Removal of Butyl Mercaptan from Gas Streams by Reactive Adsorption
Abstract
1. Introduction
2. Results and Discussion
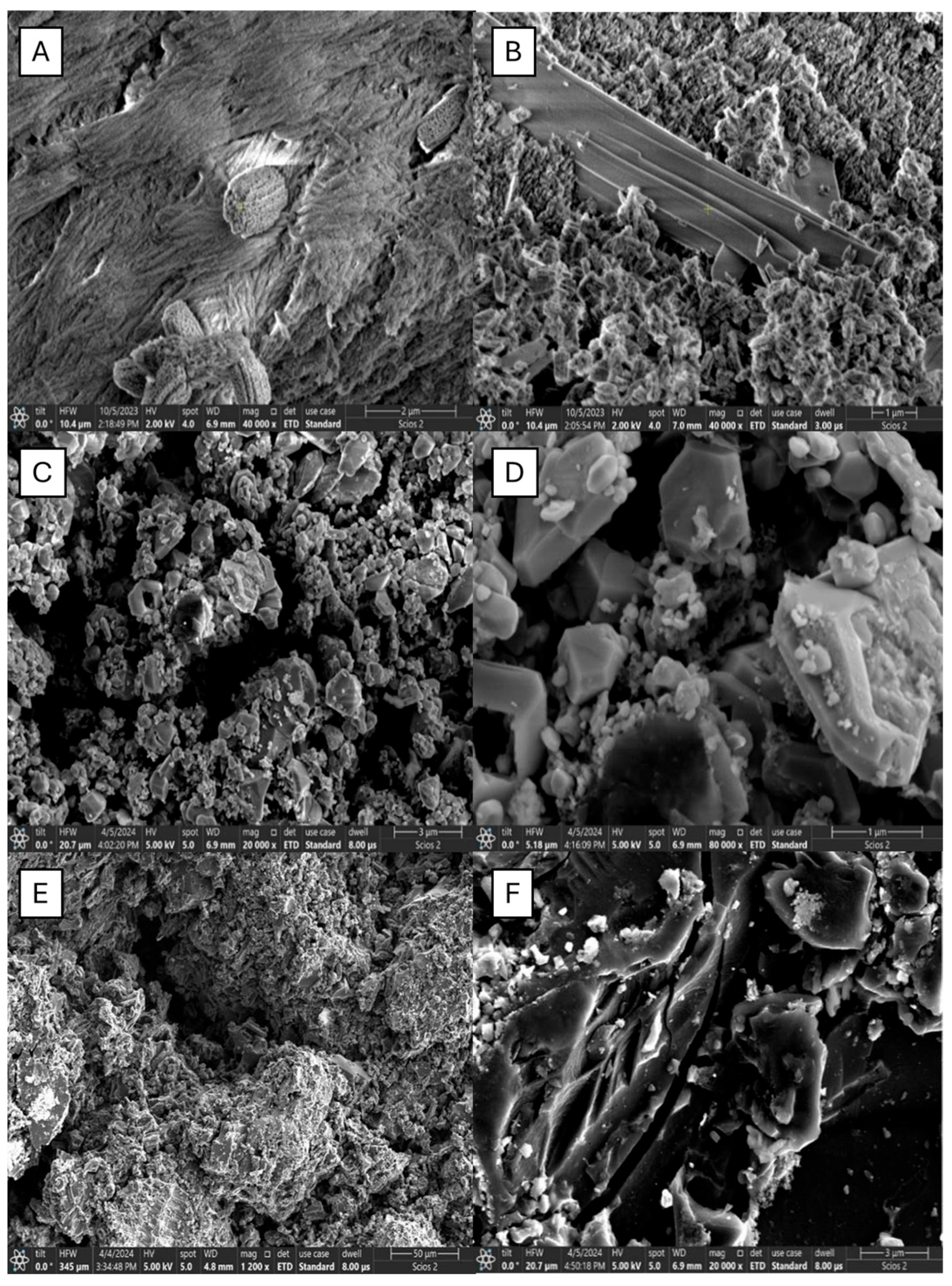
2.1. SEM Analysis
2.2. Textural Characteristics
