Study on the Catalytic Performance of Nickel(II) Complexes with Distinct Triazine Support Structures in Ethylene Oligomerization via Different Experiment Designs
Abstract
1. Introduction
2. Results and Discussion
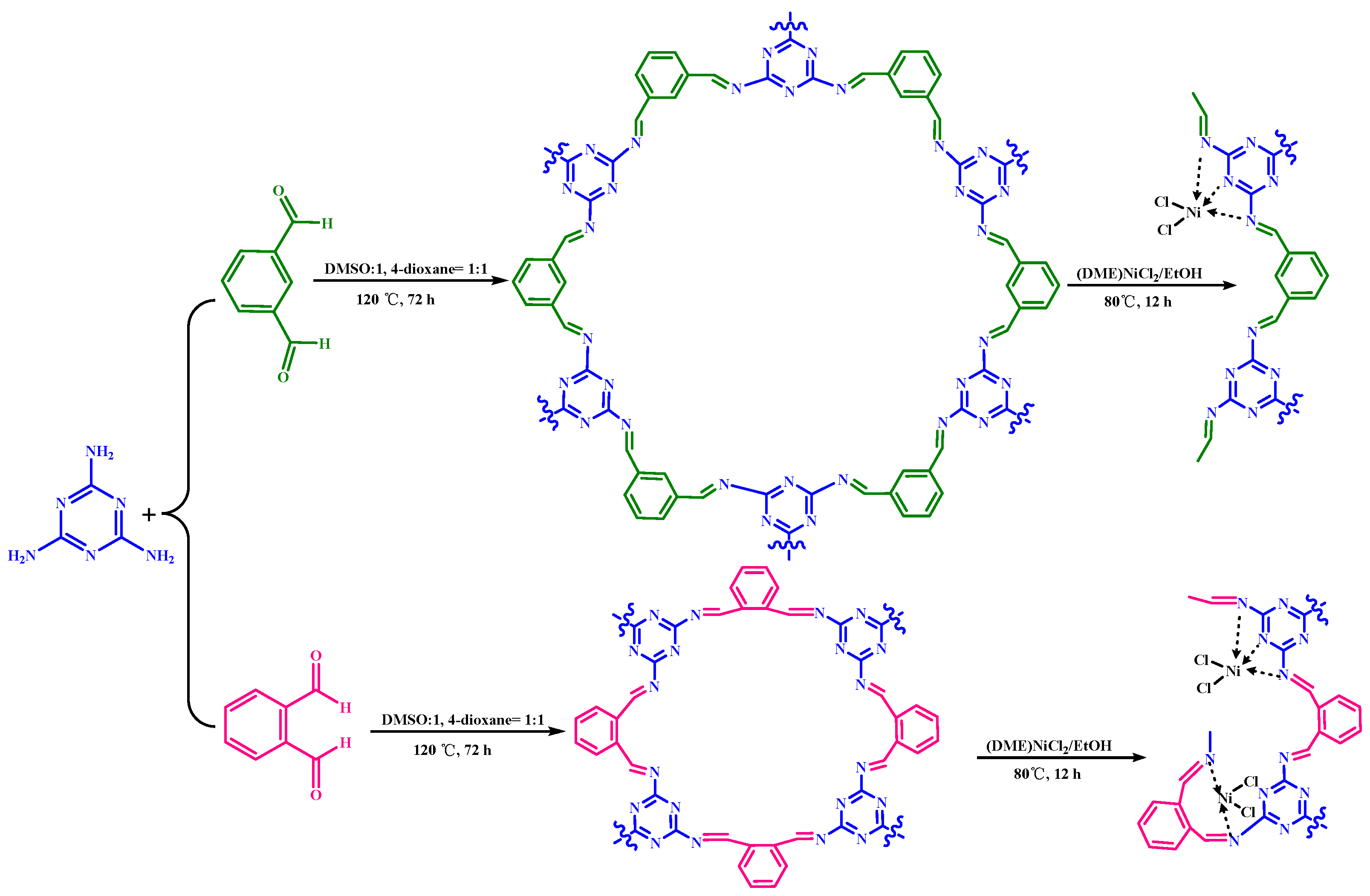
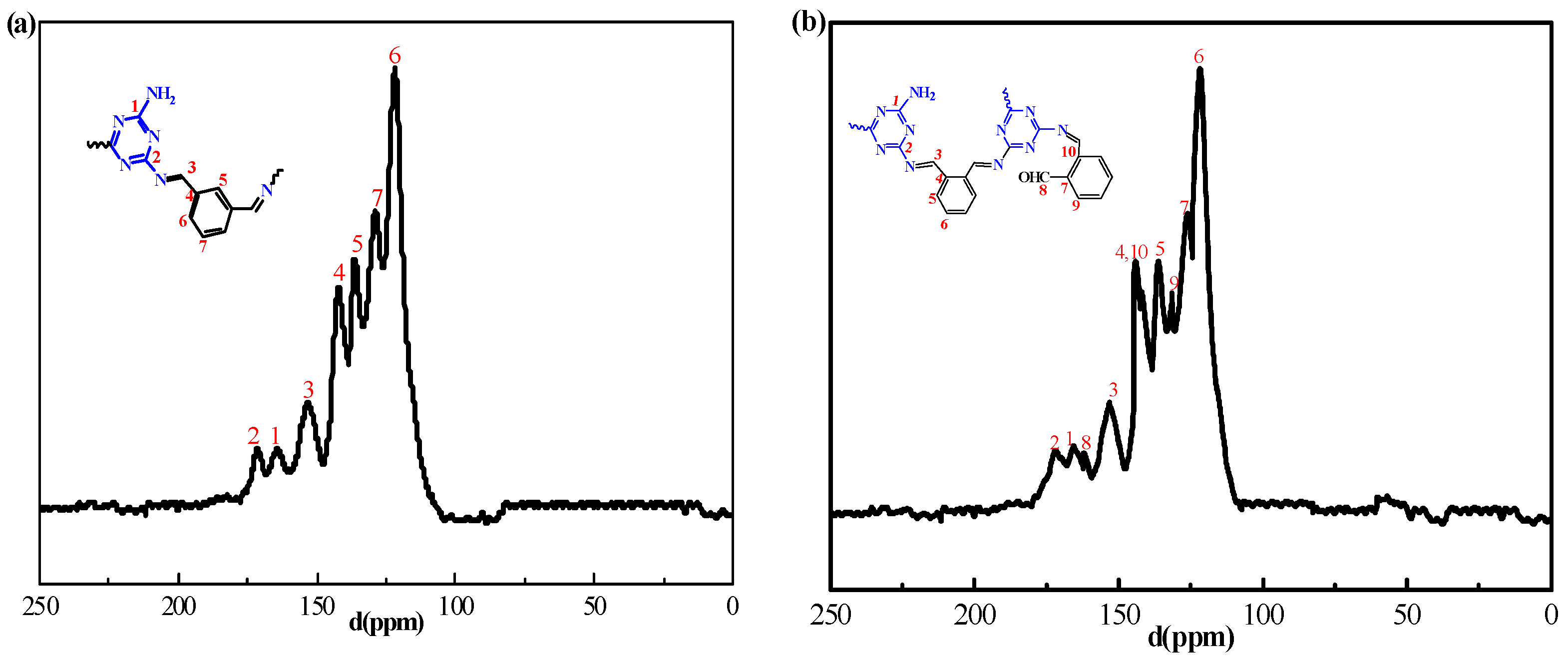
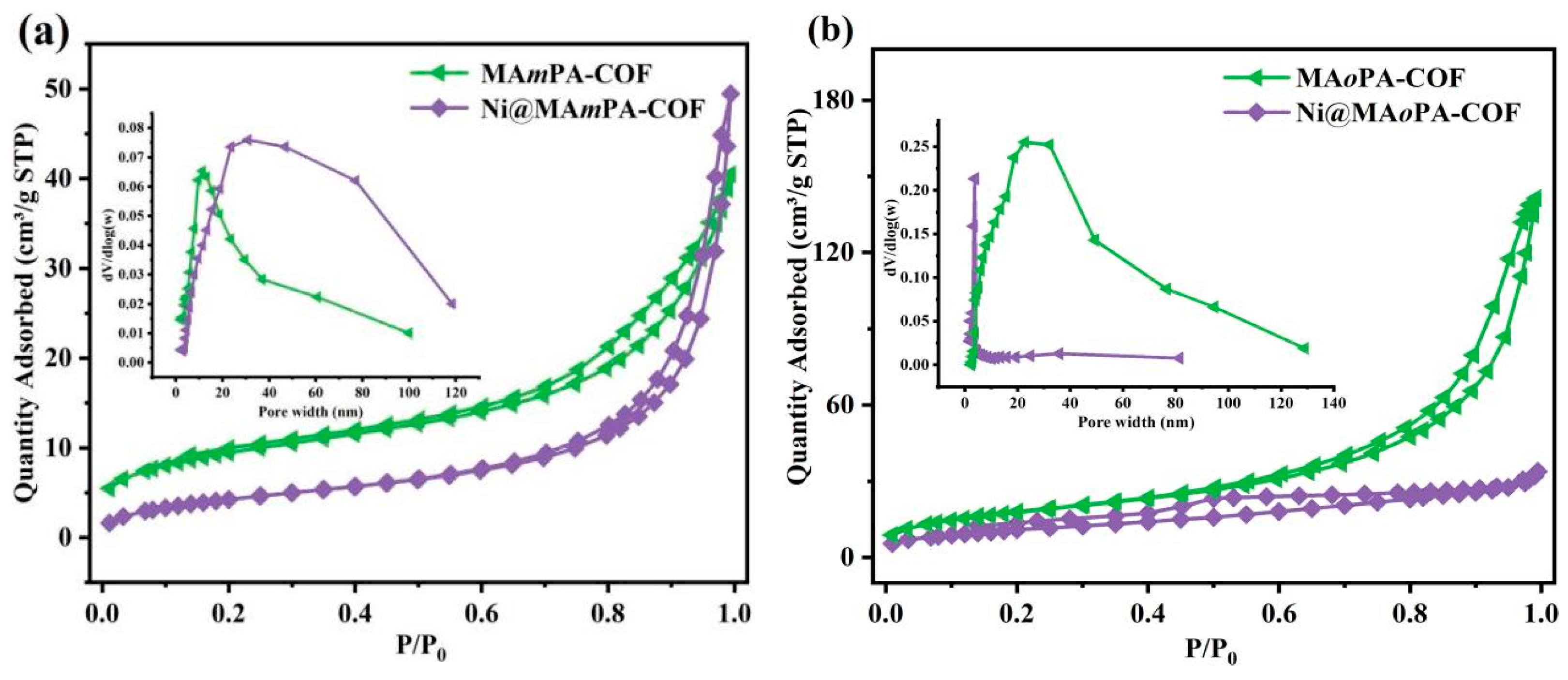
2.1. Synthesis and Characterization
2.2. Ethylene Oligomerization Catalyzed by Ni@MAmPA-COF
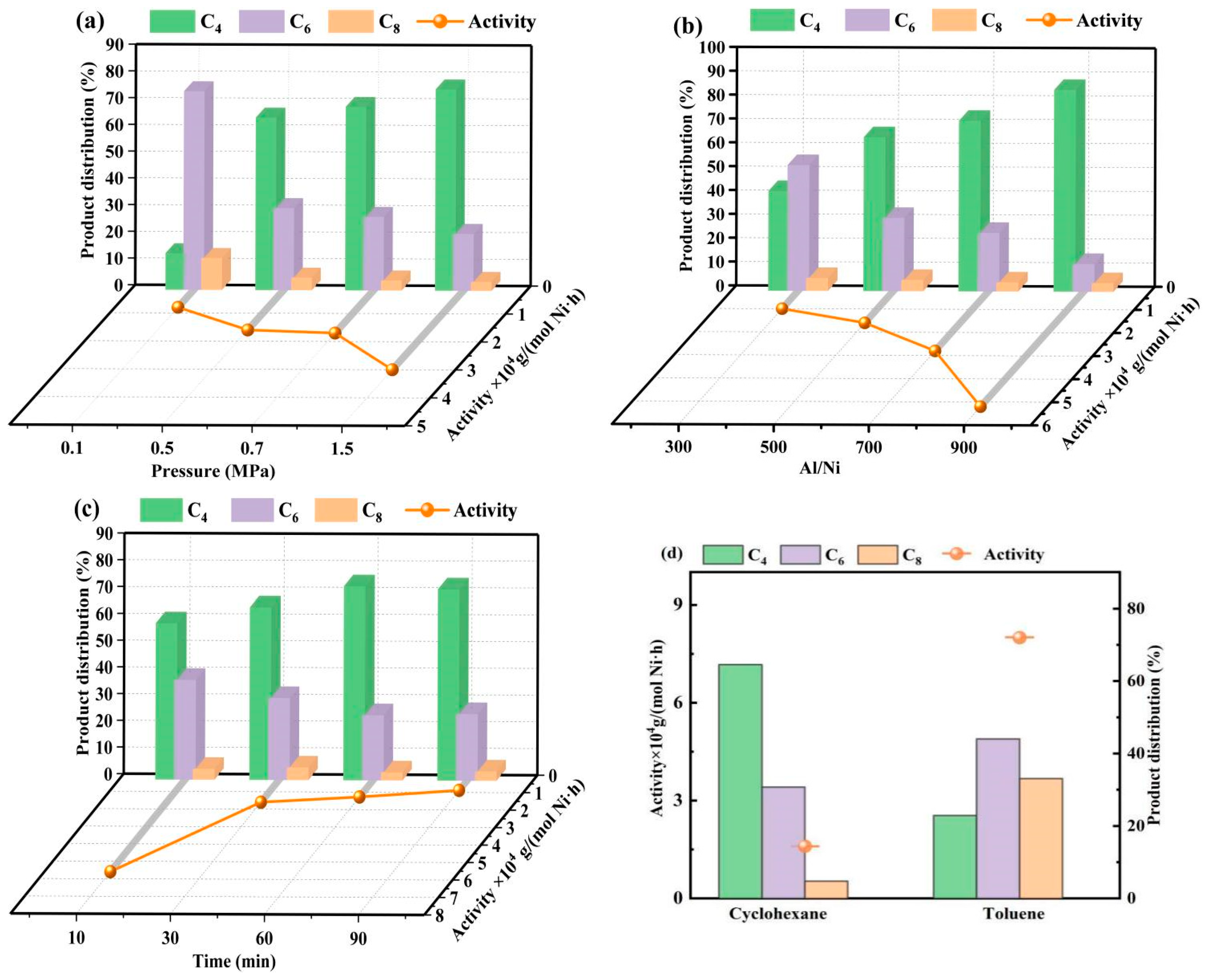
2.2.1. Influence of Reaction Parameters on the Catalytic Properties of Ni@MAmPA-COF
2.2.2. Influence of Chemical Structure on the Catalytic Properties of Catalysts
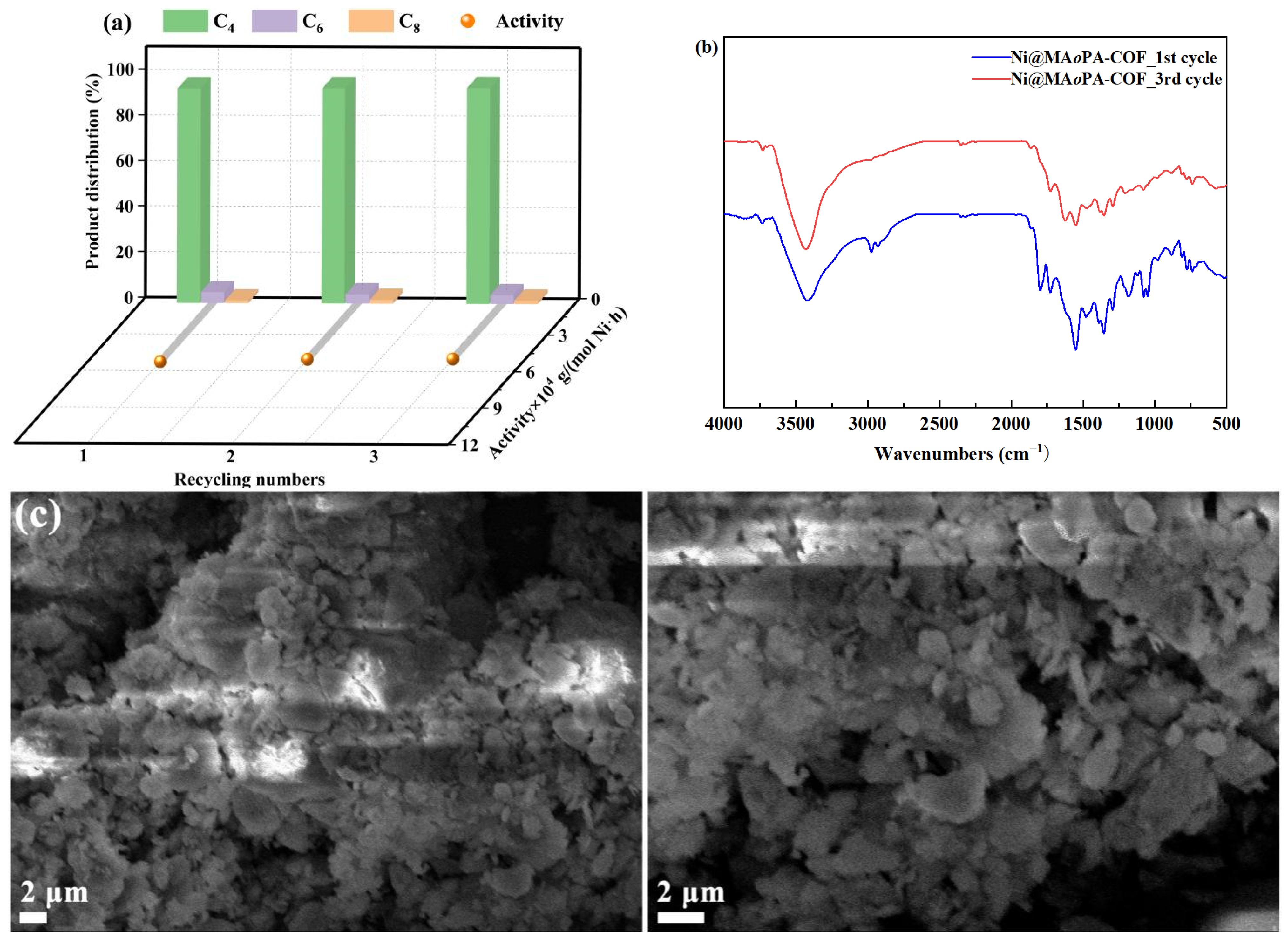
2.3. The Reusability of Ni@MAoPA-COF
3. Experimental Methods
3.1. Materials and Characterization
3.2. Synthesis of COFs Supports
3.3. Synthesis of COFs-Supported Nickel Catalysts
3.4. Catalytic Performance of Catalyst
3.5. Recycling Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouen, M.; Queval, P.; Borré, E.; Falivene, L.; Poater, A.; Berthod, M.; Hugues, F.; Cavallo, L.; Baslé, O.; Olivier-Bourbigou, H.; et al. Selective metathesis of α-olefins from bio-sourced Fischer-Tropsch feeds. ACS Catal. 2016, 6, 7970–7976. [Google Scholar] [CrossRef]
- Suerbaev, K.A.; Kudaibergenov, N.Z.; Vavasori, A. Hydroethoxycarbonylation of α-olefins at low pressure of carbon(II) oxide in the presence of the PdCl2(PPh3)2-PPh3-AlCl3 system. Russ. J. Gen. Chem. 2017, 87, 707–712. [Google Scholar] [CrossRef]
