Abstract
AgNPs have gained significant attention due to their unique physicochemical properties, making them valuable across a range of fields including medicine, textiles, and household products. With their strong antimicrobial and antiviral properties, AgNPs have shown promise in treating infections, particularly in wound care management. This review explores the mechanisms underlying the antiviral activities of AgNPs, as well as the methods used for their synthesis, which include physical, chemical, and biological approaches. The review also addresses the potential limitations of AgNPs, including their cytotoxicity to humans and the environment. The interaction between AgNPs and microorganisms, particularly viruses, varies based on synthesis methods and particle morphology. As viral infections, including resistant strains, present major global health challenges, there is a growing need for alternative antiviral therapies. Metal nanoparticles like AgNPs offer potential advantages over conventional antiviral drugs due to their broad target range, which reduces the likelihood of resistance development. This review highlights AgNPs’ effectiveness against a variety of viruses, such as HIV, hepatitis B, and respiratory syncytial virus, and discusses their potential for use in novel antiviral treatments. The review also examines AgNPs’ toxicity, offering insights into their future therapeutic roles.
1. Introduction
Viruses emerge due to changes in hosts, environments, or vectors, with several becoming widespread in humans, such as SARS, Nipah, and Chikungunya. Despite advances in detection, prevention, and treatment lag behind, with antiviral resistance posing challenges. New and recurring viruses continue to threaten public health, infecting humans, animals, and plants, leading to disease outbreaks, economic strain, and loss of life. Nanotechnology, an interdisciplinary field, offers promising solutions, particularly through metal-based nanoparticles with unique physico-chemical properties [1,2,3]. Their controlled size, shape, and surface area enable antiviral applications. While widely used in electronics and materials science, nanotechnology’s role in medicine is rapidly expanding, opening new avenues for antiviral development and disease control [4,5]. Nonetheless, the list of viral diseases for which antiviral therapies or vaccines are available is still relatively short and some viruses are developing resistance to current therapies [6]. Over the past ten years, nanotechnology has emerged as a promising approach to combat viruses. AgNPs, known for their broad-spectrum antimicrobial properties, have gained significant interest in the scientific community [7].
AgNPs exhibit antimicrobial activity in many ways and there are multiple mechanisms reported so far in the literature [6]. These mechanisms rely on multiple factors such as size, shape of nanoparticles, pH, ionic strength of media, etc. Moreover, the role of the capping agent has also proven a crucial factor. The release of the silver ions (Ag+) from AgNPs is considered a prominent mechanism behind the antimicrobial potential of AgNPs. The positively charged silver ions, in particular, are crucial for antibacterial activities and should remain in the same ionized state. Moreover, such charged ions form complexes with nucleic acids and thus, interact with nucleosides. In some cases, negatively charged cells were also attracted towards Ag+ from AgNPs. Literature is also available stating the adherence of Ag+ ions to the cytoplasm, due to affinity towards such sulfur proteins. Moreover, such free ions tend to deactivate the respiratory enzymes in the cells, giving rise to reactive oxygen species (ROS). The generations of ROS lead to DNA damage. Furthermore, it was also noted that such ROS stops the protein synthesis mechanisms. Another mechanism could also include the modulation of cellular signaling. Apart from ions, AgNPs themselves brings protein denaturation leading to microbial deaths. However, antimicrobial resistance to silver NPs is a key limitation.
Variations in chemical composition, morphology, particle size, and dispersity play a crucial role in determining the physicochemical properties of nanoparticles (NPs). These variations mainly arise from the synthesis process, which is governed by several key factors. Contemporary nanoparticle production methods focus not only on attaining accurate nanoscale dimensions but also on maintaining efficiency, cost-effectiveness, environmental sustainability, and suitability for diverse industrial and scientific applications [8,9,10,11]. Their potential applications span various biomedical fields, with sizes typically ranging from 1 to 100 nanometers [12]. There are numerous techniques available for synthesizing AgNPs, including chemical, physical, and biological methods, each of which can greatly impact their size, structure, and characteristics. However, various parameters such as dispersing agents, surfactants, and temperature can be regulated to produce AgNPs with specific sizes and properties [12]. AgNPs demonstrate significant antiviral capabilities by engaging with viral particles and preventing their replication. Their effectiveness stems from their surface characteristics, enabling them to bind to viral membranes and disrupt their structural stability. Studies have shown that AgNPs can effectively neutralize viruses like influenza and herpes simplex by interfering with viral proteins and inhibiting their entry into host cells. However, further research is necessary to confirm their safety for use in biological systems [13].
2. Synthesis
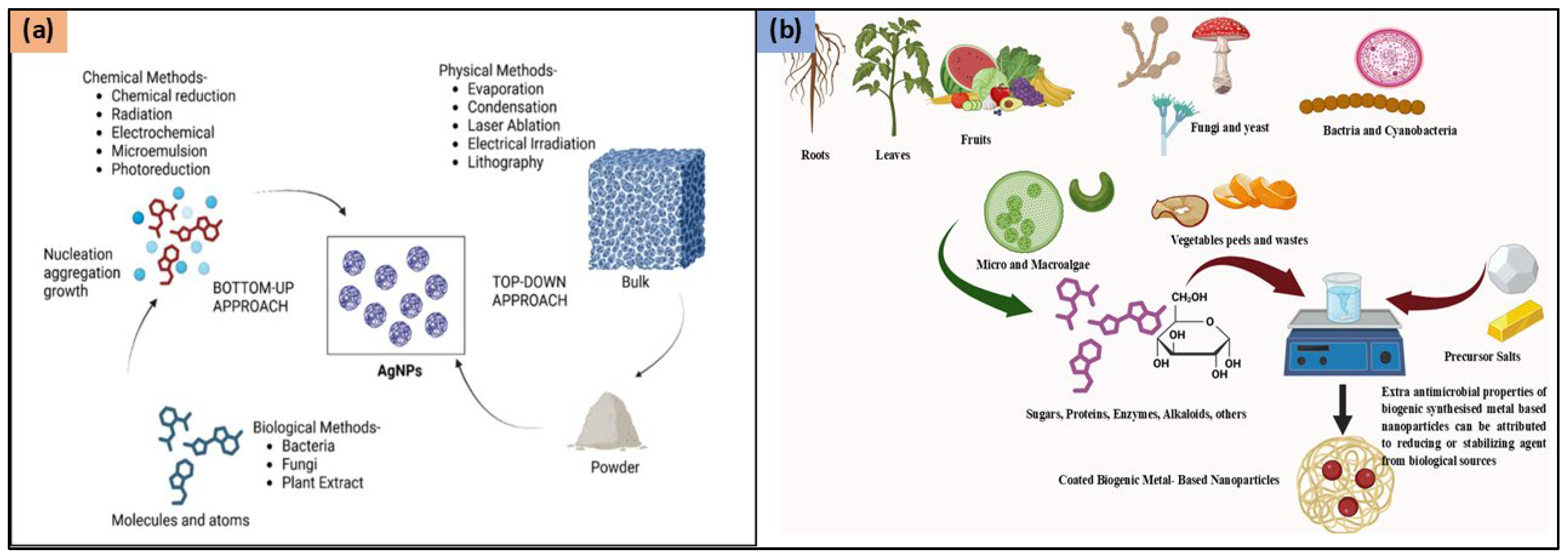
Physical, chemical, and biological approaches can be utilized for the synthesis of AgNPs (Figure 1). The process of synthesizing AgNPs includes the reduction of silver ions (Ag+) into metallic silver (Ag⁰), followed by nucleation and subsequent growth, influencing nanoparticle size, shape, and properties. The chosen synthesis method impacts the morphology and characteristics of the resulting AgNPs.
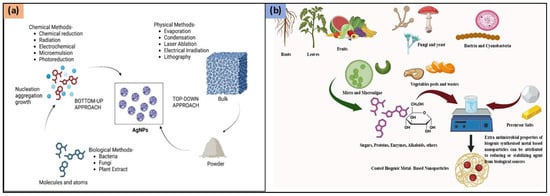
Figure 1.
Synthesis methods for AgNPs (a) Chemical, Physical, and Biological Methods and (b) Various biological sources for ‘Green synthesis’ of AgNPs. Figure 1a reproduced from Ref. [14], Copyright 2025, American Chemical Society.
2.1. Physical Methods
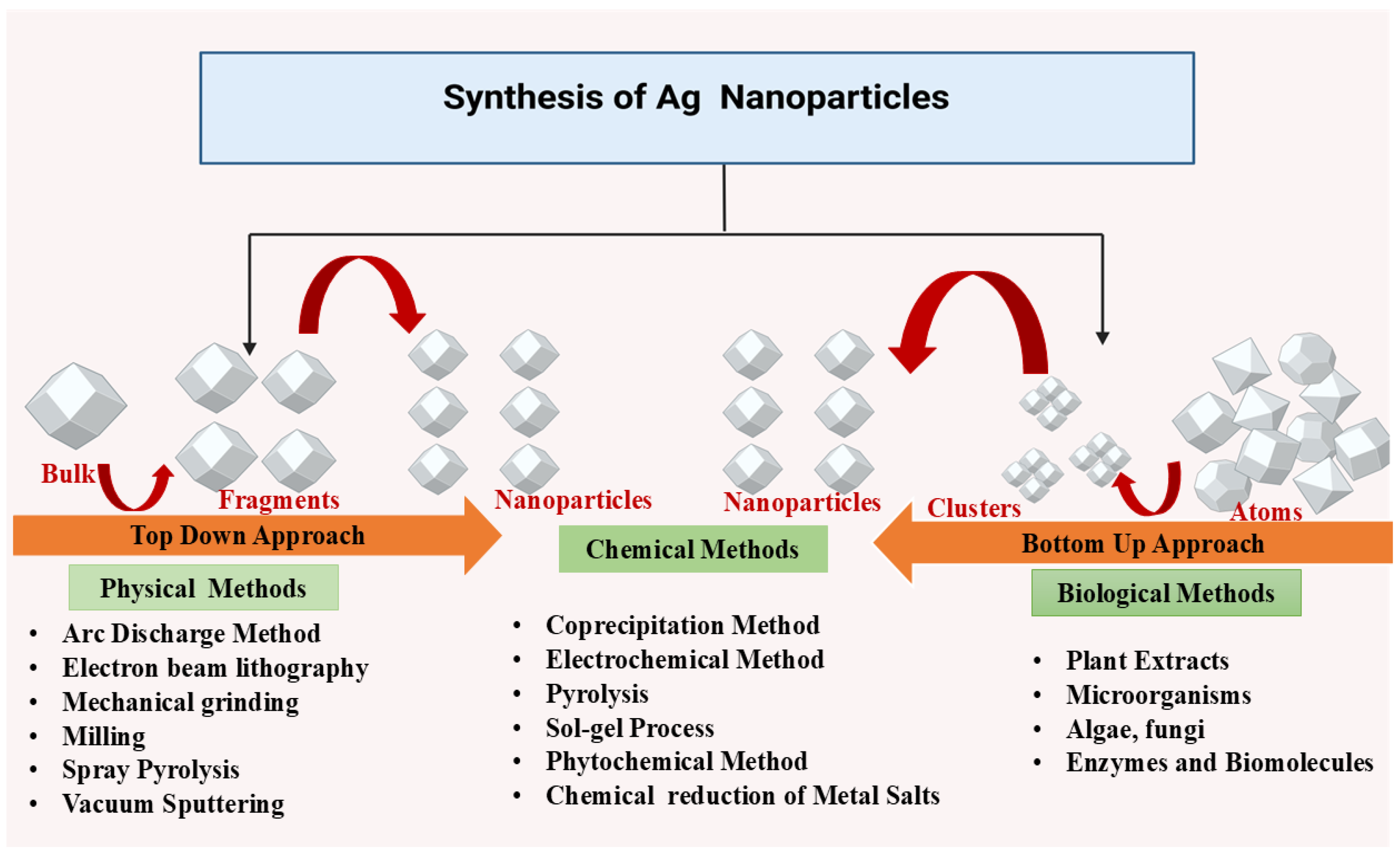
Physical methods utilize external forces to produce AgNPs from bulk silver (Figure 2). Techniques such as milling, arc discharge, and laser ablation generate nanoparticles with uniform size, high purity, and controlled morphology [14,15]. Ball milling involves mixing metal particles with milling balls in a medium of gas (e.g., inert gas or air). Parameters like milling time, rotational speed, and temperature influence particle morphology. Larger particles tend to agglomerate due to reduced surface energy, while temperature affects phase characteristics. In a study conducted by Khayati and Janghorban, nanostructured silver with a crystallite size of 28 nm was synthesized using a high-energy ball mill, followed by the reduction of Ag2O with graphite [16]. The Electrical Arc-Discharge Method utilizes a DC power source applied between silver electrodes immersed in a dielectric liquid [17]. The high temperature vaporizes silver, which then condenses into AgNPs [18]. Elwakil et al. synthesized carbon-coated AgNPs (17 nm), exhibiting strong antibacterial activity against Pseudomonas aeruginosa and cytotoxicity against normal lung cells [19]. In another study, Gharieb et al. synthesized AgNPs-CNTs (9 ± 2 nm) at −10 °C and AgNPs/C (12.4 ± 3 nm) at 25–55 °C using ethanol as a dielectric medium [20]. In the Laser Ablation Method, a pulsed laser targets bulk silver within a liquid medium, producing plasma plumes that cool and form nanoparticles [21,22]. The characteristics of the nanoparticles are influenced by factors such as laser fluence, pulse duration, and the type of solvent used. Rahmah et al. synthesized spherical AgNPs (~30 nm) with a band gap of 2.25 eV, showing anticancer activity and antibacterial effects [23]. Rafque et al. used a one-step green laser ablation method, producing smaller nanoparticles (9 nm) with enhanced thermal conductivity [24]. Kenmotsu et al. synthesized AgNPs for SERS applications using laser ablation, post-annealing, and electrostatic mobility classification [25]. Mohammed et al. conjugated AgNPs with Elettaria cardamomum seed extract, enhancing antibacterial efficacy against Escherichia coli and Staphylococcus aureus using laser ablation techniques [26]. Niaz et al. investigated the effect of confinement geometry on pulsed laser ablation [27]. Alharbi et al. combined laser ablation with RF sputtering to produce ZnO-encapsulated AgNPs, improving photodetector efficiency [28]. Raffi et al. synthesized AgNPs (8–32 nm) using inert gas condensation, demonstrating size control through evaporation temperature and helium pressure [29].