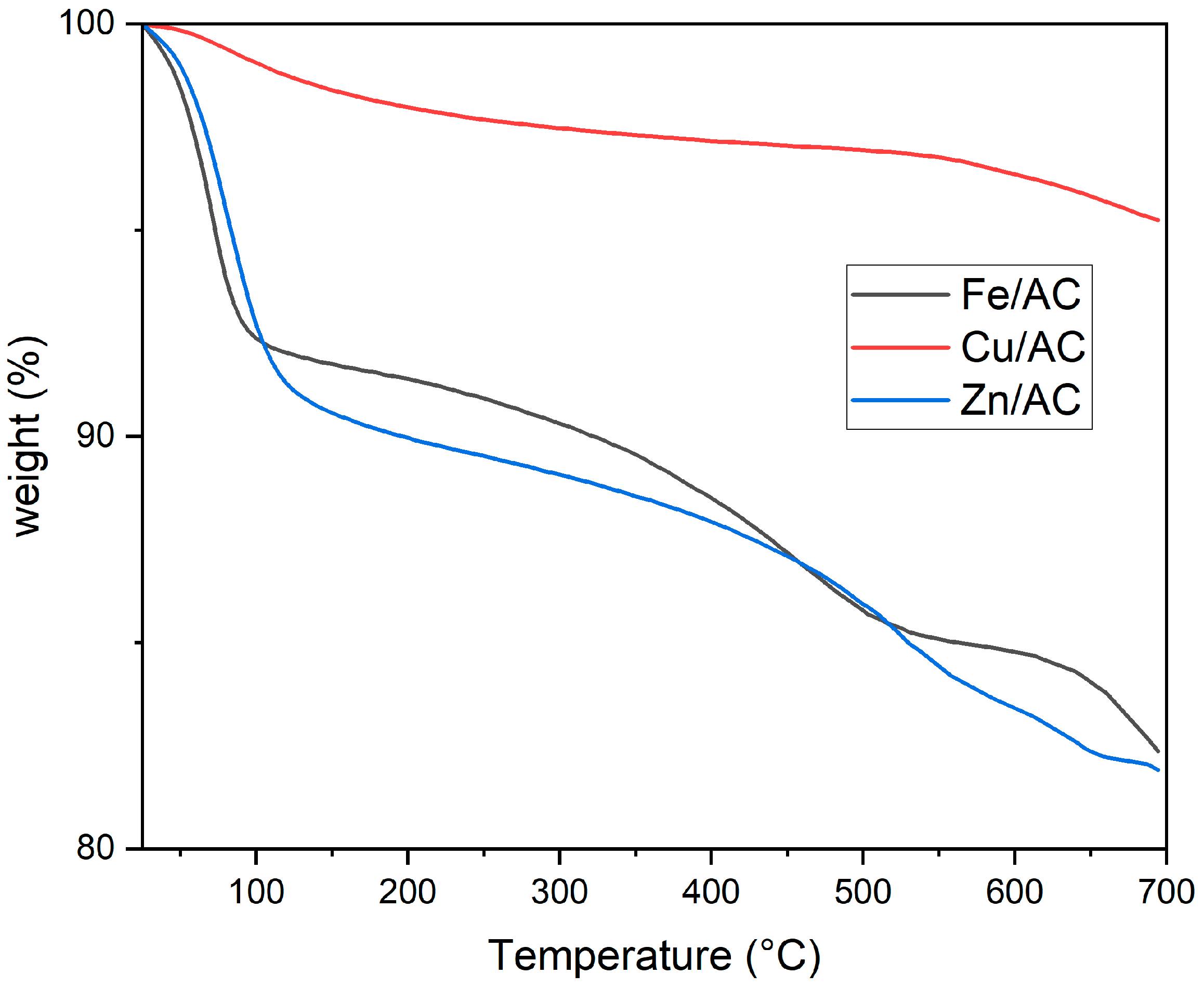
2.3. Thermogravimetric Analysis
2.4. FTIR Analysis
2.5. XRD Analysis
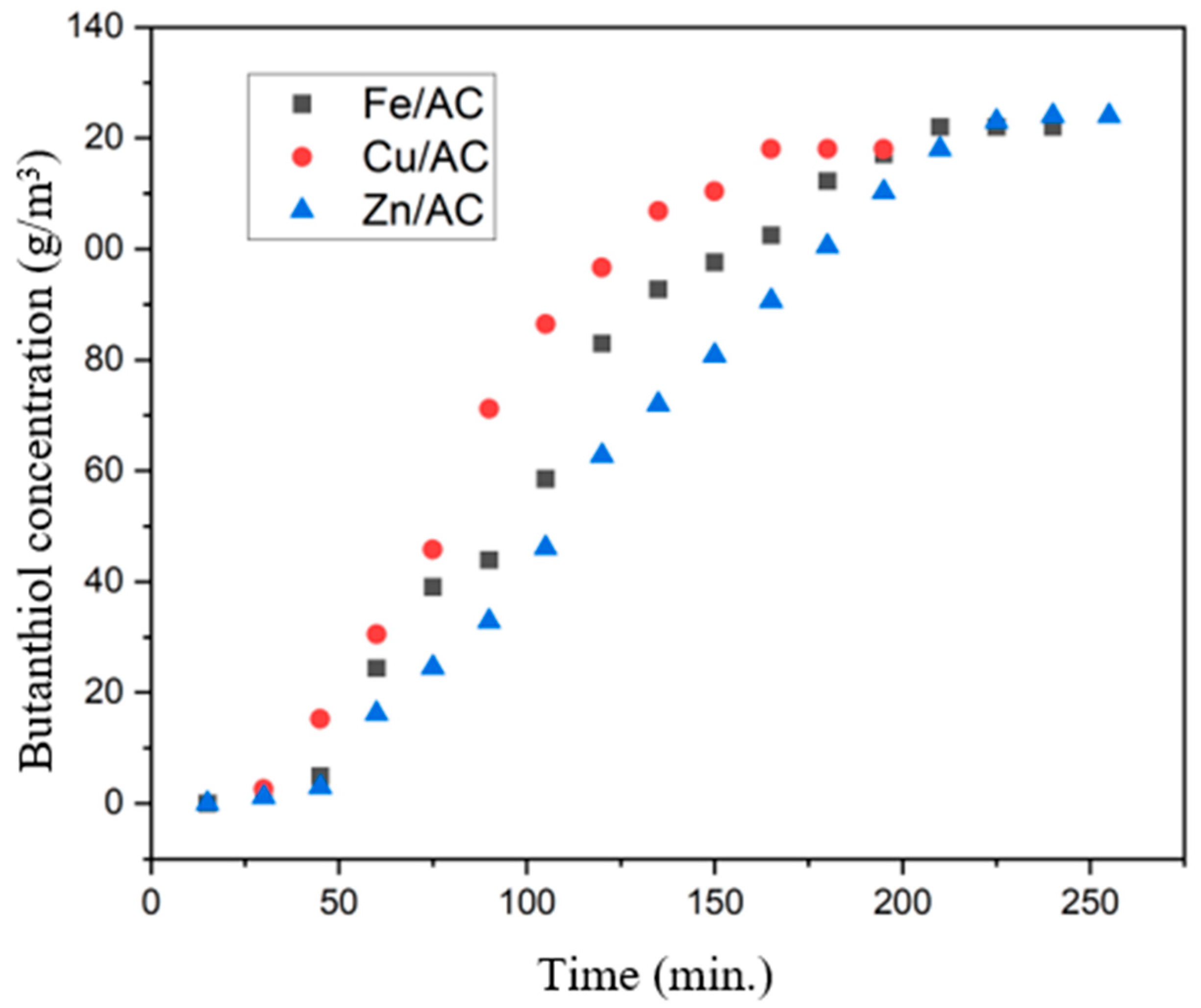
2.6. Butanethiol Adsorption Tests on Prepared Adsorbents
- (a)
- Adsorbent mass: 40 g;
- (b)
- Gas flow (nitrogen): 300 cm3/min;
- (c)
- Butanethiol concentration in the carrier gas: 121.11 g/m3;
- (d)
- Adsorber temperature: 150 °C.
