synthesis and characterisation of pure zeolite A from fly ash for removal of lead and cadmium. Int. J. Environ. Anal. Chem. 2024, 1–23. [Google Scholar] [CrossRef]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkat, M.; Trari, M. Adsorption of Zn2+ ions onto NaA and NaX zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef] [PubMed]
- El-Kamash, A.M.; Zaki, A.A.; El Geleel, M.A. Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A. J. Hazard. Mater. 2005, 127, 211–220. [Google Scholar] [CrossRef]
- Djamel, N.; Samira, A. Mechanism of Cu2+ ions uptake process by synthetic NaA zeolite from aqueous solution: Characterization, kinetic, intra-crystalline diffusion and thermodynamic studies. J. Mol. Liq. 2021, 323, 114642. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Shen, J.; Sun, Q. Core-shell Fe 3O4@zeolite NaA as an adsorbent for Cu2+. Materials 2020, 13, 5047. [Google Scholar] [CrossRef]
- Buzukashvili, S.; Hu, W.; Sommerville, R.; Brooks, O.; Kökkılıç, O.; Rowson, N.A.; Ouzilleau, P.; Waters, K.E. Magnetic zeolite: Synthesis and copper adsorption followed by magnetic separation from treated water. Crystals 2023, 13, 1369. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Guo, Y.; Ren, B.; Liu, X.; Zhu, L. A facile method to synthesize highly uniform zeolite A at near room temperature for copper (II) removal. Mater. Today Commun. 2022, 30, 103046. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Song, Q.; Wang, S.; Cui, X.; Liu, F.; Liu, X. Efficient recovery of Cu(II) by LTA-zeolites with hierarchical pores and their resource utilization in electrochemical denitrification: Environmentally friendly design and reutilization of waste in water. J. Hazard. Mater. 2020, 394, 122554. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.; He, J.; Hao, C.; Pan, Y.; Meng, C. Synthesis of zeolite A and its application as a high-capacity cadmium ion exchanger. Chin. J. Catal. 2012, 33, 1862–1869. [Google Scholar]
- Liu, J.; Huang, Z.; Sun, J.; Zou, Y.; Gong, B. Enhancing the removal performance of Cd(II) from aqueous solutions by NaA zeolite through doped thiourea reduced GO which is trapped within zeolite crystals. J. Alloys Compd. 2020, 815, 152514. [Google Scholar] [CrossRef]
