Diversity of Molecular–Network Conformations in the Over-Stoichiometric Arsenoselenides Covering a Full Thioarsenides Row As4Sen (0 ≤ n ≤ 6)
Abstract
1. Introduction
2. Results and Discussion
2.1. Mapping of Molecular- and Network-Type Clusters in AsxSe100−x Arsenoselenides (20 < x ≤ 100)
2.2. Inter-Phase Equilibria in Over-Stoichiometric Arsenoselenides AsxSe100−x (40 ≤ x ≤ 100) Governed by Molecular Network Clustering
2.3. Disproportionality Analysis of Native and Mechanoactivated Molecular Network Conformations in Over-Stoichiometric AsxSe100−x Arsenoselenides (40 ≤ x ≤ 100)
3. Methods
3.1. Cluster Modeling of Molecular Conformations in Covalent-Bonded Substances
3.2. Disproportionality Analysis of Molecular Network Conformations in Chalcogenide Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feltz, A.; Aust, H.; Blayer, A. Glass formation and properties of chalcogenide systems XXVI: Permittivity and the structure of glasses AsxSe1-x and GexSe1-x. J. Non-Cryst. Solids 1983, 55, 179–190. [Google Scholar] [CrossRef]
- Feltz, A. Amorphous Inorganic Materials and Glasses; VCH: Weinheim, Germany, 1993; pp. 1–446. [Google Scholar]
- Yang, G.; Bureau, B.; Rouxel, T.; Gueguen, Y.; Gulbiten, O.; Roiland, C.; Soignard, E.; Yarger, J.L.; Troles, J.; Sangleboeuf, J.-C.; et al. Correlation between structure and physical properties of chalcogenide glasses in the AsxSe1−x system. Phys. Rev. B 2010, 82, 195206. [Google Scholar] [CrossRef]
- Yang, G.; Gulbiten, O.; Gueguen, Y.; Bureau, B.; Sangleboeuf, J.-C.; Roiland, C.; King, E.A.; Lucas, P. Fragile-strong behavior in the AsxSe1−x glass forming system in relation to structural dimensionality. Phys. Rev. B 2012, 85, 144107. [Google Scholar] [CrossRef]
- Adam, J.-L.; Zhang, X. Chalcogenide Glasses: Preparation, Properties and Application. In Electronics and Optical Materials; Woodhead Publishing Series; Woodhead Publishing: Philadelphia, PA, USA; New Delhi, India, 2013; pp. 1–717. [Google Scholar]
- Shpotyuk, Y.; Boussard-Pledel, C.; Bureau, B.; Demchenko, P.; Szlezak, J.; Cebulski, J.; Bujňáková, Z.; Baláž, P.; Shpotyuk, O. Effect of high-energy mechanical milling on the FSDP-related XRPD correlations in Se-rich glassy arsenic selenides. J. Phys. Chem. Sol. 2019, 124, 318–326. [Google Scholar] [CrossRef]
- Shpotyuk, Y.; Demchenko, P.; Bujňáková, Z.; Baláž, P.; Boussard-Pledel, C.; Bureau, B.; Shpotyuk, O. Effect of high-energy mechanical milling on the medium-range ordering in glassy As-Se. J. Am. Ceram. Soc. 2020, 103, 1631–1646. [Google Scholar] [CrossRef]
- Shpotyuk, Y.; Demchenko, P.; Shpotyuk, O.; Balitska, V.; Boussard-Pledel, C.; Bureau, B.; Lukáčová Bujňáková, Z.; Baláž, P. High-energy mechanical milling-driven reamorphization in glassy arsenic monoselenide g-AsSe: On the path tailoring special molecular-network glasses. Materials 2021, 14, 4478. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Shpotyuk, Y.; Lukáčová Bujňáková, Z.; Baláž, P.; Demchenko, P.; Kozdras, A.; Boyko, V.; Kovalskiy, A. Molecular-Network Transformations in Tetra-Arsenic Triselenide Glassy Alloys Tuned within Nanomilling Platform. Molecules 2024, 29, 3245. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Ingram, A.; Shpotyuk, Y.; Boyko, V.; Demchenko, P.; Wojnarowska-Nowak, R.; Lukáčová Bujňáková, Z.; Baláž, P. Nanostructured Molecular-Network Arsenoselenides from the Border of a Glass-Forming Region: A Disproportionality Analysis Using Complementary Characterization Probes. Molecules 2024, 29, 3948. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Lukáčová Bujňáková, Z.; Baláž, P.; Shpotyuk, Y.; Hyla, M.; Kozdras, A.; Ingram, A.; Boyko, V.; Demchenko, P.; Kovalskiy, A. Molecular-Network Polyamorphism in Mechanically activated Arsenic Selenides under Deviation from As2Se3 Stoichiometry. Molecules 2025, 30, 642. [Google Scholar] [CrossRef]
