Electrochemiluminescence/Electrochemistry Dual-Mode Synchronous Sensing of Pb2+ Based on G4–hemin DNAzyme Complex During One-Step Scan
Abstract
1. Introduction
2. Results and Discussion
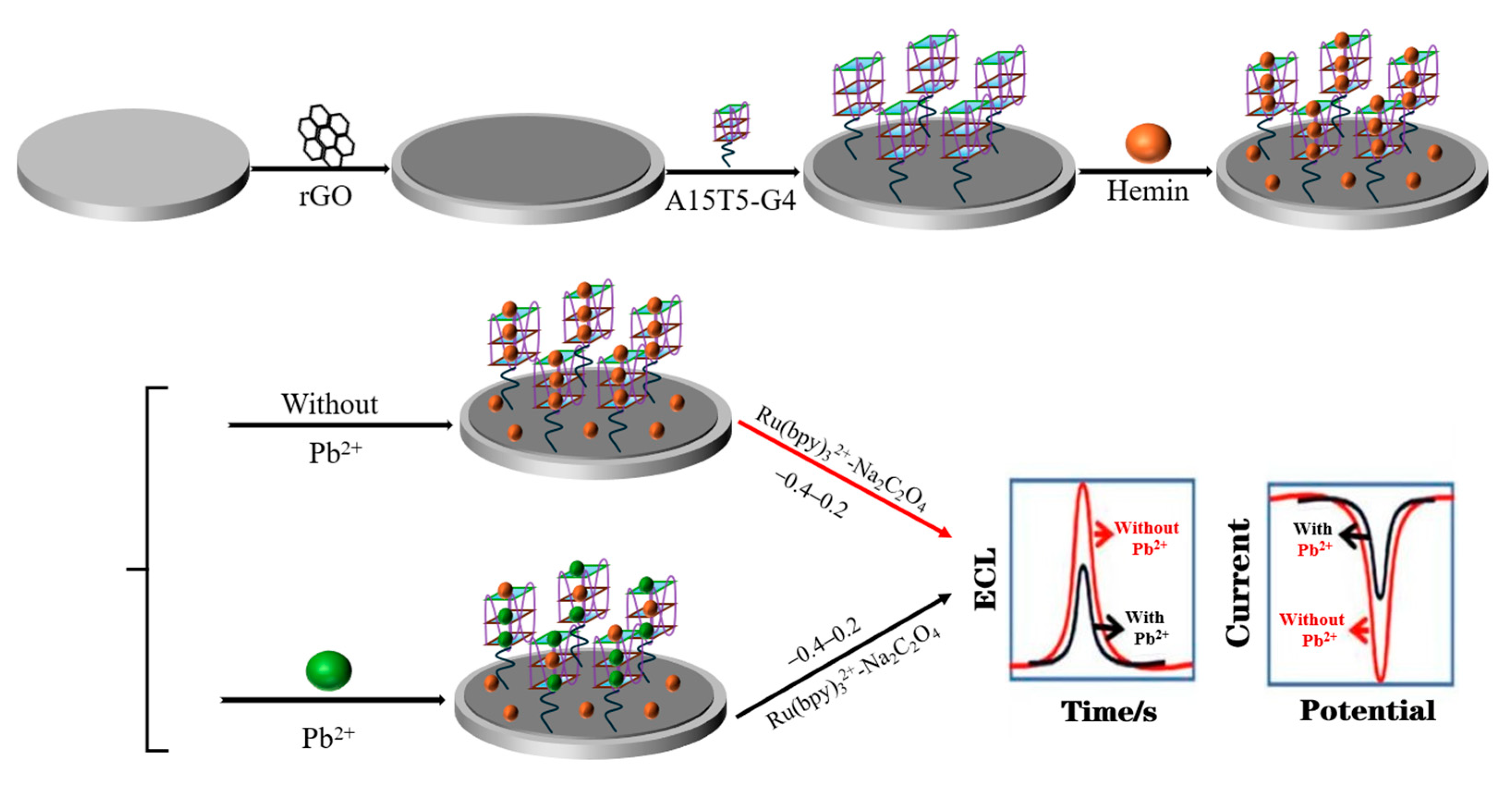
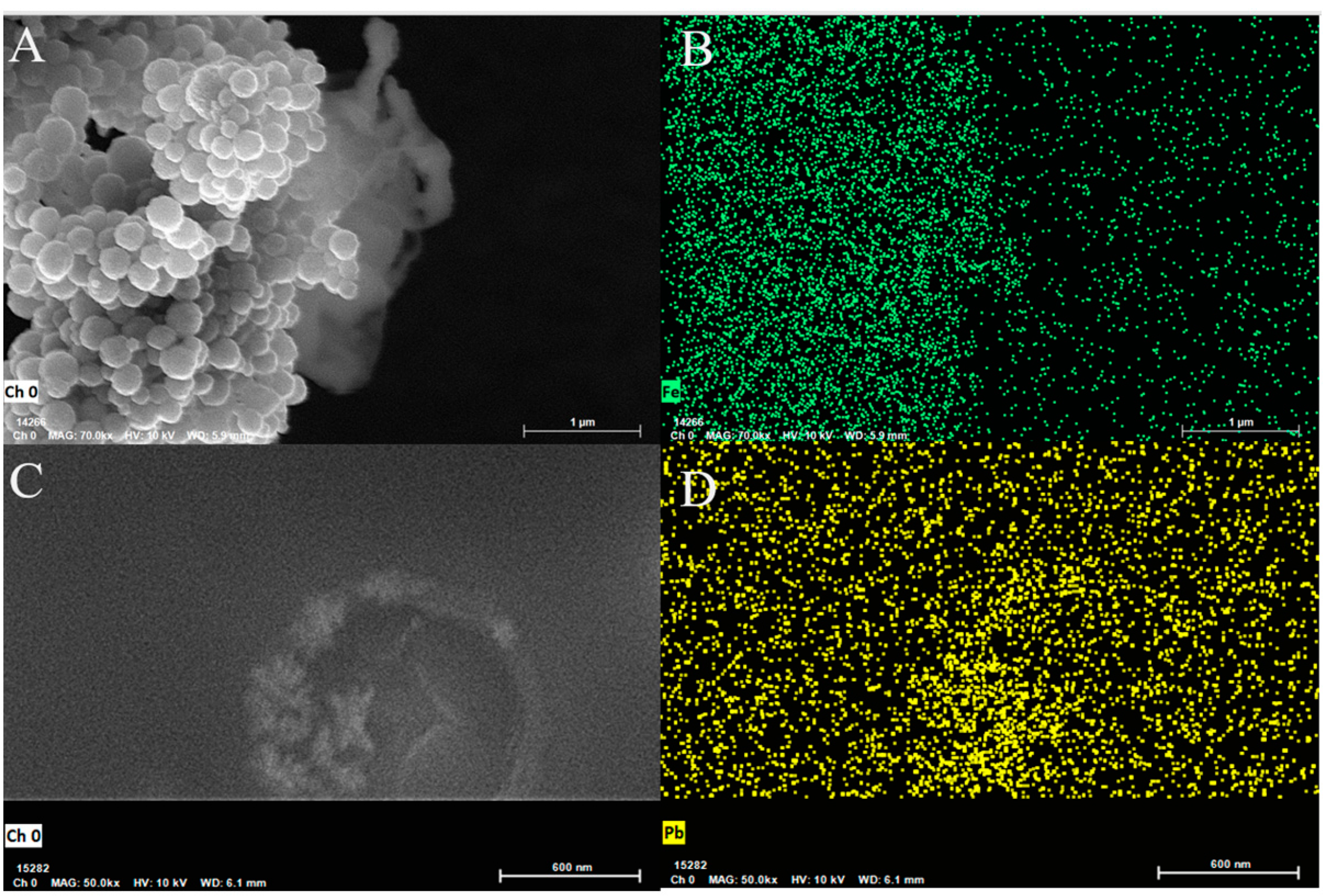
2.1. Characterization of Electrode Materials
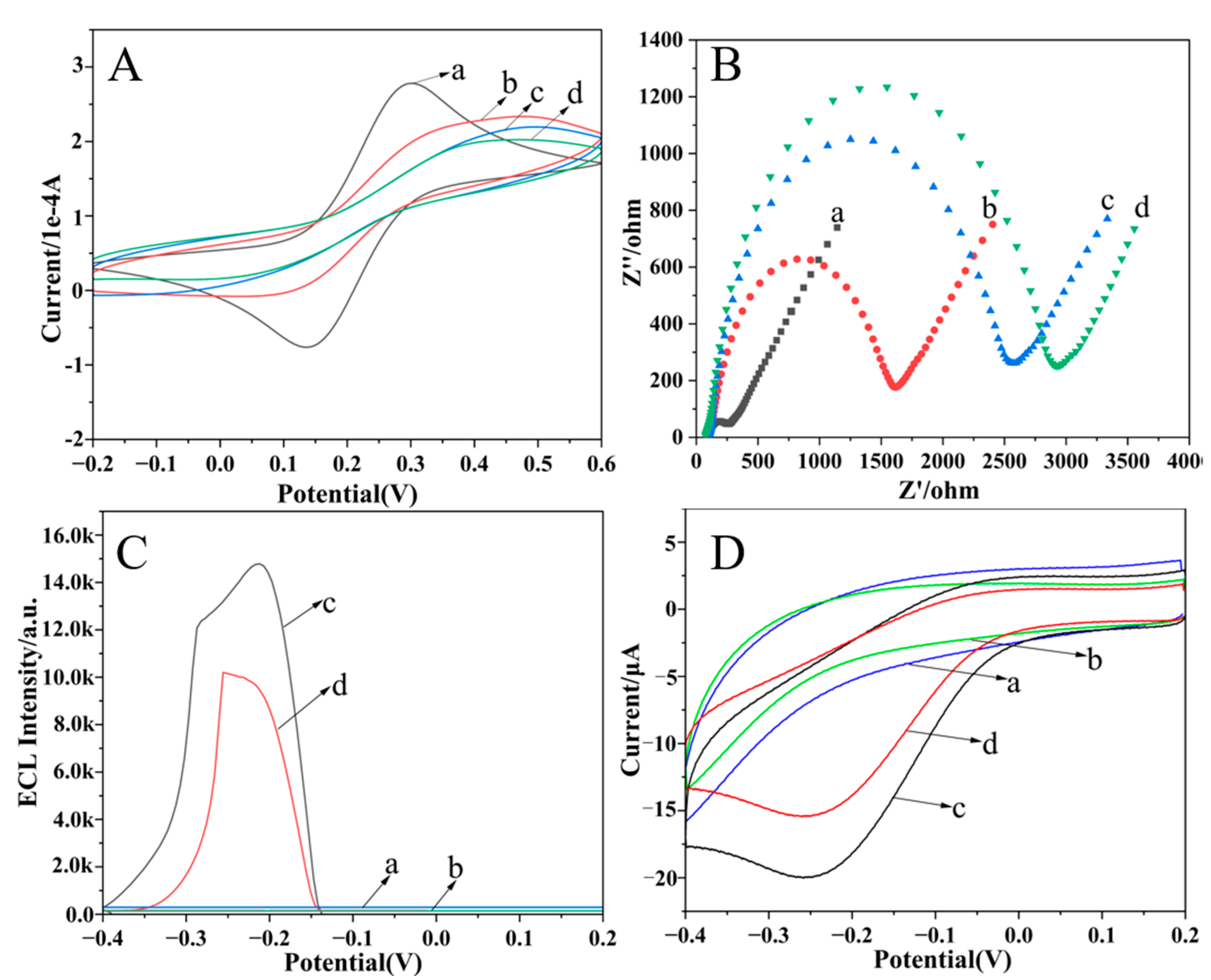
2.2. Electrochemical Characterization of the Dual-Mode Sensor
2.3. Feasibility of Dual-Mode Sensor for Pb2+ Detection
2.4. Generating Mechanism of ECL and EC Signals
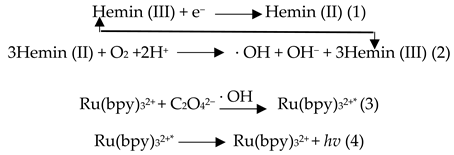 |
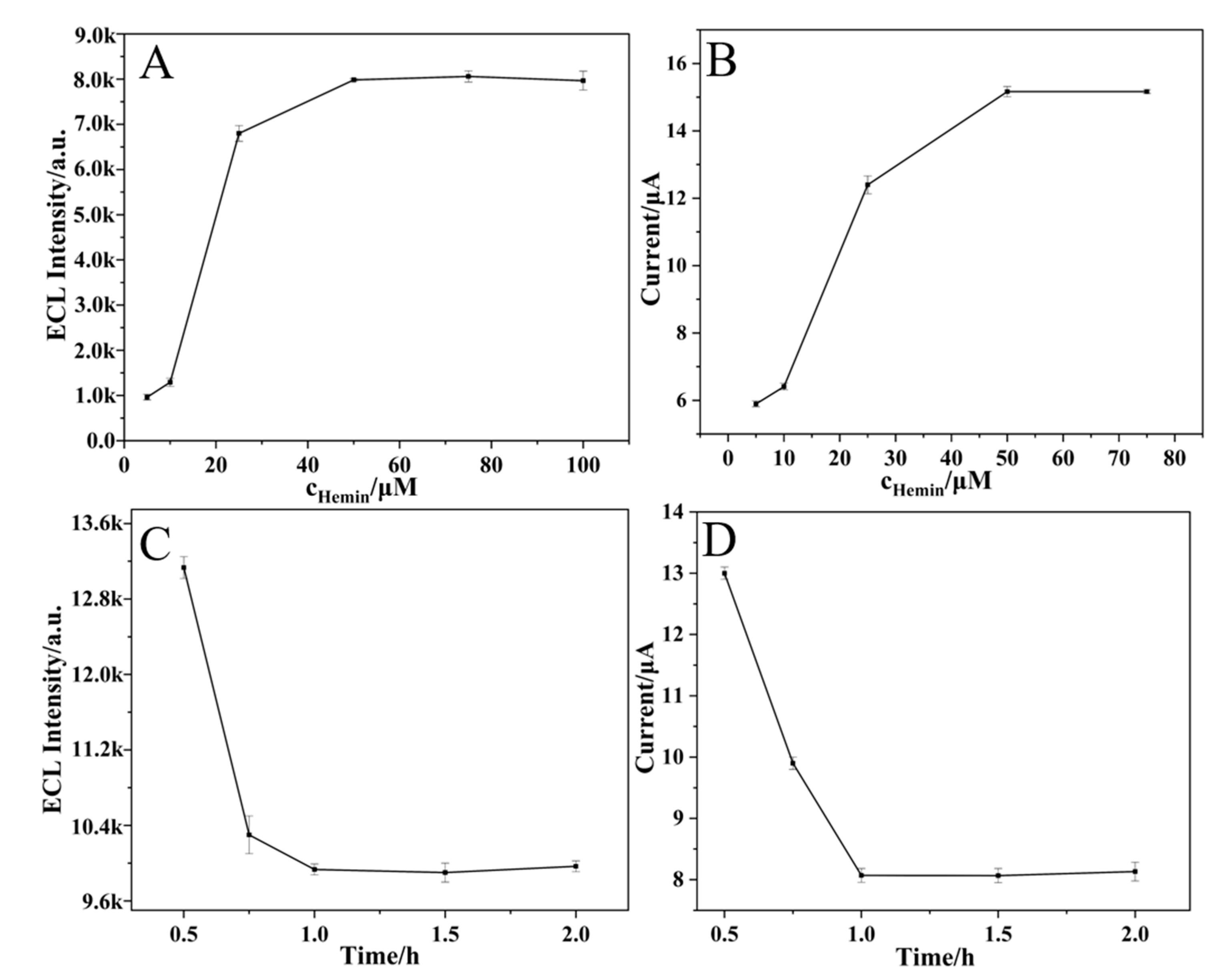
2.5. Optimization of Experimental Conditions
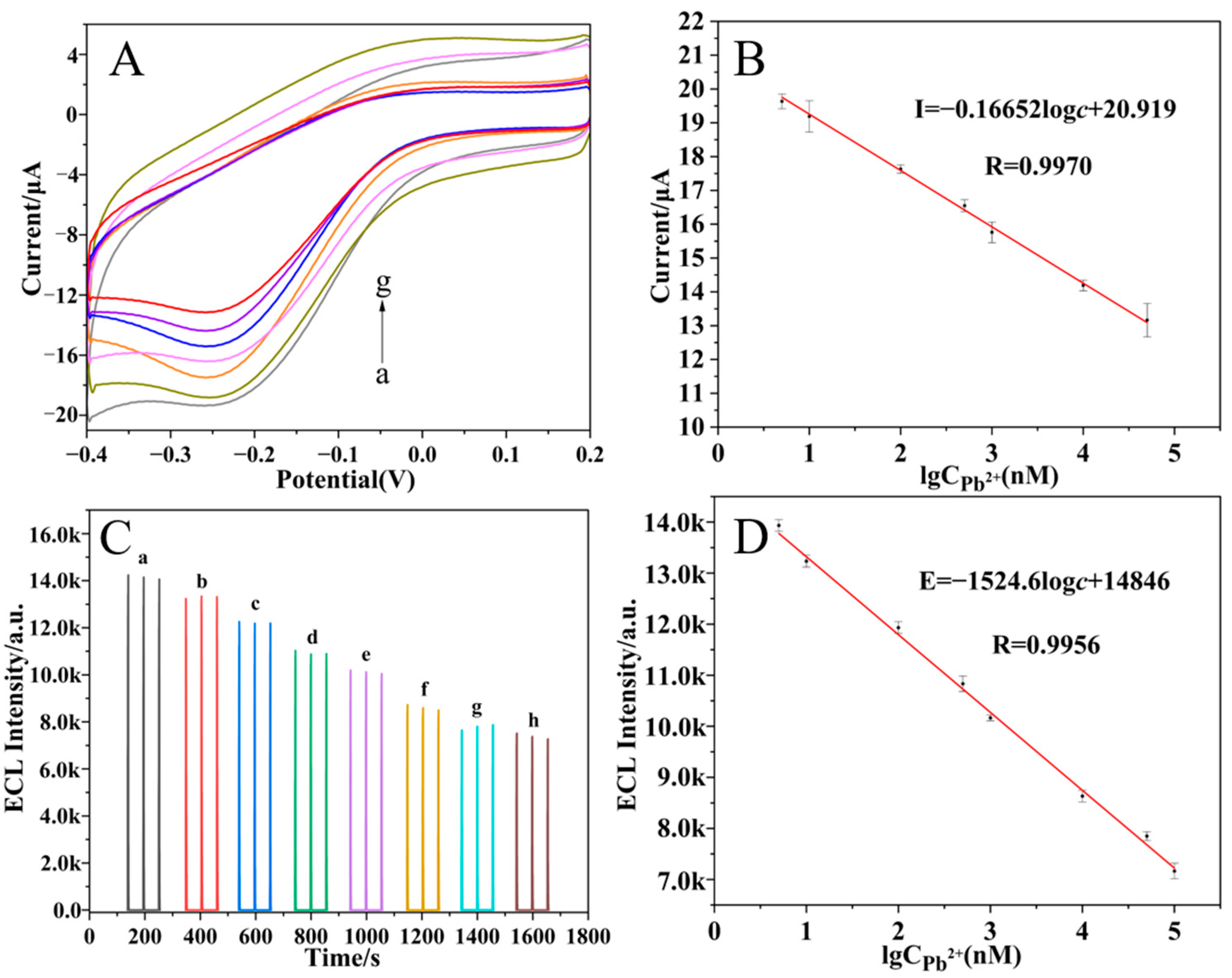
2.6. Detection of Pb2+
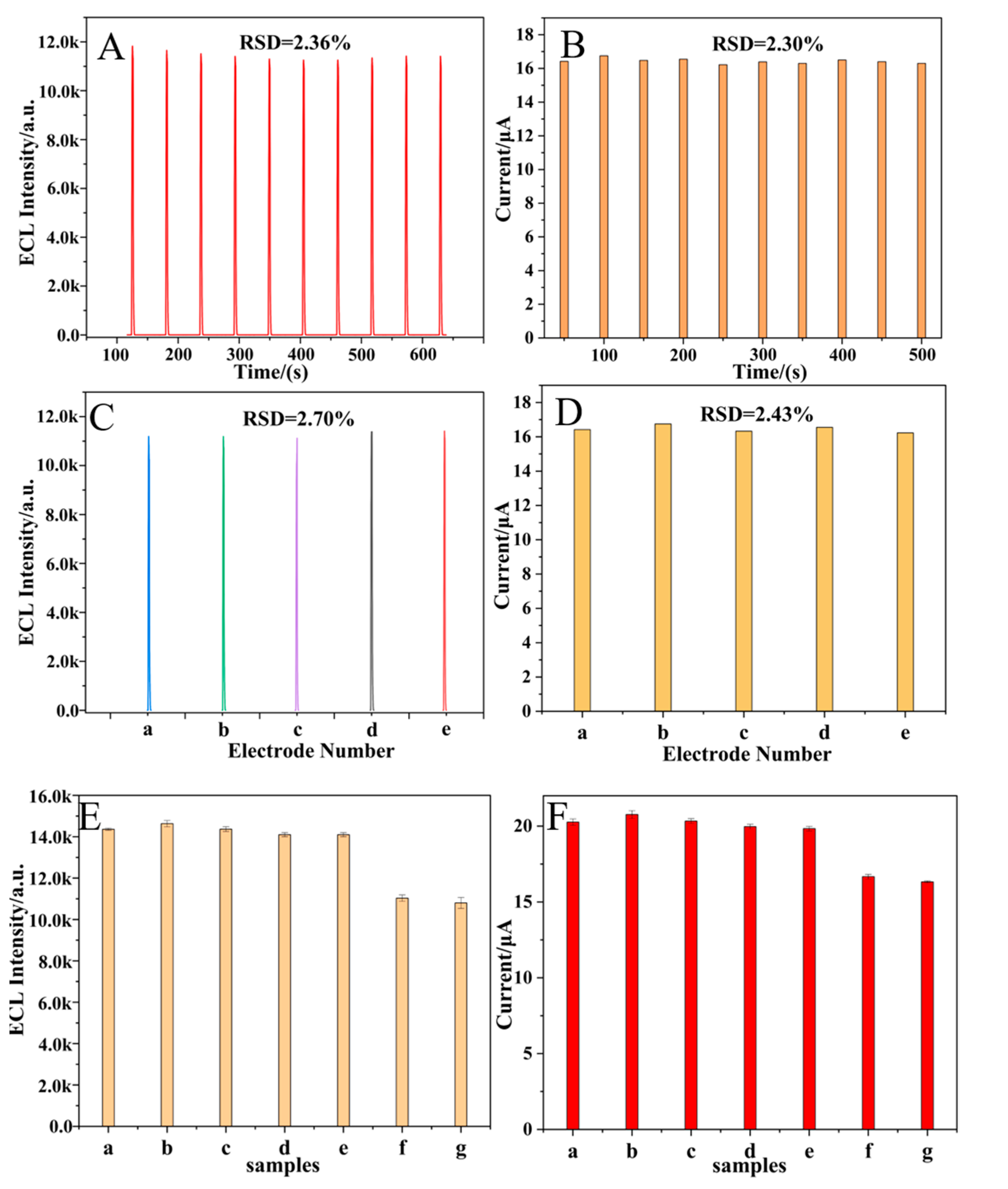
2.7. Stability, Reproducibility, and Specificity of Dual-Mode Sensor
2.8. The Practical Applicability of the Sensor
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Fabrication of ECL/EC Dual-Mode Sensor
3.4. Measurement Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, S.S.M.; Fathy, M.A. Novel paper-based potentiometric combined sensors using coumarin derivatives modified with vanadium pentoxide nanoparticles for the selective determination of trace levels of lead ions. Microchim. Acta 2024, 191, 427. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Z.; Zheng, T.; Fan, Y.; Hu, L. Label-free electrochemical aptasensor for ultrasensitive lead ion detection based on flower-like AuNPs@MoS2 and core–shell Pt@Pd bimetallic nanoparticles. Microchim. Acta 2024, 191, 358. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Wang, J.; Qi, J.; Li, J.; Ma, J.; Chen, L. A rotary multi-positioned cloth/paper hybrid microfluidic device for simultaneous fluorescence sensing of mercury and lead ions by using ion imprinted technologies. J. Hazard. Mater. 