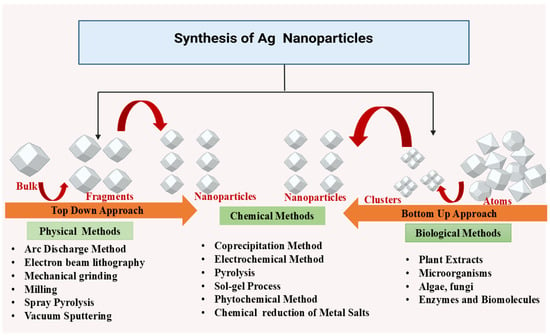
Figure 2.
Different methods of synthesizing AgNPs.
2.2. Chemical Methods
Chemical methods such as chemical reduction, are used for AgNP synthesis. These processes involve reducing the silver ions (Ag+) into metallic silver (Ag0) using organic or inorganic reducing agents (Figure 2). The resulting silver clusters exhibit a characteristic color due to surface plasmon resonance. Alternative chemical methods include microemulsion techniques and microwave-assisted synthesis, offering efficient control over nanoparticle size and properties [30,31,32]. Chemical reduction utilizes a reducing agent and a silver precursor, typically silver nitrate, to produce AgNPs. Sodium borohydride is a common reducing agent, often combined with stabilizers like trisodium citrate to control nanoparticle nucleation and growth. Agnihotri et al. synthesized AgNPs ranging from 5 to 100 nm with monodisperse characteristics using sodium borohydride and trisodium citrate [33]. The choice of stabilizing agents influences nanoparticle morphology, allowing the formation of alternative structures like rhomboidal particles and nanosheets [34]. Microemulsion synthesis involves surfactants to mix immiscible liquids, such as oil and water, enabling the production of uniform AgNPs. Reactants are separated into two phases, with the interfacial region facilitating controlled nanoparticle growth. Surfactants like sodium dodecylbenzene sulfonate, cetyltrimethylammonium bromide (CTAB), and polyvinylpyrrolidone (PVP) determine AgNP characteristics [8,35,36]. Hak et al. demonstrated the use of microemulsions for targeted breast cancer therapy, achieving controlled drug release and enhanced bioavailability in MCF-7 cells [37]. Eco-friendly AgNP synthesis can be achieved using polysaccharides and polymers as reducing and capping agents. Starch-based AgNPs, synthesized with D-glucose as a reducing agent, exhibit thermal reversibility due to weak starch-nanoparticle interactions. Other reducing agents include polyethylene glycol (PEG), polyvinyl alcohol (PVA), and chitosan with PEG-coated AgNPs demonstrating good stability in high saline solutions [38,39,40,41,42]. Singh et al. incorporated AgNPs into hydrogels using tragacanth gum and gum acacia, leading to sustained drug release and antimicrobial properties. Sarkar et al. synthesized AgNPs using bacterial polysaccharides, achieving significant photocatalytic degradation of azo dyes, highlighting AgNPs’ potential in bioremediation [43]. Chemical synthesis methods offer precise control over AgNP properties, making them valuable for applications in medicine, drug delivery, and environmental remediation.
2.3. Green Synthesis
Sustainability in silver nanoparticle (AgNP) synthesis has become increasingly important, as chemical and physical methods often leave behind harmful solvent residues like ethylene glycol, sodium citrate, oleyl amine, and liquid paraffin (Figure 2). Such residues pose risks, particularly in applications involving drug delivery, antimicrobial treatments, and human exposure. Green synthesis, an eco-friendly alternative, relies on natural sources such as microorganisms (fungi, yeasts, bacteria, and actinomycetes), and plant extracts. This method has gained traction across various sectors, including biomedicine, cosmetics, food, drug delivery, and agrochemicals, due to its sustainable approach and the unique properties of AgNPs. The green synthesis of AgNPs primarily requires a solution of silver metal ions and a biological reducing agent (Table 1). In most cases, the constituents within the cells act as natural stabilizing and capping agents, making the addition of external stabilizers unnecessary. Reducing agents are naturally present in various biological systems. AgNPs have been synthesized using organisms from four of the five kingdoms of life: Monera (prokaryotic organisms lacking a true nucleus), Protista (unicellular eukaryotic organisms with a true nucleus), Fungi (eukaryotic organisms that are either saprophytic or parasitic), Plantae (eukaryotic autotrophs), and other Animal-derived materials (eukaryotic heterotrophs) [44,45]. Ahmed et al. studied a rapid, eco-friendly synthesis of AgNPs using Azadirachta indica leaf extract as both a reducing and capping agent. Characterized by FTIR, DLS, TEM, and UV-Vis (436–446 nm), the nanoparticles showed strong antibacterial activity against Escherichia coli and Staphylococcus aureus. The one-pot green synthesis is cost-effective, stable, and excludes hazardous chemicals, making it a viable alternative to conventional methods for biomedical and optoelectronic applications [46]. In another study by Ashraf et al. the anti-glycating potential of AgNPs synthesized using Aloe vera extract was studied. Characterization through UV-Vis, EDX, TEM, XRD, and DLS confirmed that AgNPs (~30.5 nm) significantly inhibited AGE formation in a concentration-dependent manner while preserving protein structure. The study emphasizes their potential therapeutic applications in diabetes-related complications, antimicrobial activity, and antiviral effects [47]. Das et al. synthesized AgNPs using Trema orientalis leaf extract as a reducing and stabilizing agent. Characterization via UV-Vis, FTIR, TEM, XRD, and AFM confirmed their crystalline nature with sizes ranging from 14.04 to 34.38 nm. AgNPs demonstrated significant antibacterial activity against Staphylococcus aureus, with inhibition zones increasing at higher concentrations. This eco-friendly synthesis method offers a sustainable approach to developing antimicrobial agents with potential biomedical applications [48]. Singla et al. investigated the synthesis of AgNPs using Oxalis griffithii methanolic leaf. The AgNPs exhibited a spherical, crystalline structure with stability confirmed by Zeta potential. UV-Vis absorption peak appeared at 408–412 nm. The antibacterial activity was tested against Escherichia coli and Bacillus subtilis, demonstrating significant effectiveness. This eco-friendly synthesis method offers a cost-effective approach for potential biomedical applications [49]. In another study by Widatalla et al., green tea (Camellia sinensis) leaf extract was utilized as a natural reducing agent for the eco-friendly synthesis of AgNPs. UV-Vis spectroscopy confirmed their formation with a peak at 410 nm, while SEM analysis revealed sizes ranging from 15 to 33 nm. FTIR analysis identified polyphenols, polysaccharides, and proteins involved in the synthesis. Antibacterial studies demonstrated strong inhibition against Staphylococcus aureus and Klebsiella sp., highlighting their potential as antimicrobial agents [50]. Khane et al. [50], used Citrus limon zest extract as a natural reducing agent for the eco-friendly synthesis of AgNPs. Characterization through UV-Vis, FTIR, SEM, TEM, XRD, and DLS confirmed their formation, with a surface plasmon resonance peak at 535.5 nm. The crystalline, spherical AgNPs exhibited strong antimicrobial effects against E. coli, Staphylococcus aureus, and Candida albicans, along with significant antioxidant properties, highlighting their potential applications in biomedical and pharmaceutical fields.

Table 1.
Green synthesis of silvern nanoparticles (AgNPs).
3. Biophysical Properties
Nanomaterials possess unique properties distinct from their bulk counterparts, influenced by chemical composition, morphology, and surface structure. Their small size leads to enhanced reactivity, optical, and mechanical characteristics. These attributes make them valuable in diverse applications, including targeted drug delivery, bio-imaging, and disease diagnosis. Their high surface area and tunable properties enable advancements in medicine, electronics, and environmental science, driving innovation in multiple fields [58,59]. The properties of silver nanoparticles are outlined below:
3.1. Shape and Crystallinity
Various synthesis techniques enable the production of AgNPs in diverse shapes and sizes, including nanospheres, nanorods, nanobars, nanoprisms, decahedral nanoparticles, and triangular bipyramids [60]. For instance, a photo-induced approach has been employed to convert spherical AgNPs into triangular nanoprisms [61,62,63]. Research by Mirkin and Murphy investigated a seeding method for synthesizing Ag nanoprisms with controlled edge lengths [64,65]. Another study introduced a modified polyol process where ethylene glycol functions as both a solvent and a reducing agent, yielding AgNPs in different forms such as pentagonal nanowires, right bipyramids, and nano-cubes with adjustable corner truncation. Additionally, microwave heating has become a widely used method for synthesizing triangular Ag nano-plates [66,67,68,69,70]. The scanning electron microscopy (SEM) and X-ray diffraction (XRD) analyses confirmed the crystalline structure and morphological characteristics of the AgNPs, which exhibited both spherical and hexagonal geometries [71]. The morphological characteristics, dimensional parameters, and structural attributes of the synthesized nanoparticles were analyzed using scanning electron microscopy (SEM), UV-visible spectroscopy (UV-VIS), and X-ray diffraction (XRD) techniques. SEM imaging revealed that the AgNPs exhibited a predominantly spherical shape with diameters ranging from 15 to 90 nm, while triangular nanoparticles possessed edge lengths of approximately 150 nm. UV-VIS spectroscopy indicated that the surface plasmon resonance (SPR) peaks of the spherical silver colloids appeared within the 397–504 nm wavelength range. In contrast, the triangular nanoparticles displayed two distinct SPR peaks, one at 392 nm and another at 789 nm [72]. Triangular or rod-shaped AgNPs can enhance plasmonic and antibacterial effects due to their anisotropic shapes, but their higher cytotoxicity, aggregation tendencies, and limited biocompatibility often restrict their biomedical applications. For a wide range of biomedical applications, silver nanoparticles (AgNPs) in the size range of 10–30 nm are generally considered optimal, offering a good balance between antimicrobial efficacy and biocompatibility. Smaller nanoparticles (approximately 5–15 nm) are particularly advantageous for cell membrane penetration and targeted drug delivery, while larger particles (around 40–60 nm) may be better suited for applications such as wound healing and tissue regeneration, where slower release and surface interactions are beneficial [73,74].
