Exercise Attenuates Skeletal Muscle Atrophy in Senescent SAMP8 Mice: Metabolic Insights from NMR-Based Metabolomics
Abstract
1. Introduction
2. Results
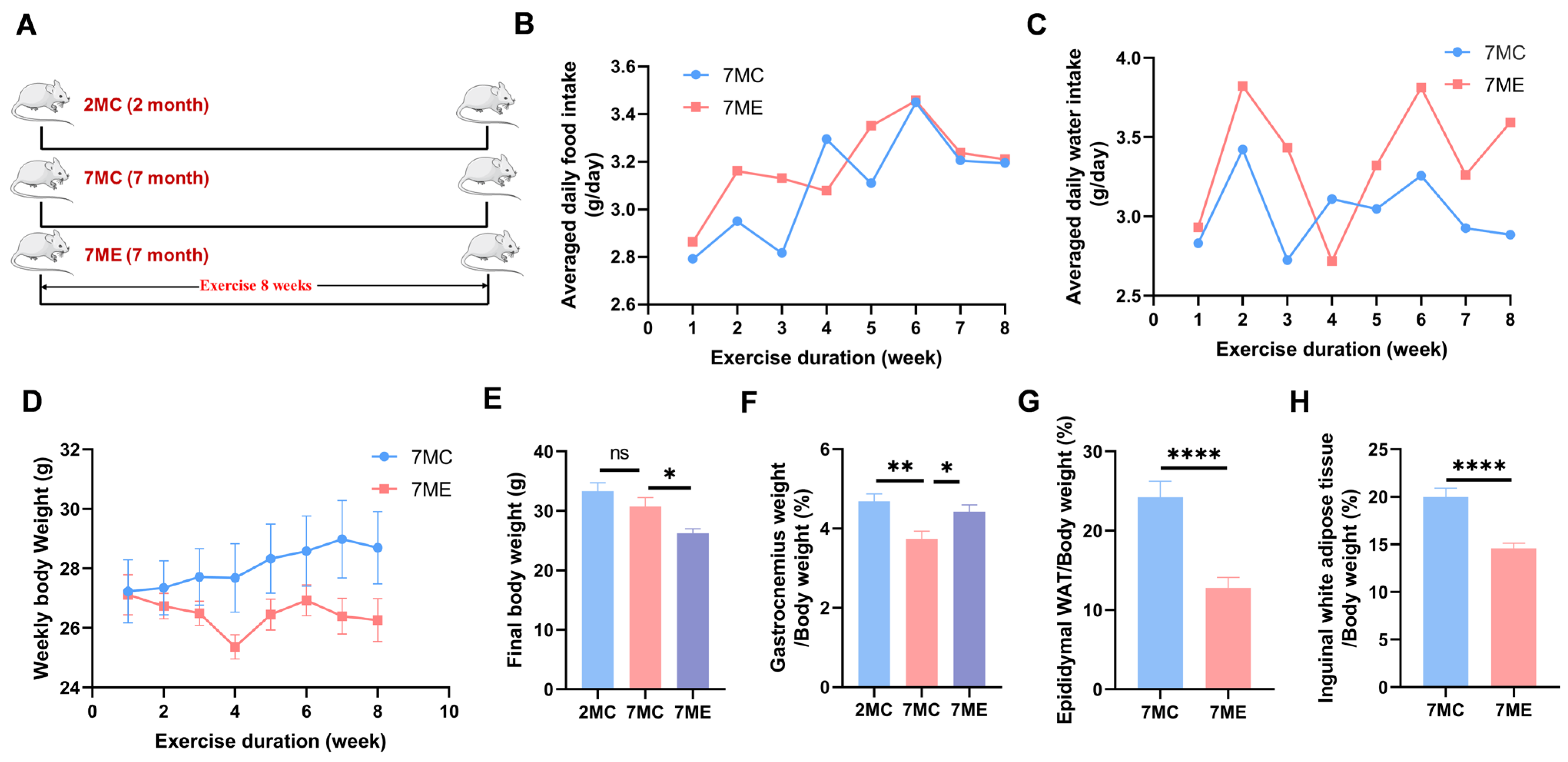
2.1. Exercise Improved Growth Performance in SAMP8 Mice
2.2. Exercise Enhanced the Strength and Muscle Fiber Area of Mouse Gastrocnemius Muscle
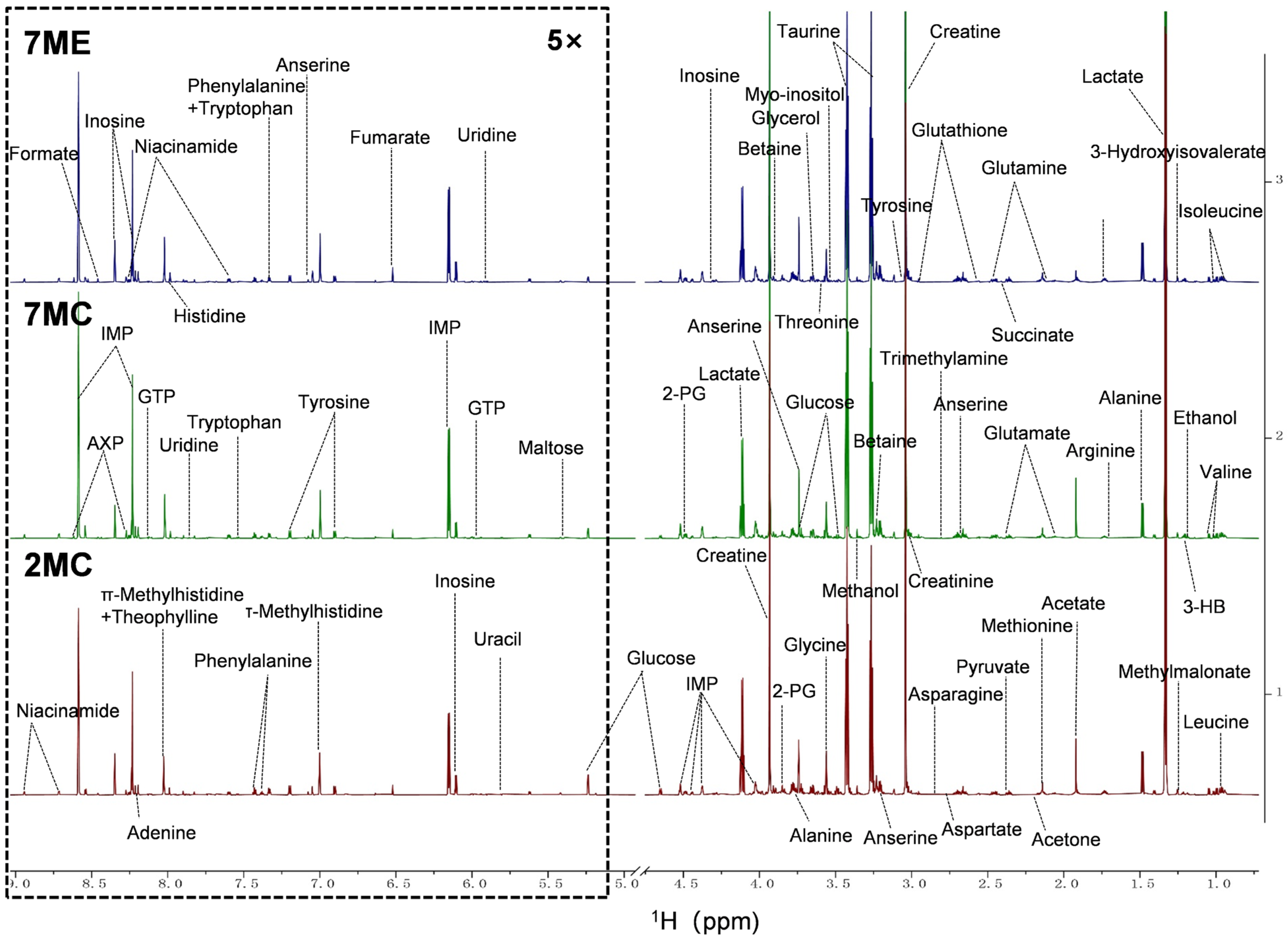
2.3. Exercise Induced Changes in the Metabolic Profile of Mouse Gastrocnemius Muscle
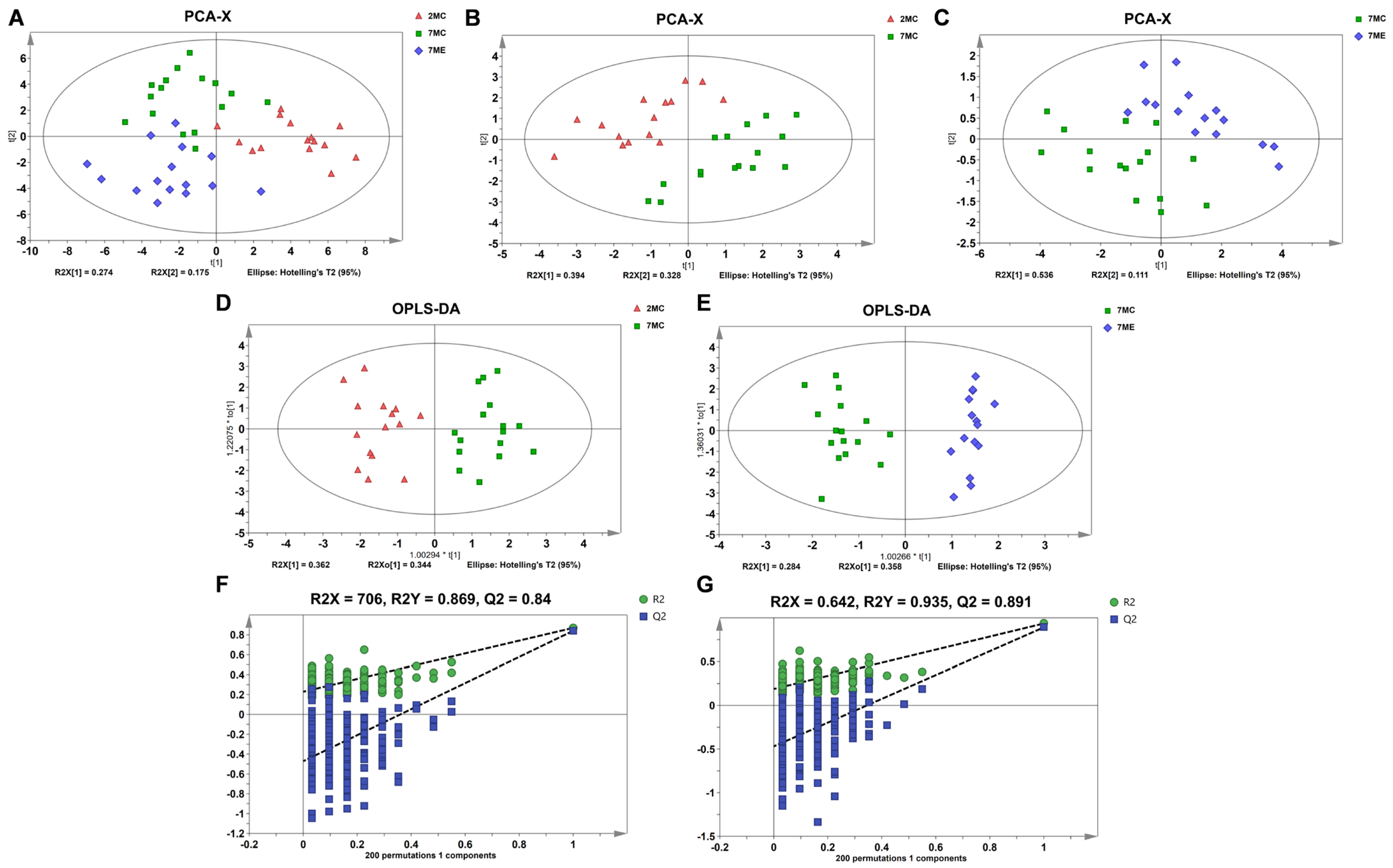
2.4. Exercise Intervention Altered the Levels of Aqueous Metabolites in Mouse Gastrocnemius Muscle
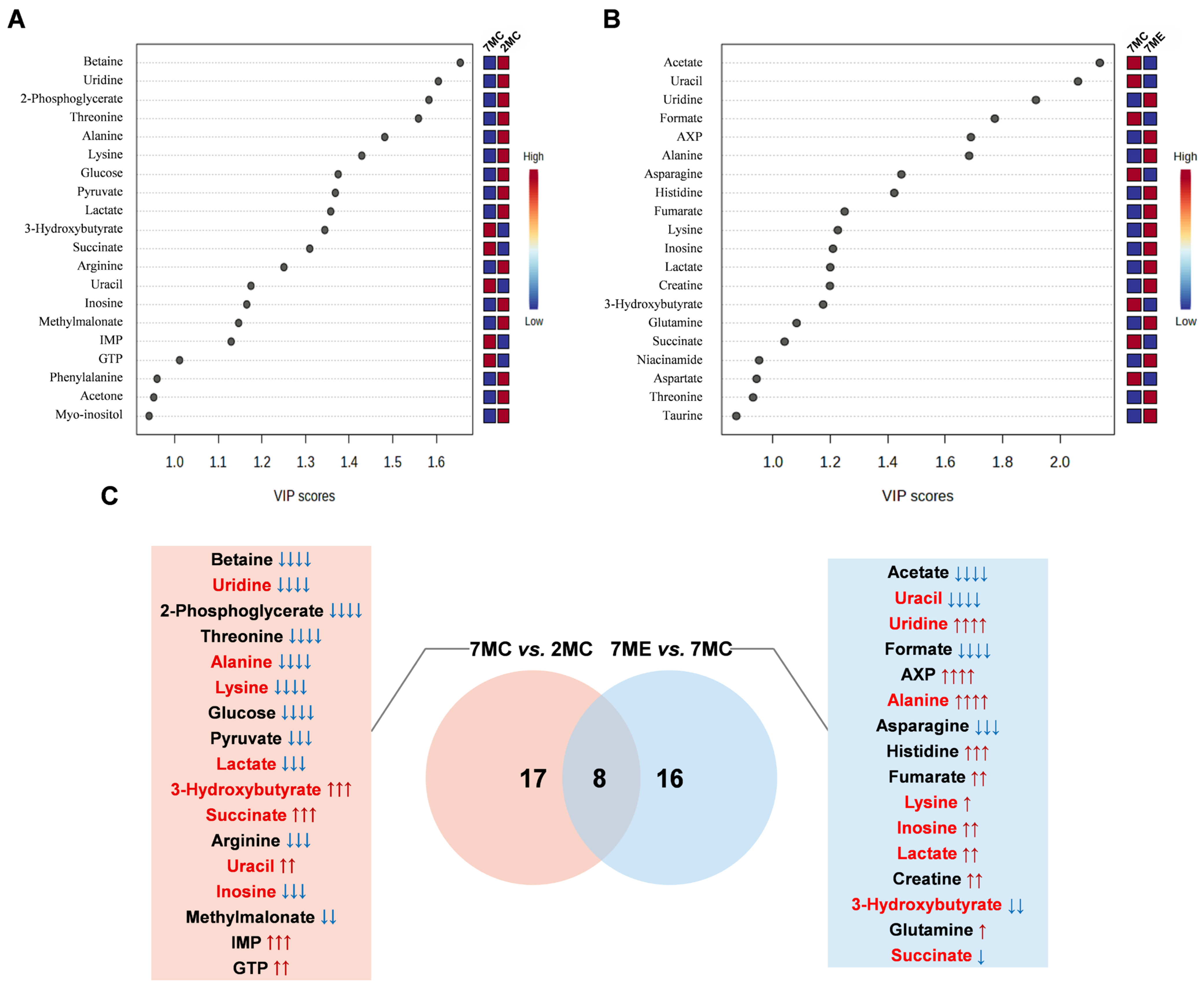
2.5. Identification of Characteristic Metabolites in Pairwise Comparisons Between Groups
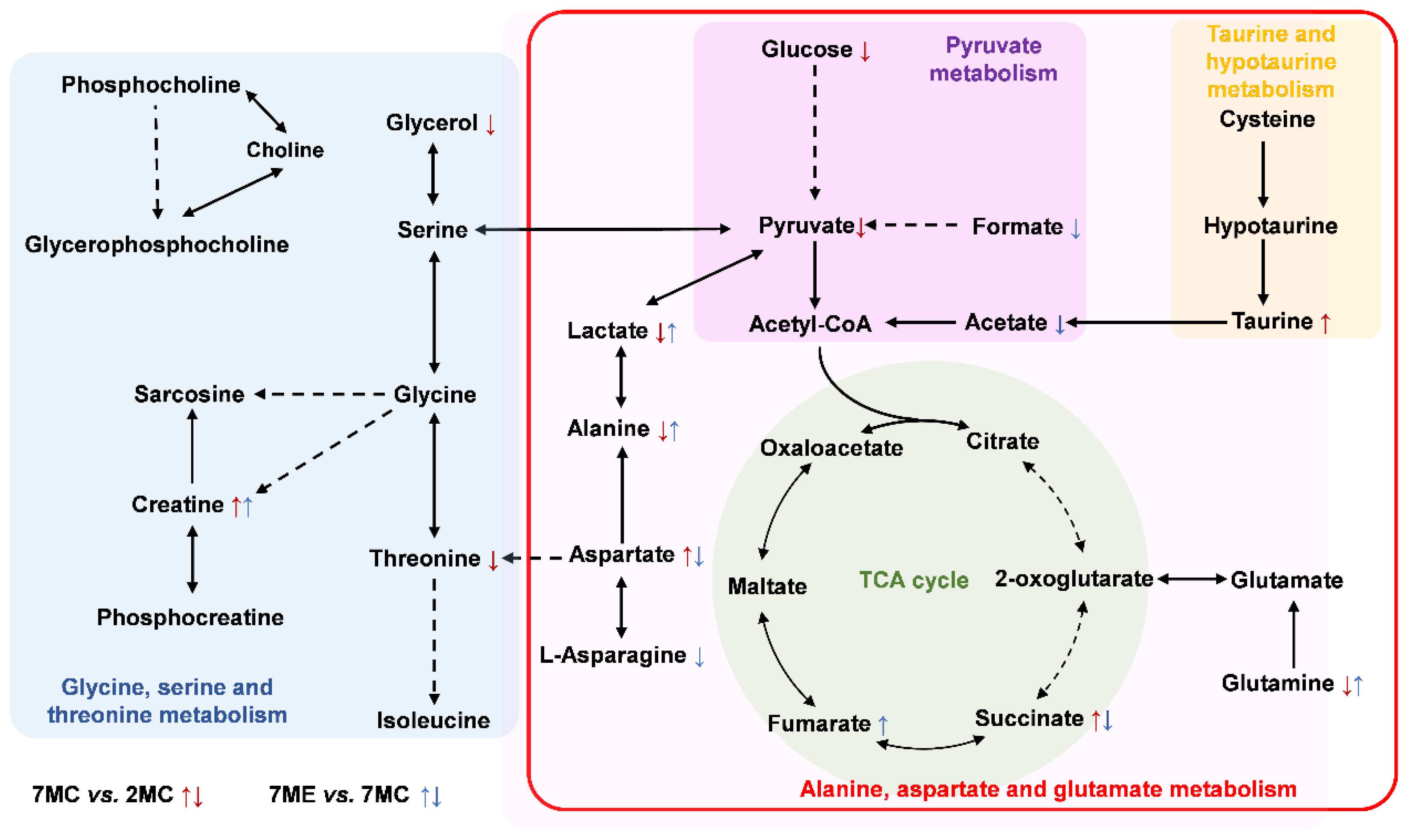
2.6. Metabolic Pathways Altered by Exercise Intervention
3. Discussion
3.1. Exercise Modulated Glycine, Serine, and Threonine Metabolism
3.2. Exercise Modulated Alanine, Aspartate, and Glutamate Metabolism
3.3. Exercise Modulated Pyruvate Metabolism
3.4. Exercise Modulated Taurine and Hypotaurine Metabolism
4. Materials and Methods
4.1. Animal Experiments
4.2. Grip Strength Test
4.3. Assessment of Gastrocnemius Muscle Contractility
4.4. Morphometric Evaluation of Gastrocnemius Muscle Fibers
4.5. Preparation of Gastrocnemius Muscle Samples for NMR Experiments
4.6. NMR Experiments
4.7. NMR Spectral Data Processing and Resonance Assignment
4.8. Multivariate Statistical Analysis and Significant Metabolite Identification
4.9. Univariate Statistical Analysis and Differential Metabolite Identification
4.10. Metabolic Pathway Analysis and Significant Pathway Identification
4.11. General Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diz, J.B.; Leopoldino, A.A.; Moreira, B.S.; Henschke, N.; Dias, R.C.; Pereira, L.S.; Oliveira, V.C. Prevalence of sarcopenia in older Brazilians: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2017, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Singh, H.; Carter, S.J.; Bryan, D.R.; Fisher, G. Sarcopenia and its implications for metabolic health. J. Obes. 2019, 2019, 8031705. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E. Sarcopenia and cardiovascular diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Yang, J.; Cao, R.Y.; Li, Q.; Zhu, F. Muscle atrophy in cancer. Muscle Atrophy 2018, 1088, 329–346. [Google Scholar]
