The Impact of Major and Minor Phytocannabinoids on the Maintenance and Function of INS-1 β-Cells Under High-Glucose and High-Lipid Conditions
Abstract
1. Introduction
2. Results
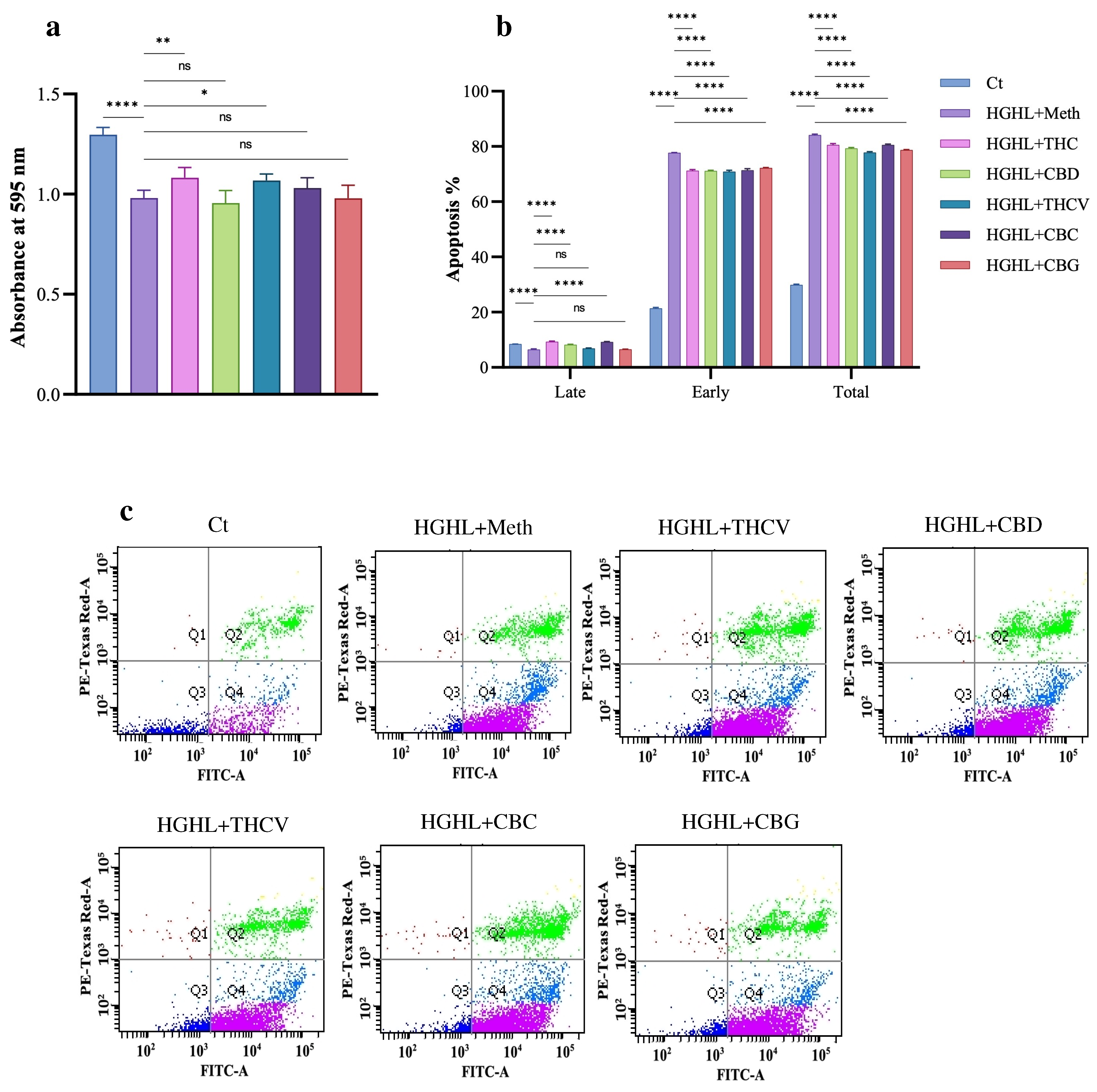
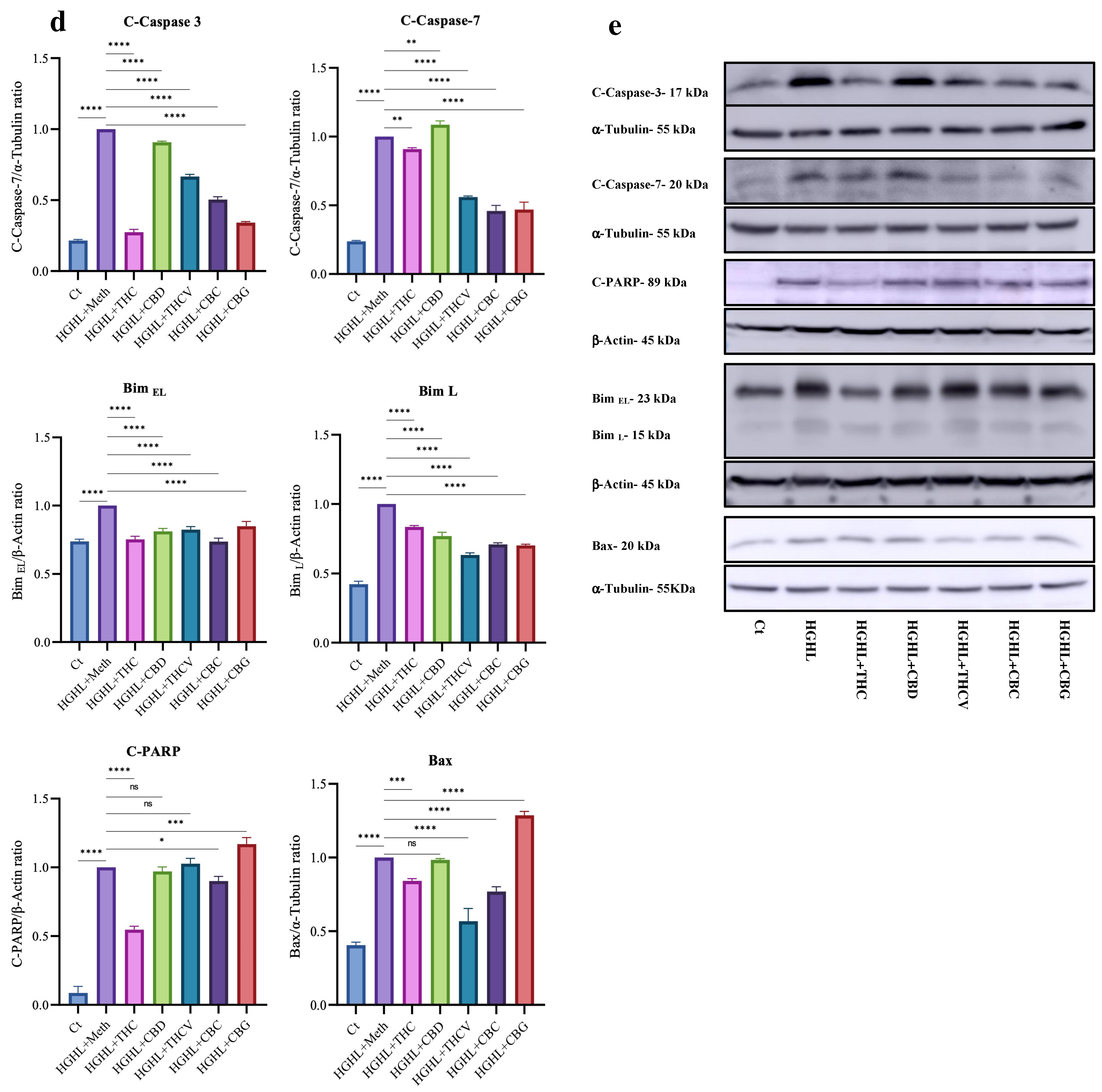
2.1. All Five Phytocannabinoids Mitigate HGHL-Induced Apoptosis in INS-1 β-Cells
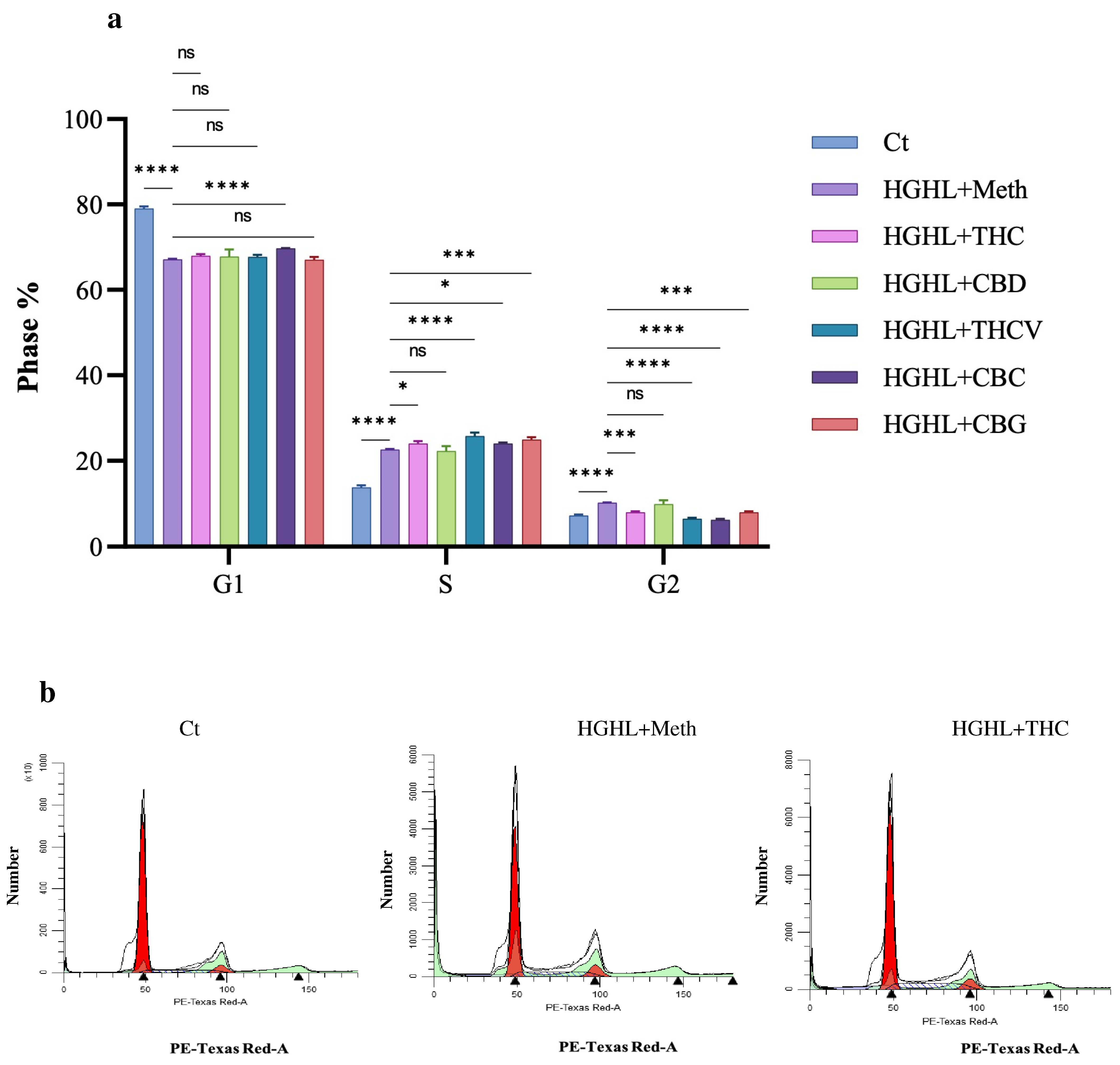
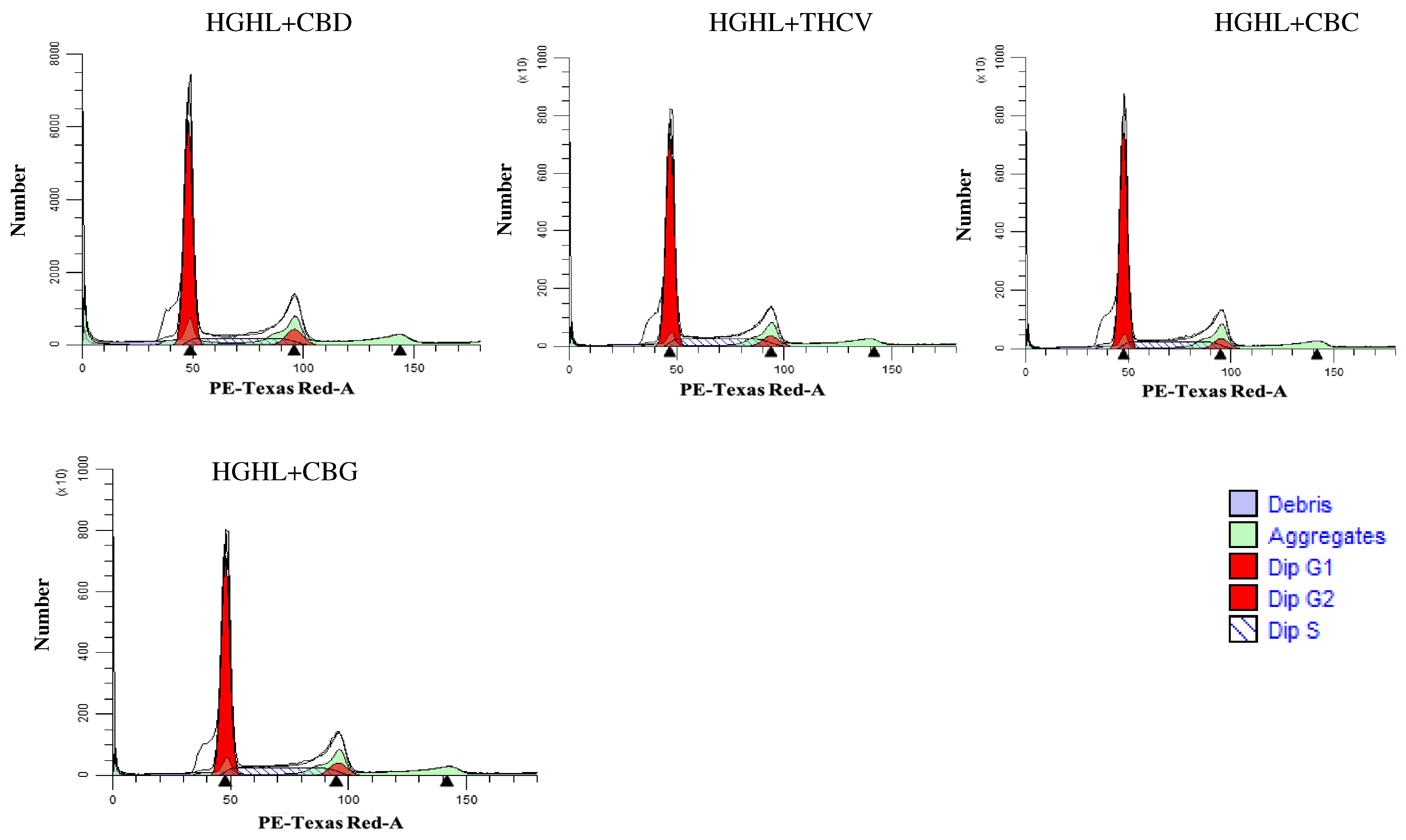
2.2. Phytocannabinoids Alter the Distribution of Cells in the G1, S, and G2 Phases of the Cell Cycle in HGHL-Induced INS-1 β-Cells
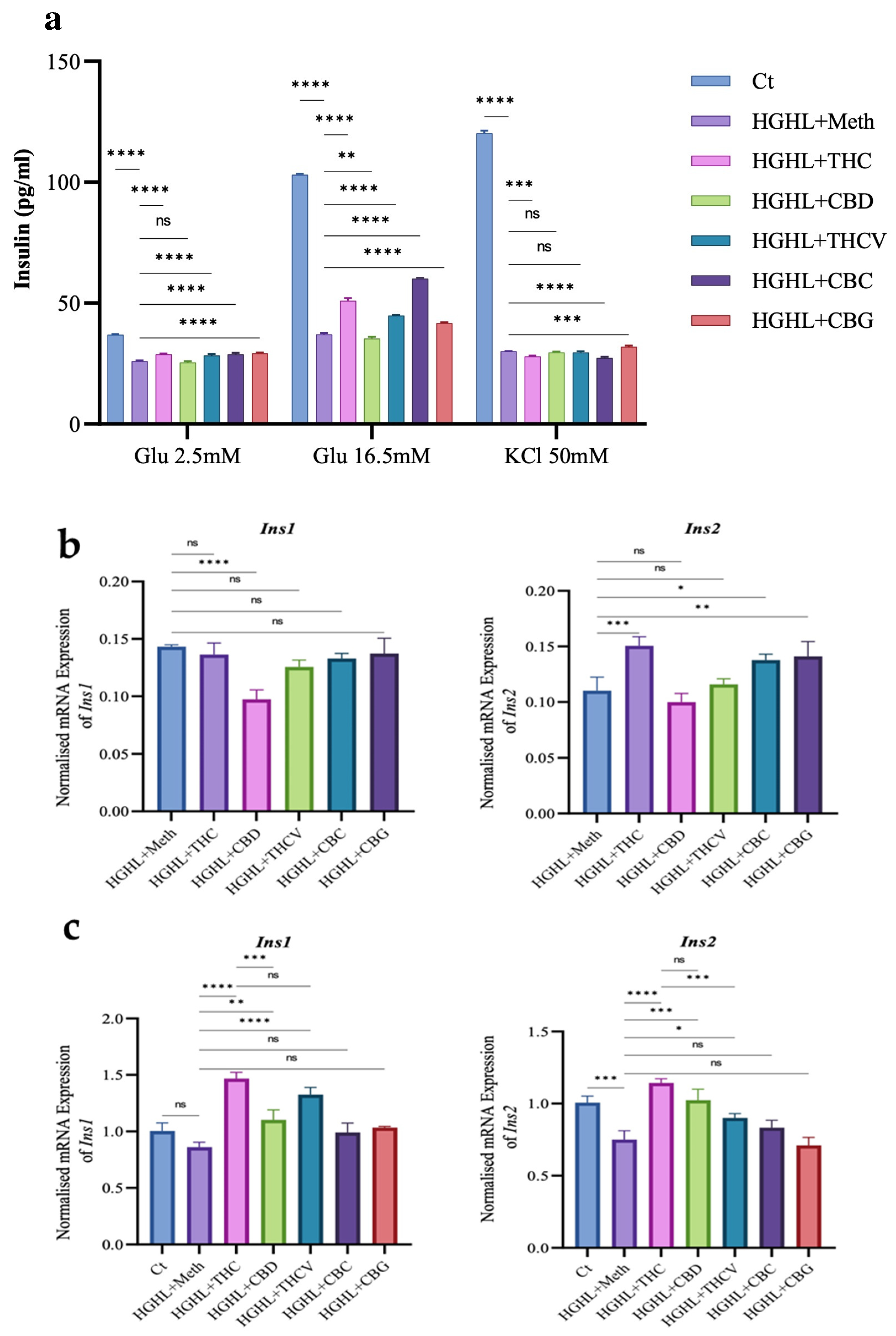
2.3. All Phytocannabinoids Except CBD Restore Impaired GSIS in HGHL-Induced INS-1 β-Cells
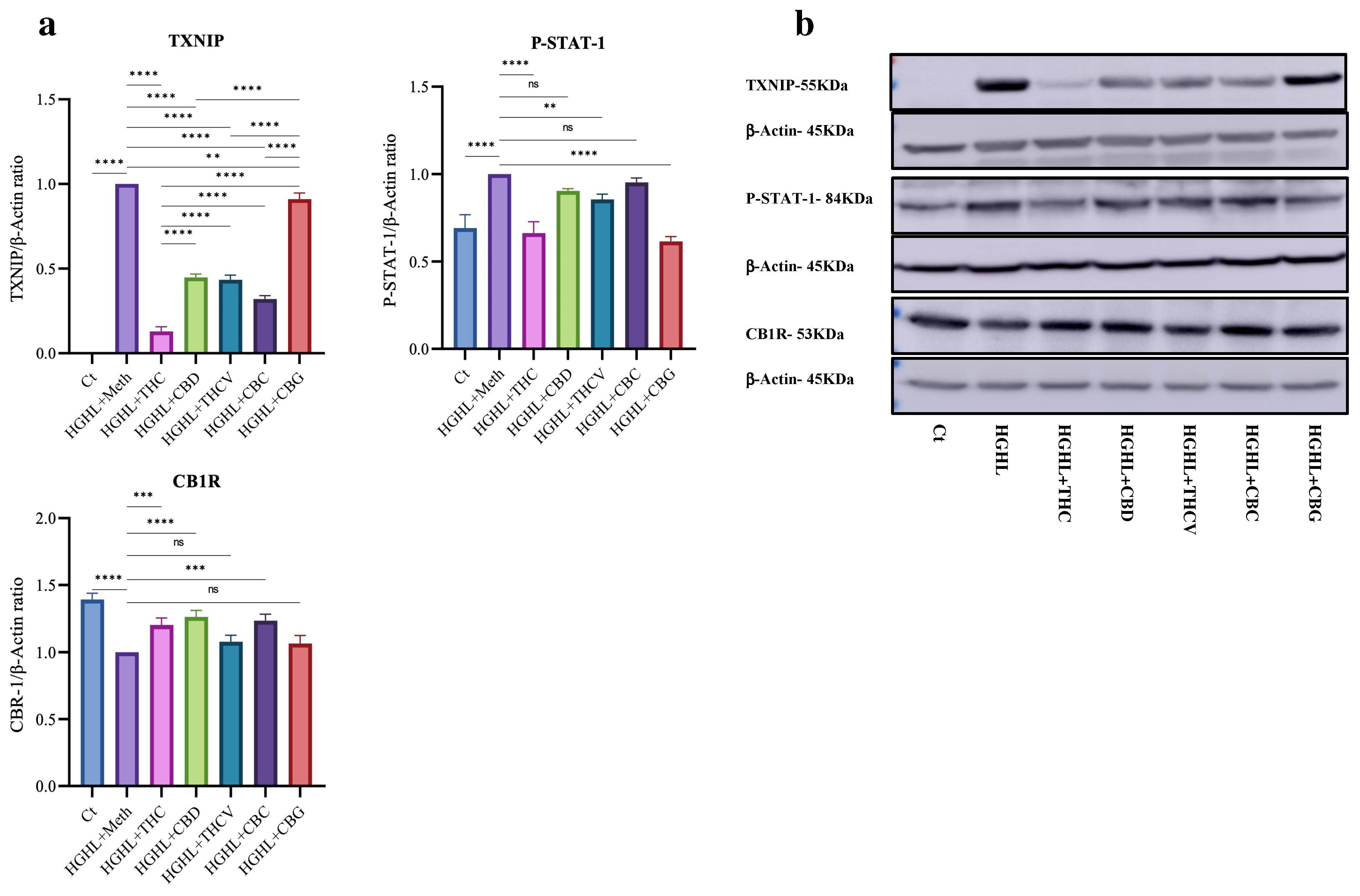
2.4. All Five Phytocannabinoids Mitigate Elevated TXNIP While Enhancing Reduced CB1R Expression
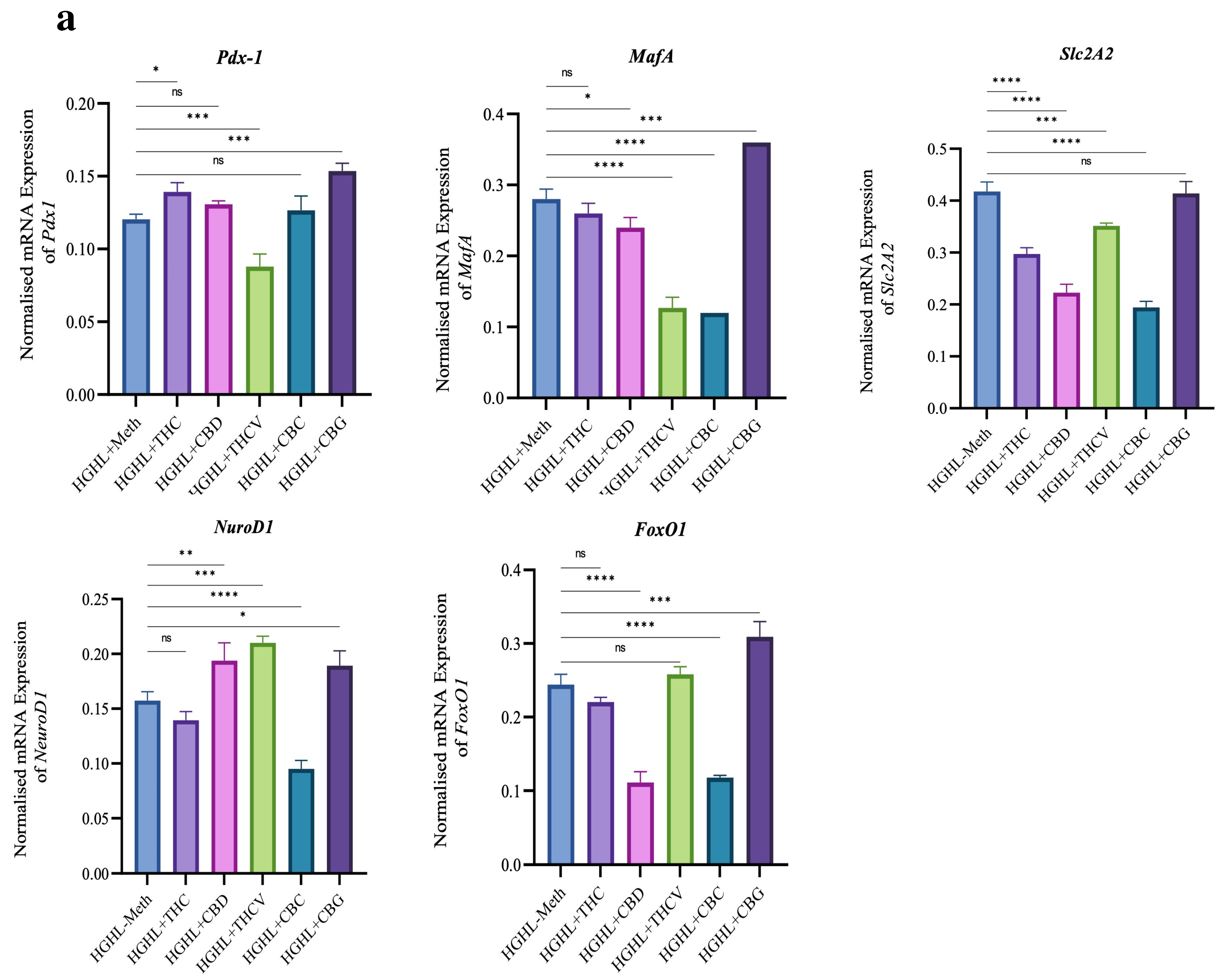
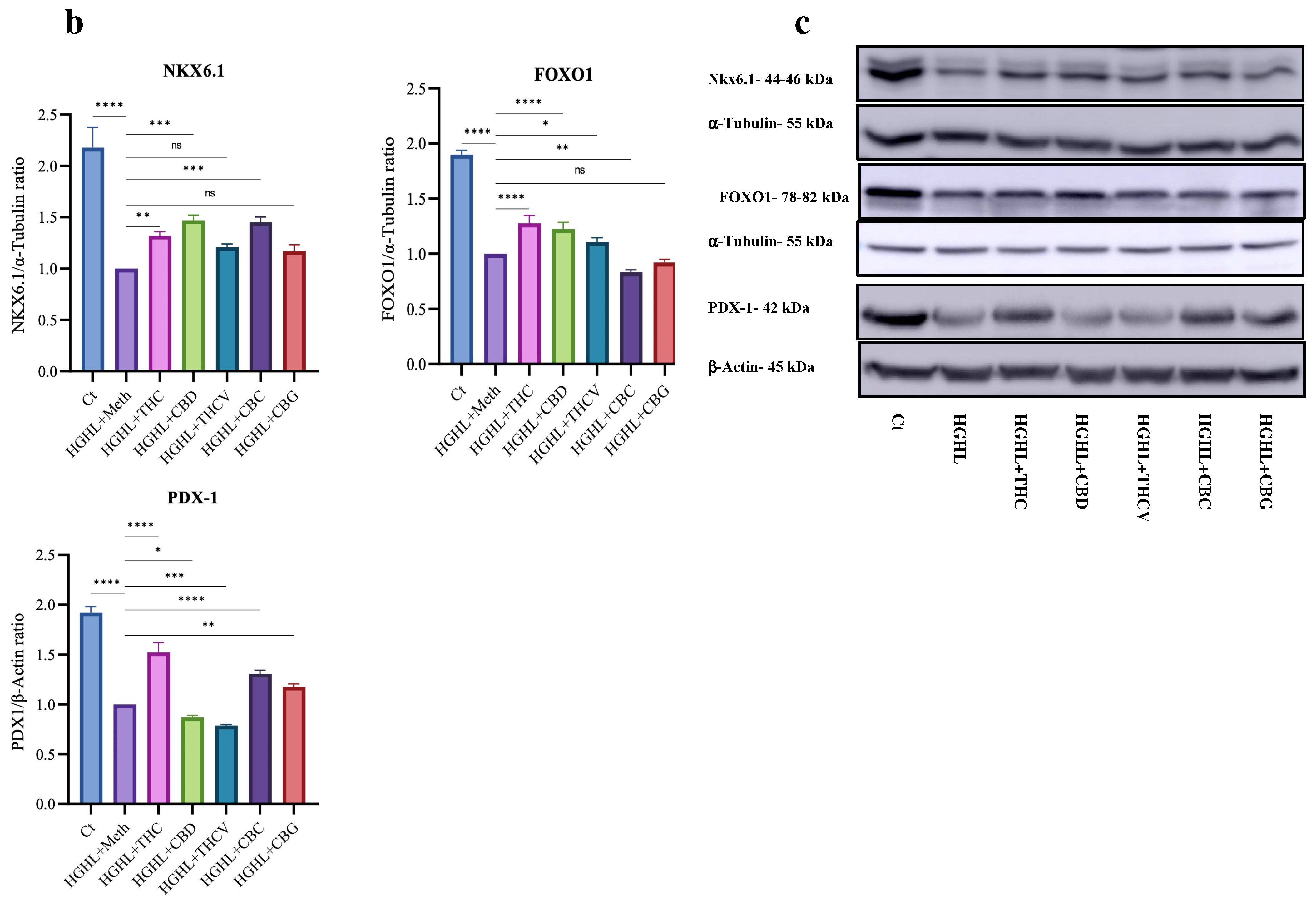
2.5. Certain Phytocannabinoids Positively Influence the Reduced Levels of β-Cell-Enriched Genes and Proteins
3. Discussion
3.1. The Impact of the Phytocannabinoids on HGHL-Induced Apoptosis in INS-1 β-Cells
3.2. The Impact of Phytocannabinoids on Cell Cycle Modification in INS-1 β-Cells
3.3. The Impact of Phytocannabinoids on the Impaired Function of HGHL-Induced INS-1 β-Cells
