Exploring the Role of Microplasma for Controlling Cellular Senescence in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results
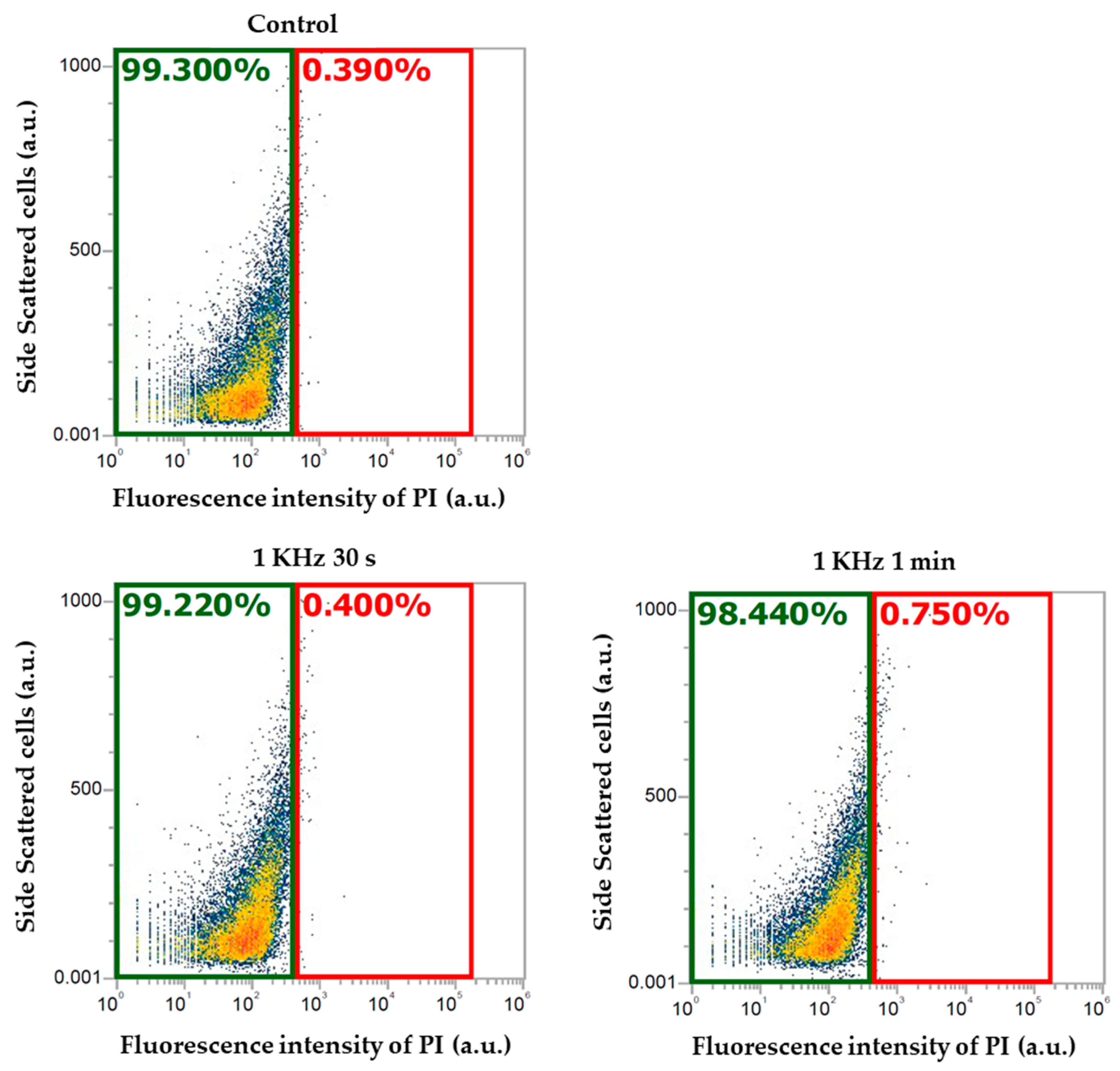
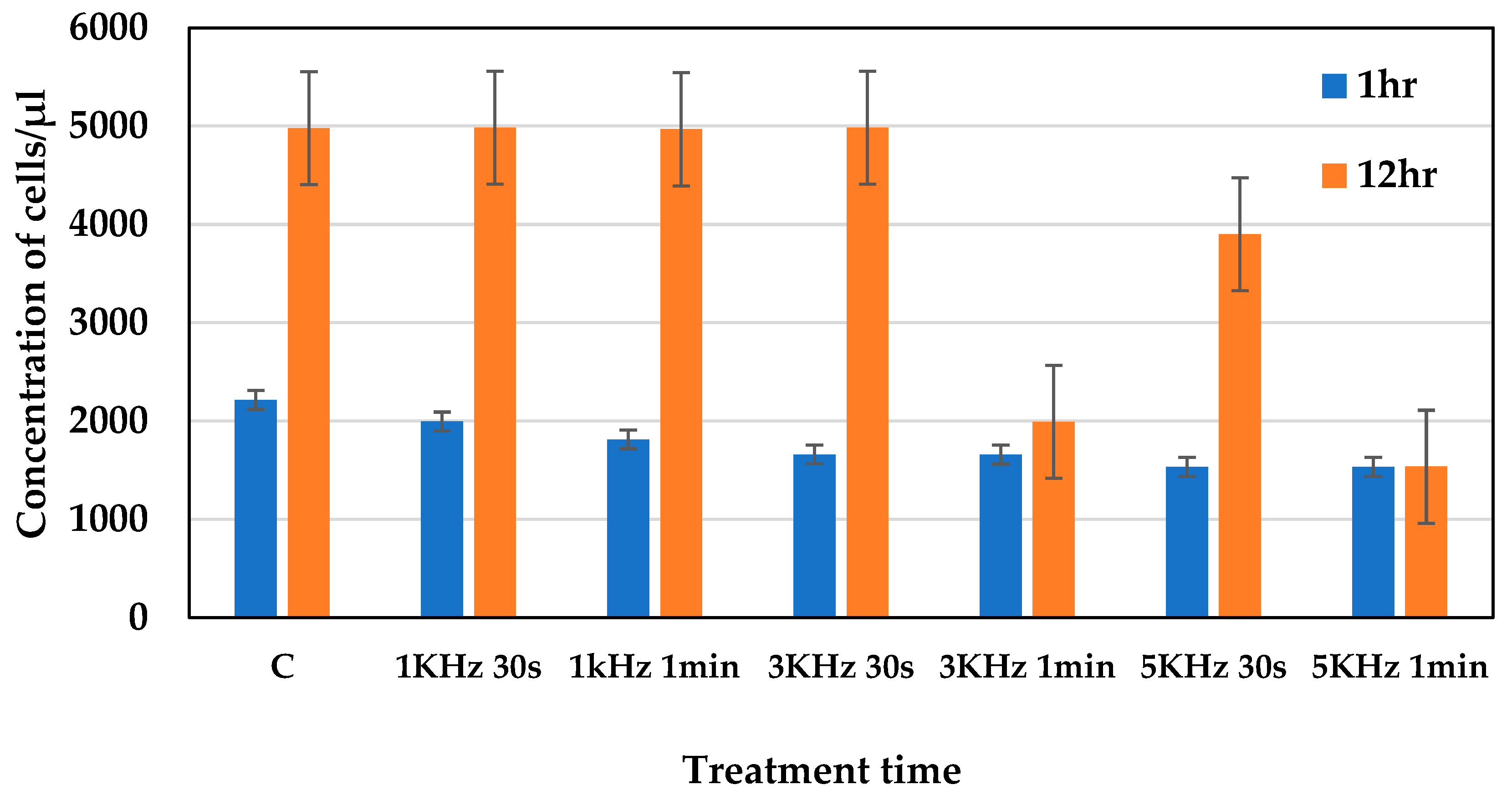
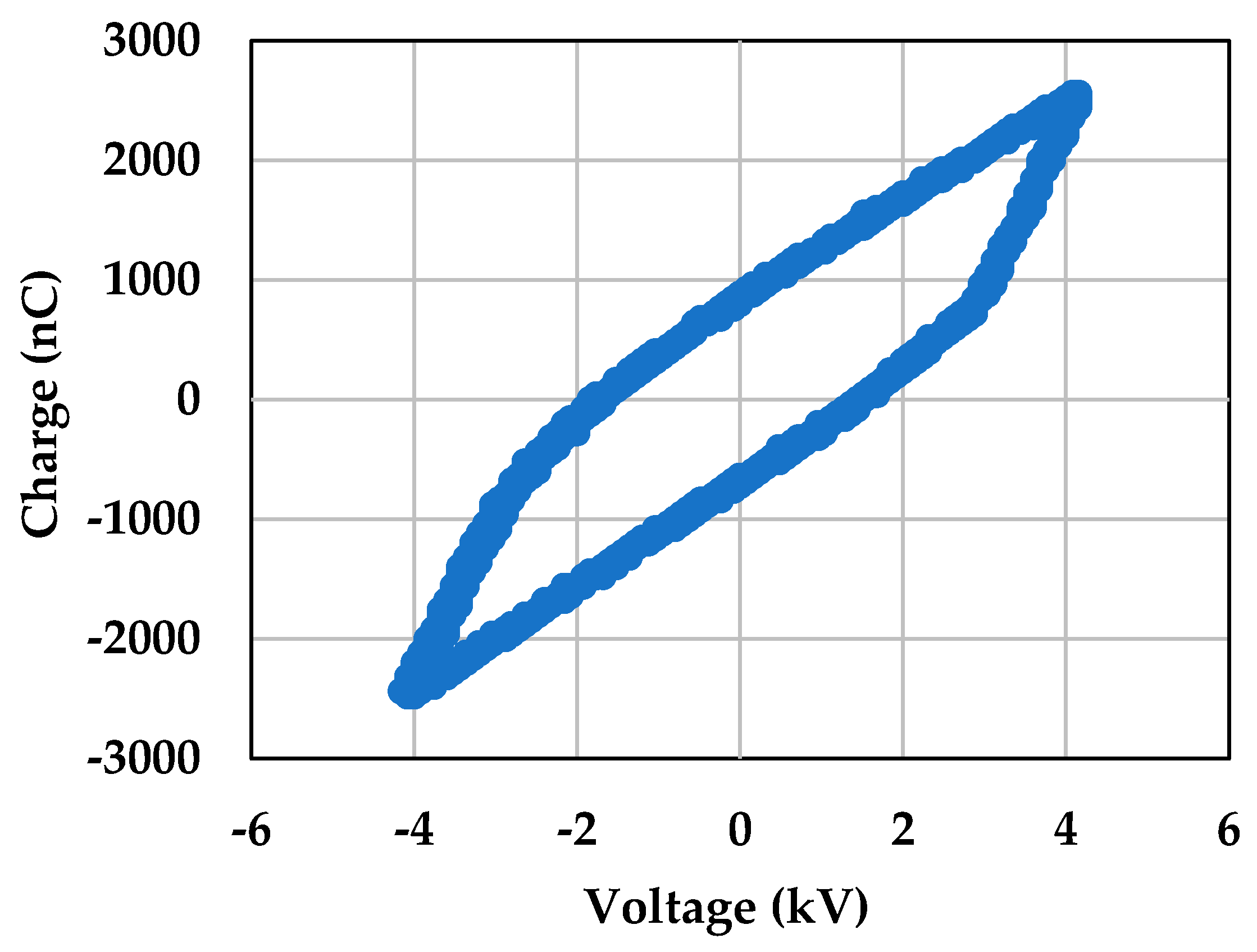
2.1. Assessing the Optimum Plasma Conditions
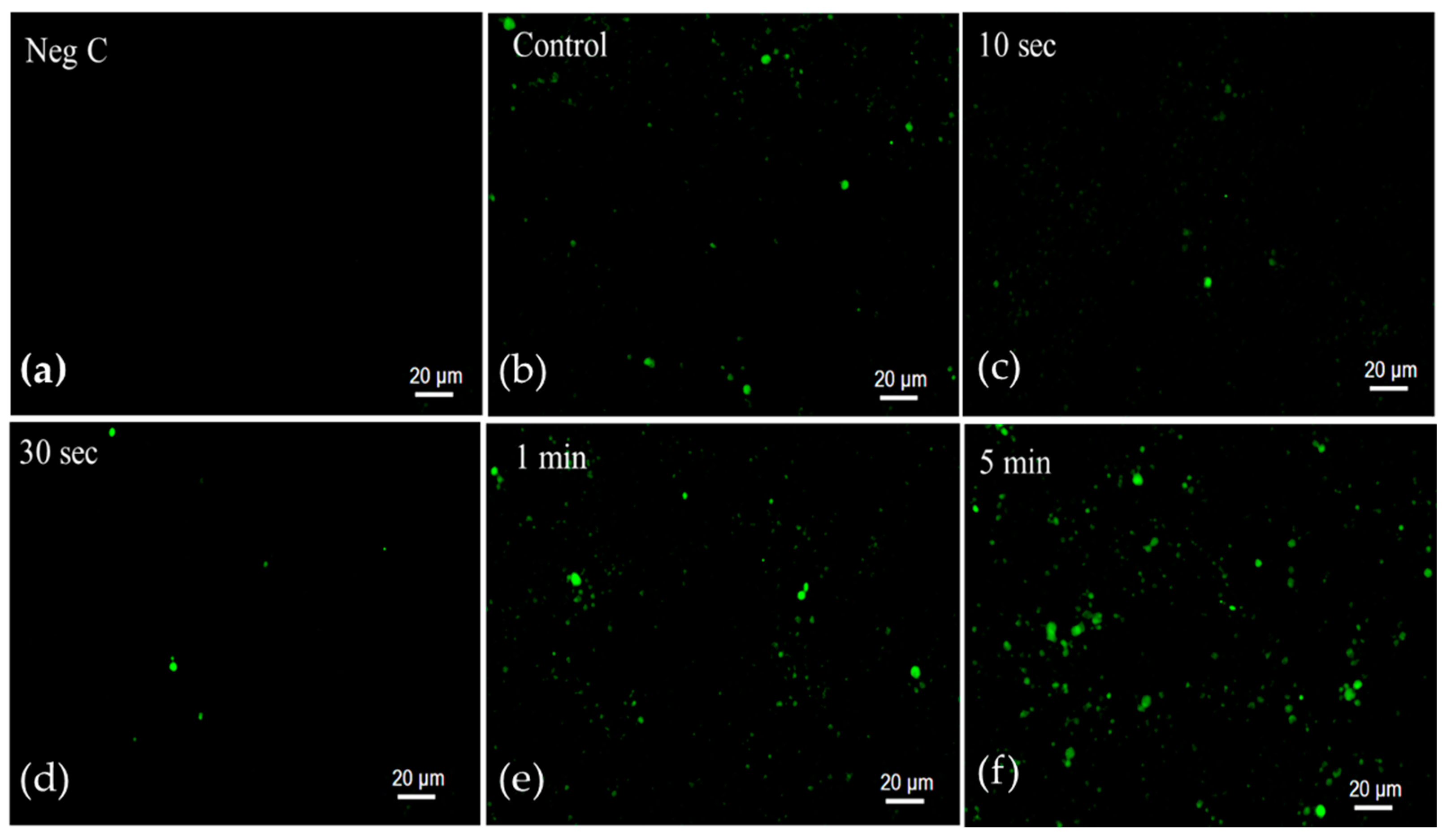
2.2. Senescence-Associated β-Galactosidase Activity
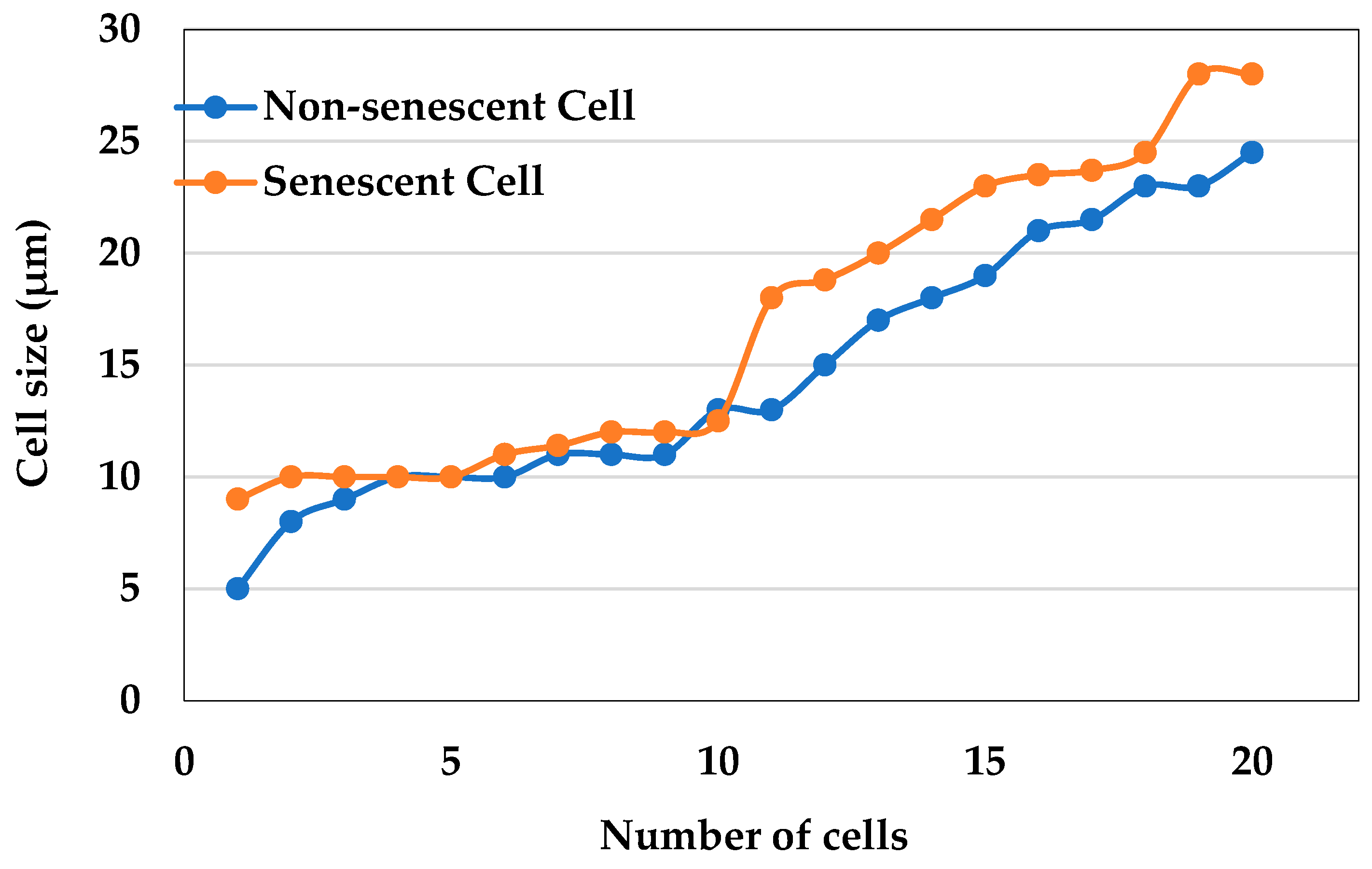
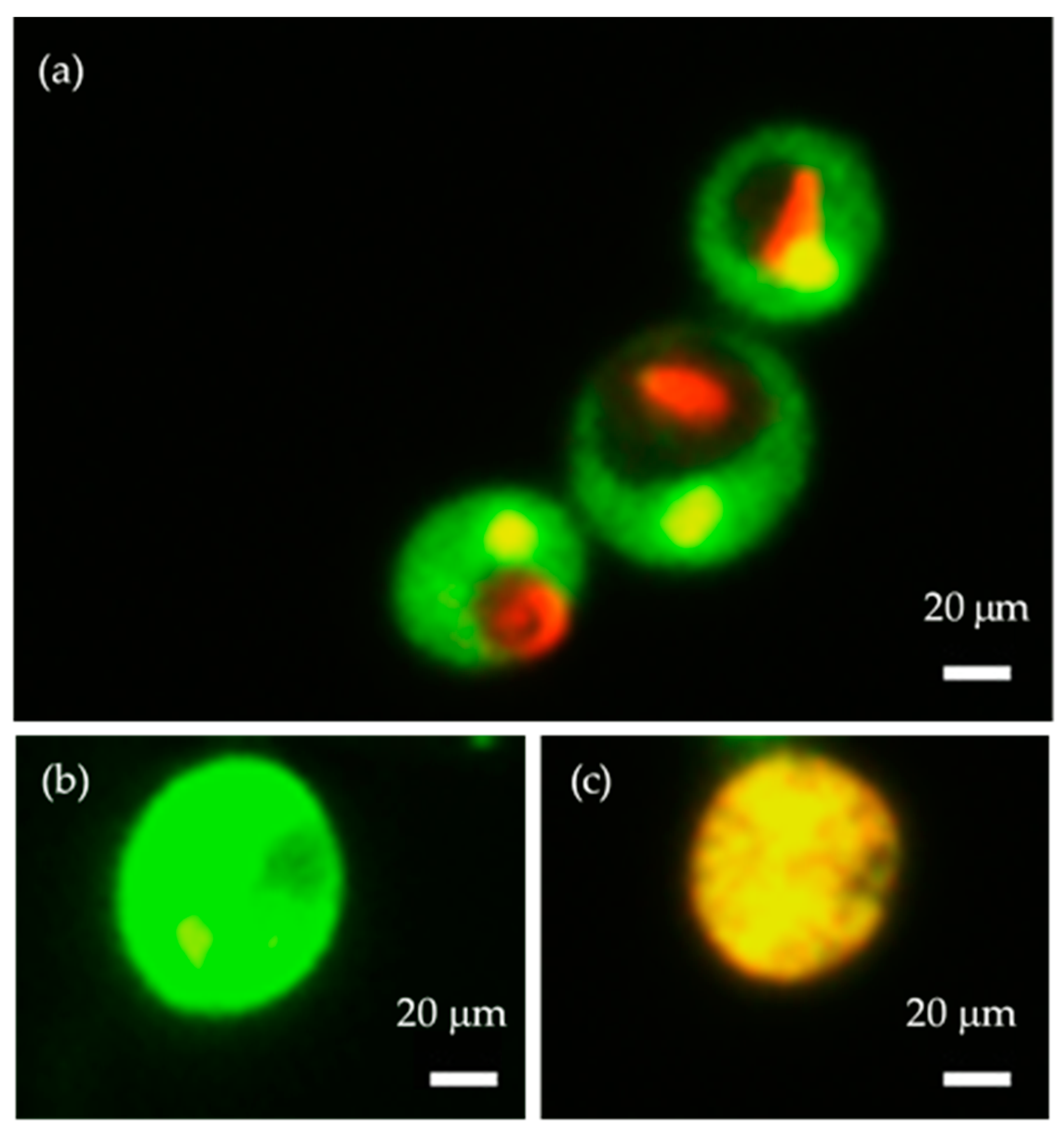
2.3. Measurement of the Cell Size
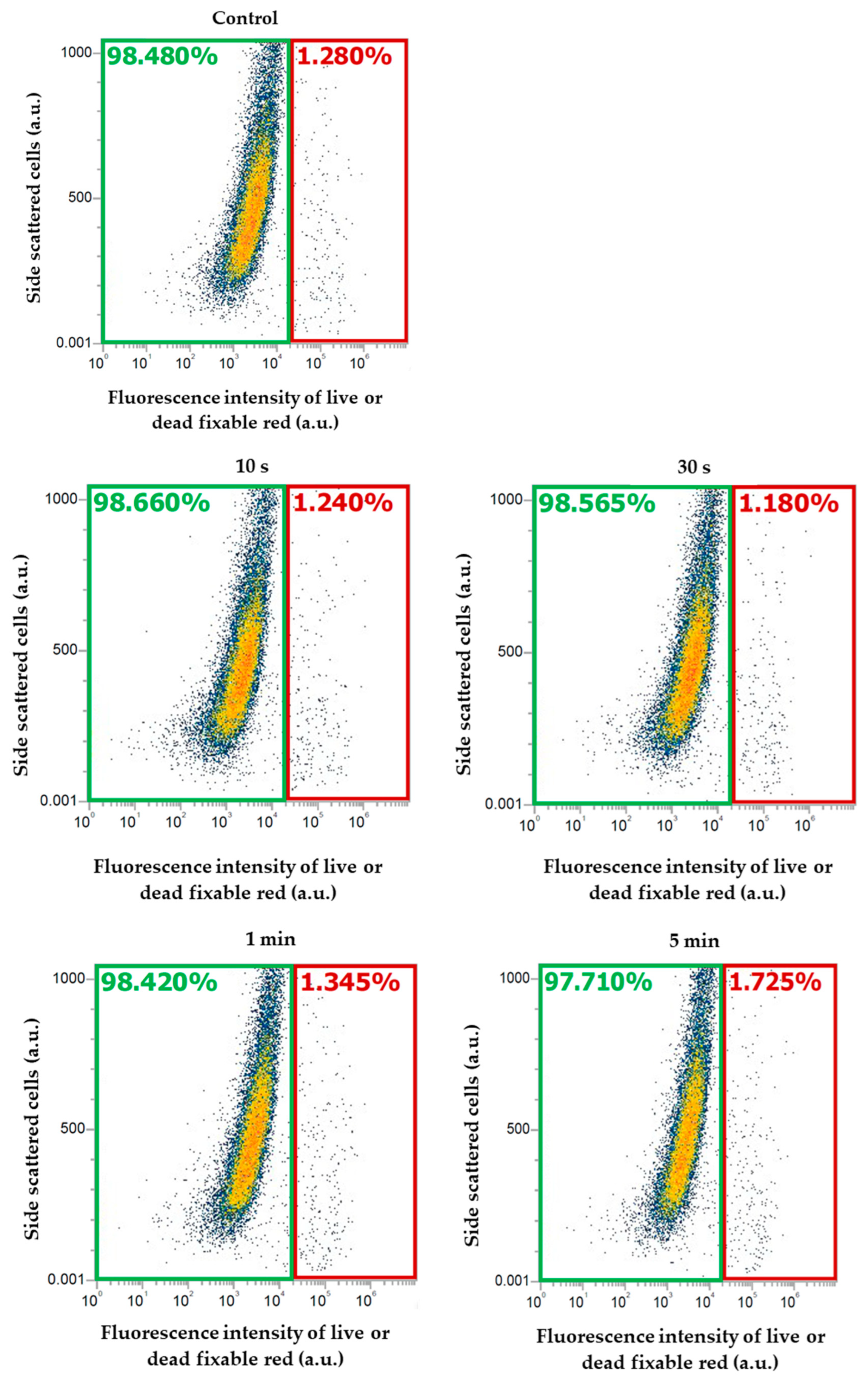
2.4. Cell Viability Measurement
2.5. Measurement of the Metabolic Activity of the Cells
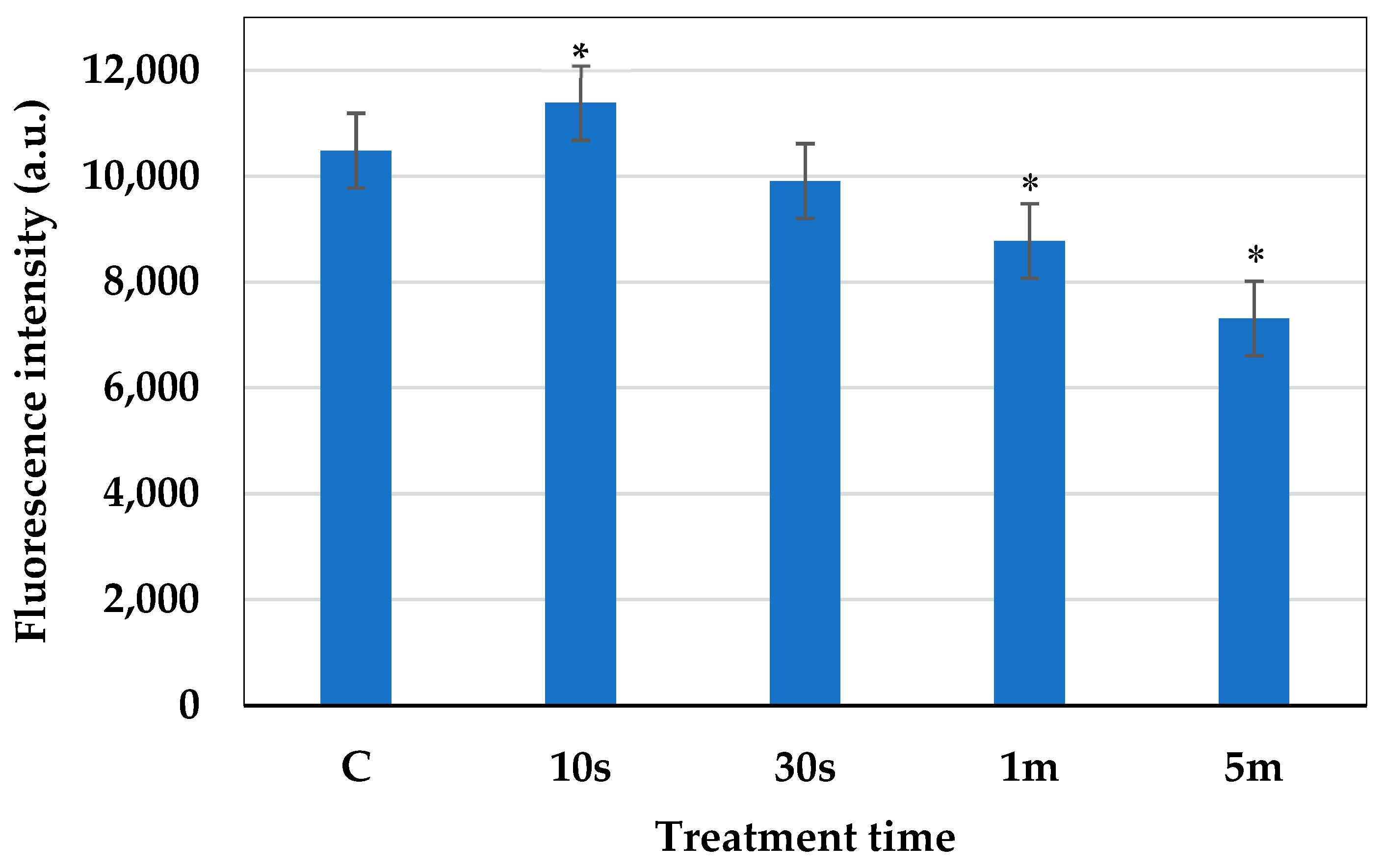
2.6. Measurement of the Cell Membrane Potential
2.7. Amount of Intracellular ROS
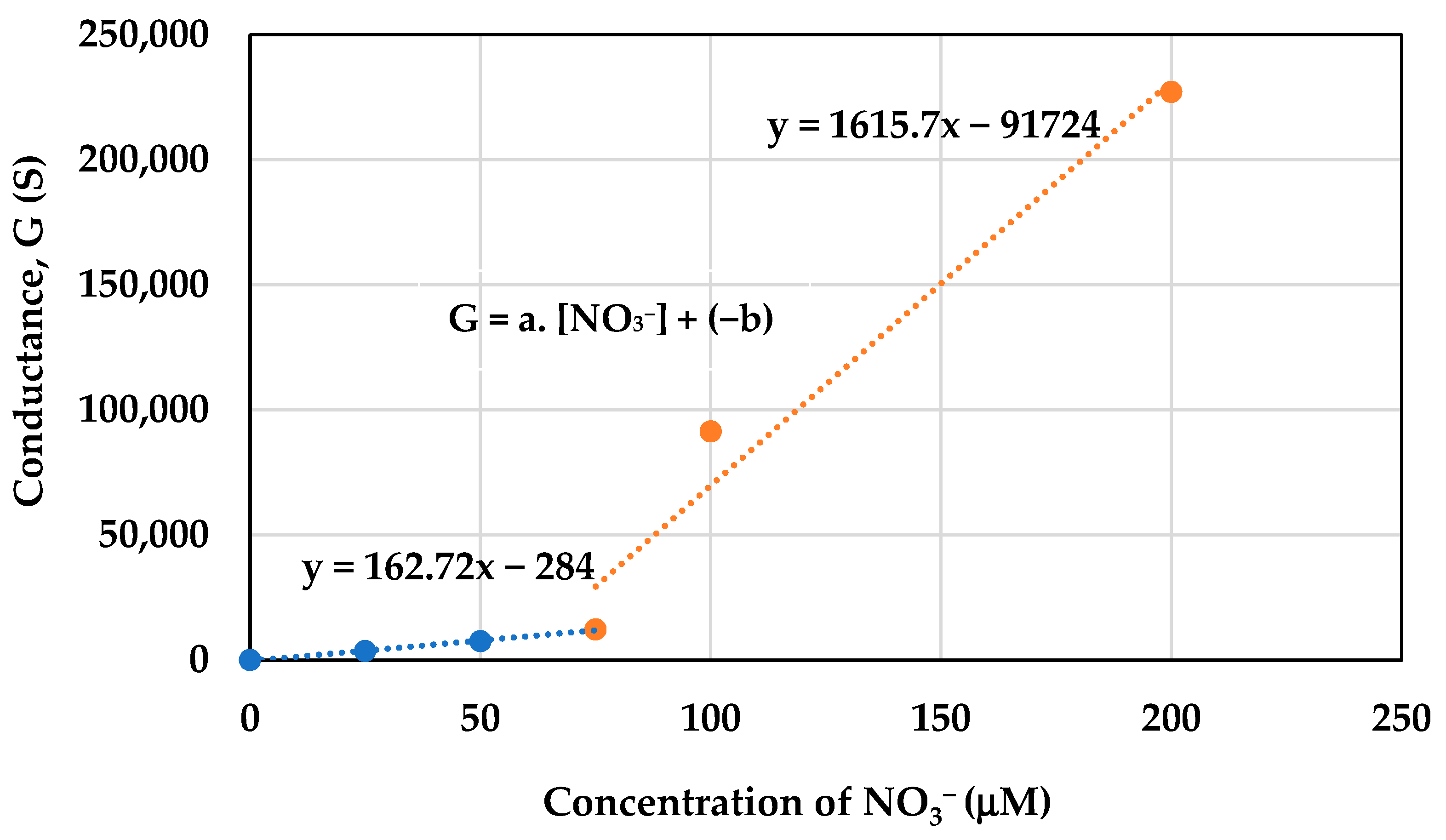
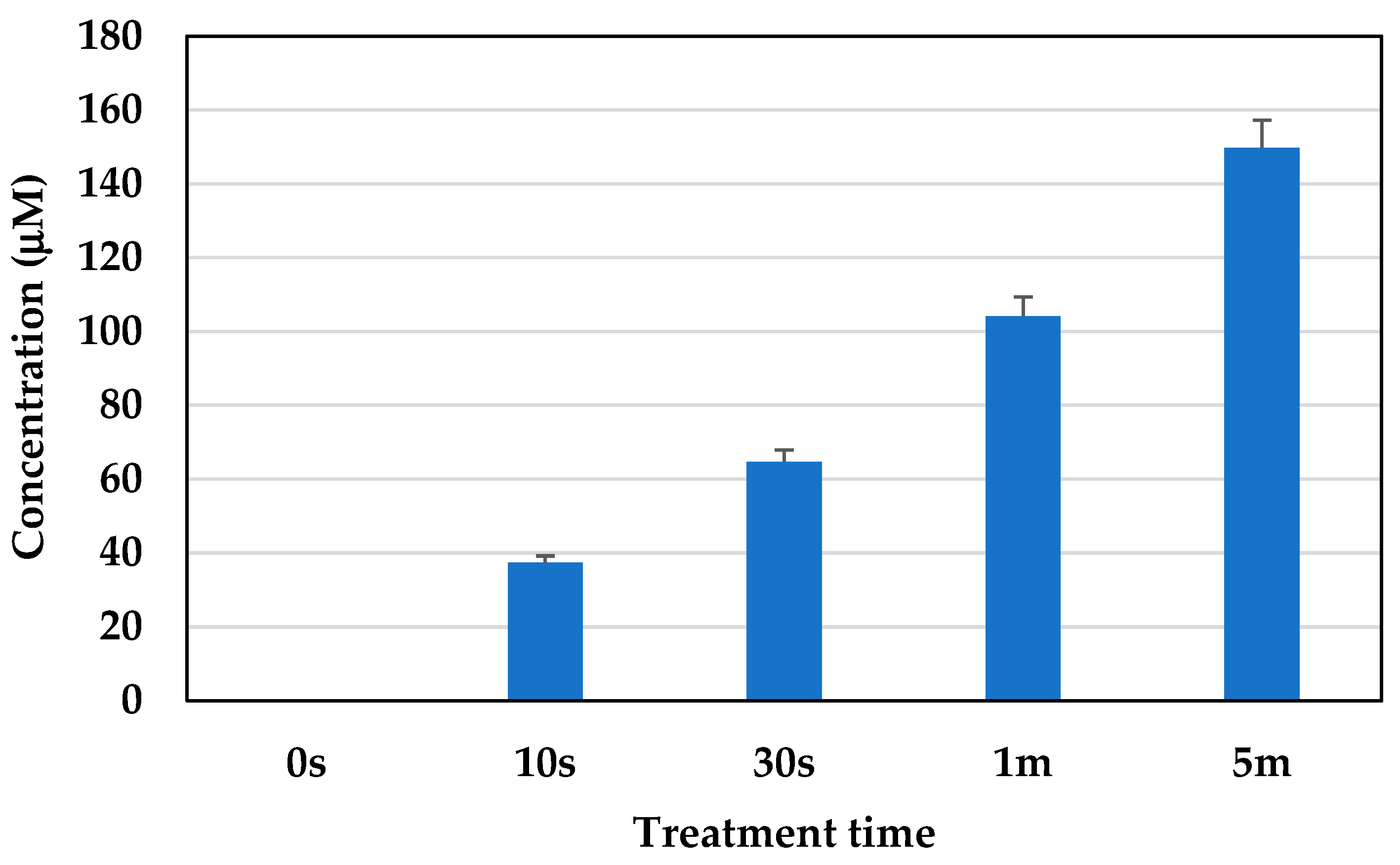
2.8. Concentration of Active Nitrogen Species
3. Discussion
4. Materials and Methods
4.1. Materials Used
4.2. Cell Culture Conditions
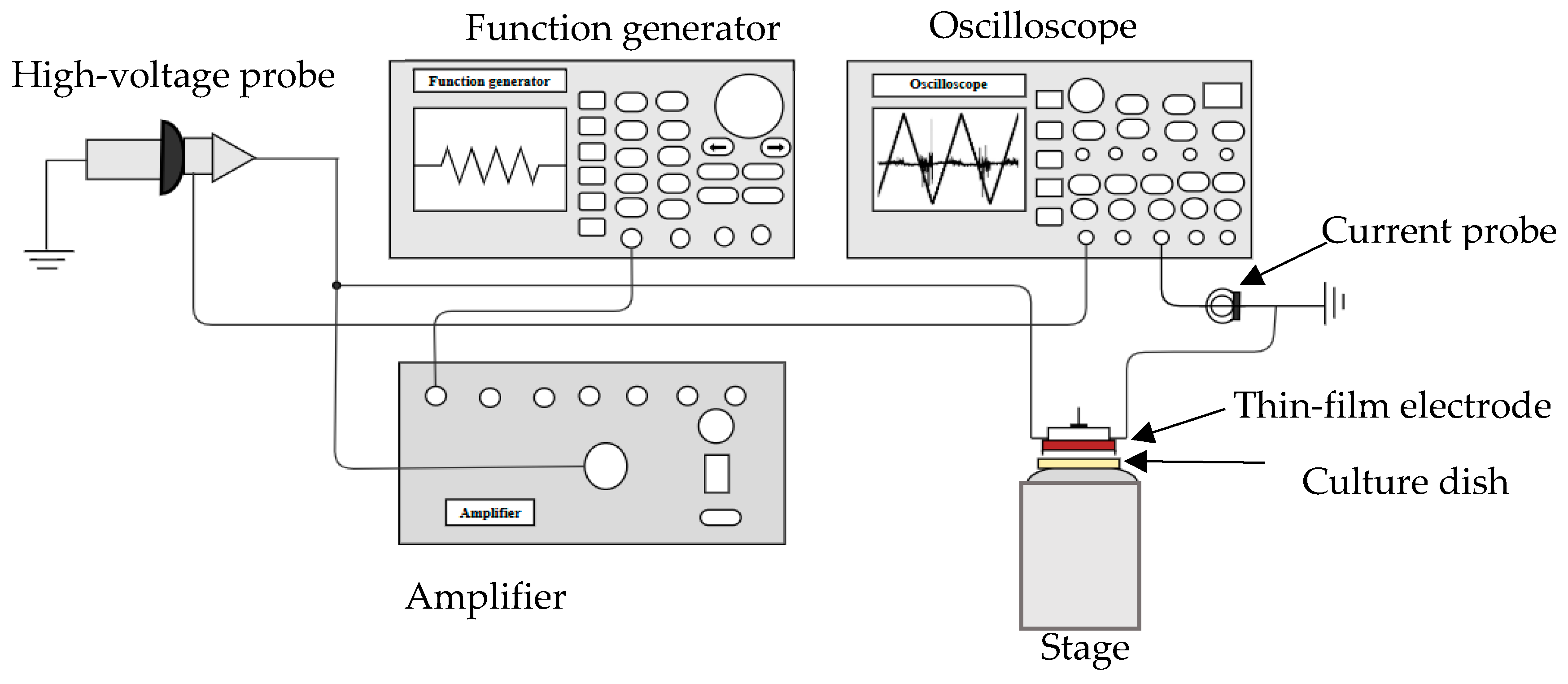
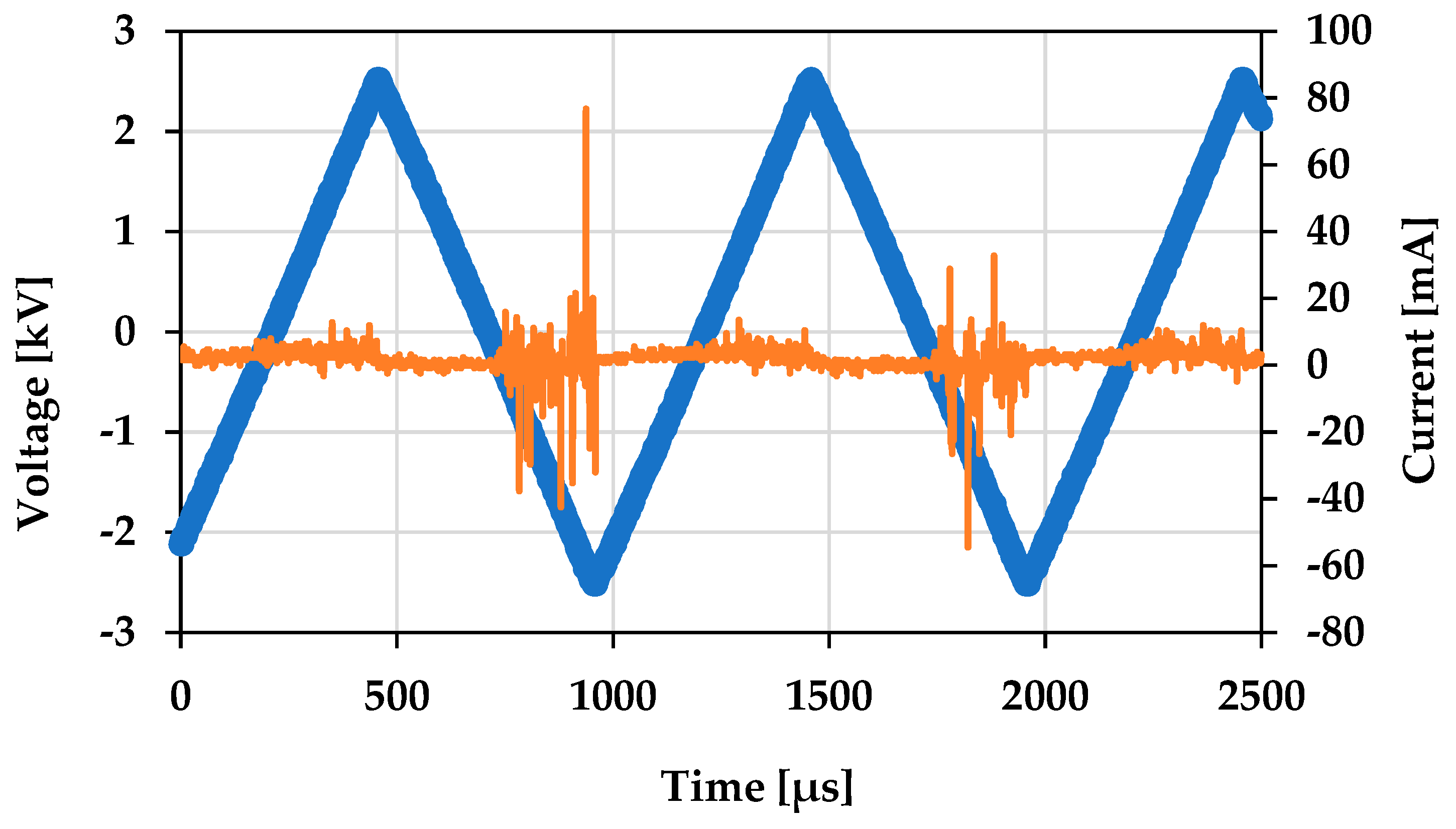
4.3. DBD Microplasma Set Up
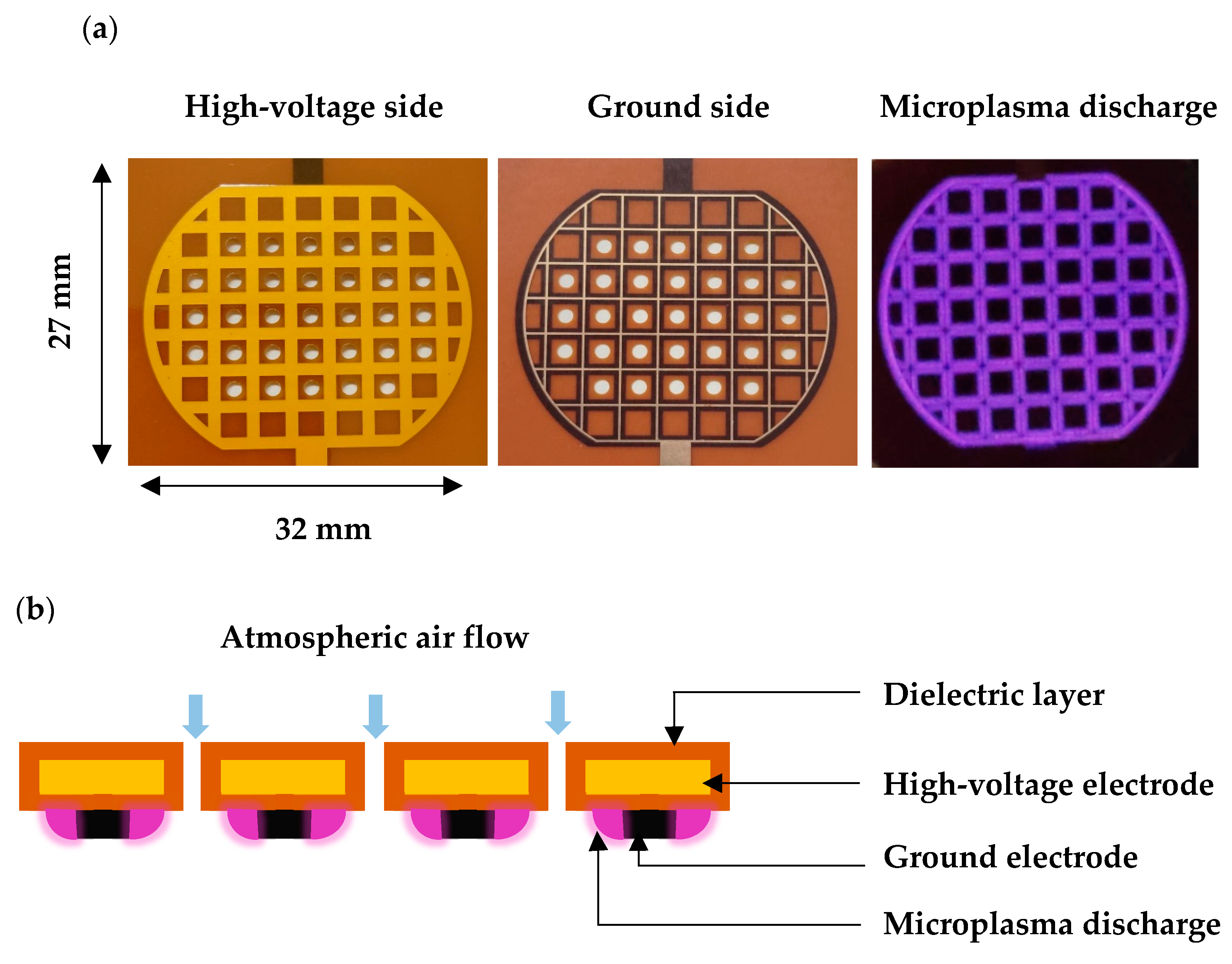
4.4. Microplasma Electrode
4.5. Plasma Conditions and Treatment
4.6. Measurement of the Cellular Senescence
4.6.1. Measurement of SA β-Galactosidase Activity
4.6.2. Cell Size
4.7. Cell Viability
4.8. Metabolic Activity of the Cells