- Li, S.; Yang, Q.; Ye, Q.; Deng, X.; Su, Q.; Cui, X. Performance of metakaolin-based geopolymer molecular sieve microspheres on dynamic recovery of Cu (II). Appl. Clay Sci. 2024, 255, 107423. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Holmes, S.M. Immobilization of cobalt ions using hierarchically porous 4A zeolite-based carbon composites: Ion-exchange and solidification. J. Water Process Eng. 2020, 33, 101059. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, F.; Wang, Y.; Luo, M.; Wang, D. Facile control of zeolite NaA dispersion into xanthan gum–alginate binary biopolymer network in improving hybrid composites for adsorptive removal of Co2+ and Ni2+. Chem. Eng. J. 2014, 255, 316–326. [Google Scholar] [CrossRef]
- Abdelbaset, H.S.; Shama, S.A.; Hegazey, R.M.; Abdelrahman, E.A. Utilisation of wastes for low-cost synthesis of chitosan composites with nanosized sodium aluminium silicate hydrate and geopolymer/zeolite A for the removal of Hg(II) and Pb(II) ions from aqueous media. Int. J. Environ. Anal. Chem. 2023, 103, 182–200. [Google Scholar] [CrossRef]
- Gao, M.; Yang, L.; Yang, S.; Jiang, T.; Wu, F.; Nagasaka, T. Simple aminated modified zeolite 4A synthesized using fly ash and its remediation of mercury contamination: Characteristics and mechanism. Sustainability 2022, 14, 15924. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, C.; Fu, Y.; Chen, F.; Hu, J. Highly efficient and selective Hg(II) adsorbent: ZnS grown on the surface of 4A zeolite and supported on starch aerogels. Environ. Sci. Pollut. Res. 2023, 30, 67059–67070. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, T.; Liu, H.; Wang, C.; Cheng, P.; Chen, D.; Xie, J. An insight into the comprehensive application of opal-palygorskite clay: Synthesis of 4A zeolite and uptake of Hg2+. Appl. Clay Sci. 2018, 165, 103–111. [Google Scholar] [CrossRef]
- Metaxas, M.; Kasselouri-Rigopoulou, V.; Galiatsatou, P.; Konstantopoulou, C.; Oikonomou, D. Thorium removal by different adsorbents. J. Hazard. Mater. 2003, 97, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.K.; Ibrahim, Z.H.; Mustafa, K.; Al-Juboori, R.A.; Nafae, T.M.; Al-Mashhadani, A.H.; Fal, M.; Alotaibi, A.M.; Alsalhy, Q.F. A modified zeolite (Na2SO4 @zeolite NaA) as a novel adsorbent for radium-226,228 from acidic radioactive wastewater: Synthesis, characterization and testing. J. Environ. Chem. Eng. 2024, 12, 112197. [Google Scholar] [CrossRef]