- Fujiwara, M.; Ferdousi, F.; Isoda, H. Investigation into Molecular Brain Aging in Senescence-Accelerated Mouse (SAM) Model Employing Whole Transcriptomic Analysis in Search of Potential Molecular Targets for Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 13867. [Google Scholar] [CrossRef]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Müller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Wei, C.; Xu, M.; Li, Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2022, 14, 2796. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, P.H.; Zhang, M.; Du, J.R. Aging-related renal injury and inflammation are associated with downregulation of Klotho and induction of RIG-I/NF-κB signaling pathway in senescence-accelerated mice. Aging Clin. Exp. Res. 2016, 28, 69–76. [Google Scholar] [CrossRef]
- Bernstein, L.R.; Mackenzie, A.C.; Kraemer, D.C.; Morley, J.E.; Farr, S.; Chaffin, C.L.; Merchenthaler, I. Shortened estrous cycle length, increased FSH levels, FSH variance, oocyte spindle aberrations, and early declining fertility in aging senescence-accelerated mouse prone-8 (SAMP8) mice: Concomitant characteristics of human midlife female reproductive aging. Endocrinology 2014, 155, 2287–2300. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Babu, S.S.; Palaniyandi, S.S.; Watanabe, K.; Cooke, J.P.; Thandavarayan, R.A. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res. Rev. 2017, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Takahashi, J.A.; Matsushita, T.; Ohnishi, K.; Higuchi, K.; Hashimoto, N.; Hosokawa, M. Tubular aggregates in the skeletal muscle of the senescence-accelerated mouse; SAM. Mech. Ageing Dev. 2000, 114, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp. Gerontol. 2005, 40, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.Y.; Leung, K.S.; Siu, P.M.; Qin, J.H.; Chow, S.K.; Qin, L.; Li, C.Y.; Cheung, W.H. Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef]