2022, 428, 128165. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; Augusto Peres, J.; Lurdes Felsner, M.; Cristiane Justi, K. Direct determination of Pb in raw milk by graphite furnace atomic absorption spectrometry (GF AAS) with electrothermal atomization sampling from slurries. Food Chem. 2017, 229, 721–725. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Chen, B.; Hu, B. Thiol-grafted magnetic polymer for preconcentration of Cd, Hg, Pb from environmental water followed by inductively coupled plasma mass spectrometry detection. Spectrochim. Acta Part B At. Spectrosc. 2021, 177, 106071. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.-X.; Lin, L. Plasma-based instant synthesis of functionalized gold nanoparticles for colorimetric detection of lead ions. Chem. Eng. Sci. 2022, 260, 117849. [Google Scholar] [CrossRef]
- Xu, S.; Wang, S.; Guo, L.; Tong, Y.; Wu, L.; Huang, X. Nanozyme-catalysed CRISPR-Cas12a system for the preamplification-free colorimetric detection of lead ion. Anal. Chim. Acta 2023, 1243, 340827. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Ali, S.; Wang, P.; Li, C.; Ouyang, Q.; Li, H.; Chen, Q. Cysteamine-mediated upconversion sensor for lead ion detection in food. J. Food Meas. Charact. 2021, 15, 4849–4857. [Google Scholar] [CrossRef]
- Gong, Q.; Xu, X.; Cheng, Y.; Wang, X.; Liu, D.; Nie, G. A novel “on-off-on” electrochemiluminescence strategy based on RNA cleavage propelled signal amplification and resonance energy transfer for Pb2+ detection. Anal. Chim. Acta 2024, 1290, 342218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, P.; Zhen, M.; Liu, X.; Luo, L.; Lv, W.; Luo, L.; You, T. PTC-NH2@MOFs-AuNCs: A novel bifunctional signal probe for ratiometric electrochemiluminescence/colorimetry dual-mode aptasensing of lead ion. Chem. Eng. J. 2024, 499, 156185. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, Y.; Yin, J.; Zhang, Y.; Guo, Q.; Sun, X.; Guo, Y.; Yang, Q.; Li, F.; Zhang, Y. Electrochemiluminescence biosensor for determination of lead(II) ions using signal amplification by Au@SiO2 and tripropylamine-endonuclease assisted cycling process. Microchim. Acta 2022, 189, 317. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Dai, C.; Mao, Z.; Zhou, Y. CRISPR/Cas12a-drived electrochemiluminescence and fluorescence dual-mode magnetic biosensor for sensitive detection of Pseudomonas aeruginosa based on iridium(III) complex as luminophore. Biosens. Bioelectron. 2024, 264, 116678. [Google Scholar] [CrossRef]
- Qin, L.; Huang, T.; Cui, H.; Cheng, M.; Wei, G.; Liao, F.; Xiong, W.; Jiang, H.; Zhang, J.; Fan, H. A fluorescence-electrochemiluminescence dual-mode sensor based on a "switch" system for highly selective and sensitive K-ras gene detection. Biosens. Bioelectron. 2023, 235, 115385. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Sun, M.; Chen, B.; Xu, H.; Fan, Y.; Zhou, H.; Liu, J. ECL and visual dual-mode detection of miRNA-21 based on HRP/Au NPs composite probe and CRISPR/Cas12a strategy. Sens. Actuators B Chem. 2025, 425, 137016. [Google Scholar] [CrossRef]
- Lai, W.; Yan, S.; Jiang, M.; Wang, M.; Qiao, X.; Hong, C. Dual-Mode Immunoassay Constructed by Water-Induced Perylene Diimide Supramolecular Self-Assembly and Enzymatic Biocatalytic Precipitation Strategy. Anal. Chem. 2024, 96, 14074–14084. [Google Scholar] [CrossRef]