3.2. Melting Temperature
Thermal behavior is a crucial factor in the production and application of materials. A notable characteristic of metal nanoparticles is their reduced melting temperature, attributed to the thermodynamic size effect, which has been widely utilized for various applications. The thermal properties of AgNPs are commonly analyzed using thermogravimetric analysis (TGA) or differential scanning calorimetry (DSC). Additionally, the Gibbs–Thomson equation serves as a theoretical approach to studying the thermal behavior of nanoparticles [75]. Metallic nanoparticles exhibit significantly lower melting points than their bulk counterparts [76]. For example, while bulk silver has a fixed melting point of 960 °C, AgNPs demonstrate a lower and size-dependent melting temperature. This behavior can be explained by the Gibbs−Thomson effect, which describes how a higher surface area-to-volume ratio in smaller nanoparticles contributes to increased surface energy [77]. Consequently, there is an inverse relationship between nanoparticle size and melting point. The Gibbs−Thomson effect also explains the tendency of smaller nanoparticles to undergo sintering or Ostwald ripening, reducing their total free energy and resulting in surface melting at lower temperatures [78,79].
3.3. Optical Properties
The optical properties of metal nanoparticles have been a significant area of study in physical chemistry since the 19th century. With advancements in lithographic techniques and wet chemistry, it is now possible to synthesize noble metal nanoparticles in a variety of sizes, shapes, and dielectric environments. Understanding their interaction with light requires solving Maxwell’s equations for light scattering, especially for particles with complex geometries. Key aspects include dipole and quadrupole plasmon resonances in spherical nanoparticles, as well as analytical and numerical methods for determining extinction and scattering cross-sections, local field distributions, and other optical characteristics in nonspherical structures. These theoretical approaches play a crucial role in exploring the behavior of triangular AgNPs and related morphologies, which are of growing interest in various applications [62]. The optical activity of AgNPs was investigated by experimentally measuring extinction, scattering, absorption cross-sections, and efficiencies in water. Measurements were conducted for 16 distinct particle sizes, ranging from 29 to 136 nm, under chemically clean conditions. Results revealed that these nanoparticles interact with light 4 to 10 times more intensely than predicted by their geometric cross-section. Additionally, the absorption and scattering contributions to the plasmon resonance were independently analyzed across the visible spectrum. To facilitate the accurate determination of particle concentrations, regardless of variations in size, shape, or aggregation state, a method called standard subtraction was introduced, offering a simple and reliable approach [80]. In metallic silver, light interaction occurs due to the confinement of conduction electrons within nanoscale dimensions and the frequency-dependent dielectric function. These factors contribute to the phenomenon of surface plasmon resonance (SPR), where the collective oscillation of conduction electrons is induced by an external electromagnetic field [61]. Numerous studies demonstrated that AgNPs absorb electromagnetic radiation within the visible spectrum, specifically in the 380–450 nm range, through a mechanism known as localized surface plasmon resonance (LSPR) excitation. The study of the optical response of embedded AgNPs reveals a strong dependence of surface plasmon resonances (SPRs) on nanoparticle morphology and dielectric environment. Using the discrete dipole approximation, extinction efficiencies were analyzed for nanoparticles of varying shapes and ambient conditions. The findings indicated that an increase in truncation leads to a blue shift in the primary SPR, with overlapping resonances at shorter wavelengths and broadening of the main resonance. As the number of facets increases, SPRs diminish, particularly when nanoparticle symmetry is enhanced. Conversely, sharper vertices result in a greater number of distinct SPRs. Environmental refractive index variations do not alter the number of SPRs but significantly influence their spectral position and width, with higher refractive indices inducing a redshift. These results provide crucial insights into the tunability of plasmonic properties for applications in sensing, imaging, and nanophotonics [81].
3.4. Electrical Properties
The electrical performance of low-temperature screen-printed AgNPs (nAg) has been evaluated at frequencies up to 220 GHz, revealing superior characteristics compared to conventional thick-film silver conductors. Notably, for frequencies above 80 GHz, coplanar waveguide structures fabricated with nAg at 350 °C exhibit lower electrical losses than those composed of micrometer-sized grains sintered at 850 °C. This enhancement is attributed to improved nanoparticle packing, leading to a threefold reduction in surface roughness. These findings demonstrate the potential of AgNPs for high-frequency applications, particularly on temperature-sensitive conformal substrates and in sub-THz metamaterials, offering new possibilities for advanced electronic and photonic devices [82]. Electrical characterization revealed that the current exhibited an increasing trend with the enhancement of the nanoparticles’ antioxidant properties. Consequently, the antioxidant potential of the synthesized nanoparticles can be estimated through an analysis of their electrical characteristics [83]. The silver nanoparticle (AgNP)-loaded silk films exhibit flexibility and demonstrate significant variations in electrical conductivity, making them highly suitable for applications in biosensors and implantable thermoelectric wireless switching devices [84].
5. Safety of AgNPs
AgNPs are widely used in various products for their antibacterial properties, but studies show they can be toxic to mammalian cells (Table 3). While green synthesis methods using plant extracts are preferred due to their eco-friendly nature, AgNPs can become toxic at concentrations above the LOAEL, leading to potential health risks. Future research should focus on understanding the factors influencing AgNP toxicity and the mechanisms behind it to minimize environmental and human health impacts while utilizing their beneficial properties [141]. Understanding toxicity mechanisms is crucial for their safe use in the future [142]. Recent research on AgNPs highlighted their antibacterial, antiviral, and anticancer properties, but concerns about their potential toxicity remain. While AgNPs are used in various products, including medical and consumer goods, their safety is a major focus. Studies emphasize the need for safer synthesis methods, such as green synthesis, and the evaluation of their biocompatibility and cytotoxicity. In-depth research is essential to ensure their safe use in therapeutic applications [143]. For instance, a study by Alwan et al. evaluates the safety of biosynthesized AgNPs using Cinnamomum zeylanicum bark extract in rats. After 14 days of oral administration at varying doses, no significant toxicity was observed. There were no changes in body weight, biochemical markers (AST, ALT, urea, creatinine), oxidative stress parameters (Mass drug administration (MDA), Superoxide Dismutase (SOD), catalase (CAT)), or histopathological features of the liver and kidneys, suggesting that AgNPs are relatively safe at the tested doses [144]. Another study by Vuković et al. investigated the safety of AgNPs with different surface coatings on the human immune system. Four types of AgNPs were tested for their effects on human peripheral blood mononuclear cells (hPBMC). Results showed that AgNPs, particularly positively charged and protein-coated ones, induced apoptosis, cell death, oxidative stress, and mitochondrial damage. The study highlighted the genotoxic potential of AgNPs, providing valuable insights for assessing the safety of nanosilver in medical applications [145]. The toxicity of AgNPs also depends on the organism’s defense mechanisms and the culture media used in testing. AgNPs and released Ag+ cause toxicity by damaging membranes, generating reactive oxygen species (ROS), and inducing protein oxidation, mitochondrial dysfunction, DNA damage, and cell proliferation inhibition. AgNPs interact with sulfur-containing macromolecules, contributing to toxicity. Their antibacterial activity and ability to penetrate cell membranes lead to cytoplasmic accumulation, oxidative damage, and apoptosis in mammalian cells [146]. Elyousfi et al. evaluated the ecotoxicity of silver nanoparticles (Ag NPs) on Ruditapes decussatus by analyzing biochemical changes in gills and digestive glands after exposure to 100 and 200 μg concentrations for 48 h and 7 days. AgNPs disrupted antioxidant and cholinergic systems, with time- and concentration-dependent effects on catalase (CAT) and glutathione S-transferase (GST) activities. Acetylcholinesterase (AChE) activity decreased, especially at higher concentrations, suggesting significant toxicity [147]. Pinheiro et al. investigated the toxicity of AgNPs in Artemia salina linked to the interaction between silver ions (Ag+) and chitin in the organism’s cuticle. AgNPs at concentrations of 50 and 100 ppm caused mortality and cellular damage after 24–48 h. Geometric optimization and SAPT0 analysis revealed that Ag3+ ions deform the chitin structure. Light and confocal microscopy confirmed AgNPs’ presence in the cuticle and the resulting cellular damage, shedding light on the toxicity mechanism [148]. Baloushi et al. synthesized AgNPs using Moringa peregrina leaf extract and demonstrated significant antioxidant and anticancer activity against human cancer cell lines (Caco-2 and MCF-7). These nanoparticles are eco-friendly and non-toxic to humans, showing promise for biological applications such as drug delivery and disease treatment. However, the study did not assess the effects on normal cells, highlighting the need for further research on AgNP toxicity, surface modifications, and underlying bioactive mechanisms [149]. Silver nanoparticles (Ag NPs) at a dose of 21.5 mg/kg effectively treated Trichinella spiralis infection in mice, showing high efficacy against adult and encapsulated larvae. The Ag NP treatment, either alone or combined with multivitamins (MM), achieved significant trichinocidal effects, surpassing reference drugs. Importantly, combining Ag NPs with MM alleviated silver-induced toxicity, improving redox parameters and liver and kidney biomarkers, thus overcoming the adverse effects of silver material while maintaining therapeutic effectiveness [150]. AgNPs exhibit concentration-dependent toxicity across biological systems. In a study, Dinç assessed their effects on Escherichia coli, Bacillus subtilis, Caenorhabditis elegans, and human vein endothelial cells (HUVECs). AgNPs inhibited bacterial growth (52% at 50 µg/mL), reduced Caenorhabditis elegans reproduction by 25% at 10 µg/mL, and caused a significant decrease in body bending frequency. HUVECs showed cytotoxicity, with an IC50 of 38 µg/mL. Findings emphasized the importance of cautious AgNP application in biomedical and environmental fields [151]. Kakakhel et al. found that Long-term exposure to high concentrations of AgNPs led to toxicity, bioaccumulation, and tissue damage in common carp (Cyprinus carpio). Fish exposed to AgNPs showed accumulation primarily in the liver, followed by the intestine, gills, and