- Böhm, A.; Hoffmann, C.; Irmler, M.; Schneeweiss, P.; Schnauder, G.; Sailer, C.; Schmid, V.; Hudemann, J.; Machann, J.; Schick, F.; et al. TGF-β Contributes to Impaired Exercise Response by Suppression of Mitochondrial Key Regulators in Skeletal Muscle. Diabetes 2016, 65, 2849–2861. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- Cunha, T.F.; Bacurau, A.V.; Moreira, J.B.; Paixão, N.A.; Campos, J.C.; Ferreira, J.C.; Leal, M.L.; Negrão, C.E.; Moriscot, A.S.; Wisløff, U.; et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS ONE 2012, 7, e41701. [Google Scholar] [CrossRef]
- Slavin, M.B.; Khemraj, P.; Hood, D.A. Exercise, mitochondrial dysfunction and inflammasomes in skeletal muscle. Biomed. J. 2024, 47, 100636. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ma, S.; Cai, Y.; Wang, S.; Ren, J.; Yang, Y.; Ping, J.; Wang, X.; Zhang, Y.; Yan, H.; et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation 2023, 4, 100380. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, N.R.; Harridge, S.D. Exercise, physiological function, and the selection of participants for aging research. J. Gerontol. Ser. A Biomed. Sci. Med. 2010, 65, 854–857. [Google Scholar] [CrossRef]
- Gremeaux, V.; Gayda, M.; Lepers, R.; Sosner, P.; Juneau, M.; Nigam, A. Exercise and longevity. Maturitas 2012, 73, 312–317. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef]
- Shur, N.; Creedon, L.; Skirrow, S.; Atherton, P.; MacDonald, I.; Lund, J.; Greenhaff, P. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 2021, 68, 101344. [Google Scholar]
- Shan, S.; Hoffman, J.M. Serine metabolism in aging and age-related diseases. GeroScience 2025, 47, 611–630. [Google Scholar] [CrossRef]
- Tsoi, B.M.; Beckhouse, A.G.; Gelling, C.L.; Raftery, M.J.; Chiu, J.; Tsoi, A.M.; Lauterbach, L.; Rogers, P.J.; Higgins, V.J.; Dawes, I.W. Essential role of one-carbon metabolism and Gcn4p and Bas1p transcriptional regulators during adaptation to anaerobic growth of Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 11205–11215. [Google Scholar] [CrossRef]
- Eller, L.; Wang, L.; Gok, M.O.; Hocaoglu, H.; Qin, S.; Gupta, P.; Sieber, M.H. GSK3 coordinately regulates mitochondrial activity and nucleotide metabolism in quiescent oocytes. Biol. Open 2025, 14, bio061815. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Adelnia, F.; Moaddel, R.; Ferrucci, L. Searching for a mitochondrial root to the decline in muscle function with ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Maj, M.C.; Levandovskiy, V.; MacKay, N.; Shelton, G.D.; Robinson, B.H. Identification of a canine model of pyruvate dehydrogenase phosphatase 1 deficiency. Mol. Genet. Metab. 2007, 90, 15–23. [Google Scholar] [CrossRef] [PubMed]