- Hercigonja, R.V.; Vranješ-Djurić, S.D.; Mirković, M.D.; Marković, B.M.; Maksin, D.D.; Marković, B.N.; Nastasović, A.B. Technetium removal from the aqueous solution using zeolites A and Y containing transition metal ions Co2+ and Zn2+. J. Radioanal. Nucl. Chem. 2018, 317, 215–225. [Google Scholar] [CrossRef]
- Nibou, D.; Khemaissia, S.; Amokrane, S.; Barkat, M.; Chegrouche, S.; Mellah, A. Removal of UO22+ onto synthetic NaA zeolite. Characterization, equilibrium and kinetic studies. Chem. Eng. J. 2011, 172, 296–305. [Google Scholar] [CrossRef]
- Barkat, M.; Nibou, D.; Amokrane, S.; Chegrouche, S.; Mellah, A. Uranium (VI) adsorption on synthesized 4A and P1 zeolites: Equilibrium, kinetic, and thermodynamic studies. Comptes Rendus Chim. 2015, 18, 261–269. [Google Scholar] [CrossRef]
- Chostak, C.L.; López-Delgado, A.; Padilla, I.; Lapolli, F.R.; Lobo-Recio, M.Á. Agarose-immobilized LTA zeolite: A novel material to use in an improved treatment process of mine-impacted water. Water Air Soil Pollut. 2023, 234, 365. [Google Scholar] [CrossRef]
- Jovanovic, M.; Arcon, I.; Kovac, J.; Tusar, N.N.; Obradovic, B.; Rajic, N. Removal of manganese in batch and fluidized bed systems using beads of zeolite a as adsorbent. Microporous Mesoporous Mater. 2016, 226, 378–385. [Google Scholar] [CrossRef]
- Zayed, A.M.; Selim, A.Q.; Mohamed, E.A.; Abdel Wahed, M.S.M.; Seliem, M.K.; Sillanpää, M. Adsorption characteristics of Na-A zeolites synthesized from Egyptian kaolinite for manganese in aqueous solutions: Response surface modeling and optimization. Appl. Clay Sci. 2017, 140, 17–24. [Google Scholar] [CrossRef]
- Yang, L.; Gao, M.; Wei, T.; Nagasaka, T. Synergistic removal of As(V) from aqueous solution by nanozero valent iron loaded with zeolite 5A synthesized from fly ash. J. Hazard. Mater. 2022, 424, 127428. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, B.; Liu, Y.; Zhou, F.; Li, D.; Zhang, Z. Gamma-FeOOH based hierarchically porous zeolite monoliths for As(V) removal: Characterisation, adsorption and response surface methodology. Microporous Mesoporous Mater. 2020, 308, 110518. [Google Scholar] [CrossRef]
- Mayta-Armas, A.F.; Canchanya-Huaman, Y.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Checca-Huaman, N.-R.; Ramos-Guivar, J.A. Enhanced removal of As(V) and Pb(II) from drinking and irrigating water effluents using hydrothermally synthesized zeolite 5A. Water 2023, 15, 1892. [Google Scholar] [CrossRef]