muscles. Histological alterations, including necrosis and tissue degeneration, were observed, particularly at higher concentrations [152]. Thwala et al. evaluated the toxicity of AgNPs on the aquatic plant Salvinia minima, focusing on size, bioaccumulation, and environmental interactions. Smaller AgNPs (10 nm) exhibited greater solubility, accumulation, and toxicity than larger ones (40 nm). Exposure reduced plant growth, chlorophyll content, and overall health, with toxicity influenced by water chemistry [153]. Polystyrene nanoplastics influence the toxicity of AgNPs in zebrafish embryos by acting as carriers in water. The release of silver ions (Ag+) from AgNPs was 4.23%. While AgNPs altered antioxidant and metabolic gene expression, nanoplastics mitigated apoptosis and immunotoxicity. Findings by Yan et al. suggested nanoplastics reduce AgNP genotoxicity by absorbing Ag+ and forming aggregates, highlighting the complex interactions between pollutants in aquatic environments [154]. AgNPs supported on zirconium dioxide (ZrO2) modified with dihydroquercetin (DHQ) exhibited strong antibacterial effects against Escherichia coli and Staphylococcus aureus, with complete bacterial reduction at all tested Ag concentrations. However, cytotoxicity tests on HeLa and MRC-5 cells showed minimal toxicity, even at high concentrations. This suggests that the modified AgNPs provide effective antimicrobial properties while maintaining biocompatibility, highlighting their potential for biomedical applications [155]. A study by Sambale et al. examined the toxic effects of AgNPs on mammalian cell lines, including human liver, lung, and fibroblast cells. Results show that AgNPs significantly reduce cell viability, with smaller particles exhibiting higher toxicity. The toxicity is attributed to nanoparticle interaction rather than silver ions alone. AgNP exposure triggers apoptosis rather than necrosis [156]. AgNPs synthesized using entomopathogenic fungi show antimicrobial properties and potential use as nanoinsecticides. However, toxicity studies are essential to assess their environmental impact and safety. AgNPs may cause cytotoxicity and genotoxicity, depending on size, shape, and concentration. In vitro methods like MTT and comet assays help evaluate their effects on cells [157]. Greulich et al. studied the toxicity of AgNPs and silver ions in bacteria and human cells. It was found that the toxic effects occur within similar concentration ranges, challenging the assumption that silver is significantly safer for mammalian cells. Both forms of silver affect Escherichia coli, Staphylococcus aureus, mesenchymal stem cells, and blood cells through ion release and reactive oxygen species. The findings raise concerns about the widespread use of silver in medical and consumer applications due to potential human toxicity [158]. Cho et al. examined the acute toxicity of AgNPs in mice, focusing on size-dependent effects. Mice administered 10 nm AgNPs exhibited reduced activity, body temperature drop, and organ damage, including liver necrosis and spleen congestion. Larger AgNPs (60 and 100 nm) showed significantly lower toxicity. Findings suggest smaller nanoparticles pose greater health risks due to higher reactivity and absorption, raising concerns about their widespread use in consumer and medical products [159]. Jian et al. investigated the toxicity of AgNPs against the fungus Fusarium graminearum, which produces harmful mycotoxins. AgNPs effectively inhibit fungal growth by damaging cell membranes, impairing metabolism, and increasing oxidative stress. However, they also trigger the production of deoxynivalenol (DON), a dangerous mycotoxin. Despite their antifungal potential, AgNPs pose risks of enhanced toxin production, necessitating careful evaluation before agricultural application to prevent unintended health and environmental hazards [160]. Similarly, Zhao et al. investigate the toxic effects of AgNPs on Chlamydomonas reinhardtii, a freshwater microalga. Exposure to AgNPs inhibits growth, damages chloroplasts, reduces photosynthetic pigment production, and increases oxidative stress. The nanoparticles disrupt membrane integrity, leading to increased permeability and cellular damage. Activation of antioxidant enzymes was observed as a defense mechanism. These findings highlight potential environmental risks of AgNP contamination in aquatic ecosystems due to their toxicity to primary producers [161]. The study by Souza et al. evaluated the toxicity of AgNPs on the aquatic plant Lemna minor, focusing on solubility, accumulation, and size-dependent effects. Smaller AgNPs (30 nm) showed higher solubility, accumulation in roots and leaves, and greater toxicity, causing 60% mortality at high concentrations. Larger AgNPs (85 and 110 nm) had lower toxicity [162]. Ke et al. investigate the toxic effects of AgNPs on Arabidopsis thaliana, focusing on their impact on flowering and offspring development. AgNP exposure reduced petal and pollen viability, delayed flowering, and impaired seed production. The toxic effects were transferred to offspring, leading to worsened plant growth and delayed flowering. Gene expression related to floral organ development was downregulated, highlighting potential risks to plant reproduction and food security due to nanoparticle contamination [163]. Marinho et al. examined the toxicity of AgNPs in Danio rerio (zebrafish) by analyzing their effects on brain, muscle, liver, and gill tissues. Exposure to AgNPs reduced acetylcholinesterase activity in the brain and muscle, inhibited catalase in the liver and gills, and caused morphological damage in gills, including lamellar fusion and epithelial lifting [164]. Maziero et al. evaluated the toxicity of AgNPs stabilized with gum arabic protein (AgNP-GP) in different species, including Daphnia similis, Danio rerio embryos, and Sprague Dawley rats. AgNP-GP caused significant toxicity in aquatic organisms, leading to immobility in Daphnia similis and developmental defects in zebrafish embryos. However, oral administration in rats up to 10 mg/kg for 28 days showed no adverse effects. These findings highlight species-specific toxicity concerns of AgNPs [165]. Abdelkhaliq et al. examined the potential developmental toxicity of AgNPs using the BeWo b30 placental transport model and the embryonic stem cell test (EST). Findings show that AgNPs can cross the placental barrier, but their transport is limited and influenced by surface chemistry. While AgNPs exhibit cytotoxicity, they do not induce developmental toxicity at non-cytotoxic concentrations. Aged silver sulfide nanoparticles (Ag2S NPs) demonstrate lower toxicity and bioavailability [166]. Chen et al. investigated the immunotoxicity of AgNPs using a zebrafish model. Exposure to AgNPs caused mortality, malformations, and immune system toxicity, affecting neutrophils and macrophages. AgNPs also disrupted immune-related gene expression and increased oxidative stress. However, pterostilbene (PTE), a natural antioxidant, reduced these toxic effects by activating immune cells and mitigating oxidative stress. The findings highlighted the potential immune risks of AgNPs and the protective role of PTE [167]. Ajdary et al. investigated the toxicity of AgNPs on endometrial receptivity in female mice. Mice exposed to AgNPs (2 and 4 mg/kg) showed increased inflammatory markers (IL-6, IL-1β), nanoparticle accumulation in endometrial tissue, and reduced pinopod and microvillus formation, affecting implantation. This suggested that AgNP exposure during pregnancy may disrupt uterine conditions, highlighting potential reproductive risks and the need for caution in nanoparticle exposure during gestation [168]. Emma et al. evaluated the sub-acute and chronic toxicity of AgNPs synthesized using Azadirachta indica extract in Swiss albino rats. While no mortality or significant weight changes were observed, higher doses (30 mg/kg) led to increased liver enzymes (ALT, AST) and portal hepatitis. Kidney function remained unaffected [169]. Khoshnamvand et al. examined the toxicity of biosynthesized silver nanoparticles (AR-AgNPs) on different aquatic organisms, including phytoplankton (Chlorella vulgaris), zooplankton (Daphnia magna), and fish (Danio rerio). Ag+ ions were more toxic than AR-AgNPs at all trophic levels, with Daphnia magna being the most sensitive. Toxicity mainly originated from nanoparticles rather than ion release. These findings highlight the potential ecological risks of AgNP contamination in aquatic food chains [170]. The study by Somda et al. investigated the biosynthesis, characterization, antimicrobial activity, and safety of AgNPs derived from Brassica carinata microgreens. AgNPs exhibited strong antimicrobial properties against various pathogens but showed minimal cytotoxicity on Vero cells, indicating potential biocompatibility [171]. Silver nanoparticles offer significant benefits but raise toxicity concerns, including oxidative stress, DNA damage, and inflammation. Their impact varies with size, concentration, and exposure duration. Comprehensive research is necessary to understand long-term effects, ensuring their safe application while minimizing potential risks to human health and environmental sustainability [172].

Table 3.
Toxicity effects of Silver Nanoparticles.
Limitations of AgNPs
AgNPs offer distinct advantages due to their unique physicochemical properties when compared to bulk silver. However, they also face certain limitations that can affect their performance and effectiveness. One significant issue is their susceptibility to oxidation. AgNPs react readily with oxygen, leading to the formation of silver ions that bind with oxygen to create strong ionic bonds. This oxidation process alters the structure of the nanoparticles, thereby modifying their physicochemical characteristics. The oxidized form of AgNPs can also reduce their antibacterial efficacy, as the activity of the nanoparticles is closely linked to the presence of silver ions. Another challenge with AgNPs is their tendency to aggregate. Research shown that AgNPs are prone to aggregation when placed in organic solvents, such as dimethylformamide and tetrahydrofuran, which can impact their stability and performance in various applications.
6. Challenges and Future Directions
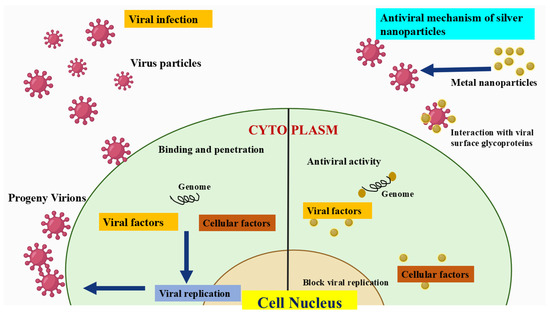
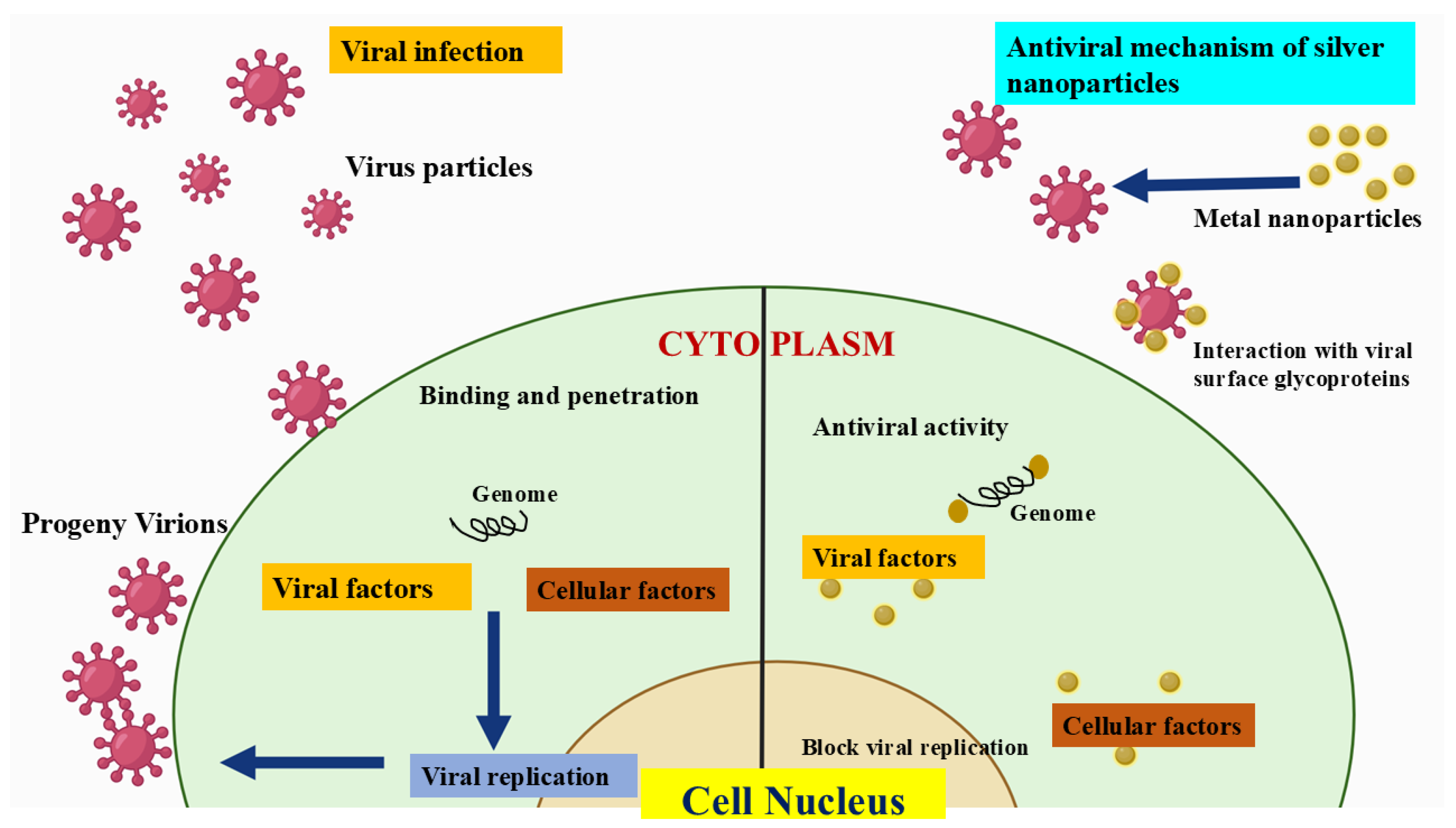
The use of AgNPs as antiviral agents holds significant promise, but several challenges remain that must be addressed to maximize their potential. Several approaches for the enhancement of the antimicrobial activity of AgNPs could be by utilizing AgNPs as antiviral agents, some aspects should be commented such as magnetic-responsive Ag NPs, light-triggered antimicrobial activity, and targeting of viral agents. Prucek et al. synthesized and characterized two magnetic silver-based nanocomposites—Ag@Fe3O4 and γ-Fe2O3@Ag—using maltose-mediated silver reduction and polyacrylate as a spacer [176]. The nanocomposites showed strong antibacterial and antifungal activity (MIC: 1.9–125 mg/L) and limited cytotoxicity in fibroblasts (toxic < 430 mg/L (Ag@Fe3O4)). Their magnetic and biocompatible properties suggest potential for targeted delivery of silver nanoparticles. In another study, Torres-Mendieta et al. achieved biofilm deterioration in bacteria using magnetic doping of AgNPs [177]. Zhang et al. reported the immobilized AgNPs (Fe3O4–SiO2–Ag) onto magnetic silica composite depicting an enhanced antibacterial activity [178]. A study conducted by Ratti and coworkers demonstrated that the antibacterial activity of laser-ablated AgNPs could be enhanced by irradiation with visible light [179].