4.9. Cell Membrane Potential
4.10. Measurement of Intracellular ROS
4.11. Measurement of Active Nitrogen Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxidative Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Tamada, Y.; Nakayama, Y.; Fukusaki, E.; Mukai, Y. Changes in Transcription and Metabolism During the Early Stage of Replicative Cellular Senescence in Budding Yeast. J. Biol. Chem. 2014, 289, 32081–32093. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Muller, M. Cellular senescence: Molecular mechanisms, in vivo significance, and redox considerations. Antioxid. Redox Signal. 2009, 11, 59–98. [Google Scholar] [CrossRef]
- Gershon, H.; Gershon, D. The budding yeast, Saccharomyces cerevisiae, as a model for aging research: A critical review. Mech. Ageing Dev. 2000, 120, 1–22. [Google Scholar] [CrossRef]
- Hartwell, L.H. Yeast and cancer. Biosci. Rep. 2002, 22, 373–394. [Google Scholar] [CrossRef]
- Coughlan, C.M.; Brodsky, J.L. Use of yeast as a model system to investigate protein conformational diseases. Mol. Biotechnol. 2005, 30, 171–180. [Google Scholar] [CrossRef]
- Lindquist, S.L. Prion proteins: One surprise after another. Harvey Lect. 2002, 98, 173–205. [Google Scholar] [CrossRef]