- Nasief, F.M.; Shaban, M.; Alamry, K.A.; Khadra, M.R.A.; Khan, A.A.P.; Asiri, A.M.; El-Salam, H.M.A. Hydrothermal synthesis and mechanically activated zeolite material for utilizing the removal of Ca/Mg from aqueous and raw groundwater. J. Environ. Chem. Eng. 2021, 9, 105834. [Google Scholar] [CrossRef]
- El-Rahman, K.M.A.; El-Kamash, A.M.; El-Sourougy, M.R.; Abdel-Moniem, N.M. Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+and Mg2+ ions from aqueous waste solutions using zeolite A. J. Radioanal. Nucl. Chem. 2006, 268, 221–230. [Google Scholar] [CrossRef]
- Soe, J.T.; Kim, S.-S.; Lee, Y.-R.; Ahn, J.-W.; Ahn, W.-S. CO2 capture and Ca2+ exchange using zeolite A and 13X prepared from power plant fly ash. Bull. Korean Chem. Soc. 2016, 37, 490–493. [Google Scholar] [CrossRef]
- Song, J.; Liu, M.; Zhang, Y. Ion-exchange adsorption of calcium ions from water and geothermal water with modified zeolite A. AIChE J. 2015, 61, 640–654. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Guo, M.; Zhang, M.; Liu, J. Feasible conversion of solid waste bauxite tailings into highly crystalline 4A zeolite with valuable application. Waste Manage. 2014, 34, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.; Sotillo, B.; Iglesias, C.; López, F.A.; Fernández, P.; Belviso, C.; Urbieta, A. LTA zeolite particles functionalized with nanomagnetite for effective recovery of dysprosium from liquid solutions. Microporous Mesoporous Mater. 2024, 363, 112843. [Google Scholar] [CrossRef]
- Malekpour, A.; Millani, M.R.; Kheirkhah, M. Synthesis and characterization of a NaA zeolite membrane and its applications for desalination of radioactive solutions. Desalination 2008, 225, 199–208. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Sadjadi, S.; Ahmadi, S.J.; Outokesh, M. Removal of heavy metals from aqueous solution using platinum nanopartcles/zeolite-4A. J. Environ. Health Sci. Eng. 2014, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Cobos, H.; Camacho, J.; López, C.M.; Valderrama, C.; Cortina, J.L. LTA and FAU-X iron-enriched zeolites: Use for phosphate removal from aqueous medium. Materials 2022, 15, 5418. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.R.; Abd El Fattah, M.; Enew, A.M. The amputation of phosphate anions by cobalt oxide nanoparticles doped functionalized nanocomposites from wastewater. Polyhedron 2024, 256, 116994. [Google Scholar] [CrossRef]