- Lai, W.; Yan, S.; Wang, M.; Jiang, M.; Liu, X.; Li, J.; Li, P.; Wei, Z.; Hong, C. S–CdIn2S4: A novel near-infrared emitter triggered by low potential for constructing a dual-mode immunosensing platform. Biosens. Bioelectron. 2023, 236, 115441. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, S.; Xi, F. Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection. Molecules 2025, 30, 746. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Li, C.; Shang, L.; Ma, R.; Jia, L.; Li, X.; Li, B.; Wang, H. Dual-mode electrochemical and electrochemiluminescence detection of dopamine based on perylene diimide self-assembly material. Microchim. Acta 2024, 191, 721. [Google Scholar] [CrossRef]
- Li, H.; Cai, Q.; Xue, Y.; Jie, G. HOF-101-based dual-mode biosensor for photoelectrochemical/electrochemiluminescence detection and imaging of oxytetracycline. Biosens. Bioelectron. 2024, 245, 115835. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, K.; Yang, X.; Nie, G. Sensitive dual-mode sensing platform for Amyloid β detection: Combining dual Z-scheme heterojunction enhanced photoelectrochemistry analysis and dual-wavelength ratiometric electrochemiluminescence strategy. Biosens. Bioelectron. 2023, 237, 115507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Dai, Y.; Ye, M.; Sun, Y.; Zhang, K.; Xu, J.-J. Dual-mode SERS-ECL biosensor for robust detection of circulating miRNAs based on in-situ synthesized probes. Chem. Eng. J. 2024, 495, 153607. [Google Scholar] [CrossRef]
- Hu, K.; Qin, L.; Ren, X.; Guo, Z.; Wang, S.; Hu, Y. Deoxyribonucleic acid-guided dual-mode electro-chemical/chemiluminescent platform for sensitive and selective examination of Pb2+. J. Electroanal. Chem. 2022, 922, 116757. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, C.; Lin, H.; Wei, W.; Yang, F.; Wu, Y.; Niu, L.; Kang, W.; Guo, Z. A One-Step Dual-Mode Aptasensor for Subnanomolar Detection of Lead Ions Based on Electrochemiluminescence and Fast Scan Voltammetry. J. Electrochem. Soc. 2020, 167, 126506. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Z.; Fang, H.; Lei, X. Unexpected sequence adsorption features of polynucleotide ssDNA on graphene oxide. Phys. Chem. Chem. Phys. 2020, 22, 11740–11746. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, W.; Shang, L.; Ma, R.; Jia, L.; Wang, H. Graphene oxide/perylene–aniline electrochemiluminescence platform for protein detection based on molecule recognition. Anal. Methods 2021, 13, 5293–5298. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Zhang, R.; Gu, L.; Yang, J.; Xing, Y.; Kou, B.; Jin, X. High-efficiency G4-hemin@peptide biomimetic nanozyme and domain-limited DNA walker for sensitive electrochemical analysis of miRNA-21. Sens. Actuators B Chem. 2025, 426, 137036. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Cheng, M.; Mergny, J.-L.; Lin, Q.; Zhou, J.; Ju, H. Highly active G-quadruplex/hemin DNAzyme for sensitive colorimetric determination of lead(II). Microchim. Acta 2019, 186, 786. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Y.; Jia, Y.; Liu, J. Heating Drives DNA to Hydrophobic Regions While Freezing Drives DNA to Hydrophilic Regions of Graphene Oxide for Highly Robust Biosensors. J. Am. Chem. Soc. 2020, 142, 14702–14709. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Hu, C.; Zhu, R.; Qiu, D.; Gong, R.; Wang, R.; Li, Q.; Yan, T.; Li, C.; Qiao, M.; et al. Low-background fluorescent biosensor based on graphene oxide and aptamer biorecognition for sensitive detection of kanamycin. J. Food Compos. Anal. 2024, 131, 106261. [Google Scholar] [CrossRef]