- Nicola, B.P.; Schwanke, A.J.; Bernardo-Gusmão, K. Porous clay nanoarchitectures as supports for heterogenized nickel complexes in catalytic reactions of ethylene oligomerization. Catal. Today 2022, 394–396, 256–267. [Google Scholar] [CrossRef]
- Shin, M.; Jeong, H.; Park, M.J.; Suh, Y.-W. Benefits of the SiO2-supported nickel phosphide catalyst on ethylene oligomerization. Appl. Catal. A Gen. 2020, 591, 117376. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization: Effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Rossetto, E.; Nicola, B.P.; de Souza, R.F.; Pergher, S.B.; Bernardo-Gusmão, K. Anchoring via covalent binding of β-diimine-nickel complexes in SBA-15 and its application in catalytic reactions. Appl. Catal. A Gen. 2015, 502, 221–229. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahamad, T.; Aldalbahi, A.; Alhokbany, N. Pyridylimine cobalt(II) and nickel(II) complex functionalized multiwalled carbon nanotubes and their catalytic activities for ethylene oligomerization. Adv. Polym. Technol. 2015, 35, 21528. [Google Scholar] [CrossRef]
- Li, C.; Zhai, X.; Cheng, M.; Li, Y.; Li, N.; Li, F.; Wang, J. Anchoring via covalent binding of a salicylaldimine-nickel complex in multi-walled carbon nanotubes and its application in ethylene oligomerization. J. Coord. Chem. 2020, 73, 1937–1953. [Google Scholar] [CrossRef]