- Xiang, L.; Guo, Z.; Yang, L.; Qin, Y.; Chen, Z.; Wang, T.; Sun, W.; Wang, C. Modification of crystal growth of NaA zeolite with steric hindrance agents for removing ammonium ion from aqueous solution. J. Ind. Eng. Chem. 2024, 132, 326–334. [Google Scholar] [CrossRef]
- Ham, K.; Kim, B.S.; Choi, K.-Y. Enhanced ammonium removal efficiency by ion exchange process of synthetic zeolite after Na+ and heat pretreatment. Water Sci. Technol. 2018, 78, 1417–1425. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Effect of Fe3O4 addition on removal of ammonium by zeolite NaA. J. Colloid Interface Sci. 2013, 390, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yang, J.; Ma, H.; Ma, X.; Yuan, J. Synthesis of pure NaA zeolites from coal fly ashes for ammonium removal from aqueous solutions. Clean Technol. Environ. Policy 2016, 18, 629–637. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Chao, C.; Zhang, B.; Liu, J. In-situ preparation of NaA zeolite/chitosan porous hybrid beads for removal of ammonium from aqueous solution. Carbohydr. Polym. 2014, 107, 103–109. [Google Scholar] [CrossRef]
- Shaban, M.; AbuKhadra, M.R.; Nasief, F.M.; Abd El-Salam, H.M. Removal of ammonia from aqueous solutions, ground water, and wastewater using mechanically activated clinoptilolite and synthetic zeolite-A: Kinetic and equilibrium studies. Water Air Soil Pollut. 2017, 228, 450. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Labik, L.K.; Nkrumah, I.; Williams, C. Removal of ammonium ions by laboratory-synthesized zeolite Linde type A adsorption from water samples affected by mining activities in Ghana. J. Water Health 2013, 12, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Talebi, S.; Farohki, M.; Mojtabaee Sabouti, R. Removal of ammonium from water by adsorption onto synthetic zeolites NaA and NaX: A comparative parametric, kinetic, and equilibrium study. Desalin. Water Treat. 2013, 51, 5710–5720. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamada, H.; Tanaka, J.; Komatsu, Y.; Moriyoshi, Y. Ammonium ion exchange of synthetic zeolites: The effect of their open-window sizes, pore structures, and cation exchange capacities. Sep. Sci. Technol. 2005, 39, 2091–2104. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Belachew, N.; Hinsene, H. Preparation of zeolite 4A for adsorptive removal of methylene blue: Optimization, kinetics, isotherm, and mechanism study. Silicon 2022, 14, 1629–1641. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Sun, X.; Huang, J. Adsorption of dye from wastewater by zeolites synthesized from fly ash: Kinetic and equilibrium studies. Chin. J. Chem. Eng. 2009, 17, 513–521. [Google Scholar] [CrossRef]
