Peas (Pisum sativum subsp. arvense Asch) and Beans (Vicia faba var. minor) as Source of Quality Plant Proteins
Abstract
1. Introduction
2. Chemical Composition of Field Bean and Field Pea
| Field Bean | Field Pea | |||||||
|---|---|---|---|---|---|---|---|---|
| References | ||||||||
| Antinutritional Factors | [35] | [24] | [34] | [17] | [64] | [50] | [55] | [65] |
| Total polyphenols | 10.9–19.86 | 1.4–5.0 | 2.66–2.81 | NR | 6.6–7.9 | NR | NR | NR |
| Total flavonoids | 5.25–6.96 | NR | NR | NR | NR | NR | NR | NR |
| Condensed tannin | 0.27–0.67 | NR | 1.12–1.24 | NR | 4.54–5.89 | NR | 5.24 | 0.34 |
| Phytate | NR | 1.12–12.81 | NR | NR | NR | NR | NR | 2.1 |
| Saponins | NR | 0.02–0.12 | NR | 0.02–0.04 | NR | NR | NR | NR |
| Vicine | NR | 0.40–7.01 | NR | 0.86–5.46 | NR | NR | NR | NR |
| Convicine | NR | 0.04–3.12 | NR | 0.52–4.02 | NR | NR | NR | NR |
| Raffinose | NR | 1.1–3.9 | NR | NR | NR | 1.7 | 37.5 | 10.1 |
| Stachyose | NR | 4.4–13.7 | NR | NR | NR | 8.1 | NR | 39.4 |
| Verbascose | NR | 8.0–15.0 | NR | NR | NR | 20 | NR | 39 |
| Trypsin inhibitor (TIU/mg) | NR | 1.2–23.1 | NR | NR | NR | NR | 1.1 | 0.4 |
| Lectin (HU/mg) | NR | 0.8–3.2 | NR | NR | NR | NR | NR | NR |
3. Extraction Process of Protein
3.1. Preprocessing: For a Better Functionality
3.2. Dry Fractionation: Air Classification and Size Reduction
3.3. Wet Extraction: Alkali Extraction/Isoelectric Precipitation (AE-IEP)
3.4. Ultrafiltration Processing
3.5. Salt Extraction and Micellization
3.6. Mild Fractionation
3.7. Ultrasound-Assisted Extraction
3.8. Enzyme-Assisted Extraction (EAE)
3.9. Fermentation
4. Protein Fractions
4.1. Globulins
4.2. Non-Globulin Proteins
4.3. Others Compounds
4.4. Protein Concentrate
4.5. Protein Isolate
5. Nutritional, Digestibility, and Amino Acid Distribution
6. Techno Functional Properties
6.1. Solubility
6.2. Water Holding Capacity (WHC)
6.3. Oil Binding Capacity (OBC)
6.4. Interfacial Properties
6.5. Emulsification Properties
6.6. Foaming Properties
6.7. Gelation Properties
6.8. Thermal Properties
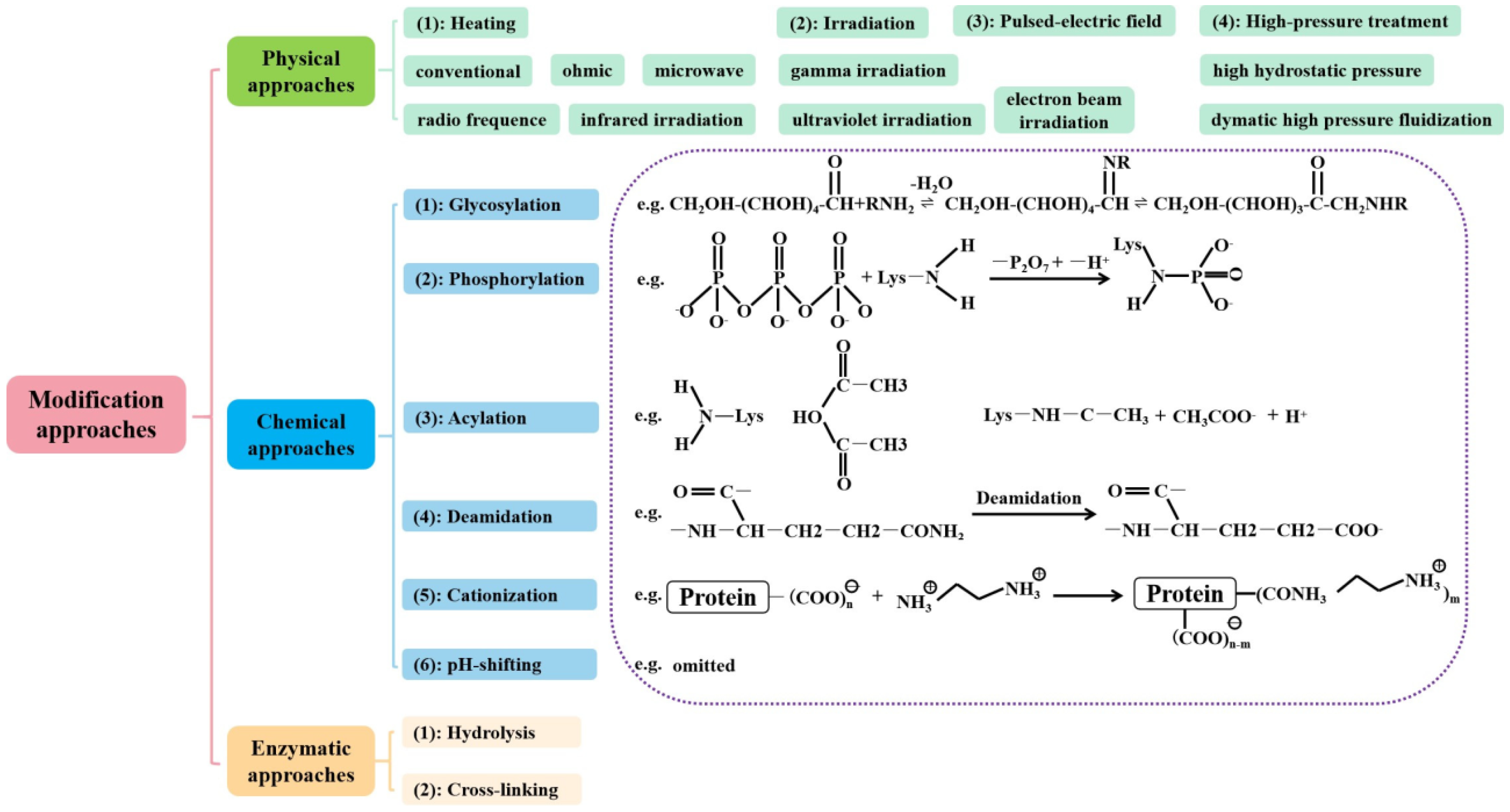
7. Structural Modification Techniques for Improvement of Functional Properties
7.1. Physical Modification
7.1.1. Heat Treatment
7.1.2. High-Pressure Processing (HPP)
7.1.3. Heat with Shear Treatment (Extrusion)
7.1.4. Cold Atmospheric Pressure Plasma Treatment
7.1.5. Ultrasonic Treatment
7.1.6. Pulsed Electric Field (PEF)
7.1.7. Electrospinning
7.2. Chemical Modification
7.2.1. Acid-Base Treatment/pH Shifting Treatment
7.2.2. Glycation
7.2.3. Acylation
7.2.4. Succinylation
7.2.5. Phosphorylation
7.2.6. Cross-Linking
7.2.7. Esterification
7.2.8. Deamidation
7.3. Biological Modification
7.3.1. Fermentation
7.3.2. Enzymatic Modification
7.3.3. Germination (Sprouting)
7.3.4. 3D Printing
8. Limitations for Human Consumption
8.1. Antinutritional Factors
8.2. Volatile Odorant Compounds
8.3. Non-Volatile Taste Compounds
8.4. Off-Flavour
8.5. Protein Bioactivity and Allergenicity
8.6. Technologies Used to Reduce Allergic Proteins
9. Food Application and Its Health Benefits
10. Concluding Remarks and Future Research Directions
Author Contributions
Funding
Conflicts of Interest
References
- Medeiros, F.; Aleman, R.S.; Gabríny, L.; You, S.W.; Hoskin, R.T.; Moncada, M. Current Status and Economic Prospects of Alternative Protein Sources for the Food Industry. Appl. Sci. 2024, 14, 3733. [Google Scholar] [CrossRef]
- Ehsani, M.; Westphalen, H.; Doan, H.; Lohi, A.; Abdelrasoul, A. Advancing Faba Bean Protein Purification Using Membrane Technology: Current State and Future Perspectives. J. Compos. Sci. 2024, 8, 15. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, P.R.; Hu, F.; Zhu, W.; Wei, Z.J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.N. Fava bean (Vicia faba L.) for food applications: From seed to ingredient processing and its effect on functional properties, antinutritional factors, flavor, and color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Liu, X.; Li, J.; Zeng, X.A.; Li, Y.; Yuan, Y.; Teng, Y.X. Pulse Protein Isolates as Competitive Food Ingredients: Origin, Composition, Functionalities, and the State-of-the-Art Manufacturing. Foods 2024, 13, 6. [Google Scholar] [CrossRef]
- Gulzar, S.; Martín-Belloso, O.; Soliva-Fortuny, R. Tailoring the Techno-Functional Properties of Fava Bean Protein Isolates: A Comparative Evaluation of Ultrasonication and Pulsed Electric Field Treatments. Foods 2024, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Karkehabadi, S.; Johansson, D.P.; Langton, M. Gelation behaviour and gel properties of the 7S and 11S globulin protein fractions from faba bean (Vicia faba var. minor) at different NaCl concentrations. Food Hydrocoll. 2023, 142, 108789. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Flavor Effects from Processing to Consumer Acceptability. Foods 2023, 12, 2237. [Google Scholar] [CrossRef]
- Vogelsang-O’dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Tachie, C.; Nwachukwu, I.D.; Aryee, A.N. Trends and innovations in the formulation of plant-based foods. Food Prod. Process. Nutr. 2023, 5, 16. [Google Scholar] [CrossRef]
- García-García, M.D.C.; Martín-Expósito, E.; Font, I.; Martínez-García, B.D.C.; Fernández, J.A.; Valenzuela, J.L.; Gómez, P.; Río-Celestino, M.D. Determination of Quality Parameters in Mangetout (Pisum sativum L. ssp. arvense) by Using Vis/Near-Infrared Reflectance Spectroscopy. Sensors 2022, 22, 4113. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and rheological properties of Vicia faba L. Protein isolates. Biomolecules 2021, 11, 178. [Google Scholar] [CrossRef]
- Karolkowski, A.; Belloir, C.; Lucchi, G.; Martin, C.; Bouzidi, E.; Levavasseur, L.; Salles, C.; Briand, L. Activation of bitter taste receptors by saponins and alkaloids identified in faba beans (Vicia faba L. minor). Food Chem. 2023, 426, 136548. [Google Scholar] [CrossRef]
- Angelis, D.D.; Latrofa, V.; Caponio, F.; Pasqualone, A.; Summo, C. Techno-functional properties of dry-fractionated plant-based proteins and application in food product development: A review. J. Sci. Food Agric. 2024, 104, 1884–1896. [Google Scholar] [CrossRef]
- Golovko, T.; Golovko, M.; Vasilenko, O.; Pertsevoi, F.; Bolgova, N.; Tischenko, V.; Prymenko, V. Technology of protein isolate from peas (Pisum sativum var. arvense). Technol. Audit Prod. Reserv. 2023, 2, 37–40. [Google Scholar] [CrossRef]