- Azizov, A.H.; Khamiyev, M.J.; Khanmetov, A.A.; Alieva, R.V.; Aliyev, B.M.; Ahmedbekova, S.F. Oligomerization of the ethylene in the presence of new heterogenized Zr-containing complex catalytic systems. Eur. Chem. Bull. 2015, 4, 503–511. [Google Scholar]
- Ngcobo, M.; Ojwach, S.O. Ethylene oligomerization reactions catalyzed by recyclable Fe(II), Ni(II) and Co(II) complexes immobilized on Fe3O4 magnetic nanoparticles. Mol. Catal. 2021, 508, 111583. [Google Scholar] [CrossRef]
- Metzger, E.D.; Comito, R.J.; Wu, Z.; Zhang, G.; Dubey, R.J.-C.; Xu, W.; Miller, J.T.; Dincă, M. Highly selective heterogeneous ethylene dimerization with a scalable and chemically robust MOF catalyst. ACS Sustain. Chem. Eng. 2019, 7, 6654–6661. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, N.; Shi, D.; Zhang, F.; Tang, J. Synthesis of UiO-66-NH2 grafted pyridineimine cobalt catalyst and its catalytic performance in ethylene oligomerization. Chin. J. Appl. Chem. 2022, 39, 258. [Google Scholar]
- Shen, A.; Luo, R.; Liao, X.; He, C.; Li, Y. Highly dispersed silver nanoparticles confined in a nitrogen-containing covalent organic framework for 4-nitrophenol reduction. Mater. Chem. Front. 2021, 5, 6923–6930. [Google Scholar] [CrossRef]
- Sharma, A.; Babarao, R.; Medhekar, N.V.; Malani, A. Methane adsorption and separation in slipped and functionalized covalent organic frameworks. Ind. Eng. Chem. Res. 2018, 57, 4767–4778. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Zakaria, M.B.; Wang, H.X.; Chen, T.; Yamauchi, Y.; Kuo, S.-W. Heteroporous bifluorenylidene-based covalent organic frameworks displaying exceptional dye adsorption behavior and high energy storage. J. Mater. Chem. A 2020, 8, 25148–25155. [Google Scholar] [CrossRef]