3.4. The Effects of Phytocannabinoids on the Expression of β-Cell-Enriched Genes and Proteins
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatments
4.3. MTT
4.4. Apoptosis Assay
4.5. Cell Cycle Assay
4.6. Western Blotting
4.7. qRT-PCR
4.8. Glucose-Stimulated Insulin Secretion (GSIS) and Potassium-Stimulated Insulin Secretion (KSIS)
4.9. Statistics
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holt, R.I.; Cockram, C.; Flyvbjerg, A.; Goldstein, B.J. Textbook of Diabetes; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2022. [Google Scholar]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef]
- Neelankal John, A.; Morahan, G.; Jiang, F.X. Incomplete Re-Expression of Neuroendocrine Progenitor/Stem Cell Markers is a Key Feature of β-Cell Dedifferentiation. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Amo-Shiinoki, K.; Tanabe, K.; Hoshii, Y.; Matsui, H.; Harano, R.; Fukuda, T.; Takeuchi, T.; Bouchi, R.; Takagi, T.; Hatanaka, M.; et al. Islet cell dedifferentiation is a pathologic mechanism of long-standing progression of type 2 diabetes. JCI Insight 2021, 6, e143791. [Google Scholar] [CrossRef] [PubMed]
- Khin, P.-P.; Lee, J.-H.; Jun, H.-S. A brief review of the mechanisms of β-cell dedifferentiation in type 2 diabetes. Nutrients 2021, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Efrat, S. Beta-cell dedifferentiation in type 2 diabetes: Concise review. Stem Cells 2019, 37, 1267–1272. [Google Scholar] [CrossRef]