- Otero, D.M.; da Rocha Lemos Mendes, G.; da Silva Lucas, A.J.; Christ-Ribeiro, A.; Ribeiro, C.D.F. Exploring alternative protein sources: Evidence from patents and articles focusing on food markets. Food Chem. 2022, 394, 133486. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.H.; Liu, J.; Fitzsimons, S.M.; Fenelon, M.A.; Murphy, E.G. Determining the influence of fava bean pre-processing on extractability and functional quality of protein isolates. Food Chem. X 2024, 21, 101200. [Google Scholar] [CrossRef]
- Narale, B.A.; Mounika, A.; Shanmugam, A. Modifications of physicochemical, functional, structural, and nutritional properties of a field bean protein isolate obtained using batch and continuous ultrasound systems. Sustain. Food Technol. 2024, 2, 470–484. [Google Scholar] [CrossRef]
- Labba, I.C.M.; Frøkiær, H.; Sandberg, A.S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Abberton, M.; Oyatomi, O.A.; Odeyemi, O.; Babalola, O.O. Potentials of underutilized legumes in food security. Front. Soil Sci. 2022, 2, 1020193. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Patil, C.; Awasthi, A.; Paul, M. Antioxidative and antimicrobial properties of pulse proteins and their applications in gluten-free foods and sports nutrition. Int. J. Food Sci. Technol. 2022, 57, 5571–5584. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules 2023, 28, 5431. [Google Scholar] [CrossRef]
- Karolkowski, A.; Martin, C.; Bouzidi, E.; Albouy, J.F.; Levavasseur, L.; Briand, L.; Salles, C. Heat Treatment, Cultivar and Formulation Modify the Sensory Properties and Consumer Acceptability of Gels Containing Faba Bean (Vicia faba L. minor) Protein Concentrates. Foods 2022, 11, 3018. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legume Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Debnath, S.; Meetei, N.T.; Rai, M. Advancement in Genomics and Molecular Marker Technologies for Breeding of Faba Bean with Low Vicine-convicine Content: A Review. Legume Res. 2024, 47, 684–694. [Google Scholar] [CrossRef]
- Auer, J.; Östlund, J.; Nilsson, K.; Johansson, M.; Herneke, A.; Langton, M. Nordic Crops as Alternatives to Soy—An Overview of Nutritional, Sensory, and Functional Properties. Foods 2023, 12, 2607. [Google Scholar] [CrossRef]
- Helios, W.; Jama-Rodzeńska, A.; Serafin-Andrzejewska, M.; Kotecki, A.; Kozak, M.; Zarzycki, P.; Kuchar, L. Depth and sowing rate as factors affecting the development, plant density, height and yielding for two faba bean (Vicia faba L. var. minor) cultivars. Agriculture 2021, 11, 820. [Google Scholar] [CrossRef]
- Serafin-Andrzejewska, M.; Helios, W.; Jama-Rodzeńska, A.; Kotecki, A.; Kozak, M.; Zarzycki, P.; Kaliska, B. Effect of the depth and rate of sowing on the yield and yield components of determinate and indeterminate faba beans (Vicia faba var. minor L.) cultivars under conditions of Southwestern Poland. Agron. Sci. 2022, 77, 27–40. [Google Scholar] [CrossRef]
- Piotrowicz-Cieślak, A.I.; Krupka, M.; Michalczyk, D.J.; Smyk, B.; Grajek, H.; Podyma, W.; Głowacka, K. Physiological characteristics of field bean seeds (Vicia faba var. minor) subjected to 30 years of storage. Agriculture 2020, 10, 545. [Google Scholar] [CrossRef]
- Yehmed, J.; Tlahig, S.; Mohamed, A.; Yahia, H.; Lachiheb, B.; Yahia, L.B.; Loumerem, M. Nutritional and Phytochemical Profiling of Vicia faba L. var. minor Seeds: A Multifaceted Exploration of Natural Antioxidants and Functional Food Potential. Appl. Biochem. Biotechnol. 2024, 196, 8471–8492. [Google Scholar] [CrossRef] [PubMed]
- Karolkowski, A.; Gourrat, K.; Bouzidi, E.; Albouy, J.F.; Levavasseur, L.; Briand, L.; Guichard, E.; Salles, C. Origins of volatile compounds and identification of odour-active compounds in air-classified fractions of faba bean (Vicia faba L. minor). Food Res. Int. 2023, 163, 112260. [Google Scholar] [CrossRef] [PubMed]
- Karolkowski, A.; Meudec, E.; Bruguière, A.; Mitaine-Offer, A.C.; Bouzidi, E.; Levavasseur, L.; Sommerer, N.; Briand, L.; Salles, C. Faba Bean (Vicia faba L. minor) Bitterness: An Untargeted Metabolomic Approach to Highlight the Impact of the Non-Volatile Fraction. Metabolites 2023, 13, 964. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Sarri, E.; Samolada, S.M.; Katsileros, A.; Tomlekova, N.; Abraham, E.M.; Tani, E. Effect of gamma-Irradiation on the Growth and Yield Response of Three Varieties of Pea (Pisum spp.). Agronomy 2024, 14, 1695. [Google Scholar] [CrossRef]
- Rasskazova, I.; Kirse-Ozolina, A. Field pea Pisum sativum L. as a perspective ingredient for vegan foods: A review. In Proceedings of the Research for Rural Development, Jelgava, Latvia, 13–15 May 2020; Latvia University of Agriculture: Jelgava, Latvia, 2020; Volume 35, pp. 125–131. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E.; Martynenko, A.; Utioh, A.; Bhowmik, P. Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications. Foods 2023, 12, 3978. [Google Scholar] [CrossRef]
- Goswami, K.; Shukla, P. Field pea (Pisum sativum) varieties: Shelf life evaluation and product development. J. Pharmacogn. Phytochem. 2020, 9, 45–50. [Google Scholar]
- Wu, D.T.; Li, W.X.; Wan, J.J.; Hu, Y.C.; Gan, R.Y.; Zou, L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023, 12, 2527. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, C.S.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein- and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- Svanes, E.; Waalen, W.; Uhlen, A.K. Environmental impacts of field peas and faba beans grown in Norway and derived products, compared to other food protein sources. Sustain. Prod. Consum. 2022, 33, 756–766. [Google Scholar] [CrossRef]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and Quantification of Major Faba Bean Seed Proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]
- Augustin, M.A.; Cole, M.B. Towards a sustainable food system by design using faba bean protein as an example. Trends Food Sci. Technol. 2022, 125, 1–11. [Google Scholar] [CrossRef]
- Schmidt, F.; Blankart, M.; Wanger, J.; Scharfe, M.; Scheuerer, T.; Hinrichs, J. Upscaling of alkaline pea protein extraction from dry milled and pre-treated peas from laboratory to pilot scale: Optimization of process parameters for higher protein yields. J. Food Meas. Charact. 2022, 16, 4904–4913. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.M.; Tran, G. Tables de Composition et de Valeur Nutritive des Matières Premières Destinées aux Animaux D’élevage: Porcs, Volailles, Bovins, Ovins, Caprins, Lapins, Chevaux, Poissons; INRA, Association Fran caise de Zootechnie: Palaiseau, France, 2002; p. 301. [Google Scholar]