- Bonazzi, P.; Bindi, L. A crystallographic review of arsenic sulfides: Effects of chemical variations and changes induced by exposure to light. Z. Kristallogr. 2008, 223, 132–147. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Wallace, A.F.; Downs, R.T.; Ross, N.L.; Cox, D.F.; Rosso, K.M. Thioarsenides: A case for long-range Lewis acid–base-directed van der Waals interactions. Phys. Chem. Miner. 2010, 38, 267–291. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Crawford, T.D.; Wallace, A.F.; Cox, D.F.; Parrish, R.M.; Hohenstein, E.G.; Sherrill, C.D. Role of Long-Range Intermolecular Forces in the Formation of Inorganic Nanoparticle Clusters. J. Phys. Chem. A 2011, 115, 12933–12940. [Google Scholar] [CrossRef] [PubMed]
- Kyono, A. Ab initio quantum chemical investigation of arsenic sulfide molecular diversity from As4S6 and As4. Phys. Chem. Minerals 2013, 40, 717–731. [Google Scholar] [CrossRef]
- Okamoto, H. As-Se (Arsenic-Selenium). J. Phase Equilibria 1998, 19, 488. [Google Scholar] [CrossRef]
- Degterov, S.A.; Pelton, A.D.; L’Ecuyer, J.D. Thermodynamic Optimization of the Selenium-Arsenic (Se-As) System. J. Phase Equilibria 1997, 18, 357–368. [Google Scholar] [CrossRef]
- Renninger, A.L.; Averbach, B.L. Crystalline structures of As2Se3 and As4Se4. Acta Cryst. B 1973, 29, 1583–1589. [Google Scholar] [CrossRef]
- Bastow, T.J.; Whitfield, H.J.J. Crystal structure of tetra-arsenic tetraselenide. Chem. Soc. Dalton Trans. 1973, 17, 1739–1740. [Google Scholar] [CrossRef]
- Smail, E.J.; Sheldrick, G.M. Tetra-arsenic tetraselenide. Acta Cryst. B 1973, 29, 2014–2016. [Google Scholar] [CrossRef]
- Bastow, T.J.; Whitfied, H.J. Crystal data and nuclear quadrupole resonance spectra of tetra-arsenic triselenide. J. Chem. Soc. Dalton Trans. 1977, 10, 959–961. [Google Scholar] [CrossRef]
- Stergiou, A.C.; Rentzeperis, P.J. The crystal structure of arsenic selenide, As2Se3. Zeitsch. Krist. 1985, 173, 185–191. [Google Scholar] [CrossRef]
- Greaves, G.N.; Elliott, S.R.; Davis, E.A. Amorphous arsenic. Adv. Phys. 1979, 28, 49–141. [Google Scholar] [CrossRef]
- Schiferl, D.; Barret, S. The Crystal Structure of Arsenic at 4.2, 78 and 299 °K. J. Appl. Cryst. 1969, 2, 30–36. [Google Scholar] [CrossRef]
- Seidl, M.; Balazs, G.; Scgeer, M. The Chemistry of Yellow Arsenic. Chem. Revs. 2019, 119, 8406–8434. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Chen, J.; Michaelides, A.; Sella, A.; Shaffer, M.S.P.; Salzmann, C.G. One-dimensional Arsenic Allotropes: Polymerization of Yellow Arsenic Inside Single-wall Carbon Nanotubes. Angewandte Chemie. 2018, 130, 11823–11827. [Google Scholar] [CrossRef]
- Blachnik, R.; Wickel, U. Thermal behaviour of A4B3 cage molecules (A = P, As; B = S, Se). Thermochim. Acta 1984, 81, 185–196. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Boyko, V. Structural-topological genesis of network-forming nano-clusters in chalcogenide semiconductor glasses. J. Optoelectron. Adv. Mater. 2013, 15, 1429–1437. Available online: https://joam.inoe.ro/articles/structural-topological-genesis-of-network-forming-nanoclusters-in-chalcogenide-semiconductor-glasses/ (accessed on 26 April 2025).