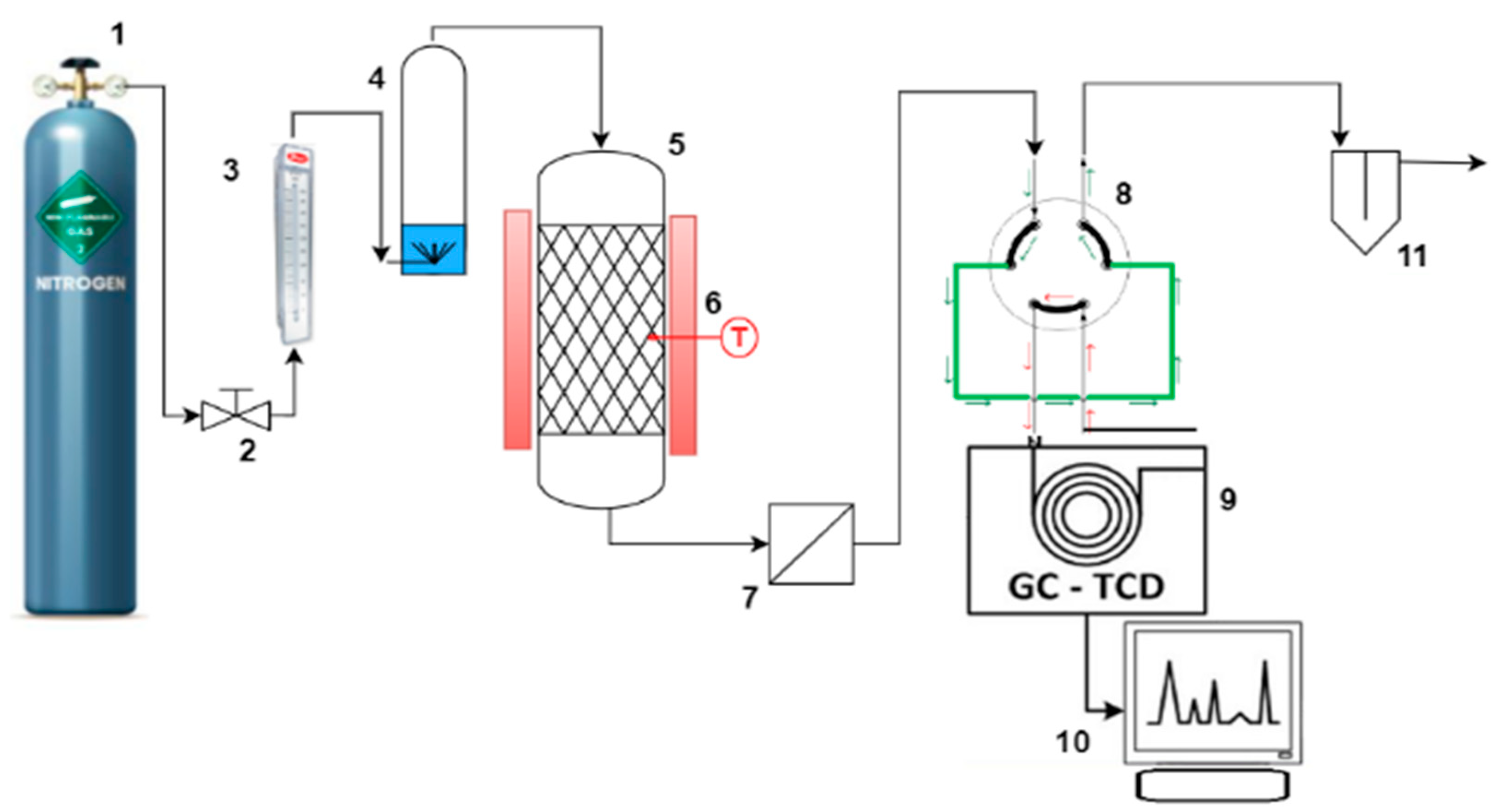
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Adsorbent
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kangas, J.; Jäppinen, P.; Savolainen, H. Exposure to hydrogen sulfide, mercaptans and sulfur dioxide in pulp industry. Am. Ind. Hyg. Assoc. J. 1984, 45, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Heaney, C.D.; Wing, S.; Campbell, R.L.; Caldwell, D.; Hopkins, B.; Richardson, D.; Yeatts, K. Relation between malodor, ambient hydrogen sulfide, and health in a community bordering a landfill. Environ. Res. 2011, 111, 847–852. [Google Scholar] [CrossRef]
- Rubright, S.L.M.; Pearce, L.L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Riveles, K.; Nagai, A. Analysis of Refinery Chemical Emissions and Health Effects; Office of Environmental Health Hazard Assessment California Environmental Protection Agency: Sacramento, CA, USA, 2019. [Google Scholar]
- Tansy, M.F.; Kendall, F.M.; Fantasia, J.; Landin, W.E.; Oberly, R.; Sherman, W. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced-sulfur compounds. J. Toxicol. Environ. Health 1981, 8, 71–88. [Google Scholar] [CrossRef]
- Fang, J.; Xu, X.; Jiang, L.; Qiao, J.; Zhou, H.; Li, K. Preliminary results of toxicity studies in rats following low-dose and short-term exposure to methyl mercaptan. Toxicol. Rep. 2019, 6, 431–438. [Google Scholar] [CrossRef]
- Bagreev, A.; Bashkova, S.; Bandosz, T.J. Dual role of water in the process of methyl mercaptan adsorption on activated carbons. Langmuir 2002, 18, 8553–8559. [Google Scholar] [CrossRef]
- Bashkova, S.; Bagreev, A.; Bandosz, T.J. Adsorption of methyl mercaptan on activated carbons. Environ. Sci. Technol. 2002, 36, 2777–2782. [Google Scholar] [CrossRef]
- Lee, S.W.; Daud, W.M.A.W.; Lee, M.G. Adsorption characteristics of methyl mercaptan, dimethyl disulfide, and trimethylamine on coconut-based activated carbons modified with acid and base. J. Ind. Eng. Chem. 2010, 16, 973–977. [Google Scholar] [CrossRef]