- Ghasemi Gojani, E.; Rai, S.; Norouzkhani, F.; Shujat, S.; Wang, B.; Li, D.; Kovalchuk, O.; Kovalchuk, I. Targeting β-cell plasticity: A promising approach for diabetes treatment. Curr. Issues Mol. Biol. 2024, 46, 7621–7667. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Butler, A.E. Alterations in beta cell identity in type 1 and type 2 diabetes. Curr. Diabetes Rep. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Ghasemi-Gojani, E.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids and terpenes for diabetes mellitus and its complications: From mechanisms to new therapies. Trends Endocrinol. Metab. 2022, 33, 828–849. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids alleviate the LPS-induced cytokine storm via attenuating NLRP3 inflammasome signaling and TYK2-mediated STAT3 signaling pathways in vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. Anti-Inflammatory Effects of Minor Cannabinoids CBC, THCV, and CBN in Human Macrophages. Molecules 2023, 28, 6487. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Accili, D. Reversing pancreatic β-cell dedifferentiation in the treatment of type 2 diabetes. Exp. Mol. Med. 2023, 55, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Lytrivi, M.; Castell, A.-L.; Poitout, V.; Cnop, M. Recent insights into mechanisms of β-cell lipo-and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Vela-Guajardo, J.E.; Garza-González, S.; García, N. Glucolipotoxicity-induced oxidative stress is related to mitochondrial dysfunction and apoptosis of pancreatic β-cell. Curr. Diabetes Rev. 2021, 17, 46–56. [Google Scholar] [CrossRef]
- Qayyum, N.; Haseeb, M.; Kim, M.S.; Choi, S. Role of thioredoxin-interacting protein in diseases and its therapeutic outlook. Int. J. Mol. Sci. 2021, 22, 2754. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.; Naamane, N.; Colli, M.L.; Bouckenooghe, T.; Ortis, F.; Gurzov, E.N.; Igoillo-Esteve, M.; Mathieu, C.; Bontempi, G.; Thykjaer, T.; et al. STAT1 is a master regulator of pancreatic β-cell apoptosis and islet inflammation. J. Biol. Chem. 2011, 286, 929–941. [Google Scholar] [CrossRef]
- González-Mariscal, I.; Pozo-Morales, M.; Romero-Zerbo, S.Y.; Espinosa-Jimenez, V.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Cobo-Vuilleumier, N.; Gauthier, B.R.; Bermúdez-Silva, F.J. Abnormal cannabidiol ameliorates inflammation preserving pancreatic beta cells in mouse models of experimental type 1 diabetes and beta cell damage. Biomed. Pharmacother. 2022, 145, 112361. [Google Scholar] [CrossRef]