- Shpotyuk, O.; Hyla, M.; Boyko, V. Compositionally-dependent structural variations in glassy chalcogenides: The case of binary As-Se system. Comput. Mater. Sci. 2015, 110, 144–151. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Boyko, V.; Shpotyuk, Y.; Balitska, V. Cluster modeling of nanostructurization driven reamorphization pathways in glassy arsenoselenides: A case study of arsenic monoselenide g-AsSe. J. Nanoparticle Res. 2022, 22, 64. [Google Scholar] [CrossRef]
- Whitfield, H.J. The crystal structure of tetra-arsenic trisulphide. J. Chem. Soc. A 1970, 0, 1800–1803. [Google Scholar] [CrossRef]
- Whitfield, H.J. Crystal structure of the β-form of tetra-arsenic trisulphide. J. Chem. Soc. Dalton 1973, 17, 1737–1738. [Google Scholar] [CrossRef]
- Wright, A.C.; Aitken, B.G.; Cuello, G.; Haworth, R.; Sinclar, R.N.; Stewart, J.R.; Taylor, J.W. Neutron studies of an inorganic plastic glass. J. Non Cryst. Solids 2011, 357, 2502–2510. [Google Scholar] [CrossRef]
- Soyer Uzun, S.; Gaudio, S.J.; Sen, S.; Mei, Q.; Benmore, C.J.; Tulk, C.A.; Xud, J.; Aitken, B.G. In situ high-pressure X-ray diffraction study of densification of a molecular chalcogenide glass. J. Phys. Chem. Sol. 2008, 69, 2336–2340. [Google Scholar] [CrossRef]
- Zarzycki, J. Phase-separated Systems. Discuss. Faraday Soc. 1970, 50, 122–134. [Google Scholar] [CrossRef]
- Köster, U. Crystallization and decomposition of amorphous semiconductors. Adv. Colloid. Interface Sci. 1970, 10, 129–172. [Google Scholar] [CrossRef]
- Myers, M.B.; Berkes, J.S. Phase separation in amorphous chalcogenides. J. Non Cryst. Solids 1972, 8–10, 804–815. [Google Scholar] [CrossRef]
- James, P.F. Review. Liquid-phase separation in glass-forming systems. J. Mater. Sci. 1975, 10, 1802–1825. [Google Scholar] [CrossRef]
- Hehre, W.J.; Stewart, R.F.; Pople, J.A. Self-consistent molecular-orbital methods. I. Use of Gaussian expansions of slater-type atomic orbitals. J. Chem. Phys. 1969, 51, 2657–2665. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11 − 18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Jackson, K. Electric fields in electronic structure calculations: Electric polarizabilities and IR and Raman spectra from first principles. Phys. Stat. Solidi B 2000, 217, 293–310. [Google Scholar] [CrossRef]
- Phillips, J.C. Topology of covalent non-crystalline solids. I: Short-range order in chalcogenide alloys. J. Non Cryst. Solids 1979, 34, 153–181. [Google Scholar] [CrossRef]
- Thorpe, M.F. Continuous deformations in random networks. J. Non Cryst. Solids 1983, 57, 355–370. [Google Scholar] [CrossRef]
- Thorpe, M.F. Bulk and surface floppy modes. J. Non Cryst. Solids 1995, 182, 135–142. [Google Scholar] [CrossRef]
- Phillips, J.C. Ideally glassy hydrogen-bonded networks. Phys. Rev. B 2006, 73, 024210. [Google Scholar] [CrossRef]
- Holomb, R.; Veres, M.; Mitsa, V. Ring-, branchy-, and cage-like AsnSm nanoclusters in the structure of amorphous semiconductors: Ab initio and Raman study. J. Optoelectron. Adv. Mater. 2009, 11, 917–923. Available online: https://dspace.uzhnu.edu.ua/jspui/bitstream/lib/4320/1/14_OptAdvMat-2009.pdf (accessed on 26 April 2025).
- Pualing, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960; pp. 1–644. Available online: https://dn790000.ca.archive.org/0/items/natureofthechemicalbondpauling/Nature-Of-The-Chemical-Bond-Pauling.pdf (accessed on 26 April 2025).