- DeBalsi, K.L.; Wong, K.E.; Koves, T.R.; Slentz, D.H.; Seiler, S.E.; Wittmann, A.H.; Ilkayeva, O.R.; Stevens, R.D.; Perry, C.G.; Lark, D.S.; et al. Targeted metabolomics connects thioredoxin-interacting protein (TXNIP) to mitochondrial fuel selection and regulation of specific oxidoreductase enzymes in skeletal muscle. J. Biol. Chem. 2014, 289, 8106–8120. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.Y.; Al-Helal, M.; Roslaini, A.M.; Fisher, N.; Bray, P.G.; Ward, S.A.; Biagini, G.A. Glycerol: An unexpected major metabolite of energy metabolism by the human malaria parasite. Malar. J. 2009, 8, 38. [Google Scholar] [CrossRef]
- Kernec, F.; Le Tallec, N.; Nadal, L.; Bégué, J.M.; Le Rumeur, E. Phosphocreatine synthesis by isolated rat skeletal muscle mitochondria is not dependent upon external ADP: A 31P NMR study. Biochem. Biophys. Res. Commun. 1996, 225, 819–825. [Google Scholar] [CrossRef]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef]
- Martino, M.R.; Habibi, M.; Ferguson, D.; Brookheart, R.T.; Thyfault, J.P.; Meyer, G.A.; Lantier, L.; Hughey, C.C.; Finck, B.N. Disruption of hepatic mitochondrial pyruvate and amino acid metabolism impairs gluconeogenesis and endurance exercise capacity in mice. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E515–E527. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Davidson, M.B. Alanine metabolism in skeletal muscle in tissue culture. Biochim. Biophys. Acta 1979, 585, 34–42. [Google Scholar] [CrossRef]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Martino, M.R.; Gutiérrez-Aguilar, M.; Yiew, N.K.H.; Lutkewitte, A.J.; Singer, J.M.; McCommis, K.S.; Ferguson, D.; Liss, K.H.H.; Yoshino, J.; Renkemeyer, M.K.; et al. Silencing alanine transaminase 2 in diabetic liver attenuates hyperglycemia by reducing gluconeogenesis from amino acids. Cell Rep. 2022, 39, 110733. [Google Scholar] [CrossRef]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef] [PubMed]