One of the key challenges is understanding the precise parameters that enhance their antiviral efficacy, such as particle size, concentration, and functionalization. While smaller AgNPs (around 10 nm) have demonstrated stronger antiviral effects, increasing the particle size can reduce efficacy. Moreover, the concentration of AgNPs in the system is critical, as higher concentrations lead to a stronger antiviral effect, though excessive amounts may cause toxicity. Functionalization of AgNPs and their incorporation into composite materials also present opportunities for improving antiviral activity by either enhancing the particles’ interaction with viruses or by incorporating antiviral agents into the material itself. Despite these promising findings, the commercial application of AgNPs, particularly for antiviral purposes, is still limited. While products such as face masks and textiles have been introduced, more advanced systems, particularly those for treating viral infections, are mostly in the prototype stage. The most promising applications of AgNPs include their use in water and air purification systems, as well as in the textile industry, with the potential to significantly impact public health and quality of life, especially during viral pandemics like COVID-19. However, the mechanisms of AgNPs’ antiviral action remain poorly understood, and further research is necessary to optimize their performance, particularly in diverse devices and applications. Additionally, environmental concerns about the toxicity and accumulation of silver nanoparticles must be addressed. Sustainable methods for collecting or recycling AgNPs could reduce waste and production costs, making these technologies more viable. Ultimately, while the antiviral applications of AgNPs are promising, their future success hinges on overcoming these challenges through further scientific investigation into their effectiveness, safety, and environmental impact.
Author Contributions
Conceptualization, A.S. and T.N.R.; methodology, S.N.M.; software, N.S.; validation, N.N.S., A.P.P. and S.Y.; formal analysis, H.K.A.Y.; investigation, H.K.A.Y.; resources, A.S.; data curation, A.S.; writing—original draft preparation, S.N.M., H.K.A.Y. and A.P.P.; writing—review, and editing, S.N.M.; visualization, H.K.A.Y.; supervision, A.P.P.; project administration, H.K.A.Y.; funding acquisition, H.K.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in the preparation of this review article is already available as published papers in the literature.
Acknowledgments
H.K.A.Y. would like to thank the Deanship of Graduate Studies and Research, Ajman University, UAE, for their support in providing assistance in article processing charges for this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tomaszewska, E.; Bednarczyk, K.; Janicka, M.; Chodkowski, M.; Krzyzowska, M.; Celichowski, G.; Grobelny, J.; Ranoszek-Soliwoda, K. The Influence of the AgNPs Ligand on the Antiviral Activity Against HSV-2. Int. J. Nanomed. 2025, 20, 2659–2671. [Google Scholar] [CrossRef]
- Idres, Y.M.; Idris, A.; Gao, W. Preclinical Testing of Antiviral SiRNA Therapeutics Delivered in Lipid Nanoparticles in Animal Models–a Comprehensive Review. Drug Deliv. Transl. Res. 2025, 1–18. [Google Scholar] [CrossRef]
- Yadav, S.; Mali, S.N.; Pandey, A. Biogenic Nanoparticles as Safer Alternatives for Gastric Ulcers: An Update on Green Synthesis Methods, Toxicity, and Their Efficacy in Controlling Inflammation. Biol. Trace Elem. Res. 2024, 1–20. [Google Scholar]
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The Challenge of Emerging and Re-Emerging Infectious Diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Gonçalves, B.C.; Lopes Barbosa, M.G.; Silva Olak, A.P.; Belebecha Terezo, N.; Nishi, L.; Watanabe, M.A.; Marinello, P.; Zendrini Rechenchoski, D.; Dejato Rocha, S.P.; Faccin-Galhardi, L.C. Antiviral Therapies: Advances and Perspectives. Fundam. Clin. Pharmacol. 2021, 35, 305–320. [Google Scholar] [CrossRef]
- Ranade, T.; Sati, A.; Pratap, A.; Mali, S.N. Curcumin-Integrated Biopolymer Films for Active Packaging: Current Trends and Future Directions. Chem. Pap. 2025, 79, 1303–1334. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Vu, K.B.; Phung, T.K.; Tran, T.T.T.; Mugemana, C.; Giang, H.N.; Nhi, T.L.P. Polystyrene Nanoparticles Prepared by Nanoprecipitation: A Recyclable Template for Fabricating Hollow Silica. J. Ind. Eng. Chem. 2021, 97, 307–315. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Orrell-Trigg, R.; Shah, D.; Cheeseman, S.; Vu, K.B.; Ngo, S.T.; Murdoch, B.J.; Choudhury, N.R.; Yin, H.; Cozzolino, D.; et al. Broad Spectrum Antibacterial Zinc Oxide-Reduced Graphene Oxide Nanocomposite for Water Depollution. Mater. Today Chem. 2023, 27, 101242. [Google Scholar] [CrossRef]
- Doan, L.; Nguyen, L.T.; Nguyen, N.T.N. Modifying Superparamagnetic Iron Oxides Nanoparticles for Doxorubicin Delivery Carriers: A Review. J. Nanoparticle Res. 2023, 25, 73. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [PubMed]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Chong, K.F.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Khayati, G.R.; Janghorban, K. The Nanostructure Evolution of Ag Powder Synthesized by High Energy Ball Milling. Adv. Powder Technol. 2012, 23, 393–397. [Google Scholar] [CrossRef]
- Tien, D.-C.; Liao, C.-Y.; Huang, J.-C.; Tseng, K.-H.; Lung, J.-K.; Tsung, T.-T.; Kao, W.-S.; Tsai, T.-H.; Cheng, T.-W.; Yu, B.-S.; et al. Novel Technique for Preparing A Nano-Silver Water Suspension by The Arc-Discharge Method. Rev. Adv. Mater. Sci. 2008, 18, 752–758. [Google Scholar]
- Tien, D.C.; Tseng, K.H.; Liao, C.Y.; Huang, J.C.; Tsung, T.T. Discovery of Ionic Silver in Silver Nanoparticle Suspension Fabricated by Arc Discharge Method. J. Alloys Compd. 2008, 463, 408–411. [Google Scholar] [CrossRef]
- Elwakil, B.H.; Eldrieny, A.M.; Almotairy, A.R.Z.; El-Khatib, M. Potent Biological Activity of Newly Fabricated Silver Nanoparticles Coated by a Carbon Shell Synthesized by Electrical Arc. Sci. Rep. 2024, 14, 5324. [Google Scholar] [CrossRef]
- Gharieb, M.A.; Khalil, A.M.; Menshawy, S.; El-Aty, A.; El-Khatib, A.M. The Impact of Different Temperatures on NanoSilver Carbon Manufacturing by Arc Discharge Method. Alfarama J. Basic. Appl. Sci. 2024, 5, 409–416. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Mahdi, M.A.; Alizadeh, F.; Rashid, S.A. Laser Ablation Technique for Synthesis of Metal Nanoparticle in Liquid. In Laser Technology and Its Applications; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Rahmah, M.I.; Ahmed, A.M.; Rashid, T.M.; Qasim, A.J. Preparation of Silver Nanoparticles Using Laser Ablation for In Vitro Treatment of MCF-7 Cancer Cells with Antibacterial Activity. Plasmonics 2024, 19, 2097–2105. [Google Scholar] [CrossRef]
- Rafique, M.; Rafique, M.S.; Kalsoom, U.; Afzal, A.; Butt, S.H.; Usman, A. Laser Ablation Synthesis of Silver Nanoparticles in Water and Dependence on Laser Nature. Opt. Quantum Electron. 2019, 51, 179. [Google Scholar] [CrossRef]
- Kenmotsu, S.; Hirasawa, M.; Tamadate, T.; Matsumoto, C.; Osone, S.; Inomata, Y.; Seto, T. Surface-Enhanced Raman Scattering on Size-Classified Silver Nanoparticles Generated by Laser Ablation. ACS Omega 2024, 9, 37716–37723. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jawad, K.H.; Çevik, S.; Sulaiman, G.M.; Albukhaty, S.; Sasikumar, P. Investigating the Antimicrobial, Antioxidant, and Anticancer Effects of Elettaria Cardamomum Seed Extract Conjugated to Green Synthesized Silver Nanoparticles by Laser Ablation. Plasmonics 2024, 19, 1187–1200. [Google Scholar] [CrossRef]
- Niaz, U.; Hemat, S.; Jamil, A.; Aziz, M.S. Exploring the Relationship between Confinement Geometry and the Formation of High-Quality Silver Nanoparticles by Laser Ablation in Liquid Media. Indian. J. Phys. 2024, 98, 4989–4995. [Google Scholar] [CrossRef]
- Alharbi, A.M.; Ahmed, N.M.; Abdul Rahman, A.; Zahirah Noor Azman, N.; Algburi, S.; Wadi, I.A.; Binzowaimil, A.M.; Aldaghri, O.; Ibnaouf, K.H. Development of ZnO and Si Semiconductor-Based Ultraviolet Photodetectors Enhanced by Laser-Ablated Silver Nanoparticles. Photonics Nanostruct 2024, 58, 101228. [Google Scholar] [CrossRef]
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the Growth Parameters for Silver Nanoparticle Synthesis by Inert Gas Condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Jeevika, A.; Shankaran, D.R. Functionalized Silver Nanoparticles Probe for Visual Colorimetric Sensing of Mercury. Mater. Res. Bull. 2016, 83, 48–55. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Haider, S.; Al-Masry, W.A.; Al-Zeghayer, Y.; Imran, M.; Haider, A.; Ullah, Z. Engineered Nanostructures: A Review of Their Synthesis, Characterization and Toxic Hazard Considerations. Arab. J. Chem. 2017, 10, S376–S388. [Google Scholar] [CrossRef]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of Silver Nanoparticles Using Mangosteen Leaf Extract and Evaluation of Their Antimicrobial Activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5-100 Nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Khan, S.U.; Saleh, T.A.; Wahab, A.; Khan, M.H.U.; Khan, D.; Khan, W.U.; Rahim, A.; Kamal, S.; Khan, F.U.; Fahad, S. Nanosilver: New Ageless and Versatile Biomedical Therapeutic Scaffold. Int. J. Nanomed. 2018, 13, 733–762. [Google Scholar]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion Method: A Novel Route to Synthesize Organic and Inorganic Nanomaterials. 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar]
- Hak, J.; Jahan, I.; Sharma, K.; Farooqui, N.A. Article in Community Practitioner: The Journal of the Community Practitioners’ & Health Visitors’ Association. Community Pract. 2024, 83, 9–26. [Google Scholar] [CrossRef]