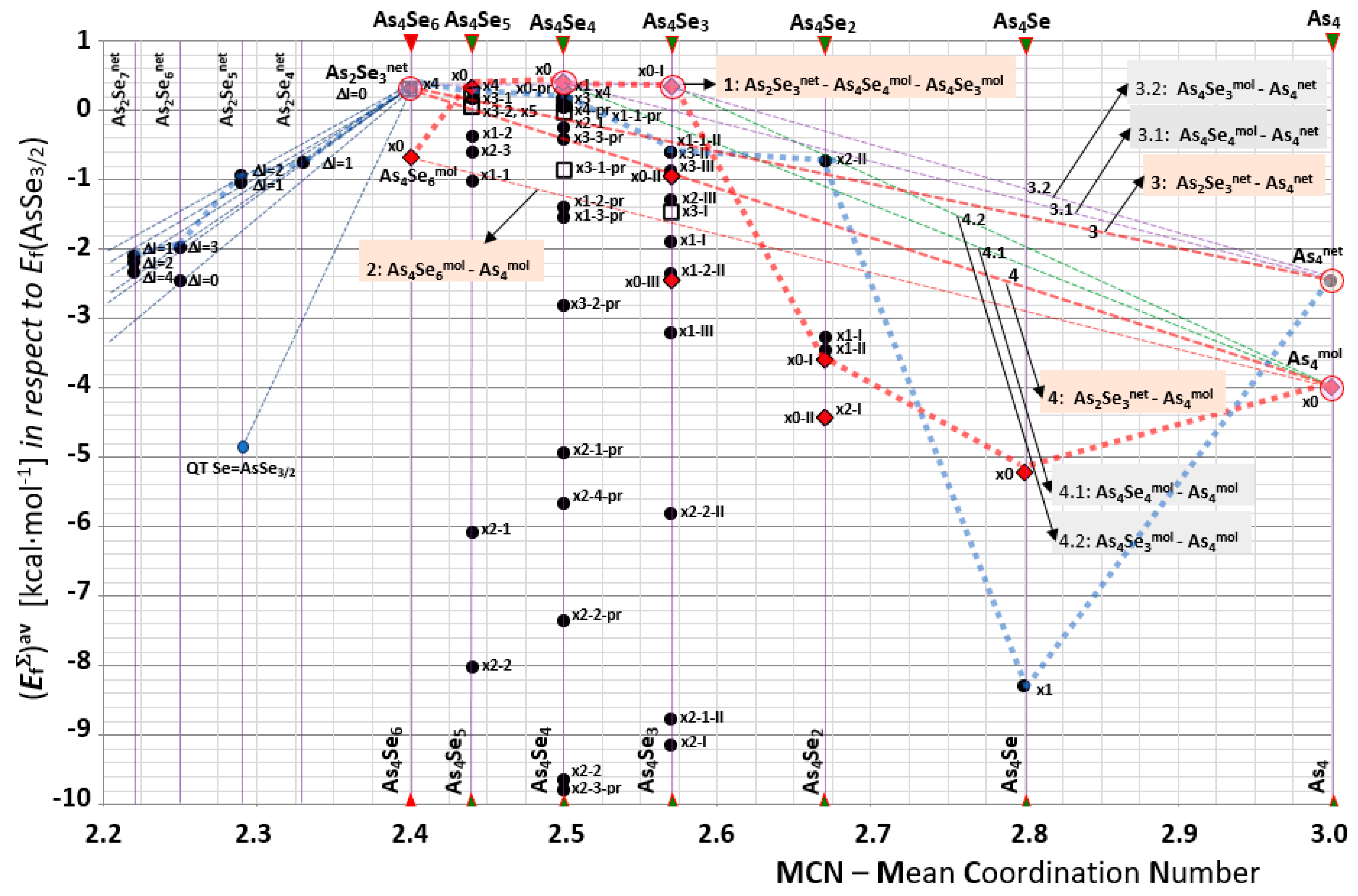
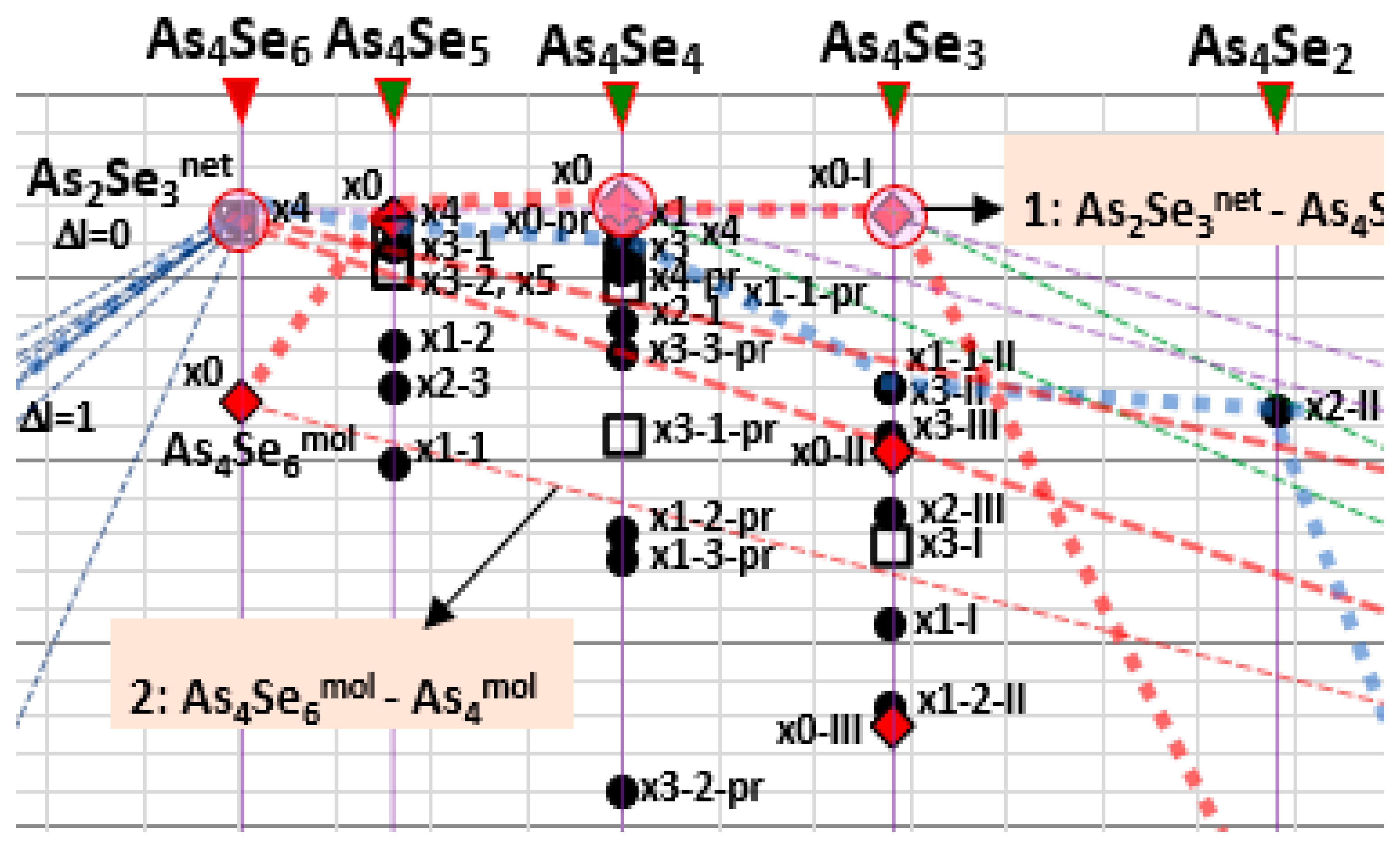
| MCN; n | Thioarsenide-Type As4Sen MFC | Thioarsenide-Type As4Sen NFC | ||
|---|---|---|---|---|
| MFC Nomenclature; nc; Small Rings | Ef, kcal·mol−1 | NFC Nomenclature; nc; Separate Units, and/or Small Rings | Ef, kcal·mol−1 | |
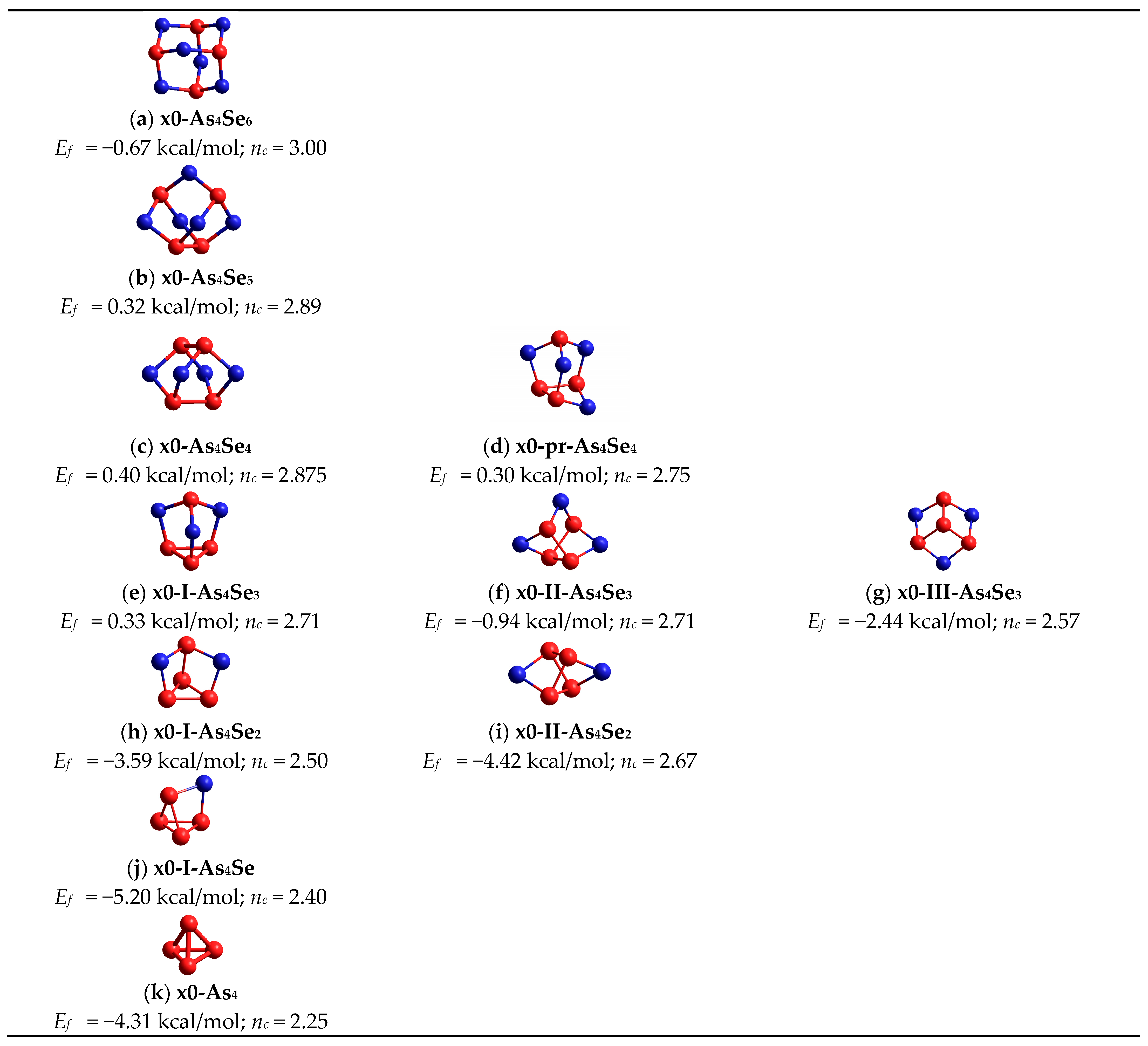
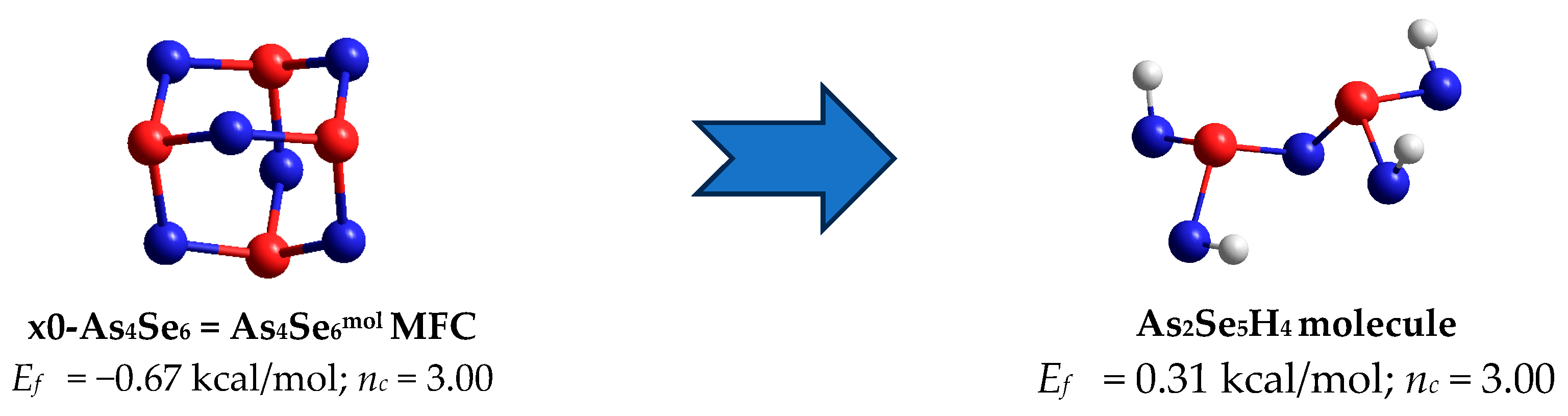
| 2.40; n = 6 | x0-As4Se6 = As4Se6mol; nc = 3.00; 4 hexagons | −0.67 | x4-As4Se6 = As2Se3net; nc = 3.00; 2 separate units, no small rings | 0.31 |
| 2.44; n = 5 | x0-As4Se5; nc = 2.89; 2 hexagons, 2 pentagons | 0.32 | x1-1-As4Se5; nc = 2.89; 2 pentagons | −1.01 |
| x1-2-As4Se5; nc = 3.00; 1 pentagon, 1 hexagon | −0.37 | |||
| x2-1-As4Se5; nc = 3.00; 1 pentagon | −6.07 | |||
| x2-2-As4Se5; nc = 3.11; no small rings | −8.02 | |||
| x2-3-As4Se5; nc = 3.11; 1 hexagon | −0.60 | |||
| x3-1-As4Se5; nc = 3.11; no small rings | 0.16 | |||
| x3-2-As4Se5; nc = 3.00; 2 separate units, 1 pentagon | 0.05 | |||