- Holopainen-Mantila, U.; Sarlin, T.; Mäkinen, O.; Laitila, A.; Sozer, N. Monitoring of early-stage water uptake by hyperspectral imaging and evaluation of nutritional and technological functionality of germinated faba bean (Vicia faba L.) var. minor and var. major as food ingredients. Legume Sci. 2022, 4, e124. [Google Scholar] [CrossRef]
- Haciseferoǧullari, H.; Gezer, I.; Bahtiyarca, Y.; Mengeş, H.O. Determination of some chemical and physical properties of Sakiz faba bean (Vicia faba L. var. major). J. Food Eng. 2003, 60, 475–479. [Google Scholar] [CrossRef]
- Goswami, K.; Shukla, P. Evaluation of improved varieties of field pea (Pisum sativum) for nutritional and functional quality. Int. J. Chem. Stud. 2019, 7, 2260–2266. [Google Scholar]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential health benefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Zduńczyk, Z.; Mikulski, D.; Jankowski, J.; Slominski, B.A.; Juśkiewicz, J. The effect of the dietary inclusion of pea seeds of colored-flowered and white-flowered varieties on gastrointestinal function in turkeys. Anim. Nutr. 2022, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Tulbek, M.C.; Lam, R.S.; Wang, Y.C.; Asavajaru, P.; Lam, A. Pea: A Sustainable Vegetable Protein Crop; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 145–164. [Google Scholar] [CrossRef]
- Pratap, V.; Sharma, V.; Kamaluddin; Shukla, G. Assessment of Genetic Variability and Relationship between Different Quantitative Traits in Field Pea (Pisum sativum var. arvense) Germplasm. Legume Res. 2024, 47, 905–910. [Google Scholar] [CrossRef]
- Haliloglu, K.; Turkoglu, A.; Tan, M.; Poczai, P. SSR-Based Molecular Identification and Population Structure Analysis for Forage Pea (Pisum sativum var. arvense L.) Landraces. Genes 2022, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Adamidou, S.; Nengas, I.; Grigorakis, K.; Nikolopoulou, D.; Jauncey, K. Chemical composition and antinutritional factors of field peas (Pisum sativum), chickpeas (Cicer arietinum), and faba beans (Vicia faba) as affected by extrusion preconditioning and drying temperatures. Cereal Chem. 2011, 88, 80–86. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Gawalko, E.J. Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem. 2008, 111, 132–138. [Google Scholar] [CrossRef]
- Ravindran, G.; Nalle, C.L.; Molan, A.; Ravindran, V. Nutritional and biochemical assessment of field peas (Pisum sativum L.) as a protein source in poultry diets. J. Poult. Sci. 2010, 47, 48–52. [Google Scholar] [CrossRef][Green Version]
- Amarakoon, D.; Thavarajah, D.; McPhee, K.; Thavarajah, P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: A potential food-based solution to global micronutrient malnutrition. J. Food Compos. Anal. 2012, 27, 8–13. [Google Scholar] [CrossRef]
- Thomsen, J.; Rao, J.; Chen, B. Faba bean protein: Chemical composition, functionality, volatile compounds, and applications in food production. Trends Food Sci. Technol. 2025, 156, 104863. [Google Scholar] [CrossRef]
- Michalczyk, D.J.; Krupka, M.; Kamiński, J.; Wierzbicka, M.; Floryańska, S.; Kopeć, W.; Piotrowicz-Cieślak, A.I. Physiological and Biochemical Parameters of Field Bean (Vicia faba var. minor) Seeds Stored for 33 Years. Agriculture 2023, 13, 2012. [Google Scholar] [CrossRef]
- Hejdysz, M.; Kaczmarek, S.A.; Rutkowski, A. Factors affecting the nutritional value of pea (Pisum sativum) for broilers. J. Anim. Feed Sci. 2015, 24, 252–259. [Google Scholar] [CrossRef]
- Neji, C.; Semwal, J.; Kamani, M.H.; Máthé, E.; Sipos, P. Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development. Processes 2022, 10, 2586. [Google Scholar] [CrossRef]
- Ayala-Rodríguez, V.A.; López-Hernández, A.A.; Lomelí, M.L.C.; González-Martínez, B.E.; Vázquez-Rodríguez, J.A. Nutritional quality of protein flours of fava bean (Vicia faba L.) and in vitro digestibility and bioaccesibility. Food Chem. X 2022, 14, 100303. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Singh, P.; Pandey, V.K.; Singh, R.; Rustagi, S. Exploring the significance of protein concentrate: A review on sources, extraction methods, and applications. Food Chem. Adv. 2024, 5, 100771. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Xia, S.; Cheong, L.; Tu, M. Recent progress in plant-based proteins: From extraction and modification methods to applications in the food industry. Food Chem. X 2024, 23, 101540. [Google Scholar] [CrossRef] [PubMed]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; Malalgoda, M.; Aluko, R.E.; Bandara, N. Novel Extraction technologies for developing plant protein ingredients with improved functionality. Trends Food Sci. Technol. 2022, 129, 492–511. [Google Scholar] [CrossRef]
- Sultan, Z.; Ashfaq, A.; Jahan, K.; Qadri, O.S.; Younis, K.; Yousuf, O. pH shift extraction technique for plant proteins: A promising technique for sustainable development. Energy Nexus 2024, 16, 100329. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Osman, A.I.; Abdelshafy, A.M.; Mo, J.; Chen, W. Plant-based proteins: Advanced extraction technologies, interactions, physicochemical and functional properties, food and related applications, and health benefits. Crit. Rev. Food Sci. Nutr. 2023, 65, 667–694. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Hansen, L.; Bu, F.; Ismail, B.P. Structure-Function Guided Extraction and Scale-Up of Pea Protein Isolate Production. Foods 2022, 11, 3773. [Google Scholar] [CrossRef]
- Emkani, M.; Moundanga, S.; Oliete, B.; Saurel, R. Protein composition and nutritional aspects of pea protein fractions obtained by a modified isoelectric precipitation method using fermentation. Front. Nutr. 2023, 10, 1284413. [Google Scholar] [CrossRef]
- Wen, C.; Liu, G.; Ren, J.; Deng, Q.; Xu, X.; Zhang, J. Current Progress in the Extraction, Functional Properties, Interaction with Polyphenols, and Application of Legume Protein. J. Agric. Food Chem. 2022, 70, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Beans Protein as an Unconventional Protein Source for the Food Industry: Processing Influence on Nutritional, Techno-Functionality, and Bioactivity. Food Rev. Int. 2024, 40, 1999–2023. [Google Scholar] [CrossRef]