- McNair, L.M.; Andersen, J.V.; Waagepetersen, H.S. Stable isotope tracing reveals disturbed cellular energy and glutamate metabolism in hippocampal slices of aged male mice. Neurochem. Int. 2023, 171, 105626. [Google Scholar] [CrossRef] [PubMed]
- Kimoloi, S. Exercise-induced fumarate accumulation: A potential mediator of mitochondrial biogenesis in mammalian skeletal muscle. Int. J. Sport Exerc. Health Res. 2023, 7, 76–82. [Google Scholar] [CrossRef]
- Henderson, G.C.; Horning, M.A.; Lehman, S.L.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Pyruvate shuttling during rest and exercise before and after endurance training in men. J. Appl. Physiol. 2004, 97, 317–325. [Google Scholar] [CrossRef]
- Consitt, L.A.; Saxena, G.; Saneda, A.; Houmard, J.A. Age-related impairments in skeletal muscle PDH phosphorylation and plasma lactate are indicative of metabolic inflexibility and the effects of exercise training. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E145–E156. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Dutka, T.L.; Lamboley, C.R.; Murphy, R.M.; Lamb, G.D. Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2014, 117, 797–805. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A regulator of cellular redox homeostasis and skeletal muscle function. Mol. Nutr. Food Res. 2019, 63, 1800569. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Zhao, H.; Gu, C.; Li, Z. Effect of taurine in muscle damage markers and inflammatory cytokines in running exercise. Front. Physiol. 2022, 13, 1008060. [Google Scholar] [CrossRef]
- Ma, Y.; Maruta, H.; Sun, B.; Wang, C.; Isono, C.; Yamashita, H. Effects of long-term taurine supplementation on age-related changes in skeletal muscle function of Sprague-Dawley rats. Amino Acids. 2021, 53, 159–170. [Google Scholar] [CrossRef]
- Liu, H.W.; Chang, Y.C.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology. 2020, 21, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut–muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Konno, M.; Honda, K.; Abe, T.; Nagata, T.; Takehara, M.; Sugasawa, T.; Takekoshi, K.; Ohmori, H. Habitual aerobic exercise diminishes the effects of sarcopenia in senescence-accelerated mice Prone8 model. Geriatrics 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Bartolacci, C.; Guescini, M.; Battistelli, M.; Stocchi, V.; Orlando, F.; Provinciali, M.; Amici, A.; Marchini, C.; Tiano, L.; et al. Combination of coenzyme Q10 intake and moderate physical activity counteracts mitochondrial dysfunctions in a SAMP8 mouse model. Oxidative Med. Cell. Longev. 2018, 2018, 8936251. [Google Scholar] [CrossRef]