- Nakano, A. Yeast Golgi apparatus–dynamics and sorting. Cell. Mol. Life Sci. 2004, 61, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Stevens, T.H. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2005, 1744, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Mirisola, M.G.; Braun, R.J.; Petranovic, D. Approaches to study yeast cell aging and death. FEMS Yeast Res. 2014, 14, 109–118. [Google Scholar] [CrossRef]
- Karathia, H.; Vilaprinyo, E.; Sorribas, A.; Alves, R. Saccharomyces cerevisiae as a model organism: A comparative study. PLoS ONE 2011, 6, e16015. [Google Scholar] [CrossRef] [PubMed]
- Jazwinski, S.M. New clues to old yeast. Mech. Ageing Dev. 2001, 122, 865–882. [Google Scholar] [CrossRef]
- Jazwinski, S.M. Yeast replicative life span—The mitochondrial connection. FEMS Yeast Res. 2004, 5, 119–125. [Google Scholar] [CrossRef][Green Version]
- Motizuki, M.; Tsurugi, K. The effect of aging on protein synthesis in the yeast Saccharomyces cerevisiae. Mech. Ageing Dev. 1992, 64, 235–245. [Google Scholar] [CrossRef]
- Rimi, S.A.; Alam, M.J.; Kristof, J.; Sadiq, A.H.; Hasan, M.; Mamun, M.A.; Setou, M.; Shimizu, K. Lipidomics of Microplasma-Irradiated Cells at Optimized Discharge Conditions for the Absorption of High-Molecule Drug. Appl. Sci. 2024, 14, 3978. [Google Scholar] [CrossRef]
- Becker, K.H.; Schoenbach, K.H.; Eden, J.G. Microplasmas and Applications. J. Phys. D Appl. Phys. 2006, 39, R55. [Google Scholar] [CrossRef]