| x4-As4Se5; nc = 3.11; 2 separate units, no small rings | 0.22 | |||
| x5-As4Se5; nc = 3.11; 3 separate units, no small rings | 0.05 | |||
| 2.50; n = 4 | x0-As4Se4; nc = 2.875; 4 pentagons, 4 hexagons | 0.40 | x1-As4Se4; nc = 3.00; 2 pentagons, 1 hexagon | 0.25 |
| x2-1-As4Se4; nc = 3.125; 1 pentagon | −0.42 | |||
| x2-2-As4Se4; nc = 3.25; 1 hexagon | −9.64 | |||
| x3-As4Se4; nc = 3.25; no small rings | 0.05 | |||
| x4-As4Se4; nc = 3.25; 2 separate units, no rings | 0.11 | |||
| x0-pr-As4Se4; nc = 2.75; 1 hexagon, 2 pentagons, 1 tetragon | 0.30 | x1-1-pr-As4Se4; nc = 3.00; 2 pentagons | −0.25 | |
| x1-2-pr-As4Se4; nc = 2.875; 1 pentagon, 1 tetragon | −1.39 | |||
| x1-3-pr-As4Se4; nc = 3.00; 1 hexagon, 1 tetragon | −1.55 | |||
| x2-1-pr-As4Se4; nc = 3.00; 1 tetragon | −4.93 | |||
| x2-2-pr-As4Se4; nc = 3.125; 1 pentagon | −7.36 | |||
| x2-3-pr-As4Se4; nc = 3.00; 1 tetragon | −9.78 | |||
| x2-4-pr-As4Se4; nc = 3.25; 1 hexagon | −5.66 | |||