- Vella, R.K.; Jackson, D.J.; Fenning, A.S. Δ9-Tetrahydrocannabinol prevents cardiovascular dysfunction in STZ-diabetic Wistar-Kyoto rats. BioMed Res. Int. 2017, 2017, 7974149. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid CB2 receptors are involved in the protection of RAW264. 7 macrophages against the oxidative stress: An in vitro study. Eur. J. Histochem. EJH 2017, 61, 2749. [Google Scholar] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.; Kovalchuk, O.; Kovalchuk, I. Single and Combined Impact of Semaglutide, Tirzepatide, and Metformin on β-Cell Maintenance and Function Under High-Glucose–High-Lipid Conditions: A Comparative Study. Int. J. Mol. Sci. 2025, 26, 421. [Google Scholar] [CrossRef]
- Fernando, M.; Duijf, P.H.G.; Proctor, M.; Stevenson, A.J.; Ehmann, A.; Vora, S.; Skalamera, D.; Adams, M.; Gabrielli, B. Dysregulated G2 phase checkpoint recovery pathway reduces DNA repair efficiency and increases chromosomal instability in a wide range of tumours. Oncogenesis 2021, 10, 41. [Google Scholar] [CrossRef]
- Löbrich, M.; Jeggo, P.A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer 2007, 7, 861–869. [Google Scholar] [CrossRef]