- VonWoedtke, T.; Laroussi, M.; Gherardi, M. Foundations of Plasmas for Medical Applications. Plasma Sources Sci. Technol. 2022, 31, 054002. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.F.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar]
- Li, L.; Li, J.; Shen, M.; Zhang, C.; Dong, Y. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 1–42. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Hatakeyama, R.; Kaneko, T. Nano-Bio Fusion Science Opened and Created with Plasmas. Plasma Fusion Res. 2011, 6, 1106011. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Kristof, J. Enhancement of percutaneous absorption on skin by plasma drug delivery method. In Advanced Technology for Delivering Therapeutics; Maiti, S., Sen, K.K., Eds.; InTech: Rijeka, Croatia, 2017; pp. 111–136. [Google Scholar]
- Shimizu, K.; Tran, A.N.; Blajan, M. Effect of microplasma irradiation on skin barrier function. Jpn. J. Appl. Phys. 2016, 55, 07LG01. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schanzer, S.; Patzelt, A.; Thiede, G.; Kramer, A.; Weltmann, K.-D.; Hartmann, B.; Lange-Asschenfeldt, B. Comparison of the Antiseptic Efficacy of Tissue-Tolerable Plasma and an Octenidine Hydrochloride-Based Wound Antiseptic on Human Skin. Ski. Pharmacol. Physiol. 2012, 25, 100–106. [Google Scholar] [CrossRef]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma medicine: Possible applications in dermatology. J. Dtsch. Dermatol. Ges. 2010, 12, 968–976. [Google Scholar] [CrossRef]