- Tamai, H.; Nagoya, H.; Shiono, T. Adsorption of methyl mercaptan on surface modified activated carbon. J. Colloid Interface Sci. 2006, 300, 814–817. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Xue, Z.; Wang, X. Electrospun graphene oxide/carbon composite nanofibers with well-developed mesoporous structure and their adsorption performance for benzene and butanone. J. Chem. Eng. 2016, 306, 99–106. [Google Scholar] [CrossRef]
- Moghadaszadeh, Z.; Toosi, M.R.; Zardoost, M.R. Adsorption of light mercaptans over metal (Co, Cu, Fe, Ni) doped hexagonal boron nitride nanosheets: A first-principles study. J. Mol. Model. 2019, 25, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, J.; Wang, J.; Hu, L. Highly efficient adsorption of ethyl mercaptan on hierarchical porous carbon derived from rice husk. Chem. Eng. Sci. 2025, 302, 120915. [Google Scholar] [CrossRef]
- Weber, G.; Benoit, F.; Bellat, J.P.; Paulin, C.; Mougin, P.; Thomas, M. Selective adsorption of ethyl mercaptan on NaX zeolite. Microporous Mesoporous Mater. 2008, 109, 184–192. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, J.; Liu, Z. Study on adsorption of low-concentration methyl mercaptan by starch-based activated carbon. Chemosphere 2022, 302, 134901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lei, Y.; Zhang, J.; Pinto, M.L.; Liu, Q.; Qian, G. Enhanced adsorption and regeneration performances for methyl mercaptan capture by a metal-organic framework incorporated in a mesoporous silica. Sep. Purif. Technol. 2025, 357, 130041. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Montes-Morán, M.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Concheso, A.; Rojas-Mayorga, C.K.; González, J. Importance of iron oxides on the carbons surface vs the specific surface for VOC’s adsorption. Ecol. Eng. 2017, 106, 400–408. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Deng, Y.; Li, W.; Ma, X.; Chen, Y. Removal of low concentration CH3SH with regenerable Cu-doped mesoporous silica. J. Colloid Interface Sci. 2018, 513, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, J.; Srinivasan, N.; Chandrasekaran, P.; Girija, E.K. Synthesis and characterization of cluster of grapes like pure and Zinc-doped CuO nanoparticles by sol-gel method. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 136, 1803–1806. [Google Scholar] [CrossRef]
- Hadkar, V.M.; Selvaraj, C.I. Characterization and investigating the potential therapeutic effects of phyto-assisted CuO and Zn-doped CuO nanoparticles from Calophyllum apetalum (Willd.) leaf extract. J. Drug Deliv. Technol. 2025, 104, 106530. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Xue, J.; Ma, Y.; Liu, Y.; Chen, Y.; Wu, L.; Zhang, Z.; et al. Efficient degradation of diclofenac sodium by periodate activation using Fe/Cu bimetallic modified sewage sludge biochar/UV system. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef]
- Nguyen-Quang, M.; Azzolina-Jury, F.; Thibault-Starzyk, F.; Travert, A.; Ziąbka, M.; Samojeden, B.; Motak, M.; Da Costa, P. Unveiling the potential of surfactant Pluronic-P123 application during the synthesis of Ni-hydrotalcitederived catalysts for low-temperature CO2 methanation: A novel approach. Appl. Mater. Today 2023, 32, 101805. [Google Scholar] [CrossRef]
- Mishra, R.K.; Lu, Q.; Mohanty, K. Thermal behaviour, kinetics and fast pyrolysis of Cynodon dactylon grass using Py-GC/MS and Py-FTIR analyser. J. Anal. Appl. Pyrolysis 2020, 150, 104887. [Google Scholar] [CrossRef]
- Chintala, V.; Kumar, S.; Pandey, J.K.; Sharma, A.K.; Kumar, S. Solar thermal pyrolysis of non-edible seeds to biofuels and their feasibility assessment. Energy Convers. Manag. 2017, 153, 482–492. [Google Scholar] [CrossRef]