- Sapawe, N.; Jalil, A.A.; Triwahyono, S.; Shah, M.I.A.; Jusoh, R.; Salleh, N.F.M.; Hameed, B.H.; Karim, A.H. Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue. Chem. Eng. J. 2013, 229, 388–398. [Google Scholar] [CrossRef]
- Belviso, C.; Guerra, G.; Abdolrahimi, M.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Ferretti, M.; Martucci, A.; Sturini, M. Efficiency in ofloxacin antibiotic water remediation by magnetic zeolites formed combining pure sources and wastes. Processes 2021, 9, 2137. [Google Scholar] [CrossRef]
- Von-Kiti, E.; Oduro, W.O.; Animpong, M.A.; Ampomah-Benefo, K.; Boafo-Mensah, G.; Kwakye-Awuah, B.; Williams, C.D. Evidence of electronic influence in the adsorption of cationic and zwitterionic dyes on zeolites. Heliyon 2023, 9, e20049. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.R.; Veličković, S.J.; Antonović, D.G. Modification of chitosan by zeolite A and adsorption of Bezactive Orange 16 from aqueous solution. Composites Part B 2013, 53, 145–151. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Zhang, C.; Huang, X.; Ma, K.; Zhang, Y. Preparation of graphene oxide/4A molecular sieve composite and evaluation of adsorption performance for Rhodamine B. Sep. Purif. Technol. 2022, 286, 120400. [Google Scholar] [CrossRef]
- Al-dahri, T.; AbdulRazak, A.A.; Rohani, S. Preparation and characterization of Linde-type A zeolite (LTA) from coal fly ash by microwave-assisted synthesis method: Its application as adsorbent for removal of anionic dyes. Int. J. Coal Prep. Util. 2022, 42, 2064–2077. [Google Scholar] [CrossRef]
- Yu, X.H.; Lü, H.L.; Zhou, G.W.; Zhou, L.G.; Zhang, Y.C. Absorption of methyl orange by modified fly zeolites. Adv. Mater. Res. 2012, 476–478, 1365–1369. [Google Scholar] [CrossRef]
- Finish, N.; Ramos, P.; Borojovich, E.J.C.; Zeiri, O.; Amar, Y.; Gottlieb, M. Zeolite performance in removal of multicomponent heavy metal contamination from wastewater. J. Hazard. Mater. 2023, 457, 131784. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.S.; Jamil, T.S.; Hegazy, E.Z. Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models. J. Hazard. Mater. 