- Lin, H.; Chen, C.; Zhou, T.; Zhang, J. Two-dimensional covalent-organic frameworks for photocatalysis: The critical roles of building block and linkage. Sol. RRL. 2020, 5, 2000458. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Langenhahn, T.; Ye, M.; Schlesiger, C.; Praetz, S.; Schmidt, J.; Thomas, A. Macro/microporous covalent organic frameworks for efficient electrocatalysis. J. Am. Chem. Soc. 2019, 141, 6623–6630. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Lai, M.Y.; Kuo, S.W. A highly fluorescent covalent organic framework as a hydrogen chloride sensor: Roles of Schiff base bonding and π-stacking. J. Mater. Chem. C. 2020, 8, 9520–9528. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Gao, R.; Zhao, Z.; Zhang, T.; Xing, G.; Chen, L. 2D covalent organic frameworks with built-in amide active sites for efficient heterogeneous catalysis. Chem. Commun. 2019, 55, 14538–14541. [Google Scholar] [CrossRef]
- Liu, Y.; Dikhtiarenko, A.; Xu, N.; Sun, J.; Tang, J.; Wang, K.; Xu, B.; Tong, Q.; Heeres, H.J.; He, S.; et al. Triphenylphosphine-based covalent organic frameworks and heterogeneous Rh-P-COFs catalysts. Chem. Eur. J. 2020, 26, 12134–12139. [Google Scholar] [CrossRef]