- Pulivarthi, M.K.; Buenavista, R.M.; Bangar, S.P.; Li, Y.; Pordesimo, L.O.; Bean, S.R.; Siliveru, K. Dry fractionation process operations in the production of protein concentrates: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4670–4697. [Google Scholar] [CrossRef]
- Schutyser, M.; Novoa, S.C.; Wetterauw, K.; Politiek, R.; Wilms, P. Dry Fractionation for Sustainable Production of Functional, Nutritional and Palatable Grain Legume Protein Ingredients. Food Eng. Rev. 2025. [Google Scholar] [CrossRef]
- Stone, A.K.; Shi, D.; Marinangeli, C.P.F.; Carlin, J.; Nickerson, M.T. Current review of faba bean protein fractionation and its value-added utilization in foods. Sustain. Food Proteins 2024, 2, 101–124. [Google Scholar] [CrossRef]
- Cheng, S.; Langrish, T.A.G. A Review of the Treatments to Reduce Anti-Nutritional Factors and Fluidized Bed Drying of Pulses. Foods 2025, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Sharma, A.; Sarkar, P.K. Conventional and emerging processing techniques for the post-harvest reduction of antinutrients in edible legumes. Appl. Food Res. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Rashid, S.; Kaur, S.; Ali, N.; Chawla, P.; Sharma, M. Effect of Sustainable Pretreatments on the Nutritional and Functionality of Chickpea Protein: Implication for Innovative Food Product Development. J. Food Biochem. 2024, 2024, 5173736. [Google Scholar] [CrossRef]
- Rivera, J.; Siliveru, K.; Li, Y. A comprehensive review on pulse protein fractionation and extraction: Processes, functionality, and food applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 4179–4201. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6390–6420. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Rohtagi, B.; Khandelwal, A.; Shrivastava, M. Protein for Human Consumption From Oilseed Cakes: A Review. Front. Sustain. Food Syst. 2022, 6, 856401. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Lie-Piang, A.; Yang, J.; Schutyser, M.A.I.; Nikiforidis, C.V.; Boom, R.M. Mild Fractionation for More Sustainable Food Ingredients. Annu. Rev. Food Sci. Technol. 2024, 14, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Fernando, S. Pulse protein ingredient modification. J. Sci. Food Agric. 2022, 102, 892–897. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Industrial processing of chickpeas (Cicer arietinum) for protein production. Crop Sci. 2024, 65, e21361. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Ntone, E.; Meijers, M.G.; van den Hoek, I.A.; Sagis, L.M.; Venema, P.; Meinders, M.B.; Berton-Carabin, C.C.; Nikiforidis, C.V.; et al. Plant protein aggregates induced by extraction and fractionation processes: Impact on techno-functional properties. Food Hydrocoll. 2024, 155, 110223. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Kornet, R.; Yang, J.; Venema, P.; van der Linden, E.; Sagis, L.M. Optimizing pea protein fractionation to yield protein fractions with a high foaming and emulsifying capacity. Food Hydrocoll. 2022, 126, 107456. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Optimization of ultrasound-assisted extraction of faba bean protein isolate: Structural, functional, and thermal properties. Part 2/2. Ultrason. Sonochem. 2024, 110, 107030. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Yildiz, S.; Karaca, A.C.; Yemiş, O. Ultrasound and enzyme-pretreated extraction for the valorization of pea pod proteins. J. Food Process Eng. 2023, 46, e14452. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Jahan, K.; Ashfaq, A.; Younis, K.; Yousuf, O.; Islam, R.U. A review of the effects of ultrasound-assisted extraction factors on plant protein yield and functional properties. J. Food Meas. Charact. 2022, 16, 2875–2883. [Google Scholar] [CrossRef]
- Kibar, E.A.A.; Aslan, Ö. Ultrasound-Assisted Extraction of Chickpea Proteins and Their Functional and Technological Properties. Food Technol. Biotechnol. 2024, 62, 480–487. [Google Scholar] [CrossRef]
- Yildiz, S.; Karabulut, G.; Sıçramaz, H. High-intensity ultrasound-assisted extraction for functionalized pistachio meal protein concentrate. J. Food Sci. 2025, 90, e70031. [Google Scholar] [CrossRef]
- Kleekayai, T.; Khalesi, M.; Amigo-Benavent, M.; Cermeño, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Enzyme-Assisted Extraction of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Hernández-Álvarez, A.J., Mondor, M., Nosworthy, M.G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 131–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, R.; Xu, Z.; Jiang, L.; Sui, X. Recent advances in soy protein extraction technology. J. Am. Oil Chem. Soc. 2023, 100, 187–195. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Pea protein extraction assisted by lactic fermentation: Impact on protein profile and thermal properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Dangi, P.; Chaudhary, N.; Paul, A.; Prabha, S.; Kumar, R.; Poonia, A. Faba Bean Proteins: Extraction Methods, Properties and Applications. In Faba Bean: Chemistry, Properties and Functionality; Springer International Publishing: Cham, Switzerland, 2022; pp. 245–273. [Google Scholar] [CrossRef]
- Farshi, P.; Mirmohammadali, S.N.; Rajpurohit, B.; Smith, J.S.; Li, Y. Pea protein and starch: Functional properties and applications in edible films. J. Agric. Food Res. 2024, 15, 100927. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- D’Alessio, G.; Flamminii, F.; Faieta, M.; Prete, R.; Michele, A.D.; Pittia, P.; Mattia, C.D.D. High pressure homogenization to boost the technological functionality of native pea proteins. Curr. Res. Food Sci. 2023, 6, 100499. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Zotzel, J.; Stadtmüller, J.; Bonerz, D.; Aschoff, J.; Olsen, K.; Rinnan, Å.; Saint-Eve, A.; Maillard, M.N.; Orlien, V. Effect of industrial process conditions of fava bean (Vicia faba L.) concentrates on physico-chemical and functional properties. Innov. Food Sci. Emerg. Technol. 2022, 81, 103142. [Google Scholar] [CrossRef]
- Zha, F.; Rao, J.; Chen, B. Modification of pulse proteins for improved functionality and flavor profile: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.S.; Anjum, R.; Yang, Y.; Tu, M.; Zhang, T.; Pan, D.; Sun, Y.; Wu, Z. Recent trends in fermented plant-based analogues and products, bioactive peptides, and novel technologies-assisted fermentation. Trends Food Sci. Technol. 2024, 149, 104529. [Google Scholar] [CrossRef]