2010, 182, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Prelot, B.; Araïssi, M.; Gras, P.; Marchandeau, F.; Zajac, J. Contribution of calorimetry to the understanding of competitive adsorption of calcium, strontium, barium, and cadmium onto 4A type zeolite from two-metal aqueous solutions. Thermochim. Acta 2018, 664, 39–47. [Google Scholar] [CrossRef]
- Araissi, M.; Ayed, I.; Elaloui, E.; Moussaoui, Y. Removal of barium and strontium from aqueous solution using zeolite 4A. Water Sci. Technol. 2015, 73, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Breck, D.W.; Eversole, W.G.; Milton, R.M.; Reed, T.B.; Thomas, T.L. Crystalline zeolites. I. The properties of a new synthetic zeolite, type A. J. Am. Chem. Soc. 1956, 78, 5963–5972. [Google Scholar] [CrossRef]
- de Morais França, A.M.; Sousa, F.W.; Loiola, A.R.; de Luna, F.M.T.; Vidal, C.B.; do Nascimento, R.F. Study of Cu2+, Ni2+, and Zn2+ competitive adsorption on synthetic zeolite: An experimental and theoretical approach. Desalin. Water Treat. 2021, 227, 263–277. [Google Scholar] [CrossRef]
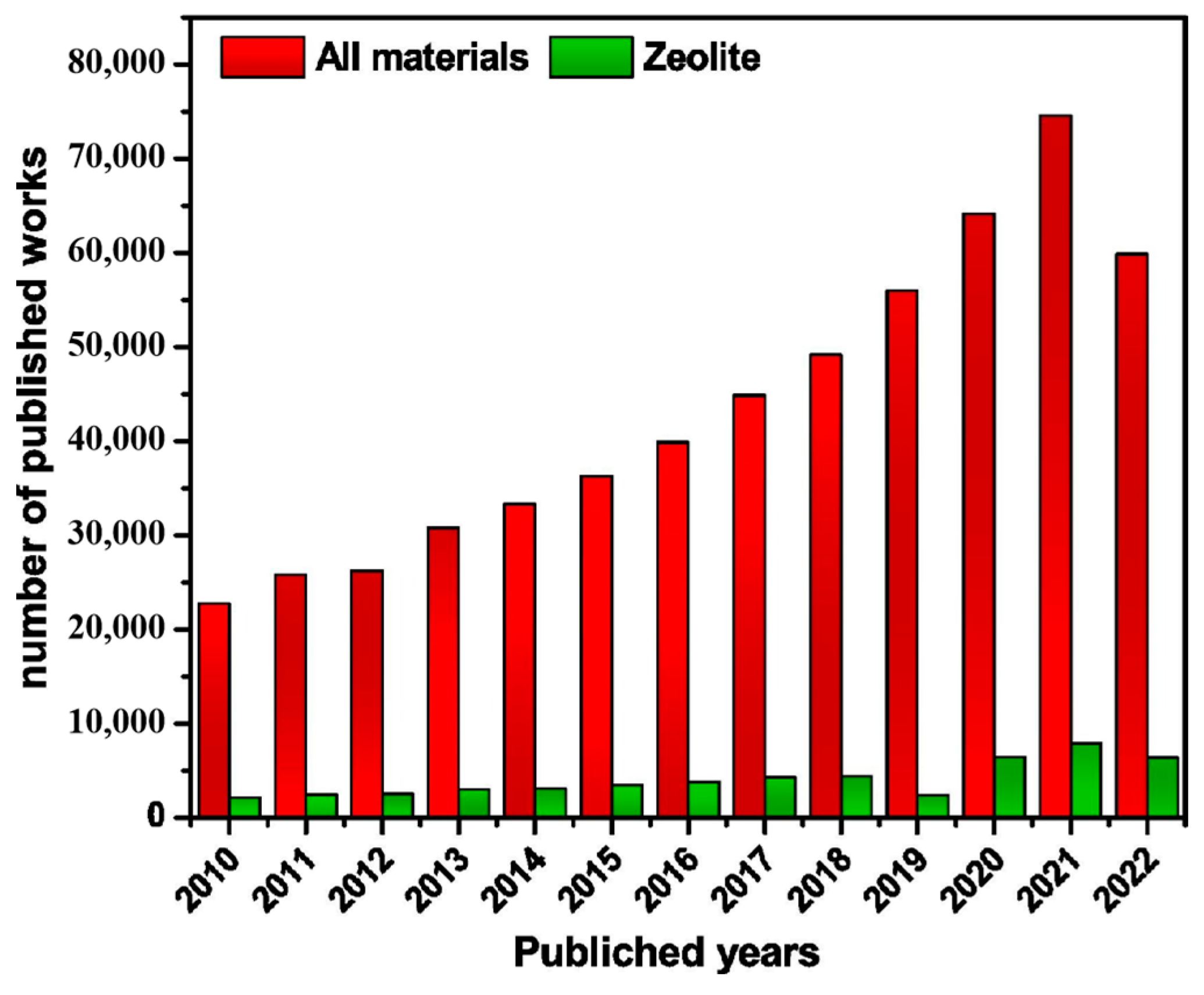


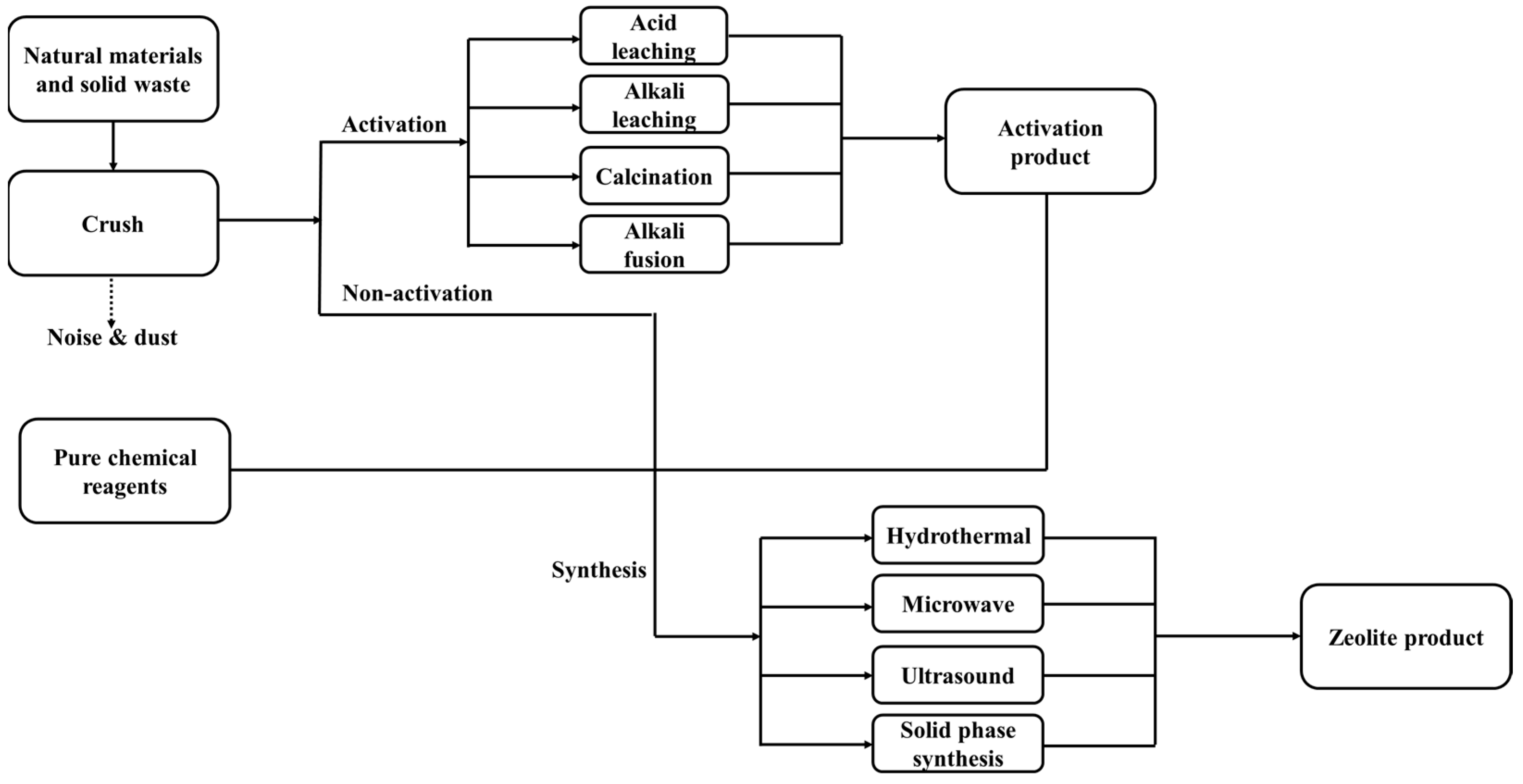
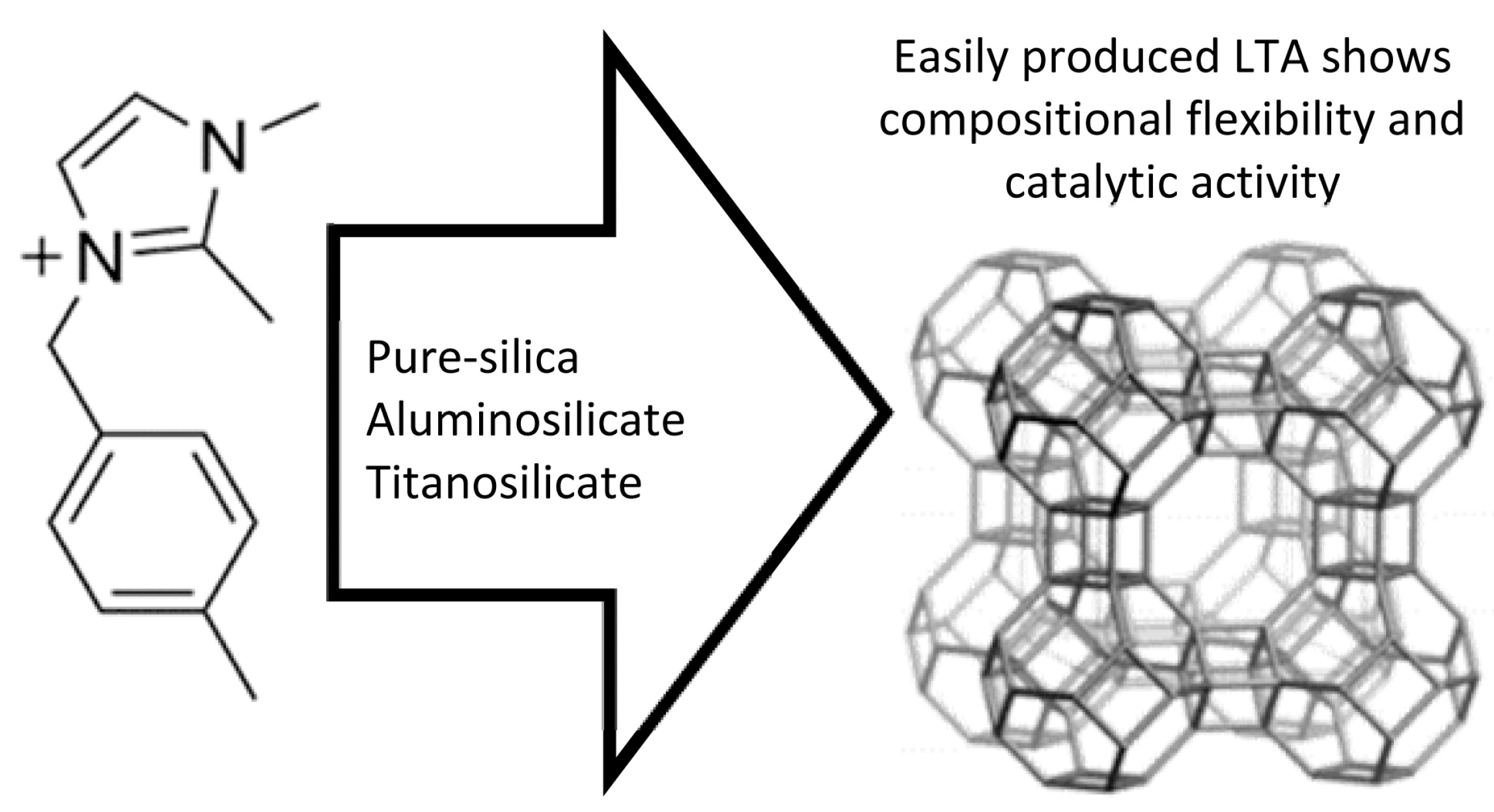
| Adsorption Reaction | ΔE (eV) |
|---|---|
| LTA + 2H2O + NH4+·(H2O)4 → LTA-NH4 + Na+·(H2O)6 | −0.65 |
| LTA + K+·(H2O)6 → LTA-K + Na+·(H2O)6 | −2.13 |
| LTA + Cs+·(H2O)6 → LTA-Cs + Na+·(H2O)6 | −0.11 |
| LTA + 6H2O + Mg2+·(H2O)6 → LTA-Mg + 2Na+·(H2O)6 | −1.94 |
| LTA + 6H2O + Ca2+·(H2O)6 → LTA-Ca + 2Na+·(H2O)6 | −6.17 |
| LTA + 6H2O + Sr2+·(H2O)6 → LTA-Sr + 2Na+·(H2O)6 | −8.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Wang, B.; Liu, S.; Liu, S.; Yan, W. Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review. Molecules 2025, 30, 554. https://doi.org/10.3390/molecules30030554
Pan J, Wang B, Liu S, Liu S, Yan W. Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review. Molecules. 2025; 30(3):554. https://doi.org/10.3390/molecules30030554
Chicago/Turabian StylePan, Junyao, Binyu Wang, Simiao Liu, Shanshan Liu, and Wenfu Yan. 2025. "Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review" Molecules 30, no. 3: 554. https://doi.org/10.3390/molecules30030554
APA StylePan, J., Wang, B., Liu, S., Liu, S., & Yan, W. (2025). Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review. Molecules, 30(3), 554. https://doi.org/10.3390/molecules30030554