- Fan, M.; Wang, W.D.; Zhu, Y.; Sun, X.; Zhang, F.; Dong, Z. Palladium clusters confined in triazinyl-functionalized COFs with enhanced catalytic activity. Appl. Catal. B. 2019, 257, 117942. [Google Scholar] [CrossRef]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Rozhko, E.; Bavykina, A.; Osadchii, D.; Makkee, M.; Gascon, J. Covalent organic frameworks as supports for a molecular Ni based ethylene oligomerization catalyst for the synthesis of long chain olefins. J. Catal. 2017, 345, 270–280. [Google Scholar] [CrossRef]
- Li, D.; Guo, L.; Li, F.; Huang, J.; Li, J.; Li, M.; Li, C. Synthesis and catalytic behavior of nickel heterogenized in covalent organic frameworks as precatalysts in ethylene oligomerization. Microporous Mesoporous Mater. 2022, 338, 111979. [Google Scholar] [CrossRef]
- Mu, X.; Zhan, J.; Feng, X.; Yuan, B.; Qiu, S.; Song, L.; Hu, Y. A novel melamine/o-phthalaldehyde covalent organic frameworks nanosheets: Enhancement flame retardant and mechanical performances of thermoplastic polyurethanes. ACS Appl. Mater. Interfaces 2017, 9, 23017–23026. [Google Scholar] [CrossRef]
- Qu, A.; Xu, X.; Zhang, Y.; Li, Y.; Zha, W.; Wen, S.; Xie, H.; Wang, J. A nitrogen-rich mesoporous polymer for photocatalytic hydrogen evolution from water. React. Funct. Polym. 2016, 102, 93–100. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, P.; An, W.; Ma, M.; Yang, M.; Luan, T.; Tang, R.; Wang, B.; Hao, A. pH-Responsive dipeptide-based dynamic covalent chemistry systems whose products and self-assemblies depend on the structure of isomeric aromatic dialdehydes. Langmuir 2018, 34, 13725–13734. [Google Scholar] [CrossRef]