- Lademann, O.; Kramer, A.; Richter, H.; Patzelt, A.; Meinke, M.C.; Czaika, V.; Weltmann, K.D.; Hartmann, B.; Koch, S. Skin Disinfection by Plasma-Tissue Interaction: Comparison of the Effectivity of Tissue-Tolerable Plasma and a Standard Antiseptic. Ski. Pharmacol. Physiol. 2011, 24, 284–288. [Google Scholar] [CrossRef]
- Yahaya, A.G.; Okuyama, T.; Kristof, J.; Blajan, M.G.; Shimizu, K. Direct and Indirect Bactericidal Effects of Cold Atmospheric-Pressure Microplasma and Plasma Jet. Molecules 2021, 26, 2523. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.W.; Moy, R.L.; Fincher, E.F. Advances in Plasma Skin Regeneration. J. Cosmet. Dermatol. 2008, 7, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Evans, M.M.; Ostrikov, K.K. Effect of atmospheric gas plasmas on cancer cell signaling. Int. J. Cancer 2014, 134, 1517–1528. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric pressure air plasma. PLoS ONE 2014, 9, 1517–1528. [Google Scholar] [CrossRef]
- Dias, C.; Nylandsted, J. Plasma membrane integrity in health and disease: Significance and therapeutic potential. Cell Discov. 2021, 4, 4. [Google Scholar] [CrossRef]