- Kalsbeek, D.; Golsteyn, R.M. G2/M-phase checkpoint adaptation and micronuclei formation as mechanisms that contribute to genomic instability in human cells. Int. J. Mol. Sci. 2017, 18, 2344. [Google Scholar] [CrossRef]
- Kim, W.; Lao, Q.; Shin, Y.K.; Carlson, O.D.; Lee, E.K.; Gorospe, M.; Kulkarni, R.N.; Egan, J.M. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci. Signal. 2012, 5, ra23. [Google Scholar] [CrossRef]
- Jourdan, T.; Godlewski, G.; Kunos, G. Endocannabinoid regulation of β-cell functions: Implications for glycaemic control and diabetes. Diabetes Obes. Metab. 2016, 18, 549–557. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Anand, U.; Jones, B.; Korchev, Y.; Bloom, S.R.; Pacchetti, B.; Anand, P.; Sodergren, M.H. CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons. J. Pain Res. 2020, 13, 2269–2278. [Google Scholar] [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-N.; Shi, Y.; Zhao, K.; Yang, G.; Yu, J.; Berggren, P.-O. Pancreatic β cell CaV channels in health and disease. In Voltage-Gated Calcium Channels; Zamponi, G.W., Weiss, N., Eds.; Springer: Cham, Switzerland, 2022; pp. 425–448. [Google Scholar]
- Ali, R.M.; Al Kury, L.T.; Yang, K.H.; Qureshi, A.; Rajesh, M.; Galadari, S.; Shuba, Y.M.; Howarth, F.C.; Oz, M. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium 2015, 57, 290–299. [Google Scholar] [CrossRef]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Straub, S.G.; Sharp, G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/Metab. Res. Rev. 2002, 18, 451–463. [Google Scholar] [CrossRef]
- Bratanova-Tochkova, T.K.; Cheng, H.; Daniel, S.; Gunawardana, S.; Liu, Y.J.; Mulvaney-Musa, J.; Schermerhorn, T.; Straub, S.G.; Yajima, H.; Sharp, G.W. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 2002, 51, S83–S90. [Google Scholar] [CrossRef]
- Shin, H.; Han, J.H.; Yoon, J.; Sim, H.J.; Park, T.J.; Yang, S.; Lee, E.K.; Kulkarni, R.N.; Egan, J.M.; Kim, W. Blockade of cannabinoid 1 receptor improves glucose responsiveness in pancreatic beta cells. J. Cell Mol. Med. 2018, 22, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, I.; Montoro, R.A.; Doyle, M.E.; Liu, Q.R.; Rouse, M.; O’Connell, J.F.; Santa-Cruz Calvo, S.; Krzysik-Walker, S.M.; Ghosh, S.; Carlson, O.D.; et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 2018, 61, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, K.; Thomas, M.A.; Glick, S.; Fung, E.N.; Wang, V.; Watson, L.; Gregory, P.; Antel, J.; Pelleymounter, M.A. Ibipinabant attenuates beta-cell loss in male Zucker diabetic fatty rats independently of its effects on body weight. Diabetes Obes. Metab. 