- Ma, K.; Li, X.; Xu, B.; Tian, W. Label-free bioassay with graphene oxide-based fluorescent aptasensors: A review. Anal. Chim. Acta 2021, 1188, 338859. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, E.; Dong, S. Potassium−Lead-Switched G-Quadruplexes: A New Class of DNA Logic Gates. J. Am. Chem. Soc. 2009, 131, 15082–15083. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bai, W.; Zheng, J. A novel self-cleaning electrochemical biosensor integrating copper porphyrin-derived metal-organic framework nanofilms, G-quadruplex, and DNA nanomotors for achieving cyclic detection of lead ions. Biosens. Bioelectron. 2022, 197, 113801. [Google Scholar] [CrossRef]
- Cao, W.; Xu, G.; Zhang, Z.; Dong, S. Novel tris(2,2′-bipyridine)ruthenium(ii) cathodic electrochemiluminescence in aqueous solution at a glassy carbon electrode. Chem. Commun. 2002, 14, 1540–1541. [Google Scholar] [CrossRef]
- Alinejad, F.; Khoshbin, Z.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A label-free DNAzyme-Mediated biosensor for fluorescent detection of Lead (II) ion. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2025, 329, 125627. [Google Scholar] [CrossRef]
- Singh, G.; Gupta, N.; Kumar, A.; Prasher, P.; Mudila, H. Systematic development of PPY/GO/ZnO hybrid composite for electrochemical detection of Pb2+ in aqueous solution. Ionics 2025, 31, 3105–3119. [Google Scholar] [CrossRef]
- Ali, I.; Zhang, J.; Bian, L. A Novel Pb2+and Cys Induced Switch Off–On Strategy and Its Application in Detection Based on the Platform of N-Methylmesoporphyrin IX Fluorescence Being Amplied by G-Quadruplex. J. Fluoresc. 2025, 35, 1–13. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Ranjini, A.; Haware, D.J.; Manjunath, M.N.; Manonmani, H.K. Immunoassay for the detection of lead ions in environmental water samples. Int. J. Environ. Anal. Chem. 2012, 92, 334–343. [Google Scholar] [CrossRef]