- Yang, J.; Lorenzetti, R.L.; Bing, D.; Zhang, S.; Lu, J.; Chen, L. Composition, functionalities, and digestibility of proteins from high protein and normal pea (Pisum sativum) genotypes. Sustain. Food Proteins 2023, 1, 4–15. [Google Scholar] [CrossRef]
- Oluwajuyitan, T.D.; Aluko, R.E. Structural and functional properties of fava bean albumin, globulin and glutelin protein fractions. Food Chem. X 2025, 25, 102104. [Google Scholar] [CrossRef]
- Messina, V.; Skylas, D.J.; Roberts, T.H.; Valtchev, P.; Whiteway, C.; Li, Z.; Hopf, A.; Dehghani, F.; Quail, K.J.; Kaiser, B.N. Pulse Proteins: Processing, Nutrition, and Functionality in Foods. Foods 2025, 14, 1151. [Google Scholar] [CrossRef]
- Shen, Y.; Du, Z.; Wu, X.; Li, Y. Modulating molecular interactions in pea protein to improve its functional properties. J. Agric. Food Res. 2022, 8, 100313. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; Goot, A.J.V.D. Modifying faba bean protein concentrate using dry heat to increase water holding capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef]
- Mesfin, N.; Belay, A.; Amare, E. Effect of germination, roasting, and variety on physicochemical, techno-functional, and antioxidant properties of chickpea (Cicer arietinum L.) protein isolate powder. Heliyon 2021, 7, e08081. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Beyrer, M. Impact of native pea proteins on the gelation properties of pea protein isolates. Food Struct. 2023, 37, 100340. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharan, S.; Rinnan, Å.; Orlien, V. Survey on Methods for Investigating Protein Functionality and Related Molecular Characteristics. Foods 2021, 10, 2848. [Google Scholar] [CrossRef]
- Samal, I.; Bhoi, T.K.; Raj, M.N.; Majhi, P.K.; Murmu, S.; Pradhan, A.K.; Kumar, D.; Paschapur, A.U.; Joshi, D.C.; Guru, P.N. Underutilized legumes: Nutrient status and advanced breeding approaches for qualitative and quantitative enhancement. Front. Nutr. 2023, 10, 1110750. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, F.; Adhikari, B. Recent advances in plant protein modification: Spotlight on hemp protein. Sustain. Food Technol. 2024, 2, 893–907. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, J.; Wang, Z.; Wang, Y.; Wang, X.; Chen, Z. Improving the Gelation Properties of Pea Protein Isolates Using Psyllium Husk Powder: Insight into the Underlying Mechanism. Foods 2024, 13, 3413. [Google Scholar] [CrossRef]
- Shi, D.; Nickerson, M.T. Comparative evaluation of the functionality of faba bean protein isolates with major legume proteins in the market. Cereal Chem. 2022, 99, 1246–1260. [Google Scholar] [CrossRef]
- Keivaninahr, F.; Gadkari, P.; Zoroufchi Benis, K.; Tulbek, M.; Ghosh, S. Prediction of emulsification behaviour of pea and faba bean protein concentrates and isolates from structure–functionality analysis. RSC Adv. 2021, 11, 12117–12135. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Pradhan, P.C.; Bhushan, B.; et al. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ahmed, M.A.; Verma, A.K.; Umaraw, P.; Mehta, N.; Abubakar, A.A.; Hayat, M.N.; Kaka, U.; Lee, S.J.; et al. Technological interventions in improving the functionality of proteins during processing of meat analogs. Front. Nutr. 2022, 9, 1044024. [Google Scholar] [CrossRef]
- Zhang, W.; Boateng, I.D.; Xu, J.; Zhang, Y. Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods 2024, 13, 1974. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Z.; Liu, Y. Effects of modification on plant protein digestion and absorption. Food Biosci. 2025, 63, 105761. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Xue, F. Effects of high-pressure homogenization treatment on physiochemical properties of novel plant proteins. Appl. Food Res. 2023, 3, 100285. [Google Scholar] [CrossRef]
- Liu, C.; Pei, R.; Heinonen, M. Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022, 369, 130879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, Q.; Chen, J. Effect of dry- and moist-heat treatment processes on the structure, solubility, and in vitro digestion of macadamia protein isolate. J. Food Sci. 2024, 89, 4671–4687. [Google Scholar] [CrossRef]
- Paramita, V.D.; Panyoyai, N.; Kasapis, S. Molecular functionality of plant proteins from low-to high-solid systems with ligand and co-solute. Int. J. Mol. Sci. 2020, 21, 2550. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Castagnini, J.M.; Berrada, H.; Barba, F.J. Pulsed electric field (PEF) recovery of biomolecules from Chlorella: Extract efficiency, nutrient relative value, and algae morphology analysis. Food Chem. 2023, 404, 134615. [Google Scholar] [CrossRef]
- Sim, S.Y.; Hua, X.Y.; Henry, C.J. A novel approach to structure plant-based yogurts using high pressure processing. Foods 2020, 9, 1126. [Google Scholar] [CrossRef]
- Ma, X.; Feng, R.; Ahrné, L.; Orlien, V. Pressure-induced gelation of blended milk and pea protein suspensions. Food Hydrocoll. 2024, 146, 109284. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of Legumes in Extrusion Cooking: A Review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Guerrero, M.; Stone, A.K.; Singh, R.; Lui, Y.C.; Koksel, F.; Nickerson, M.T. Effect of Extrusion Conditions on the Characteristics of Texturized Vegetable Protein from a Faba Bean Protein Mix and Its Application in Vegan and Hybrid Burgers. Foods 2025, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Nyaisaba, B.M.; Koddy, J.K.; Chen, M.; Hatab, S.; Deng, S. Effect of cold atmospheric plasma on the physicochemical and functional properties of myofibrillar protein from Alaska pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2020, 55, 517–525. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, M.D.P.P.; Caracciolo, P.C.; Abraham, G.A. Latest advances in electrospun plant-derived protein scaffolds for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 18, 100243. [Google Scholar] [CrossRef]
- Aghababaei, F.; McClements, D.J.; Martinez, M.M.; Hadidi, M. Electrospun plant protein-based nanofibers in food packaging. Food Chem. 2024, 432, 137236. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional properties and structural characteristics of phosphorylated pea protein isolate. Int. J. Food Sci. Technol. 2020, 55, 2002–2010. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ezeocha, V.C.; Adedeji, O.E.; Jolayemi, O.S.; Onwuka, Q.I.; Ilowefah, M.A.; Adebo, J.A.; Rosell, C.M.; Bamidele, O.P.; Adebo, O.A. Germinated/fermented legume flours as functional ingredients in wheat-based bread: A review. J. Food Sci. 2025, 90, e70022. [Google Scholar] [CrossRef]