- Leng, W.; Peng, Y.; Zhang, J.; Lu, H.; Feng, X.; Ge, R.; Dong, B.; Wang, B.; Hu, X.; Gao, Y. Sophisticated Design of Covalent Organic Frameworks with Controllable Bimetallic Docking for a Cascade Reaction. Chem. Eur. J. 2016, 22, 9087–9091. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Rafiee, Z. Fabrication of efficient Zn-MOF/COF catalyst for the Knoevenagel condensation reaction. J. Iran. Chem. Soc. 2021, 18, 2657–2664. [Google Scholar] [CrossRef]
- Fan, M.; Long, Y.; Zhu, Y.; Hu, X.; Dong, Z. Two-dimensional covalent-organic-framework-derived nitrogen-rich carbon nanosheets modified with small Pd nanoparticles for the hydrodechlorination of chlorophenols and hydrogenation of phenol. Appl. Catal. A Gen. 2018, 568, 130–138. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R.; Zhu, W.; Zhang, Z.; Chen, Y.; Wang, S.; Deng, Q. Desirability of position 2, 2′-bipyridine group into COFs for the fluorescence sensing of Ni(II). Sens. Actuators B Chem. 2021, 344, 130216. [Google Scholar] [CrossRef]
- Chen, L.; Huo, H.; Wang, L.; Kuang, Q.; Shi, W.; Zhang, N.; Li, Z.; Wang, J. Ethylene oligomerization studies utilizing nickel complexes bearing pyridine-imine ligands. Inorganica Chim. Acta. 2019, 491, 67–75. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Chen, L.; Lan, T.; Wang, L. Nickel complexes based on hyperbranched bispyridylamine ligands as catalyst precursors for ethylene oligomerization. J. Chem. Res. 2019, 44, 206–211. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Pastor, M.F.E.; Delcroix, D. Nickel catalyzed olefin oligomerization and dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Lin, W.; Ma, Y.; Hu, X.; Flisak, Z.; Sun, W.H. Ethylene oligomerization with 2-hydroxymethyl-5,6,7- trihydroquinolinyl-8-ylideneamine-Ni(II) chlorides. J. Organomet. Chem. 2021, 937, 121720. [Google Scholar] [CrossRef]
- Bekmukhamedov, G.E.; Sukhov, A.V.; Kuchkaev, A.M.; Yakhvarov, D.G. Ni-based complexes in selective ethylene oligomerization processes. Catalysts 2020, 10, 498. [Google Scholar] [CrossRef]

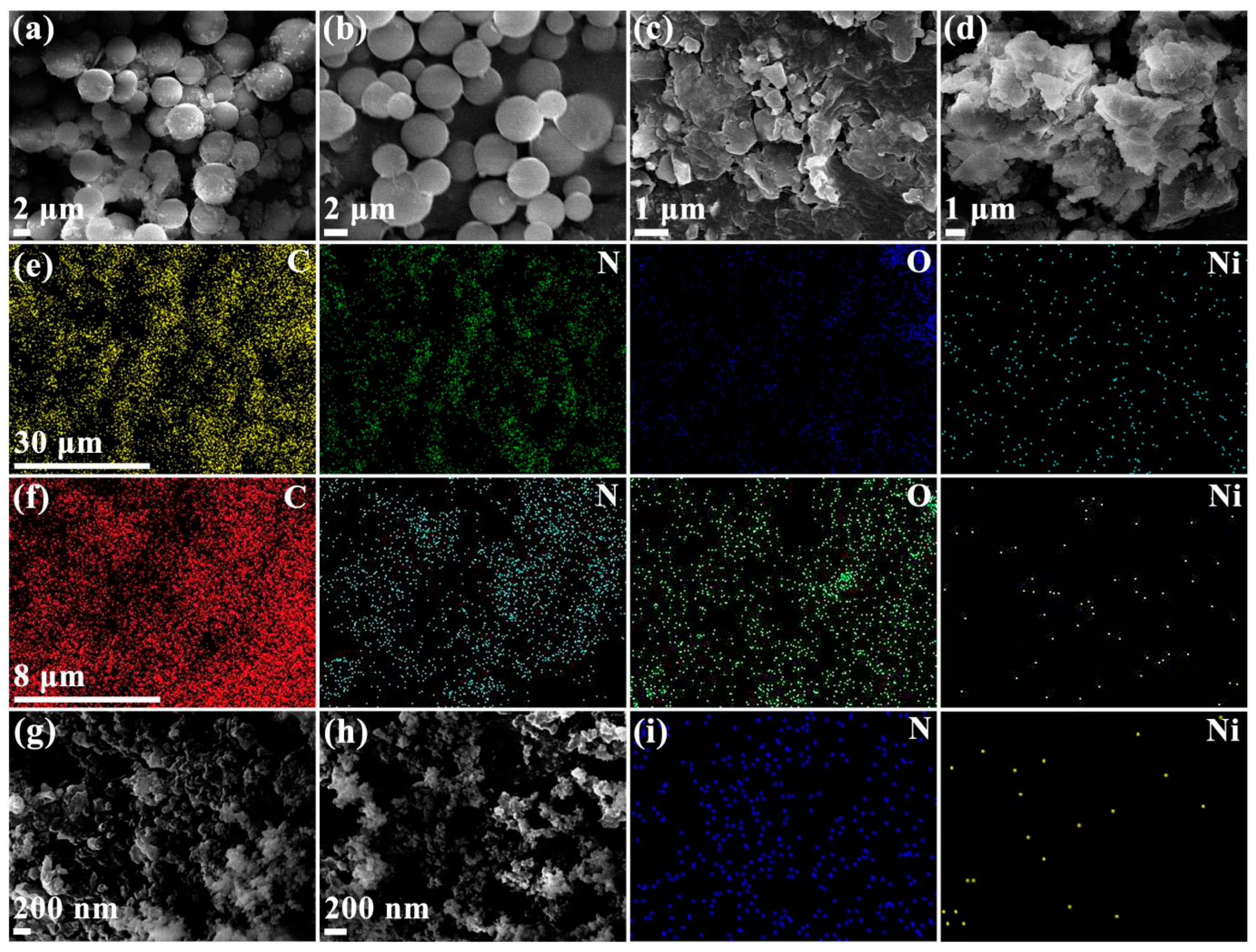

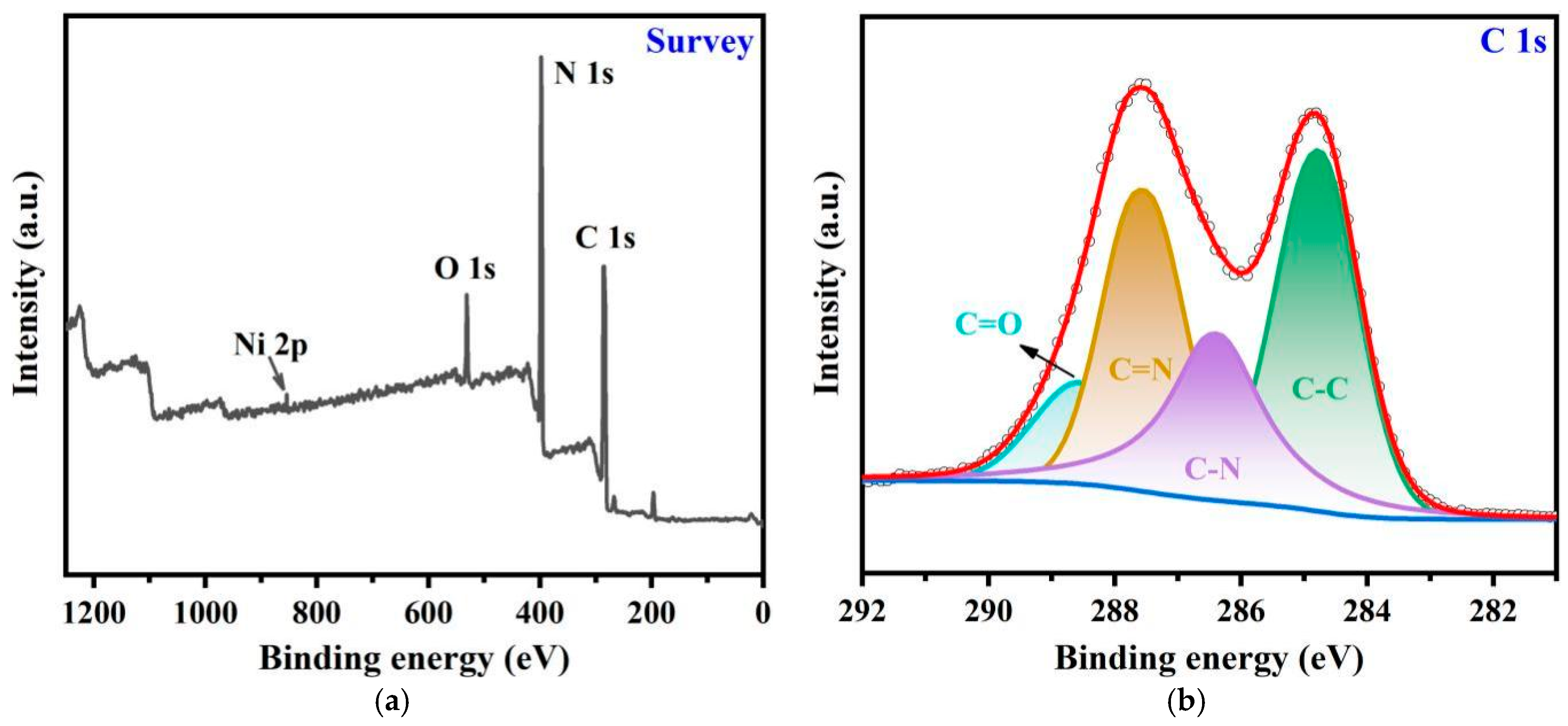
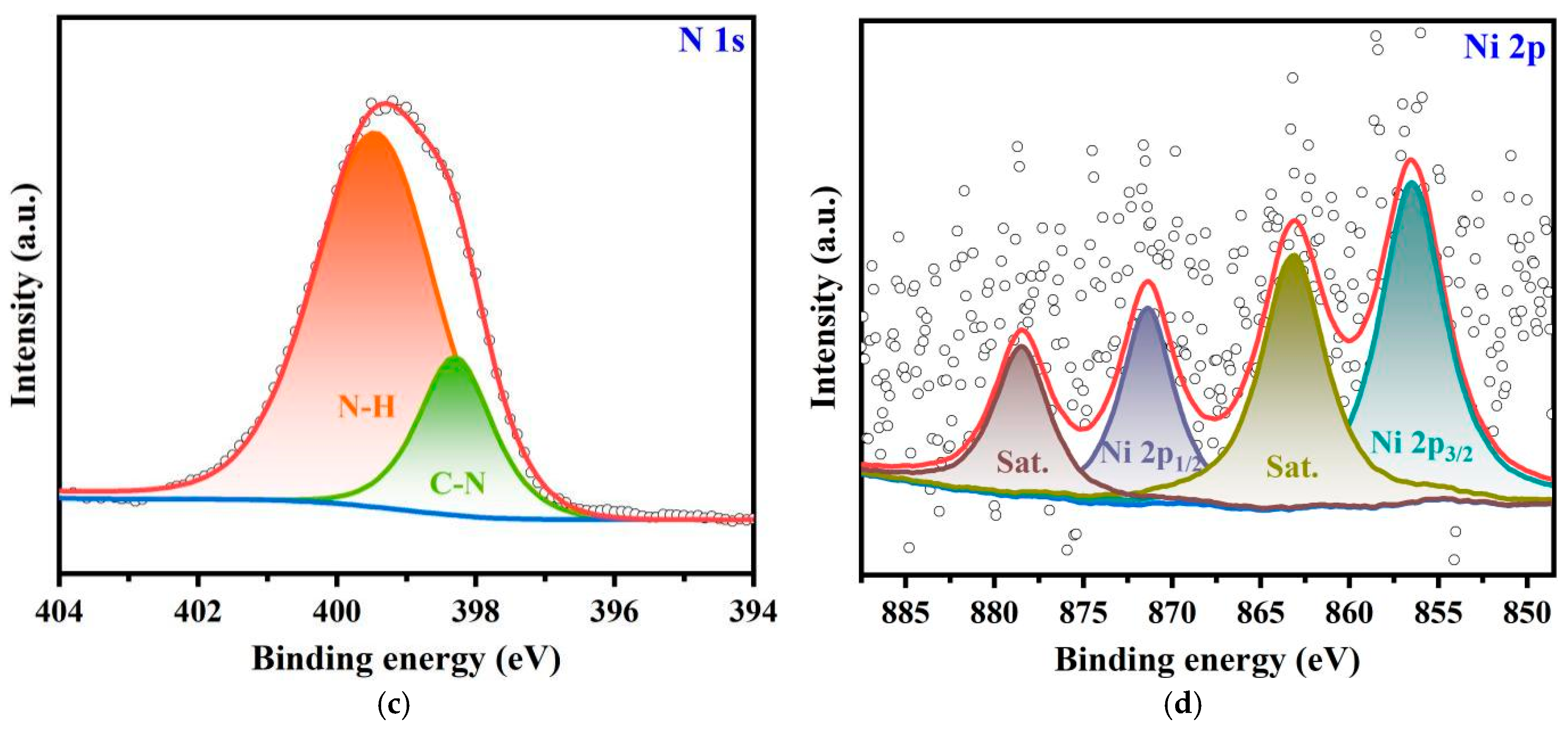
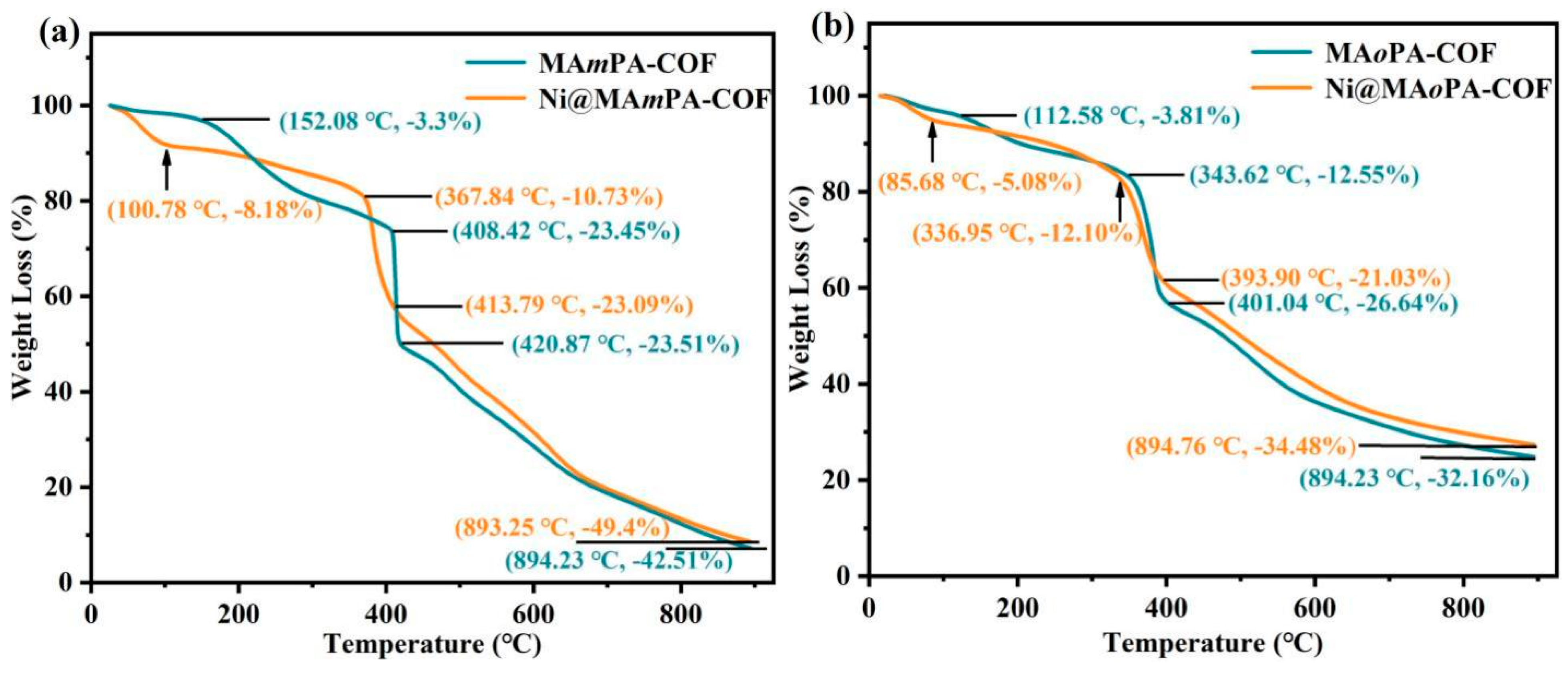

| Samples | EDX | ICP | |||
|---|---|---|---|---|---|
| C | N | O | Ni | Ni | |
| Ni@MAPA-COF | 81.35 | 8.94 | 8.38 | 1.33 | 7.58 |
| Ni@MAmPA-COF | 44.48 | 45.95 | 8.44 | 1.13 | 5.76 |
| Ni@MAoPA-COF | 58.93 | 30.32 | 9.95 | 0.80 | 4.91 |
| Sample Name | BET Surface Area (m2 g−1) | Pore Width (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| MAmPA-COF a | 32.8 | 10.0 | 0.06 |
| MAoPA-COF a | 64.9 | 13.7 | 0.23 |
| MAPA-COF b | 234.7 | 17.5 | 0.33 |
| Ni@MAmPA-COF | 16.3 | 18.3 | 0.08 |
| Ni@MAoPA-COF | 40.3 | 1.9 | 0.06 |