2012, 14, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. The impact of psilocybin on high glucose/lipid-induced changes in INS-1 cell viability and dedifferentiation. Genes 2024, 15, 183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. The Impact of Major and Minor Phytocannabinoids on the Maintenance and Function of INS-1 β-Cells Under High-Glucose and High-Lipid Conditions. Molecules 2025, 30, 1991. https://doi.org/10.3390/molecules30091991
Gojani EG, Wang B, Li D-P, Kovalchuk O, Kovalchuk I. The Impact of Major and Minor Phytocannabinoids on the Maintenance and Function of INS-1 β-Cells Under High-Glucose and High-Lipid Conditions. Molecules. 2025; 30(9):1991. https://doi.org/10.3390/molecules30091991
Chicago/Turabian StyleGojani, Esmaeel Ghasemi, Bo Wang, Dong-Ping Li, Olga Kovalchuk, and Igor Kovalchuk. 2025. "The Impact of Major and Minor Phytocannabinoids on the Maintenance and Function of INS-1 β-Cells Under High-Glucose and High-Lipid Conditions" Molecules 30, no. 9: 1991. https://doi.org/10.3390/molecules30091991
APA StyleGojani, E. G., Wang, B., Li, D.-P., Kovalchuk, O., & Kovalchuk, I. (2025). The Impact of Major and Minor Phytocannabinoids on the Maintenance and Function of INS-1 β-Cells Under High-Glucose and High-Lipid Conditions. Molecules, 30(9), 1991. https://doi.org/10.3390/molecules30091991