- Wang, Y.; Irudayaraj, J. A SERS DNAzyme biosensor for lead ion detection. Chem. Commun. 2011, 47, 4394–4396. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Lin, X.; Waheed, A.; Ravikumar, A.; Zhang, Z.; Chen, C. A self-assembled G-quadruplex/hemin DNAzyme-driven DNA walker strategy for sensitive and rapid detection of lead ions based on rolling circle amplification. Biosensors 2023, 13, 761. [Google Scholar] [CrossRef]
- Han, Y.; Du, Y.; Xu, K.; Ren, X.; Zhao, G.; Ru, Z.; Jia, Y.; Wei, Q. Sulfur Quantum Dot-Based Electrochemiluminescence Resonance Energy Transfer System for a Dual-Mode Ultrasensitive Detection of MC-LR Using Ag+ as a Signal Amplification Switch. Anal. Chem. 2023, 95, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, M.; Lai, W.; Song, X.; Li, J.; Liu, D.; Wei, Z.; Hong, C. Construction of electrochemical and electrochemiluminescent dual-mode aptamer sensors based on ferrocene dual-functional signal probes for the sensitive detection of Alternariol. Anal. Chim. Acta 2023, 1272, 341476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zuo, M.; Yang, Y.; Zhao, N.-N.; Wang, X.; Cui, L.; Zhang, C.-Y. Construction of a Dual-Mode Biosensor with Ferrocene as Both a Signal Enhancer and a Signal Tracer for Electrochemiluminescent and Electrochemical Enantioselective Recognition. Anal. Chem. 2023, 95, 17920–17927. [Google Scholar] [CrossRef] [PubMed]

| Detection Method | Linear Range (nM) | Detection Limit (nM) | Ref. |
|---|---|---|---|
| FL | 100–6 × 105 | 18 | [35] |
| EC | 300–3 × 103 | 50 | [36] |
| FL | 400–1.6 × 103 | 450 | [37] |
| ELISA | 20–1 × 106 | 200 | [38] |
| SERS | 50–1 × 103 | 20 | [39] |
| ECL | 5–1 × 105 | 1.51 | This work |
| EC | 5–5 × 104 | 2.03 | This work |
| Detection Method | ECL Potential Range (V) | EC Potential Range (V) | Ref. |
|---|---|---|---|