- Du, T.; Huang, J.; Xu, X.; Xiong, S.; Zhang, L.; Xu, Y.; Zhao, X.; Huang, T.; Xiao, M.; Xiong, T.; et al. Effects of fermentation with Lactiplantibacillus plantarum NCU116 on the antihypertensive activity and protein structure of black sesame seed. Int. J. Biol. Macromol. 2024, 262, 129811. [Google Scholar] [CrossRef] [PubMed]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, D.; Torley, P.J.; Chandrapala, J.; Terefe, N.S. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation 2023, 9, 635. [Google Scholar] [CrossRef]
- Hernández-López, I.; Ortiz-Solà, J.; Alamprese, C.; Barros, L.; Shelef, O.; Basheer, L.; Rivera, A.; Abadias, M.; Aguiló-Aguayo, I. Valorization of Local Legumes and Nuts as Key Components of the Mediterranean Diet. Foods 2022, 11, 3858. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Shi, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. LWT 2021, 150, 111927. [Google Scholar] [CrossRef]
- Youssef, C.E.; Bonnarme, P.; Fraud, S.; Péron, A.C.; Helinck, S.; Landaud, S. Sensory improvement of a pea protein-based product using microbial co-cultures of lactic acid bacteria and yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef]
- Narciso, J.O.; Gulzar, S.; Soliva-Fortuny, R.; Martín-Belloso, O. Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients. Foods 2024, 13, 2180. [Google Scholar] [CrossRef]
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. The potentials and challenges of using fermentation to improve the sensory quality of plant-based meat analogs. Front. Microbiol. 2023, 14, 1267227. [Google Scholar] [CrossRef]
- He, Y.; Deng, Z.; Chai, T.; Yang, M.; Liu, J.; Liu, H. Germination affects structural and techno-functional properties of proteins from quinoa seeds with increased realease of antioxidant peptides by gastrointestinal digestion. Food Chem. 2025, 469, 142532. [Google Scholar] [CrossRef]
- Bagarinao, N.C.; King, J.; Leong, S.Y.; Agyei, D.; Sutton, K.; Oey, I. Effect of Germination on Seed Protein Quality and Secondary Metabolites and Potential Modulation by Pulsed Electric Field Treatment. Foods 2024, 13, 1598. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.; Sagis, L.M.; Bitter, J.H.; Nikiforidis, C.V. Jammed Emulsions with Adhesive Pea Protein Particles for Elastoplastic Edible 3D Printed Materials. Adv. Funct. Mater. 2021, 31, 2101749. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, R.K. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legume Sci. 2022, 4, e111. [Google Scholar] [CrossRef]
- Pandey, P.K.; Bhowmik, P.; Kagale, S. Optimized methods for random and targeted mutagenesis in field pea (Pisum sativum L.). Front. Plant Sci. 2022, 13, 995542. [Google Scholar] [CrossRef] [PubMed]
- Rajhi, I.; Boulaaba, M.; Baccouri, B.; Rajhi, F.; Hammami, J.; Barhoumi, F.; Flamini, G.; Mhadhbi, H. Assessment of dehulling effect on volatiles, phenolic compounds and antioxidant activities of faba bean seeds and flours. S. Afr. J. Bot. 2022, 147, 741–753. [Google Scholar] [CrossRef]
- Akkad, R.; Buchko, A.; Johnston, S.P.; Han, J.; House, J.D.; Curtis, J.M. Sprouting improves the flavour quality of faba bean flours. Food Chem. 2021, 364, 130355. [Google Scholar] [CrossRef]
- Krause, M.; Sørensen, J.C.; Petersen, I.L.; Duque-Estrada, P.; Cappello, C.; Tlais, A.Z.A.; Cagno, R.D.; Ispiryan, L.; Sahin, A.W.; Arendt, E.K.; et al. Associating Compositional, Nutritional and Techno-Functional Characteristics of Faba Bean (Vicia faba L.) Protein Isolates and Their Production Side-Streams with Potential Food Applications. Foods 2023, 12, 919. [Google Scholar] [CrossRef]
- Akgun, D.; Canci, H. Selection of Faba Bean (Vicia faba L.) Genotypes for High Yield, Essential Amino Acids and Low Anti-Nutritional Factors. Agriculture 2023, 13, 932. [Google Scholar] [CrossRef]
- Rajpurohit, B.; Li, Y. Overview on pulse proteins for future foods: Ingredient development and novel applications. J. Future Foods 2023, 3, 340–356. [Google Scholar] [CrossRef]

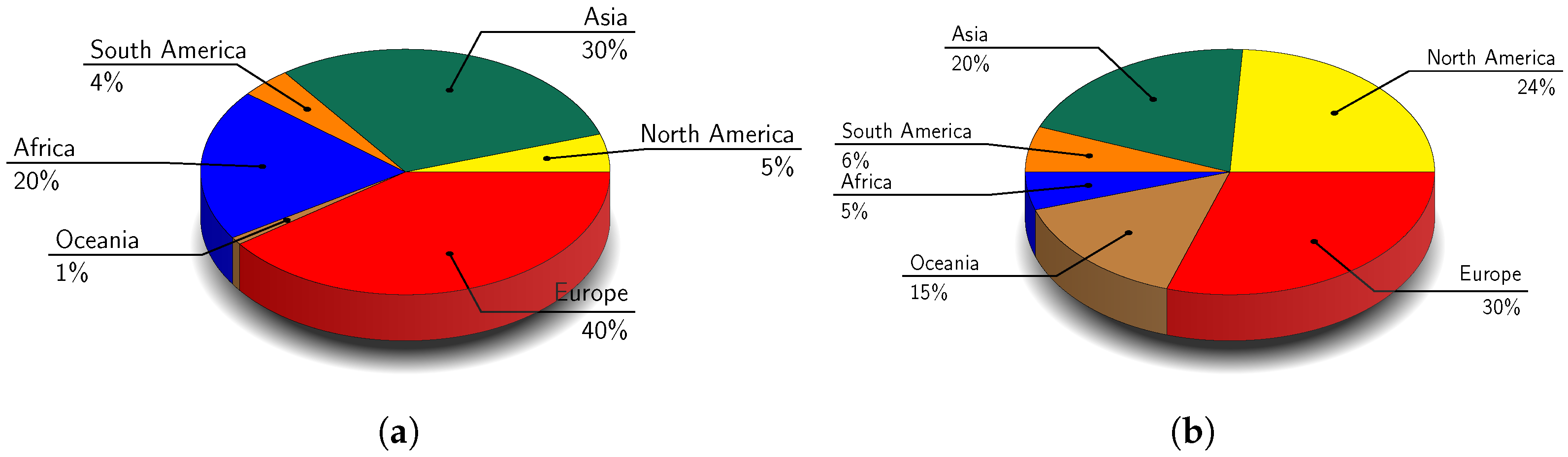
| Pulse | Protein | Ash | Fat | Fibre | Carbohydrates | Energy (kJ/100 g) | Moisture | References |
|---|---|---|---|---|---|---|---|---|
| Field bean | 22.7–28.3 | 2.72–3.41 | 1.0–2.0 | 11.37–16.59 | NR | NR | NR | [24] |
| 30.4–31.6 | NR | NR | NR | 51.3–51.9 | NR | NR | [47] | |
| 25.78–29.13 | NR | 2.52–2.61 | NR | NR | NR | NR | [36] | |
| 25.4–26.8 | 3.3–3.6 | 1.1–1.3 | 7.9–7.5 | 41.0–41.3 | 1610.0–1620.0 | NR | [49] | |
| 35.3 | NR | NR | NR | 43.2 | NR | NR | [50] | |
| 29.63 | 2.90 | 1.06 | NR | NR | 451.0 | NR | [51] | |
| Field pea | 19.21 | 3.41 | 1.61 | NR | NR | NR | 75.60 | [19] b |
| 16.14–20.32 | 2.86–3.22 | 0.90–2.17 | 1.56–3.39 | 58.46–64.08 | 1376.5–1418.4 | 12.31–13.44 | [52] | |
| 23.0 | 3.0 | 4.0 | NR | 56.0 | NR | NR | [44] | |
| 20.0–25.0 | NR | NR | 10.0–20.0 | 40.0–50.0 | NR | NR | [53] | |
| 20.5–22.6 | 3.2 | 2.0–3.0 | 2.5 | 17.0–22.0 | NR | NR | [54] | |
| 20.37 | 2.3 | 1.57 | 8.72 | 51.16 | NR | NR | [55] | |
| 21.0–24.0 | 1.9–2.2 | 1.5–2.0 | NR | 42.0–46.0 | NR | 10.0–15.0 | [56] |
| Field Bean | Field Pea | ||||
|---|---|---|---|---|---|
| References | |||||
| Minerals and Vitamins | [24] | [49] | [51] | [19] | [13] b |
| Ca | NR | 140 | 72.86 | 26 | NR |
| Fe | 1.8–21.3 | 5.9–7.3 | 8.92 | 1.57 | NR |
| Zn | 0.9–5.2 | 3.1 | 5.28 | 1.54 | NR |
| Mg | NR | 160.0–170.0 | 142.63 | 35 | NR |
| Mn | NR | 0.7 | 1.59 | 0.46 | NR |
| K | NR | 980.0–1000.0 | 1548.61 | 255 | NR |
| P | NR | 460.0–470.0 | 654.55 | 110 | NR |
| Cu | NR | 1.1–1.2 | NR | 0.196 | NR |
| Vitamin C | NR | NR | NR | NR | 43.82 |
| Vitamin E | NR | 0.5 | NR | NR | NR |
| Vitamin B1 | NR | 0.5 | NR | NR | NR |
| Vitamin B2 | NR | 0.3 | NR | NR | NR |
| Niacin | NR | 2.5 | NR | NR | NR |
| Pantothenic acid | NR | 0.3 | NR | NR | NR |
| Choline | NR | NR | NR | NR | |
| Extraction Methods | Advantages | Limitations | Effect on Techno-Functional Properties | Nutritional Value | Applications in Food Industry | Future Perspectives & Research Trends | Examples | References |
|---|---|---|---|---|---|---|---|---|