| x3-1-pr-As4Se4; nc = 3.00; 2 separate units, 1 tetragon | −0.85 | |||
| x3-2-pr-As4Se4; nc = 3.25; no small rings | −2.81 | |||
| x3-3-pr-As4Se4; nc = 3.25; no small rings | −0.47 | |||
| x4-pr-As4Se4; nc = 3.25; 2 separate units, no rings | −0.03 | |||
| 2.57; n = 3 | x0-I-As4Se3; nc = 2.71; 3 pentagons, 1 triangle | 0.33 | x1-I-As4Se3; nc = 2.86; 1 pentagon + 1 triangle | −1.90 |
| x2-I-As4Se3; nc = 3.00; 1 triangle | −9.13 | |||
| x3-I-As4Se3; nc = 3.00; 2 separate units, 1 triangle | −1.47 | |||
| x0-II-As4Se3; nc = 2.71; 2 pentagons, 2 tetragons | −0.94 | x1-1-II-As4Se3; nc = 3.00; 1 tetragon, 1 pentagon | −0.60 | |
| x1-2-II-As4Se3; nc = 2.86; 2 tetragons | −2.36 | |||
| x2-1-II-As4Se3; nc = 3.29; 1 pentagon | −8.76 | |||
| x2-2-II-As4Se3; nc = 3.14; 1 tetragon | −5.80 | |||
| x3-II-As4Se3; nc = 3.43; no small rings | −0.60 | |||
| x0-III-As4Se3; nc = 2.57; 1 hexagon, 3 tetragons | −2.44 | x1-III-As4Se3; nc = 2.86; 2 tetragons | −3.22 | |