- Park, K.H.; Brotto, L.; Lehoang, O.; Brotto, M.; Ma, J.; Zhao, X. Ex vivo assessment of contractility, fatigability and alternans in isolated skeletal muscles. J. Vis. Exp. 2012, 69, 4198. [Google Scholar] [CrossRef]
- Le Gall, G. Sample collection and preparation of biofluids and extracts for NMR spectroscopy. Methods Mol. Biol. 2015, 1277, 15–28. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts Magn. Reson. Part A 2011, 38, 197–220. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Bingol, K.; Bruschweiler-Li, L.; Li, D.W.; Brüschweiler, R. Customized metabolomics database for the analysis of NMR 1H-1H TOCSY and 13C-1H HSQC-TOCSY spectra of complex mixtures. Anal. Chem. 2014, 86, 5494–5501. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]


| Metabolites | Mean ± SD | Multiple Comparisons | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| 2MC | 7MC | 7ME | 7MC vs. 2MC | 7ME vs. 7MC | 7ME vs. 2MC | F-Value | p-Value | |
| Leucine | 1.199 ± 0.149 | 1.072 ± 0.169 | 0.947 ± 0.223 | ns | ns | ↓↓ | 7.156 | 0.002 |
| Isoleucine | 0.402 ± 0.054 | 0.361 ± 0.062 | 0.342 ± 0.073 | ns | ns | ↓ | 3.489 | 0.039 |
| Valine | 0.603 ± 0.080 | 0.522 ± 0.099 | 0.506 ± 0.112 | ns | ns | ↓ | 4.28 | 0.02 |
| 3-Hydroxybutyrate | 0.152 ± 0.064 | 0.327 ± 0.124 | 0.199 ± 0.074 | ↑↑↑ | ↓↓ | ns | 33.071 | <0.0001 |
| 3-Hydroxyisovalerate | 0.150 ± 0.007 | 0.144 ± 0.009 | 0.141 ± 0.008 | ns | ns | ↓ | 4.94 | 0.012 |
| Methylmalonate | 0.132 ± 0.014 | 0.108 ± 0.020 | 0.121 ± 0.024 | ↓↓ | ns | ns | 5.887 | 0.005 |
| Alanine | 2.603 ± 0.237 | 2.130 ± 0.174 | 2.487 ± 0.168 | ↓↓↓↓ | ↑↑↑↑ | ns | 24.889 | <0.0001 |
| Lysine | 1.362 ± 0.308 | 0.885 ± 0.129 | 1.149 ± 0.285 | ↓↓↓↓ | ↑ | ns | 14.026 | <0.0001 |
| Acetate | 1.468 ± 0.103 | 1.401 ± 0.072 | 0.490 ± 0.078 | ns | ↓↓↓↓ | ↓↓↓↓ | 620.643 | <0.0001 |
| Arginine | 0.471 ± 0.079 | 0.360 ± 0.052 | 0.362 ± 0.089 | ↓↓↓ | ns | ↓↓↓ | 10.924 | <0.001 |