| Dry Fractionation (Air Classification & Size Reduction) | Maintains native protein functionality. Energy-efficient, chemical-free treatment. Clean-label process. | Lower protein purity (50–60%). Limited protein solubility and functionality. Off-flavours and relatively high level of antinutritional factors. Particle-particle collisions may happen. | Higher emulsification and foaming and water-holding capacity. Low solubility. | Preserves bioactive compounds, micronutrients and fibre content. Moderate protein yield. | Pea protein concentrates. Protein-enriched bakery and snack products. | Improving solubility through milling/thermal treatment. Exploring hybrid methods combining air classification/electrostatic, or dry/wet techniques | Common for producing protein concentrates. Pea and faba bean protein concentrate, lentil protein flour | [10,14,41,79,80] |
| Wet Fractionation (Alkaline Extraction/Isoelectric Precipitation—AEIEP) | High protein purity (up to 90%) and high yield. Little off-flavours and a low level of antinutritional factors. Low fat content. | Intensive use of energy and water. Chemical use may denature proteins or affect native functionality. Loss of micronutirnts. | High solubility at specific pH levels. Improved emulsifying and gelling properties. | High protein content but potential loss of heat-sensitive nutrients (e.g., vitamins) High digestibility and bioavailability | Plant-based protein isolates for dairy/meat alternatives. Beverages, protein bars. | Reducing chemical use, optimizing protein extraction yield. Researching cost-effective, eco-friendly extraction methods. | Common for producing protein isolates. Fava bean, pea and soy protein isolate | [10,41,75,78,79] |
| Salt Extraction | Produces high-purity protein (80–90%). Reduce processing time and cost. Reduce solvent consumption | Aggregation of protein may occur. Low protein extraction rate and purity. Water-intensive and high waste streams. | High solubility and binding properties of proteins to improve texture. Increasing hydration and water oil binding and foaming capacity. | Retains essential amino acids but may lose minerals due to dialysis. | Functional foods and beverages, and specialized food products with high bioactivity. | Research into cost-effective and scalable salt extraction methods. Exploring protein-specific optimization. | Pea, fava bean, chickpea, and lentil protein isolate. | [10,41,83,88] |
| Ultrafiltration | Relatively simple and produce high purity and quality (90–95%). Maintains native protein structure. Minimal product degradation. | Fouling and concentration polarization challenges occur and cause low efficiency. Time-consuming and high operational cost. High level of antinutritional factors. | High solubility, emulsifying, foaming and gelling functionality. Improved water absorption and oil binding capacity. | Retains bioactive peptides and functional proteins. | High-functional protein products for beverages and protein supplements. | Researching to address fouling and concentration polarization challenges. | Pea, chickpea and lentil protein isolates. | [41,76,83] |
| Ultrasound-Assisted Extraction | Enhances protein yield and extraction efficiency. Reduces extraction time and energy. | Expensive equipment. Potential for protein denaturation and reduce the protein concentration if conditions are not optimized. | Enhances solubility, emulsification, foaming and oil holding capacity. Shortens extraction time. | Improves digestibility and reduces anti-nutritional factors. | Applied for protein enrichment in beverages and bakery products. | Scaling up and optimizing ultrasound settings for improved yield. Research on protein bioactivity preservation. | Ultrasound-extracted pea and faba bean proteins. | [68,98,99] |
| Enzyme-Assisted Extraction | Increases the protein yield and purity. Low energy consumption and decreased waste formation. Reduces anti-nutritional factors. | High enzyme costs. Risk of incomplete protein extraction if not optimized. | Improves solubility and emulsification. | Enhances protein digestibility. Increases bioactive peptides. | Functional protein ingredients for nutraceutical and functional food products. | Exploring enzyme blends to maximize extraction efficiency. Optimizing conditions to enhance protein functionality. | Lupin and soybean protein extraction. | [68,98,102] |
| Field Bean | Field Pea | |||
|---|---|---|---|---|
| References | ||||
| Essential Amino Acids | [24] | [49] | [19] | [53] |
| Lysine | 44.8–74.8 | 16.6–17.2 | 3.2 | 47 |
| Threonine | 26.6–38.0 | 9.1–9.5 | 2.2 | 25 |
| Leucine | 50.8–72.1 | 19.3–20.3 | 3.3 | 57 |
| Isoleucine | 20.7–33.1 | 10.3–10.9 | 2 | 23 |
| Methionine | NR | 1.8–1.9 | 0.9 | 3 |
| Phenylalanine | 23.0–36.6 | 10.7–11.2 | 2.1 | 37 |
| Valine | 32.9–42.3 | 11.5–12.1 | 2.4 | NR |
| Histidine | 13.3–35.9 | 6.4–6.8 | 1.1 | 16 |
| Non-Essential Amino Acids | ||||
| Alanine | 45.6–57.9 | 10.5–11.0 | 0.24 | NR |
| Asparginine | 84.1–120.5 | 30.4–32.2 | NR | NR |
| Aspartic Acid | 86.4–109.5 | NR | 5.00 | NR |
| Glutamic Acid | 125.6–187.9 | 44.8–47.2 | 7.5 | NR |
| Arginine | 52.1–121.0 | 24.1–26.1 | 4.3 | NR |
| Proline | 32.9–48.2 | 10.5–11.1 | 1.8 | NR |
| Serine | 39.5–52.1 | 12.9–13.6 | 1.9 | NR |
| Tyrosine | 18.6–41.5 | 8.1–8.5 | 1.5 | NR |
| Fatty Acids (mg/g) | [36] | [49] | ||
| Palmitic acid C16:0 | NR | 1.5–1.8 | ||
| Stearic acid C18:0 | NR | 0.2–0.3 | ||
| Oleic d | 3.91–4.52 | 2.3–2.8 | ||
| Omega-3 (-Linolenic acid) | 7.31–9.02 | 4.4–5.3 | ||
| Omega-6 (Linoleic acid) | 0.41–0.58 | 0.3–0.4 | ||
| Modification Technique | Advantages | Limitations | Effect on Techno-Functional Properties | Examples | References |
|---|---|---|---|---|---|
| Physical Modification | |||||
| Thermal Treatment | Denatures proteins, improves digestibility and reduces anti-nutritional factors | May cause loss of sensitive amino acids and bioactive compounds | Enhances emulsification, solubility, water-holding capacity, foaming and gelling | Heating of faba bean, kidney bean and pea proteins | [76,77,125,130] |
| Extrusion | Reduce heat-labile ANFs, increase protein and starch digestibility | Nutritional loss | Enhances gelation and water-holding capacity | Pea, faba bean, and soy protein, chickpea flour | [31,66,90,126,139] |
| High-pressure Processing | Enhances protein solubility, emulsification, and gelation | Expensive and may not eliminate all anti-nutritional factors | Improves solubility, gel formation, foaming and protein structure | HPP-treated fava bean, lentil protein | [21,103,130] |