| x2-III-As4Se3; nc = 3.14; 1 tetragon | −1.29 | |||
| x3-III-As4Se3; nc = 3.43; no small rings | −0.88 | |||
| 2.67; n = 2 | x0-I-As4Se2; nc = 2.50; 1 pentagon, 2 tetragons, 1 triangle | −3.59 | x1-I-As4Se2; nc = 2.83; 1 tetragon, 1 triangle | −3.27 |
| x2-I-As4Se2; nc = 3.17; 1 triangle | −4.42 | |||
| x0-II-As4Se2; nc = 2.67; 5 tetragons | −4.42 | x1-II-As4Se2; nc = 3.00; 3 tetragons | −3.46 | |
| x2-II-As4Se2; nc = 3.33; 1 tetragon | −0.72 | |||
| 2.80; n = 1 | x0-As4Se; nc = 2.40; 2 tetragons, 2 triangles | −5.20 | x1-As4Se; nc = 2.80; 2 triangles, 1 tetragon | −8.30 |
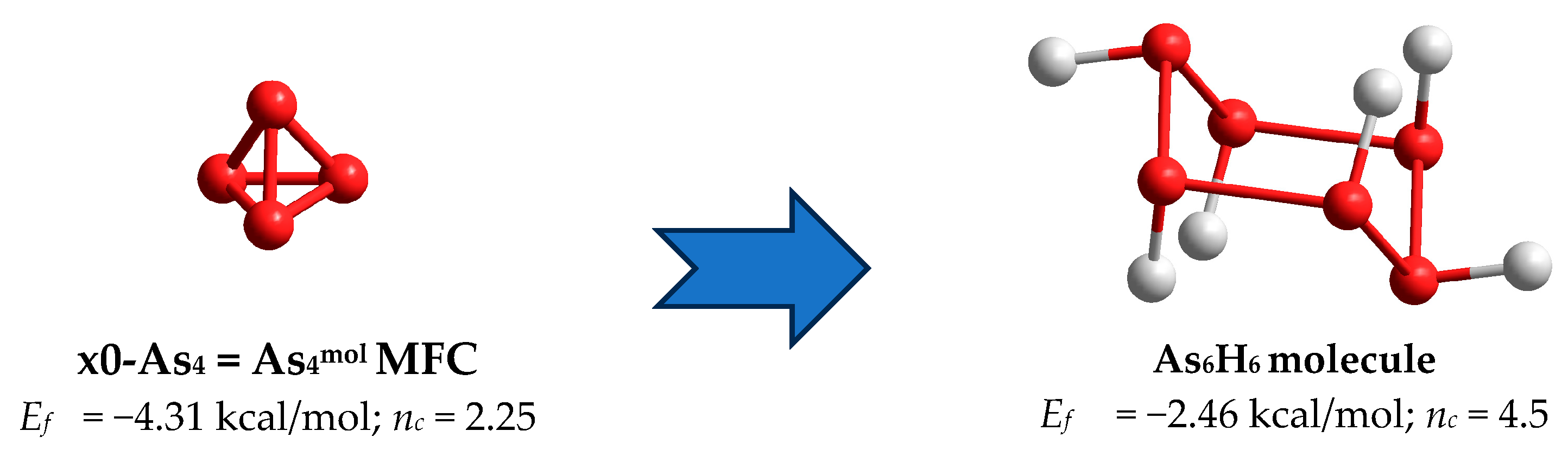
| 3.00; n = 0 | x0-As4 = As4mol; nc = 2.25; 4 triangels | −4.31 | As4net; nc = 4.50; 1 hexagon | −2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shpotyuk, O.; Hyla, M.; Lukáčová Bujňáková, Z.; Shpotyuk, Y.; Boyko, V. Diversity of Molecular–Network Conformations in the Over-Stoichiometric Arsenoselenides Covering a Full Thioarsenides Row As4Sen (0 ≤ n ≤ 6). Molecules 2025, 30, 1963. https://doi.org/10.3390/molecules30091963
Shpotyuk O, Hyla M, Lukáčová Bujňáková Z, Shpotyuk Y, Boyko V. Diversity of Molecular–Network Conformations in the Over-Stoichiometric Arsenoselenides Covering a Full Thioarsenides Row As4Sen (0 ≤ n ≤ 6). Molecules. 2025; 30(9):1963. https://doi.org/10.3390/molecules30091963
Chicago/Turabian StyleShpotyuk, Oleh, Malgorzata Hyla, Zdenka Lukáčová Bujňáková, Yaroslav Shpotyuk, and Vitaliy Boyko. 2025. "Diversity of Molecular–Network Conformations in the Over-Stoichiometric Arsenoselenides Covering a Full Thioarsenides Row As4Sen (0 ≤ n ≤ 6)" Molecules 30, no. 9: 1963. https://doi.org/10.3390/molecules30091963
APA StyleShpotyuk, O., Hyla, M., Lukáčová Bujňáková, Z., Shpotyuk, Y., & Boyko, V. (2025). Diversity of Molecular–Network Conformations in the Over-Stoichiometric Arsenoselenides Covering a Full Thioarsenides Row As4Sen (0 ≤ n ≤ 6). Molecules, 30(9), 1963. https://doi.org/10.3390/molecules30091963