| Methionine | 0.462 ± 0.046 | 0.412 ± 0.050 | 0.400 ± 0.037 | ↓↓ | ns | ↓↓ | 8.169 | 0.001 |
| Glutamate | 0.737 ± 0.090 | 0.692 ± 0.110 | 0.679 ± 0.105 | ns | ns | ns | 1.309 | 0.281 |
| Glutamine | 1.075 ± 0.127 | 0.948 ± 0.109 | 1.064 ± 0.101 | ↓↓ | ↑ | ns | 6.119 | 0.005 |
| Acetone | 0.038 ± 0.010 | 0.028 ± 0.009 | 0.023 ± 0.007 | ↓↓ | ns | ↓↓↓ | 11.259 | <0.001 |
| Pyruvate | 0.037 ± 0.017 | 0.014 ± 0.005 | 0.016 ± 0.006 | ↓↓↓ | ns | ↓↓ | 19.601 | <0.0001 |
| Succinate | 0.019 ± 0.007 | 0.049 ± 0.023 | 0.031 ± 0.009 | ↑↑↑ | ↓ | ↑↑ | 23.158 | <0.0001 |
| Glutathione | 0.332 ± 0.034 | 0.300 ± 0.054 | 0.268 ± 0.058 | ns | ns | ↓↓ | 6.14 | 0.005 |
| Anserine | 0.783 ± 0.085 | 0.869 ± 0.090 | 0.893 ± 0.143 | ns | ns | ↑ | 4.29 | 0.02 |
| Aspartate | 0.091 ± 0.026 | 0.122 ± 0.036 | 0.093 ± 0.027 | ↑ | ↓ | ns | 5.014 | 0.011 |
| Asparagine | 0.053 ± 0.028 | 0.081 ± 0.044 | 0.029 ± 0.008 | ns | ↓↓↓ | ↓ | 26.998 | <0.001 |
| Creatine | 19.392 ± 1.127 | 20.484 ± 1.373 | 22.057 ± 1.169 | ↑ | ↑↑ | ↑↑↑↑ | 17.763 | <0.0001 |
| Betaine | 0.628 ± 0.065 | 0.452 ± 0.049 | 0.496 ± 0.087 | ↓↓↓↓ | ns | ↓↓↓↓ | 27.575 | <0.0001 |
| Taurine | 18.223 ± 1.225 | 19.458 ± 1.162 | 20.447 ± 1.143 | ↑ | ns | ↑↑↑↑ | 13.458 | <0.0001 |
| Glycine | 1.085 ± 0.327 | 0.939 ± 0.110 | 0.866 ± 0.071 | ns | ns | ↓ | 4.58 | 0.016 |
| Threonine | 0.140 ± 0.015 | 0.097 ± 0.012 | 0.110 ± 0.018 | ↓↓↓↓ | ns | ↓↓↓↓ | 29.985 | <0.0001 |
| Glycerol | 1.047 ± 0.086 | 0.954 ± 0.089 | 0.895 ± 0.124 | ↓ | ns | ↓↓↓ | 8.603 | 0.001 |
| 2-Phosphoglycerate | 1.452 ± 0.106 | 1.117 ± 0.125 | 1.086 ± 0.167 | ↓↓↓↓ | ns | ↓↓↓↓ | 34.294 | <0.0001 |
| Creatinine | 0.037 ± 0.007 | 0.030 ± 0.005 | 0.033 ± 0.008 | ↓ | ns | ns | 3.914 | 0.027 |
| Lactate | 7.931 ± 0.602 | 6.285 ± 1.044 | 7.681 ± 1.249 | ↓↓↓ | ↑↑ | ns | 12.224 | <0.0001 |
| Myoinositol | 0.141 ± 0.018 | 0.122 ± 0.015 | 0.123 ± 0.021 | ↓ | ns | ↓ | 5.374 | 0.008 |
| IMP | 1.205 ± 0.113 | 1.373 ± 0.111 | 1.430 ± 0.117 | ↑↑↑ | ns | ↑↑↑↑ | 15.945 | <0.0001 |
| Glucose | 0.281 ± 0.066 | 0.154 ± 0.067 | 0.146 ± 0.081 | ↓↓↓↓ | ns | ↓↓↓↓ | 17.077 | <0.0001 |
| Uracil | 0.009 ± 0.001 | 0.011 ± 0.002 | 0.003 ± 0.001 | ↑↑ | ↓↓↓↓ | ↓↓↓↓ | 36.899 | <0.0001 |
| GTP | 0.014 ± 0.002 | 0.017 ± 0.002 | 0.016 ± 0.002 | ↑↑ | ns | ↑↑↑ | 6.242 | 0.004 |