- Chalmers, J.M. Mid-infrared spectroscopy: Anomalies, artifacts and common errors. Handb. Vib. Spectrosc. 2006, 3, 2326–2347. [Google Scholar]
- González-Suárez, B.W.; Pantoja-Espinoza, J.C.; Lardizábal-Gutierrez, D.; Flores, M.U.; Paraguay-Delgado, F. Effect of [Zn acetate]/[KOH] ratio on ZnO particles synthesis and photocatalytic Rhodamine B degradation. JMR&T 2024, 30, 8092–8107. [Google Scholar]
- Saussey, J.; Lavalley, J.C.; Bovet, C. Infrared study of CO2 adsorption on ZnO. Adsorption sites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1982, 78, 1457–1463. [Google Scholar] [CrossRef]
- Ma, J.; Cui, S.; Zhang, J.; Huang, W.; Wang, Y. Efficient degradation of methyl orange with Fe/Co@PC particle electrodes in a three dimensional electrochemical reactor: Performance and mechanism. J. Water Process Eng. 2025, 69, 106765. [Google Scholar] [CrossRef]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.; Ko, F.K. X-Ray Diffraction Analysis of Kraft Lignins and Lignin-Derived Carbon Nanofibers. ASME J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Annamalai, S.; Kumar, A.V.N.; Shin, W.S. Revisiting the persulfate activation performance of seaweed derived biochars: The composition and origin of pollutant degradation activity. Process Saf. Environ. Prot. 2025, 193, 195–204. [Google Scholar] [CrossRef]
- Jose, L.M.; Kuriakose, S.; Thomas, S. Fabrication, Characterization and In Vitro Antifungal Property Evaluation of Biocompatible Lignin-Stabilized Zinc Oxide Nanoparticles Against Selected Pathogenic Fungal Strains. BioNanoScience 2020, 10, 583–596. [Google Scholar] [CrossRef]
- Deng, L.; Du, P.; Yu, W.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; Zhou, J. Novel hierarchically porous allophane/diatomite nanocomposite for benzene adsorption. Appl. Clay Sci. 2019, 168, 155–163. [Google Scholar] [CrossRef]