- dos Santos, M.A.; Paterno, L.G.; Moreira, S.G.C.; Sales, M.J.A. Original Photochemical Synthesis of Ag Nanoparticles Mediated by Potato Starch. SN Appl. Sci. 2019, 1, 554. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-Coated Silver Nanoparticles–A Comprehensive Toxicological Profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Pencheva, D.; Bryaskova, R.; Kantardjiev, T. Polyvinyl Alcohol/Silver Nanoparticles (PVA/AgNps) as a Model for Testing the Biological Activity of Hybrid Materials with Included Silver Nanoparticles. Mater. Sci. Eng. C 2012, 32, 2048–2051. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; El-Rafie, M.H.; Al-Deyab, S.S. Polyacrylamide/Guar Gum Graft Copolymer for Preparation of Silver Nanoparticles. Carbohydr. Polym. 2011, 85, 692–697. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A.; Kumar, S. Designing Silver Nanoparticles Impregnated Acacia and Tragacanth Gum Based Copolymeric Hydrogels for Drug Delivery Applications. Results Surf. Interfaces 2024, 16, 100256. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green. Sustain. Chem. 2016, 06, 34–56. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of Silver and Gold Nanoparticles by Novel Sundried Cinnamomum Camphora Leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green Synthesis of Silver Nanoparticles Using Azadirachta Indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green Synthesis of Silver Nanoparticles and Characterization of Their Inhibitory Effects on AGEs Formation Using Biophysical Techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef]
- Das, R.; Kumar, P.; Singh, A.K.; Agrawal, S.; Albukhaty, S.; Bhattacharya, I.; Tiwari, K.N.; Mishra, S.K.; Tripathi, A.K.; AlMalki, F.A.; et al. Green Synthesis of Silver Nanoparticles Using Trema Orientalis (L.) Extract and Evaluation of Their Antibacterial Activity. Green. Chem. Lett. Rev. 2025, 18, 2444679. [Google Scholar] [CrossRef]
- Singla, S.; Jana, A.; Thakur, R.; Kumari, C.; Goyal, S.; Pradhan, J. Green Synthesis of Silver Nanoparticles Using Oxalis Griffithii Extract and Assessing Their Antimicrobial Activity. OpenNano 2022, 7, 100047. [Google Scholar] [CrossRef]
- Widatalla, H.A.; Yassin, L.F.; Alrasheid, A.A.; Rahman Ahmed, S.A.; Widdatallah, M.O.; Eltilib, S.H.; Mohamed, A.A. Green Synthesis of Silver Nanoparticles Using Green Tea Leaf Extract, Characterization and Evaluation of Antimicrobial Activity. Nanoscale Adv. 2022, 4, 911–915. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Elshafie, H.S.; Pohl, P. Green Synthesis of Silver Nanoparticles (AgNPs) by Lallemantia Royleana Leaf Extract: Their Bio-Pharmaceutical and Catalytic Properties. J. Photochem. Photobiol. A Chem. 2024, 448, 115318. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Sánchez-Ante, G.; Cerro-López, M.; Minutti-Calva, Y.; Navarro-López, D.E.; Lozada-Ramírez, J.D.; Bach, H.; López-Mena, E.R.; Sánchez-Arreola, E. Green Synthesis of Silver Nanoparticles with Extracts from Kalanchoe Fedtschenkoi: Characterization and Bioactivities. Biomolecules 2024, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Dabirian, S.; Kariminejad, F.; Koohi, D.E.; Nemattalab, M.; Majidimoghadam, S.; Zamani, E.; Yousefbeyk, F. Process Optimization for Green Synthesis of Silver Nanoparticles Using Rubus Discolor Leaves Extract and Its Biological Activities against Multi-Drug Resistant Bacteria and Cancer Cells. Sci. Rep. 2024, 14, 4130. [Google Scholar] [CrossRef]
- Taleb Safa, M.A.; Koohestani, H. Green Synthesis of Silver Nanoparticles with Green Tea Extract from Silver Recycling of Radiographic Films. Results Eng. 2024, 21, 101808. [Google Scholar] [CrossRef]
- Losetty, V.; Devanesan, S.; AlSalhi, M.S.; Velu, P.P.; Muthupillai, D.; Kumar, K.A.; Lakkaboyana, S.K. Green Synthesis of Silver Nanoparticles Using Malachra Alceifolia (Wild Okra) for Wastewater Treatment and Biomedical Applications with Molecular Docking Approach. Environ. Sci. Pollut. Res. 2024, 31, 55562–55576. [Google Scholar] [CrossRef]
- Gangal, A.; Bachhar, V.; Joshi, V.; Akhtar, N.; Duseja, M.; Sethiya, N.K.; Shukla, R.K. Green Synthesis of Silver Nanoparticles from the Essential Oil of Curcuma Amada and Their Antihyperglycemic Effect in STZ Induced Diabetic Rats. Inorg. Chem. Commun. 2024, 168, 112873. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Vieira, Í.R.S.; Souza, L.M.d.S.; Florêncio, I.; Silva, I.G.M.d.; Tavares Junior, A.G.; Machado, Y.A.A.; Santos, L.C.d.; Taube, P.S.; Nakazato, G.; et al. Green Synthesis of Silver Nanoparticles Using Paullinia Cupana Kunth Leaf Extract Collected in Different Seasons: Biological Studies and Catalytic Properties. Pharmaceutics 2025, 17, 356. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.H.; Hosseinzadeh, H.; Cho, J.Y. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Maaz, K. Silver Nanoparticles: Fabrication, Characterization and Applications; IntechOpen: Rijeka, Croatia, 2018; ISBN 9781789234787. [Google Scholar]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and Characterization of Size- and Shape-Controlled Silver Nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Haes, A.J.; Haynes, C.L.; McFarland, A.D.; Schatz, G.C.; Van Duyne, R.P.; Zou, S. Plasmonic Materials for Surface-Enhanced Sensing and Spectroscopy. MRS Bull. 2005, 30, 368–375. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Bastys, V.; Pastoriza-Santos, I.; Rodríguez-González, B.; Vaisnoras, R.; Liz-Marzán, L.M. Formation of Silver Nanoprisms with Surface Plasmons at Communication Wavelengths. Adv. Funct. Mater. 2006, 16, 766–773. [Google Scholar] [CrossRef]
- Métraux, G.S.; Mirkin, C.A. Rapid Thermal Synthesis of Silver Nanoprisms with Chemically Tailorable Thickness. Adv. Mater. 2005, 17, 412–415. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Room Temperature, High-Yield Synthesis of Multiple Shapes of Gold Nanoparticles in Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of Polychrome Silver Nanoparticles in Different Solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyamae, N.; Lim, S.; Kimura, K.; Zhang, X.; Hikino, S.; Nishio, M. Crystal Structures and Growth Mechanisms of Au@Ag Core−Shell Nanoparticles Prepared by the Microwave−Polyol Method. Cryst. Growth Des. 2006, 6, 1801–1807. [Google Scholar] [CrossRef]
- Xiao, J.P.; Xie, Y.; Tang, R.; Chen, M.; Tian, X.B. Novel Ultrasonically Assisted Templated Synthesis of Palladium and Silver Dendritic Nanostructures. Adv. Mater. 2001, 13, 1887–1891. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-Assisted Synthesis of Metallic Nanostructures in Solution. Chem.–A Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the Surface Plasmon Resonance of Silver Nanostructures through Shape-Controlled Synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Murtaza, G.; Mehmood, A.; Bhatti, T.M. Green Synthesis of Silver Nanoparticles Using Leaves Extract of Skimmia Laureola: Characterization and Antibacterial Activity. Mater. Lett. 2015, 153, 10–13. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar]
- Dhayalan, M.; Riyaz, S.M.; Karikalan, P.; Srinivasan, N. Biomedical Applications of Silver Nanoparticles. In Silver Micro-Nanoparticles—Properties, Synthesis, Characterization, and Applications; Kumar, S., Kumar, P., Pathak, C.S., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-660-3. [Google Scholar]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Roduner, E. Nanoscopic Materials: Size-Dependent Phenomena and Growth Principles; RSC: London, UK, 2015; ISBN 9781782624943. [Google Scholar]
- Attarian Shandiz, M. Effective Coordination Number Model for the Size Dependency of Physical Properties of. J. Phys. Condens. Matter 2008, 20, 325237. [Google Scholar] [CrossRef]
- Allen, G.L.; Bayles, R.A.; Gile, W.W.; Jesser, W.A. Small Particle Melting of Pure Metals. Thin Solid. Film. 1986, 144, 297–308. [Google Scholar] [CrossRef]
- Ide, E.; Angata, S.; Hirose, A.; Kobayashi, K.F. Metal–Metal Bonding Process Using Ag Metallo-Organic Nanoparticles. Acta Mater. 2005, 53, 2385–2393. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-Controlled Synthesis of Nanoparticles. 2. Measurement of Extinction, Scattering, and Absorption Cross Sections. J. Phys. Chem. B 2004, 108, 13957–13962. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C. Optical Properties of Silver Nanoparticles. Phys. Status Solidi C 2007, 4, 4118–4126. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Bhagat, M.; Rajput, S.; Arya, S.; Khan, S.; Lehana, P. Biological and Electrical Properties of Biosynthesized Silver Nanoparticles. Bull. Mater. Sci. 2015, 38, 1253–1258. [Google Scholar]
- Shivananda, C.S.; Lakshmeesha Rao, B. Sangappa Structural, Thermal and Electrical Properties of Silk Fibroin–Silver Nanoparticles Composite Films. J. Mater. Sci. Mater. Electron. 2020, 31, 41–51. [Google Scholar] [CrossRef]
- Yamari, I.; Abchir, O.; Mali, S.N.; Errougui, A.; Talbi, M.; Kouali, M.E.; Chtita, S. The Anti-SARS-CoV-2 Activity of Novel 9, 10-Dihydrophenanthrene Derivatives: An Insight into Molecular Docking, ADMET Analysis, and Molecular Dynamics Simulation. Sci. Afr. 2023, 21, e01754. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis Approach-Dependent Antiviral Properties of Silver Nanoparticles and Nanocomposites. J. Nanostructure Chem. 2022, 12, 809–831. [Google Scholar]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Demchenko, V.; Mamunya, Y.; Sytnyk, I.; Iurzhenko, M.; Krivtsun, I.; Rybalchenko, N.; Naumenko, K.; Artiukh, L.; Kowalczuk, M.; Demchenko, O.; et al. Fabrication of Polylactide Composites with Silver Nanoparticles by Sputtering Deposition and Their Antimicrobial and Antiviral Applications. Polym. Int. 2025, 74, 207–216. [Google Scholar] [CrossRef]
- de Souza, T.B.; Rosa, A.S.; Constantino-Teles, P.; Ferreira, V.N.S.; Archanjo, B.S.; Soares, C.A.G.; Picciani, P.H.S.; Allão Cassaro, R.A.; Miranda, M.D.; Poneti, G. Silver Nanoparticles-Functionalized Textile against SARS-CoV-2: Antiviral Activity of the Capping Oleylamine Molecule. ACS Appl. Mater. Interfaces 2025, 17, 4. [Google Scholar] [CrossRef]
- Martín-Faivre, L.; Prince, L.; Cornu, C.; Villeret, B.; Sanchez-Guzman, D.; Rouzet, F.; Sallenave, J.M.; Garcia-Verdugo, I. Pulmonary Delivery of Silver Nanoparticles Prevents Influenza Infection by Recruiting and Activating Lymphoid Cells. Biomaterials 2025, 312, 122721. [Google Scholar] [CrossRef]
- EL Bagoury, G.F.; Mahmoud, A.H.; Kassem, S.; Elhabashy, R. Green Synthesis of Silver Nanoparticles Using Green Tea Extract and Evaluation of Their Antiviral Potential against Foot-and-Mouth Disease Virus Serotype O: An In-Vitro Study. Egypt. J. Vet. Sci. 2025, 1–11. [Google Scholar] [CrossRef]