| ECL/EC | −2.0~0 | −0.6~0.2 | [41] |
| ECL/EC | −1.2~0 | 0~0.6 | [42] |
| ECL/EC | 0~1.5 | −0.40~−0.8 | [22] |
| ECL/EC | 0~1.5 | 0~0.5 | [43] |
| ECL/EC | 1.0~1.8 | −1.0~1.0 | [23] |
| ECL/EC | 0.2~−0.4 | 0.2~−0.4 | This work |
| Method | Added (nM) | Found (nM) | Recovery (%) (n = 3) | RSD % (n = 3) |
|---|---|---|---|---|
| ECL | 0 | 10.13 | - | 0.60 |
| 10.00 | 20.31 | 101.6 | 4.40 | |
| 100.0 | 105.3 | 95.51 | 3.60 | |
| 1000 | 993.4 | 99.44 | 7.10 | |
| EC | 0 | 9.978 | - | 1.23 |
| 10.00 | 20.47 | 103.1 | 4.78 | |
| 100.0 | 108.8 | 98.69 | 2.24 | |
| 1000 | 997.8 | 99.63 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, R.; Shang, L.; Zhang, W.; Li, X.; Jia, L.; Ma, R.; Wang, H. Electrochemiluminescence/Electrochemistry Dual-Mode Synchronous Sensing of Pb2+ Based on G4–hemin DNAzyme Complex During One-Step Scan. Molecules 2025, 30, 1951. https://doi.org/10.3390/molecules30091951
Wei R, Shang L, Zhang W, Li X, Jia L, Ma R, Wang H. Electrochemiluminescence/Electrochemistry Dual-Mode Synchronous Sensing of Pb2+ Based on G4–hemin DNAzyme Complex During One-Step Scan. Molecules. 2025; 30(9):1951. https://doi.org/10.3390/molecules30091951
Chicago/Turabian StyleWei, Rukai, Lei Shang, Wei Zhang, Xiaojian Li, Liping Jia, Rongna Ma, and Huaisheng Wang. 2025. "Electrochemiluminescence/Electrochemistry Dual-Mode Synchronous Sensing of Pb2+ Based on G4–hemin DNAzyme Complex During One-Step Scan" Molecules 30, no. 9: 1951. https://doi.org/10.3390/molecules30091951
APA StyleWei, R., Shang, L., Zhang, W., Li, X., Jia, L., Ma, R., & Wang, H. (2025). Electrochemiluminescence/Electrochemistry Dual-Mode Synchronous Sensing of Pb2+ Based on G4–hemin DNAzyme Complex During One-Step Scan. Molecules, 30(9), 1951. https://doi.org/10.3390/molecules30091951