| Ultrasonication | Reduces particle size, improves solubility and foaming ability | Limited impact on anti-nutritional compounds, high-energy consumption | Enhances emulsification, foaming, and solubility | Ultrasound-treated faba bean, lentil protein | [2,21,24,43] |
| Cold plasma | Microbial inactivation, quick processing and no thermal damage | May affect the overall flavour and colour | Enhances solubility, gelling properties and water-binding capacity | CP-modified pea protein | [22,90,119] |
| Chemical Modification | |||||
| pH shifting treatment | Solubilizes proteins, improves emulsifying properties | Degrades amino acids, may create off-fflavours, and reduce nutritional quality | Alters solubility, emulsion stability, foaming, and protein structure | Alkali-modified pea and soy proteins | [2,47,71,127] |
| Glycation (Maillard Reaction) | Minimize adverse effects on flavour and colour, improves digestibility and inactivate enzyme inhibitors | Potential to form harmful advanced glycation end products | Improves solubility, emulsification, and thermal stability | Glycated pea, soy and yellow pea proteins | [110,127] |
| Phosphorylation | Improves digestibility | Less desirable for food applications, possibility of toxicity of residues | Improves emulsifying, solubility, gelling, foaming, and oil absorption ability | Phosphorylated pea and soy protein isolate | [90,127,145] |
| Acetylation and Succinylation | Improves water solubility, emulsifying properties. | Chemical reagents may leave residues and alter protein structure | Alters surface properties, increasing solubility and functional properties like foaming and emulsifying ability | Applied in mung bean, pea and faba bean proteins | [90,110] |
| Deamidation | Enhance the nutritional quality and sensory properties | Chemical reagents may leave residues | Improves solubility, emulsifying capacity | Deamidated pea and soy protein | [10,90,110] |
| Biological Modification | |||||
| Fermentation | Increase bioavailability, producing bioactive peptides, enhances flavour, reduces anti-nutritional compounds | Time-consuming and requires specialized microbes | Improves solubility, oil-holding capacity, and foaming properties | Fermented lupin, pea, and faba bean proteins | [90,153,154] |
| Enzyme treatment | Reduce anti-nutritional factors like off-flavours, improves nutritional value and sensory properties, and produce bioactive peptides | Complex processing, high enzyme and energy cost | Improves solubility, emulsification, oil absorption, foaming and bioactive peptide content | Enzyme treated pea and faba bean proteins | [5,9,69,121] |
| Germination, Sprouting | Reduces anti-nutritional factors, enhances digestibility and improves nutritional value | Limited large-scale applicability, variability in results | Enhances water-binding capacity, solubility, foam stability and gelling | Germinated chickpea, faba beans and lentils | [69,156] |
| Limitation | Field Pea | Field Bean | Details | References |
|---|---|---|---|---|
| Antinutritional factors | Contains phytates, tannins, trypsin inhibitors, oxalates, and saponins, lectins, and etc. | Contains tannins, lectins, trypsin inhibitors, vicine and convicine, which can cause favism (especially in individuals with G6PD deficiency). | These compounds can reduce the bioavailability of iron, calcium, and other essential nutrients and protein digestion. | [12,14,21,75,107] |
| Allergenic potential | Low but possible allergenic potential, particularly in individuals sensitive to legume proteins. | Potential allergenicity, especially in individuals with legume allergies or faba bean sensitivities. | Field pea proteins have a lower allergenic risk compared to other legumes, but may still trigger reactions. | [9,21,93] |
| Digestibility | Moderate digestibility due to the presence of fibres and antinutritional factors. | Lower digestibility because of high levels of fibres, tannins, and lectins. | Pea proteins may cause bloating and indigestion due to fibre content. | [21,53,77] |
| Flavour and sensory issues | Grassy, beany flavours may be undesirable in certain food applications. | Strong bitter and astringent flavours limit consumer acceptability | Both proteins require flavour masking or removal strategies in processed foods. | [14,17,36] |
| Gastrointestinal effects | High fibre content can lead to flatulence, bloating, and gas formation. | Can cause gastrointestinal discomfort, such as bloating, especially in sensitive individuals. | Enzyme treatments or fermentation can help reduce these effects. | [8,9,15,24,75] |
| Vicine and convicine (Favism risk) | Not present in field pea, so no risk of causing favism. | Contains vicine and convicine, which can trigger favism in genetically predisposed individuals. | Favism occurs due to the consumption of fava beans by individuals with G6PD deficiency. | [16,21,164,165] |
| Heat-induced changes | Proteins may undergo denaturation during high-temperature processing, reducing solubility and functionality. | Field bean proteins may lose functionality under excessive heat, affecting food texture. | Both proteins are heat-sensitive and may require mild heat processing to retain functionality. | [27,76,130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiruneh, A.; Ptaszek, P.; Żmudziński, D.; Tarko, T. Peas (Pisum sativum subsp. arvense Asch) and Beans (Vicia faba var. minor) as Source of Quality Plant Proteins. Molecules 2025, 30, 2009. https://doi.org/10.3390/molecules30092009
Tiruneh A, Ptaszek P, Żmudziński D, Tarko T. Peas (Pisum sativum subsp. arvense Asch) and Beans (Vicia faba var. minor) as Source of Quality Plant Proteins. Molecules. 2025; 30(9):2009. https://doi.org/10.3390/molecules30092009
Chicago/Turabian StyleTiruneh, Abebaw, Paweł Ptaszek, Daniel Żmudziński, and Tomasz Tarko. 2025. "Peas (Pisum sativum subsp. arvense Asch) and Beans (Vicia faba var. minor) as Source of Quality Plant Proteins" Molecules 30, no. 9: 2009. https://doi.org/10.3390/molecules30092009
APA StyleTiruneh, A., Ptaszek, P., Żmudziński, D., & Tarko, T. (2025). Peas (Pisum sativum subsp. arvense Asch) and Beans (Vicia faba var. minor) as Source of Quality Plant Proteins. Molecules, 30(9), 2009. https://doi.org/10.3390/molecules30092009