- Gattucci, F.; Lallukka, M.; Grifasi, N.; Piumetti, M.; Miola, M. Tannic Acid-Assisted Green Functionalization of Clinoptilolite: A Step-by-Step Characterization of Silver Nanoparticles in Situ Reduction. Ceram. Int. 2025, 51, 13051–13057. [Google Scholar] [CrossRef]
- Amaral, M.V.M.V.; Carraro, C.B.; Antoniêto, A.C.C.; Costa, M.N.; Fraga-Silva, T.F.C.; Cipriano, U.G.; Abuná, R.P.F.; Rodrigues, T.S.; Martins, R.B.; Luzenti, A.M.; et al. Biogenic Silver Nanoparticles Produced by Trichoderma Reesei Inhibit SARS-CoV-2 Infection, Reduce Lung Viral Load and Ameliorate Acute Pulmonary Inflammation. Curr. Res. Biotechnol. 2025, 9, 100277. [Google Scholar] [CrossRef]
- Sahu, S.K.; Sahoo, P.R.; Dash, S.; Mishra, S.R.; Behera, P.C. Antimicrobial Activity of Silver Nanoparticles Against Common Bovine Mastitis Pathogens: A Comparative Analysis. Curr. Microbiol. 2025, 82, 121. [Google Scholar] [CrossRef]
- Obasi, D.E.; Nebolisa, N.M.; Akinwunmi, A.R.; Abimbolu, A.K.; Ezeorah, M.C.; Areola, O.M.; Donatus, U.D.; Oladipupo, V.T.; Ohiani, J.J.; Ayanleke, T.A.; et al. Eco-Friendly and Facile Production Method, Natural Products Chemistry, and Pharmacological Properties of Silver Nanoparticles Using Telfaria Occidentalis Leaf and Stem Extracts. Eur. J. Sustain. Dev. Res. 2025, 9, em0280. [Google Scholar] [CrossRef]
- Fereydani, M.; Jalalian, A.; Saber, N. Green Synthesis of Silver Nanoparticles from Cuscuta Epithymum Extract, Evaluation of Antibacterial, Antioxidant Activity, Cytotoxic Effect on MCF-7 Cell Line; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Długosz, O.; Żebracka, A.; Sochocka, M.; Franz, D.; Ochnik, M.; Chmielowiec-Korzeniowska, A.; Banach, M. Selective and Complementary Antimicrobial and Antiviral Activity of Silver, Copper, and Selenium Nanoparticle Suspensions in Deep Eutectic Solvent. Env. Environ. Res. 2025, 264, 120351. [Google Scholar] [CrossRef]
- Barabadi, H.; Vahidi, H.; Karami, K.; Kamali, M.; Jounaki, K.; Jahani, R.; Hosseini, O.; Amidi, S.; Ashouri, F. Cephalosporium Aphidicola-Derived Silver Nanoparticles: In Vitro Physicochemical, Antibacterial, Antifungal, Biofilm Inhibition, Biofilm Degradation, Antioxidant, Alpha-Amylase, and Urease Inhibitory Properties. Bionanoscience 2025, 15, 48. [Google Scholar] [CrossRef]
- Srikhao, N.; Ounkaew, A.; Srichiangsa, N.; Phanthanawiboon, S.; Boonmars, T.; Artchayasawat, A.; Theerakulpisut, S.; Okhawilai, M.; Kasemsiri, P. Green-Synthesized Silver Nanoparticle Coating on Paper for Antibacterial and Antiviral Applications. Polym. Bull. 2023, 80, 9651–9668. [Google Scholar] [CrossRef]
- Naumenko, K.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Antiviral Activity of Silver Nanoparticles against the Influenza A Virus. J. Virus Erad. 2023, 9, 100330. [Google Scholar] [CrossRef]
- Elnosary, M.E.; Aboelmagd, H.A.; Sofy, M.R.; Sofy, A.R.; Elshazly, E.H. Antiviral and Antibacterial Properties of Synthesis Silver Nanoparticles with Nigella Arvensis Aqueous Extract. Egypt. J. Chem. 2023, 66, 209–223. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Soliman, A.M.; Ismail, A.M.; Sattar, M.N.; Farroh, K.Y.; Shafie, R.M. Antiviral Activity of Chitosan Nanoparticles and Chitosan Silver Nanocomposites against Alfalfa Mosaic Virus. Polymers 2023, 15, 2961. [Google Scholar] [CrossRef]
- Doszpoly, A.; Shaalan, M.; El-Matbouli, M. Silver Nanoparticles Proved to Be Efficient Antivirals In Vitro against Three Highly Pathogenic Fish Viruses. Viruses 2023, 15, 1689. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Aseel, D.G.; El-Gendi, H.; Sobhy, S.; Samy, M.A.; Hamdy, E.; El-Messeiry, S.; Behiry, S.I.; Elbeaino, T.; Abdelkhalek, A. Antiviral Activity of Biosynthesized Silver Nanoparticles from Pomegranate (Punica Granatum L.) Peel Extract against Tobacco Mosaic Virus. Plants 2023, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral Activity of Mycosynthesized Silver Nanoparticles against Herpes Simplex Virus and Human Parainfluenza Virus Type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef]
- Makhlof, M.E.M.; Diab, H.A.; Mabrouk, M.E.; Abd El Kareem, M.S. Antiviral and Antioxidant Activity, Green Synthesis, and Optimization of Silver Nanoparticles Derived from Ulva Lactuca. Egypt. J. Phycol. 2024, 25, 1–42. [Google Scholar] [CrossRef]
- Butler, M.R.; Hrncirova, J.; Jacot, T.A.; Dutta, S.; Clark, M.R.; Doncel, G.F.; Cooper, J.B. Detection and Quantification of Antiviral Drug Tenofovir Using Silver Nanoparticles and Surface Enhanced Raman Spectroscopy (SERS) with Spatially Resolved Hotspot Selection. Front. Nanotechnol. 2023, 5, 1270474. [Google Scholar] [CrossRef]
- Abdulsattar Ali, A.; Tahir Maher, F.; Ahmed Al-Bajari, S. Green biosynthesis of silver nanoparticles from taraxacum officinale roots plant and studying its antiviral properties to coronavirus (SARS-CoV-2) infected lung cells. J. Hyg. Eng. Des. 2023, 42, 361–369. [Google Scholar]
- Khan, R.; Naureen, H.; Javed, A.; Khalid, M.; Khan, H. Alocasia Odora–Mediated Synthesis of Silver Nanoparticles, Their Cytotoxicity, and Virucidal Potential. Appl. Microbiol. Biotechnol. 2023, 107, 111–123. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Bosch, R.; Morey, J.; Bastidas-Caldes, C.; Torres, M.; Toscano, F.; Debut, A.; Pazmiño-Viteri, K.; de las Nieves Piña, M. High in Vitro Activity of Gold and Silver Nanoparticles from Solanum Mammosum L. against SARS-CoV-2 Surrogate Phi6 and Viral Model PhiX174. Nanotechnology 2023, 34, 175705. [Google Scholar] [CrossRef]
- 111. Rybalchenko, N.P.; Hnatiuk; Artiukh, L.O.; Naumenko, S.; Zaremba, P.Y.; Demchenko, V.L.; Kokhtych, L.M.; Iurzhenko, M.V.; Rybalchenko, T.V.; Ovsyankina, V.; et al. Antimicrobial and Antiviral Activity of Nanocomposites Based on Polyelectrolyte Complexes with Silver Nanoparticles. Mikrobiolohichnyi Zhurnal 2024, 86, 36–50. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Van Den Hengel, S.K.; Raza, B.G.; Rutjes, S.A.; De Roda Husman, A.M.; Peijnenburg, W.J.G.M.; Roesink, H.D.W.; De Vos, W.M. Surface Chemistry-Dependent Antiviral Activity of Silver Nanoparticles. Nanotechnology 2021, 32, 365101. [Google Scholar] [CrossRef]
- Bharti, S.; Mukherji, S.; Mukherji, S. Antiviral Application of Colloidal and Immobilized Silver Nanoparticles. Nanotechnology 2021, 32, 205102. [Google Scholar] [CrossRef]
- Emam, M.H.; Elezaby, R.S.; Swidan, S.A.; Loutfy, S.A.; Hathout, R.M. Enhancing Polyacrylonitrile Nanofibers Antiviral Activity Using Greenly Synthesized Silver Nanoparticles. Arch. Pharm. 2025, 358, e202400943. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An Overview of Functional Nanoparticles as Novel Emerging Antiviral Therapeutic Agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef]
- Park, S.J.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G.P. Antiviral Properties of Silver Nanoparticles on a Magnetic Hybrid Colloid. Appl. Env. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral Surfaces and Coatings and Their Mechanisms of Action. Commun. Mater. 2021, 2, 53. [Google Scholar]
- Sadiq, S.; Khan, I.; Shen, Z.; Wang, M.; Xu, T.; Khan, S.; Zhou, X.; Bahadur, A.; Rafiq, M.; Sohail, S.; et al. Recent Updates on Multifunctional Nanomaterials as Antipathogens in Humans and Livestock: Classification, Application, Mode of Action, and Challenges. Molecules 2023, 28, 7674. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Hadinejad, F.; Morad, H.; Jahanshahi, M.; Zarrabi, A.; Pazoki-Toroudi, H.; Mostafavi, E. A Novel Vision of Reinforcing Nanofibrous Masks with Metal Nanoparticles: Antiviral Mechanisms Investigation. Adv. Fiber Mater. 2023, 5, 1273–1317. [Google Scholar]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Manisekaran, R.; Chettiar, A.-D.R.; Marasamy, L.; Ibarra, V.C.; Lopez-Ayuso, C.A.; Chavez-Granados, P.A.; Kandasamy, G.; Acosta-Torres, L.S.; Arthikala, M.-K. Silver-Nanoparticles-Based Composites for Antimicrobial Applications: An Update. ChemistrySelect 2024, 9, e202403772. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity─A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobełny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef]
- Frippiat, T.; Art, T.; Delguste, C. Silver Nanoparticles as Antimicrobial Agents in Veterinary Medicine: Current Applications and Future Perspectives. Nanomaterials 2025, 15, 202. [Google Scholar] [CrossRef]
- Wang, D.; Yin, C.; Bai, Y.; Zhou, M.; Wang, N.; Tong, C.; Yang, Y.; Liu, B. Chitosan-Modified AgNPs Efficiently Inhibit Swine Coronavirus-Induced Host Cell Infections via Targeting the Spike Protein. Biomolecules 2024, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
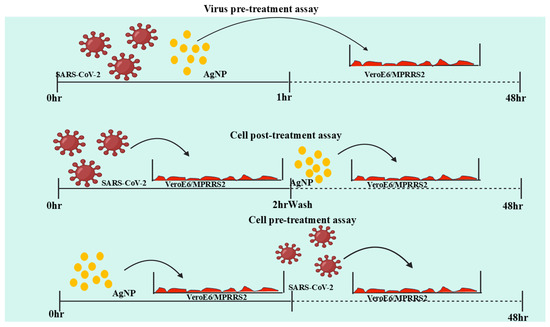
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.N.; Pandey, A. Multiple QSAR and Molecular Modelling for Identification of Potent Human Adenovirus Inhibitors. J. Indian. Chem. Soc. 2021, 98, 100082. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Rodriguez-Padilla, C. PVP-Coated Silver Nanoparticles Block the Transmission of Cell-Free and Cell-Associated HIV-1 in Human Cervical Culture. J. Nanobiotechnology 2010, 8, 15. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of Antiviral Action of Silver Nanoparticles against HIV-1. J. Nanobiotechnology 2010, 8, 1. [Google Scholar]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 Attachment, Entry, and Cell-to-Cell Spread by Functionalized Multivalent Gold Nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Sun, L.; Singh, A.; Vig, K.; Pillai, S.; Singh, S. Silver Nanoparticles Inhibit Replication of Respiratory Syncytial Virus. J. Biomed. Nanotechnol. 2008, 4, 149–158. [Google Scholar]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A Preliminary Assessment of Silver Nanoparticle Inhibition of Monkeypox Virus Plaque Formation. Nanoscale Res. Lett. 2008, 3, 129. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of Influenza Virus Infection by Multivalent Sialic-Acid- Functionalized Gold Nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Mali, S.N.; Pratap, A.P.; Thorat, B.R. The Rise of New Coronavirus Infection (COVID-19): A Recent Update and Potential Therapeutic Candidates. Eurasian J. Med. Oncol. 2020, 4, 35–41. [Google Scholar]