| Ni@MAPA-COF | 192.6 | 17.0 | 0.30 |
| Target | Molar Ratio of Al/Ni | Reaction Time | Reaction Pressure |
|---|---|---|---|
| Activity | 0.6764 | 0.5776 | 0.7238 |
| Selectivity of C4 + C6 | 0.6824 | 0.5818 | 0.6536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, J.; Li, D.; Guo, L.; Xiao, Y.; Li, C. Study on the Catalytic Performance of Nickel(II) Complexes with Distinct Triazine Support Structures in Ethylene Oligomerization via Different Experiment Designs. Molecules 2025, 30, 1977. https://doi.org/10.3390/molecules30091977
Wei X, Li J, Li D, Guo L, Xiao Y, Li C. Study on the Catalytic Performance of Nickel(II) Complexes with Distinct Triazine Support Structures in Ethylene Oligomerization via Different Experiment Designs. Molecules. 2025; 30(9):1977. https://doi.org/10.3390/molecules30091977
Chicago/Turabian StyleWei, Xiaobing, Jiahui Li, Dan Li, Lijun Guo, Yanling Xiao, and Cuiqin Li. 2025. "Study on the Catalytic Performance of Nickel(II) Complexes with Distinct Triazine Support Structures in Ethylene Oligomerization via Different Experiment Designs" Molecules 30, no. 9: 1977. https://doi.org/10.3390/molecules30091977
APA StyleWei, X., Li, J., Li, D., Guo, L., Xiao, Y., & Li, C. (2025). Study on the Catalytic Performance of Nickel(II) Complexes with Distinct Triazine Support Structures in Ethylene Oligomerization via Different Experiment Designs. Molecules, 30(9), 1977. https://doi.org/10.3390/molecules30091977