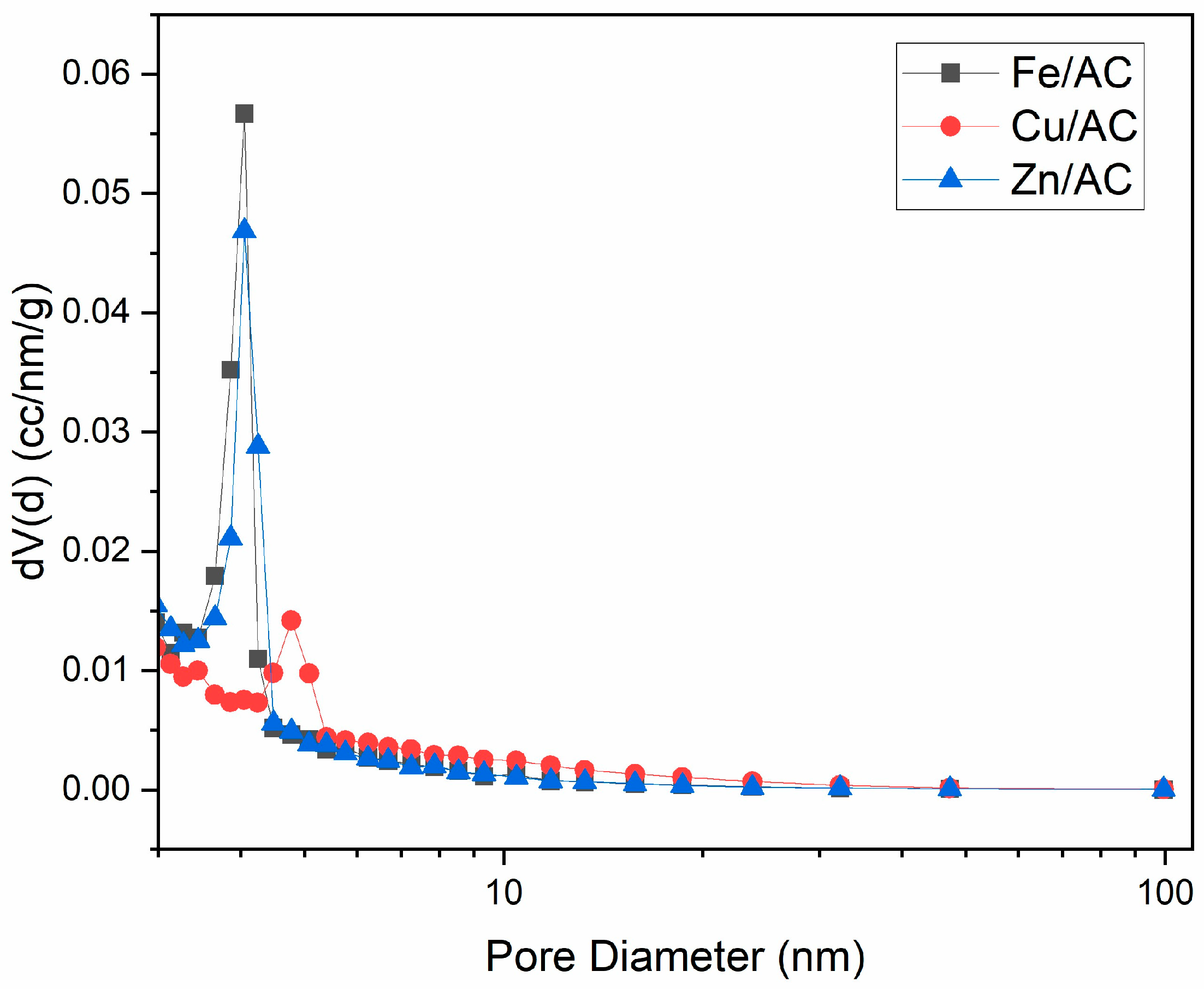


| No. | Adsorbent | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| 1 | Cu/AC | 443.7 | 0.2797 | 2.521 |
| 2 | Fe/AC | 836.1 | 0.4717 | 2.256 |
| 3 | Zn/AC | 816.2 | 0.4627 | 2.268 |
| Sample | Compound | Crystallite Size (nm) | Crystallinity Degree, Xc % |
|---|---|---|---|
| Cu/AC | copper oxide | 124.7 | 96.37 |
| copper sulphide | 82.4 | 96.35 | |
| copper | 54.7 | 99.09 | |
| Fe/AC | iron oxide | 35.01 | 80.72 |
| iron | 85.41 | 99.21 | |
| Zn/AC | zinc | 56.6 | 90.05 |
| zinc oxide | 97.4 | 98.94 |
| No. | Adsorbent | Amount Adsorbed (g Butanethiol/m3) | Maximum Adsorption Capacity (cm3 Butanethiol/g Adsorbent) |
|---|---|---|---|
| 1 | Cu/AC | 9368.79 | 1.696 |
| 2 | Fe/AC | 12,579.42 | 1.790 |
| 3 | Zn/AC | 13,662.28 | 1.601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanda, M.; Onuțu, I.; Dușescu-Vasile, C.M.; Vasilievici, G.; Bomboș, D.; Băjan, M.; Brănoiu, G. Removal of Butyl Mercaptan from Gas Streams by Reactive Adsorption. Molecules 2025, 30, 1962. https://doi.org/10.3390/molecules30091962
Sanda M, Onuțu I, Dușescu-Vasile CM, Vasilievici G, Bomboș D, Băjan M, Brănoiu G. Removal of Butyl Mercaptan from Gas Streams by Reactive Adsorption. Molecules. 2025; 30(9):1962. https://doi.org/10.3390/molecules30091962
Chicago/Turabian StyleSanda, Mia, Ion Onuțu, Cristina Maria Dușescu-Vasile, Gabriel Vasilievici, Dorin Bomboș, Marian Băjan, and Gheorghe Brănoiu. 2025. "Removal of Butyl Mercaptan from Gas Streams by Reactive Adsorption" Molecules 30, no. 9: 1962. https://doi.org/10.3390/molecules30091962
APA StyleSanda, M., Onuțu, I., Dușescu-Vasile, C. M., Vasilievici, G., Bomboș, D., Băjan, M., & Brănoiu, G. (2025). Removal of Butyl Mercaptan from Gas Streams by Reactive Adsorption. Molecules, 30(9), 1962. https://doi.org/10.3390/molecules30091962