- Habibi, S.; Ghajarieh, A. Application of Nanofibers in Virus and Bacteria Filtration. Russ. J. Appl. Chem. 2022, 95, 486–498. [Google Scholar]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver Nanoparticles Synthesis Mediated by New Isolates of Bacillus Spp., Nanoparticle Characterization and Their Activity against Bean Yellow Mosaic Virus and Human Pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnology 2005, 3, 6. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory Effects of Silver Nanoparticles against Adenovirus Type 3 in Vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Kamarudin, D.; Hashim, N.A.; Ong, B.H.; Che Hassan, C.R.; Abdul Manaf, N. Synthesis of Silver Nanoparticles Stabilised by PVP for Polymeric Membrane Application: A Comparative Study. Mater. Technol. 2022, 37, 289–301. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Env. Environ. Saf. 2023, 253, 114636. [Google Scholar]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2021, 81, 859–863. [Google Scholar]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.; Al-Saeed, M.; Abid, H. Safety Assessment and Biochemical Evaluation of the Effect of Biogenic Silver Nanoparticles (Using Bark Extract of C. Zeylanicum) on Rattus Norvegicus Rats. Baghdad J. Biochem. Appl. Biol. Sci. 2021, 2, 133–145. [Google Scholar] [CrossRef]
- Vuković, B.; Milić, M.; Dobrošević, B.; Milić, M.; Ilić, K.; Pavičić, I.; Šerić, V.; Vrček, I.V. Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells. Nanomaterials 2020, 10, 1390. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar]
- Elyousfi, S.; Dellali, M.; Mezni, A.; Ben Ali, M.; Hedfi, A.; Almalki, M.; Mezni, A.; Rohal-Lupher, M.; Dervishi, A.; Boufahja, F. Toxicity of Silver Nanoparticles on the Clam Ruditapes Decussatus Assessed through Biomarkers and Clearance Rate. Mater. Res. Express 2021, 8, 105005. [Google Scholar] [CrossRef]
- Pinheiro, S.K.d.P.; Lima, A.K.M.; Miguel, T.B.A.R.; Filho, A.G.S.; Ferreira, O.P.; Pontes, M.d.S.; Grillo, R.; Miguel, E.d.C. Assessing Toxicity Mechanism of Silver Nanoparticles by Using Brine Shrimp (Artemia Salina) as Model. Chemosphere 2024, 347, 140673. [Google Scholar] [CrossRef]
- Al Baloushi, K.S.Y.; Senthilkumar, A.; Kandhan, K.; Subramanian, R.; Kizhakkayil, J.; Ramachandran, T.; Shehab, S.; Kurup, S.S.; Alyafei, M.A.M.; Al Dhaheri, A.S.; et al. Green Synthesis and Characterization of Silver Nanoparticles Using Moringa Peregrina and Their Toxicity on MCF-7 and Caco-2 Human Cancer Cells. Int. J. Nanomed. 2024, 19, 3891–3905. [Google Scholar] [CrossRef]
- Taha, N.M.; Youssef, F.S.; Auda, H.M.; El-Bahy, M.M.; Ramadan, R.M. Efficacy of Silver Nanoparticles against Trichinella Spiralis in Mice and the Role of Multivitamin in Alleviating Its Toxicity. Sci. Rep. 2024, 14, 5843. [Google Scholar] [CrossRef]
- Dinç, B. Comprehensive Toxicity Assessment of Silver Nanoparticles on Bacteria, Human Vein Endothelial Cells, and Caenorhabditis Elegans. Results Chem. 2025, 14, 102092. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-Term Exposure to High-Concentration Silver Nanoparticles Induced Toxicity, Fatality, Bioaccumulation, and Histological Alteration in Fish (Cyprinus Carpio). Env. Environ. Sci. Eur. 2021, 33, 14. [Google Scholar] [CrossRef]
- Thwala, M.; Klaine, S.; Musee, N. Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia Minima. Molecules 2021, 26, 2305. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhou, Y.; Zhu, P.; Bao, X.; Su, P. Polystyrene Nanoplastics Mediated the Toxicity of Silver Nanoparticles in Zebrafish Embryos. Front. Mar. Sci. 2023, 10, 1195125. [Google Scholar] [CrossRef]
- Sredojević, D.; Lazić, V.; Pirković, A.; Periša, J.; Murafa, N.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Zarubica, A.; Jovanović Krivokuća, M.; et al. Toxicity of Silver Nanoparticles Supported by Surface-Modified Zirconium Dioxide with Dihydroquercetin. Nanomaterials 2022, 12, 3195. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 136765. [Google Scholar] [CrossRef]
- Santos, T.S.; Silva, T.M.; Cardoso, J.C.; de Albuquerque-Júnior, R.L.C.; Zielinska, A.; Souto, E.B.; Severino, P.; Mendonça, M.D.C. Biosynthesis of Silver Nanoparticles Mediated by Entomopathogenic Fungi: Antimicrobial Resistance, Nanopesticides, and Toxicity. Antibiotics 2021, 10, 852. [Google Scholar]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The Toxic Effect of Silver Ions and Silver Nanoparticles towards Bacteria and Human Cells Occurs in the Same Concentration Range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Cho, Y.M.; Mizuta, Y.; Akagi, J.I.; Toyoda, T.; Sone, M.; Ogawa, K. Size-Dependent Acute Toxicity of Silver Nanoparticles in Mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and Action Mechanisms of Silver Nanoparticles against the Mycotoxin-Producing Fungus Fusarium Graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, L.; Wang, Y.; Li, B.; Zhang, W.; Li, X. Toxicity Mechanism of Silver Nanoparticles to Chlamydomonas Reinhardtii: Photosynthesis, Oxidative Stress, Membrane Permeability, and Ultrastructure Analysis. Environ. Sci. Pollut. Res. 2020, 28, 15032–15042. [Google Scholar] [CrossRef]
- Souza, L.R.R.; Corrêa, T.Z.; Thaís Bruni, A.; Da Veiga, M.A.M.S. The Effects of Solubility of Silver Nanoparticles, Accumulation, and Toxicity to the Aquatic Plant Lemna Minor. Environ. Sci. Pollut. Res. 2021, 28, 16720–16733. [Google Scholar] [CrossRef]
- Ke, M.; Li, Y.; Qu, Q.; Ye, Y.; Peijnenburg, W.J.G.M.; Zhang, Z.; Xu, N.; Lu, T.; Sun, L.; Qian, H. Offspring Toxicity of Silver Nanoparticles to Arabidopsis Thaliana Flowering and Floral Development. J. Hazard. Mater. 2020, 386, 121975. [Google Scholar] [CrossRef]
- Marinho, C.S.; Matias, M.V.F.; Toledo, E.K.M.; Smaniotto, S.; Ximenes-da-Silva, A.; Tonholo, J.; Santos, E.L.; Machado, S.S.; Zanta, C.L.P.S. Toxicity of Silver Nanoparticles on Different Tissues in Adult Danio Rerio. Fish. Physiol. Biochem. 2021, 47, 239–249. [Google Scholar] [CrossRef]
- Maziero, J.S.; Thipe, V.C.; Rogero, S.O.; Cavalcante, A.K.; Damasceno, K.C.; Ormenio, M.B.; Martini, G.A.; Batista, J.G.S.; Viveiros, W.; Katti, K.K.; et al. Species-Specific in Vitro and in Vivo Evaluation of Toxicity of Silver Nanoparticles Stabilized with Gum Arabic Protein. Int. J. Nanomed. 2020, 15, 7359–7376. [Google Scholar] [CrossRef]
- Abdelkhaliq, A.; Van Der Zande, M.; Peters, R.J.B.; Bouwmeester, H. Combination of the BeWo B30 Placental Transport Model and the Embryonic Stem Cell Test to Assess the Potential Developmental Toxicity of Silver Nanoparticles. Part. Fibre Toxicol. 2020, 17, 11. [Google Scholar] [CrossRef]
- Chen, R.J.; Huang, C.C.; Pranata, R.; Lee, Y.H.; Chen, Y.Y.; Wu, Y.H.; Wang, Y.J. Modulation of Innate Immune Toxicity by Silver Nanoparticle Exposure and the Preventive Effects of Pterostilbene. Int. J. Mol. Sci. 2021, 22, 2536. [Google Scholar] [CrossRef] [PubMed]
- Michalec, S.; Nieckarz, W.; Klimek, W.; Lange, A.; Matuszewski, A.; Piotrowska, K.; Hotowy, A.; Kunowska-Slósarz, M.; Sosnowska, M. Green Synthesis of Silver Nanoparticles from Chlorella vulgaris Aqueous Extract and Their Effect on Salmonella enterica and Chicken Embryo Growth. Molecules 2025, 30, 1521. [Google Scholar] [CrossRef]
- Emma, N.; Judith, S.; Peter, M.; Naomi, M. Sub-Acute and Chronic Toxicity of Silver Nanoparticles Synthesized by Azadirachta Indica Extract. Afr. J. Biotechnol. 2020, 19, 320–331. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hao, Z.; Fadare, O.O.; Hanachi, P.; Chen, Y.; Liu, J. Toxicity of Biosynthesized Silver Nanoparticles to Aquatic Organisms of Different Trophic Levels. Chemosphere 2020, 258, 127346. [Google Scholar] [CrossRef]
- Somda, D.; Bargul, J.L.; Wesonga, J.M.; Wachira, S.W. Green Synthesis of Brassica Carinata Microgreen Silver Nanoparticles, Characterization, Safety Assessment, and Antimicrobial Activities. Sci. Rep. 2024, 14, 29273. [Google Scholar] [CrossRef]
- Sati, A.; Nandiwdekar, O.; Ratnaparkhi, A.; Doke, R.B.; Pinjari, D.V.; Mali, S.N.; Pratap, A.P. Bio-Based Alkyd–Polyesteramide–Polyurethane Coatings from Castor, Neem, and Karanja Oils with Inherent Antimicrobial Properties for Enhanced Hygiene. Coatings 2025, 15, 370. [Google Scholar] [CrossRef]
- Haghighat, F.; Kim, Y.; Sourinejad, I.; Yu, I.J.; Johari, S.A. Titanium Dioxide Nanoparticles Affect the Toxicity of Silver Nanoparticles in Common Carp (Cyprinus Carpio). Chemosphere 2021, 262, 127805. [Google Scholar] [CrossRef]
- Chen, F.; Aqeel, M.; Maqsood, M.F.; Khalid, N.; Irshad, M.K.; Ibrahim, M.; Akhter, N.; Afzaal, M.; Ma, J.; Hashem, M.; et al. Mitigation of Lead Toxicity in Vigna Radiata Genotypes by Silver Nanoparticles. Environ. Pollut. 2022, 308, 119606. [Google Scholar] [CrossRef]
- Chi, Z.; Lin, H.; Li, W.; Zhang, X.; Zhang, Q. In vitro assessment of the toxicity of small silver nanoparticles and silver ions to the red blood cells. Environ. Sci. Pollut. Res. 2018, 25, 32373–32380. [Google Scholar]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The Targeted Antibacterial and Antifungal Properties of Magnetic Nanocomposite of Iron Oxide and Silver Nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mendieta, R.; Nguyen, N.H.A.; Guadagnini, A.; Semerad, J.; Łukowiec, D.; Parma, P.; Yang, J.; Agnoli, S.; Sevcu, A.; Cajthaml, T.; et al. Growth Suppression of Bacteria by Biofilm Deterioration Using Silver Nanoparticles with Magnetic Doping. Nanoscale 2022, 14, 18143–18156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, H.; Yan, J.; Cai, Y. Immobilizing Silver Nanoparticles onto the Surface of Magnetic Silica Composite to Prepare Magnetic Disinfectant with Enhanced Stability and Antibacterial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 186–192. [Google Scholar] [CrossRef]
- Ratti, M.; Naddeo, J.J.; Tan, Y.; Griepenburg, J.C.; Tomko, J.; Trout, C.; O’Malley, S.M.; Bubb, D.M.; Klein, E.A. Irradiation with Visible Light Enhances the Antibacterial Toxicity of Silver Nanoparticles Produced by Laser Ablation. Appl. Phys. A Mater. Sci. Process 2016, 122, 346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).