| Inosine | 0.250 ± 0.042 | 0.194 ± 0.031 | 0.242 ± 0.041 | ↓↓↓ | ↑↑ | ns | 10.1 | <0.001 |
| Fumarate | 0.050 ± 0.006 | 0.047 ± 0.006 | 0.060 ± 0.012 | ns | ↑↑ | ↑ | 28.687 | 0.001 |
| Tyrosine | 0.132 ± 0.016 | 0.122 ± 0.011 | 0.112 ± 0.019 | ns | ns | ↓↓ | 6.116 | 0.005 |
| 1-Methylhistidine | 0.509 ± 0.065 | 0.557 ± 0.063 | 0.580 ± 0.101 | ns | ns | ↑ | 3.218 | 0.05 |
| Phenylalanine | 0.162 ± 0.019 | 0.141 ± 0.016 | 0.136 ± 0.026 | ↓ | ns | ↓↓ | 6.265 | 0.004 |
| Niacinamide | 0.090 ± 0.006 | 0.089 ± 0.005 | 0.094 ± 0.006 | ns | ↑ | ns | 4.222 | 0.021 |
| Tryptophan | 0.016 ± 0.002 | 0.016 ± 0.002 | 0.014 ± 0.002 | ns | ns | ↓↓↓ | 3.153 | 0.053 |
| Uridine | 0.013 ± 0.002 | 0.005 ± 0.002 | 0.018 ± 0.004 | ↓↓↓↓ | ↑↑↑↑ | ↑↑ | 28.277 | <0.0001 |
| Histidine | 0.083 ± 0.014 | 0.084 ± 0.012 | 0.107 ± 0.017 | ns | ↑↑↑ | ↑↑ | 14.498 | <0.0001 |
| Adenine | 0.068 ± 0.012 | 0.068 ± 0.008 | 0.064 ± 0.013 | ns | ns | ↓↓ | 0.639 | 0.533 |
| AXP | 0.050 ± 0.004 | 0.056 ± 0.006 | 0.069 ± 0.005 | ↑↑ | ↑↑↑↑ | ↑↑ | 53.753 | <0.0001 |
| Formate | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.004 ± 0.001 | ns | ↓↓↓↓ | ↓↓↓ | 31.689 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Zhang, L.; Chen, Y.; Huang, C.; Yang, L.; Lin, D. Exercise Attenuates Skeletal Muscle Atrophy in Senescent SAMP8 Mice: Metabolic Insights from NMR-Based Metabolomics. Molecules 2025, 30, 2003. https://doi.org/10.3390/molecules30092003
Wu W, Zhang L, Chen Y, Huang C, Yang L, Lin D. Exercise Attenuates Skeletal Muscle Atrophy in Senescent SAMP8 Mice: Metabolic Insights from NMR-Based Metabolomics. Molecules. 2025; 30(9):2003. https://doi.org/10.3390/molecules30092003
Chicago/Turabian StyleWu, Wenfang, Linglin Zhang, Yifen Chen, Caihua Huang, Longhe Yang, and Donghai Lin. 2025. "Exercise Attenuates Skeletal Muscle Atrophy in Senescent SAMP8 Mice: Metabolic Insights from NMR-Based Metabolomics" Molecules 30, no. 9: 2003. https://doi.org/10.3390/molecules30092003
APA StyleWu, W., Zhang, L., Chen, Y., Huang, C., Yang, L., & Lin, D. (2025). Exercise Attenuates Skeletal Muscle Atrophy in Senescent SAMP8 Mice: Metabolic Insights from NMR-Based Metabolomics. Molecules, 30(9), 2003. https://doi.org/10.3390/molecules30092003