- Chrysafides, S.M.; Bordes, S.J.; Sharma, S. Physiology. Resting Potential. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gary, R.K.; Kindell, S.M. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem. 2005, 343, 329–334. [Google Scholar] [CrossRef]
- Ghanem, N.Z.; Malla, S.R.L.; Araki, N.; Lewis, L.K. Quantitative assessment of changes in cell growth, size and morphology during telomere-initiated cellular senescence in Saccharomyces cerevisiae. Exp. Cell Res. 2019, 381, 18–28. [Google Scholar] [CrossRef]
- Molon, M.; Szajwaj, M.; Tchorzewski, M.; Skoczowski, A.; Niewiadomska, E.; Zadrag-Tecza, R. The rate of metabolism as a factor determining longevity of the Saccharomyces cerevisiae yeast. Age 2016, 38, 11. [Google Scholar] [CrossRef]
- Frasca, D.; Saada, Y.B.; Garcia, D.; Friguet, B. Effects of cellular senescence on metabolic pathways in non-immune and immune cells. Mech. Ageing Dev. 2021, 194, 111428. [Google Scholar] [CrossRef]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep. 2019, 52, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, A.J. Membrane Potential: Concepts. Encycl. Cell Biol. 2016, 1, 218–236. [Google Scholar]
- Bao, L.; Kefaloyianni, E.; Lader, J.; Hong, M.; Morley, G.; Fishman, G.I.; Sobie, E.A.; Coetzee, W.A. Unique properties of the ATP-sensitive K+ channel in the mouse ventricular cardiac conduction system. Circ. Arrhythmia Electrophysiol. 2011, 4, 926–935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bräuner, T.; Hülser, D.F.; Strasser, R.J. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim. Biophys. Acta. 1984, 771, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Blajan, M.G.; Ciorita, A.; Surducan, E.; Surducan, V.; Shimizu, K. Biological Decontamination by Microplasma. Appl. Sci. 2025, 15, 2527. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins (Review). Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Ngoi, N.Y.; Liew, A.Q.; Chong, S.J.F.; Davids, M.S.; Clement, M.V.; Pervaiz, S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021, 45, 102032. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Furuta, S. Basal S-Nitrosylation Is the Guardian of Tissue Homeostasis. Trends Cancer 2017, 3, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.A.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants–maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Šimek, M.; Homola, T. Plasma-assisted agriculture: History, presence, and prospects—A review. Eur. Phys. J. D 2021, 75, 210. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of Apoptosis by Propidium Iodide Staining and Flow Cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 647–651. [Google Scholar] [CrossRef]
- Millard, P.J.; Roth, B.L.; Thi, H.P.; Yue, S.T.; Haugland, R.P. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl. Environ. Microbiol. 1997, 63, 2897–2905. [Google Scholar] [CrossRef]
- Epps, D.E.; Wolfe, M.L.; Groppi, V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol (Dibac4(3)) in model systems and cells. Chem. Phys. Lipids 1994, 69, 137–150. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, F.; Kristof, J.; Alam, M.J.; Sadiq, A.H.; Hasan, M.; Soichiro, K.; Shimizu, K. Exploring the Role of Microplasma for Controlling Cellular Senescence in Saccharomyces cerevisiae. Molecules 2025, 30, 1970. https://doi.org/10.3390/molecules30091970
Begum F, Kristof J, Alam MJ, Sadiq AH, Hasan M, Soichiro K, Shimizu K. Exploring the Role of Microplasma for Controlling Cellular Senescence in Saccharomyces cerevisiae. Molecules. 2025; 30(9):1970. https://doi.org/10.3390/molecules30091970
Chicago/Turabian StyleBegum, Farhana, Jaroslav Kristof, Md Jahangir Alam, Abubakar Hamza Sadiq, Mahedi Hasan, Kinoshita Soichiro, and Kazuo Shimizu. 2025. "Exploring the Role of Microplasma for Controlling Cellular Senescence in Saccharomyces cerevisiae" Molecules 30, no. 9: 1970. https://doi.org/10.3390/molecules30091970
APA StyleBegum, F., Kristof, J., Alam, M. J., Sadiq, A. H., Hasan, M., Soichiro, K., & Shimizu, K. (2025). Exploring the Role of Microplasma for Controlling Cellular Senescence in Saccharomyces cerevisiae. Molecules, 30(9), 1970. https://doi.org/10.3390/molecules30091970





