Valorization of Beetroot Waste via Subcritical Water Extraction for Developing Active Food Packaging Materials
Abstract
1. Introduction
2. Results and Discussion
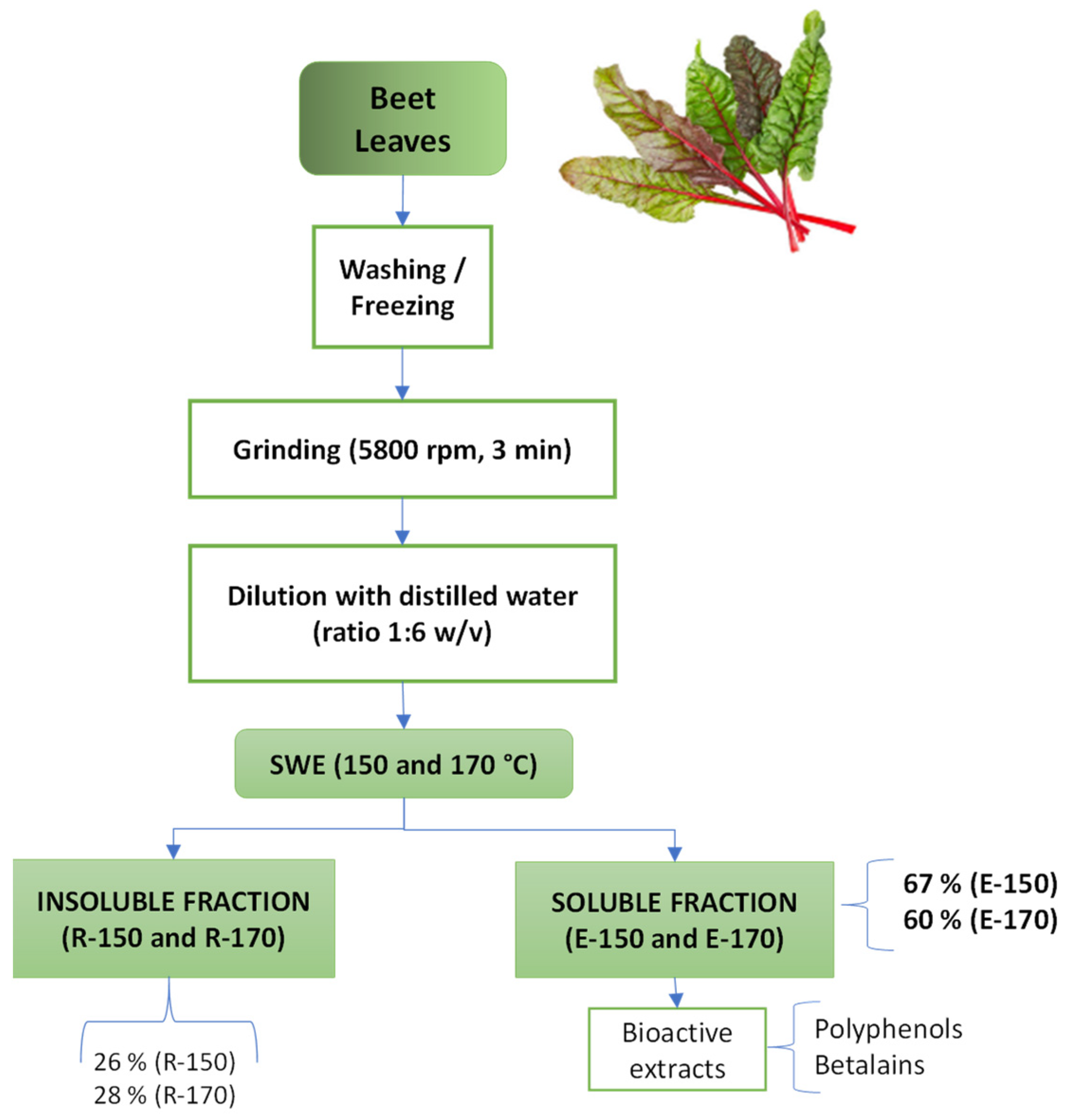
2.1. Extraction Yields and Bioactive Properties of the Extracts
2.2. Optical Properties of the Films Containing BLS Extracts
2.3. Barrier Properties of the Films Containing BLS Extracts
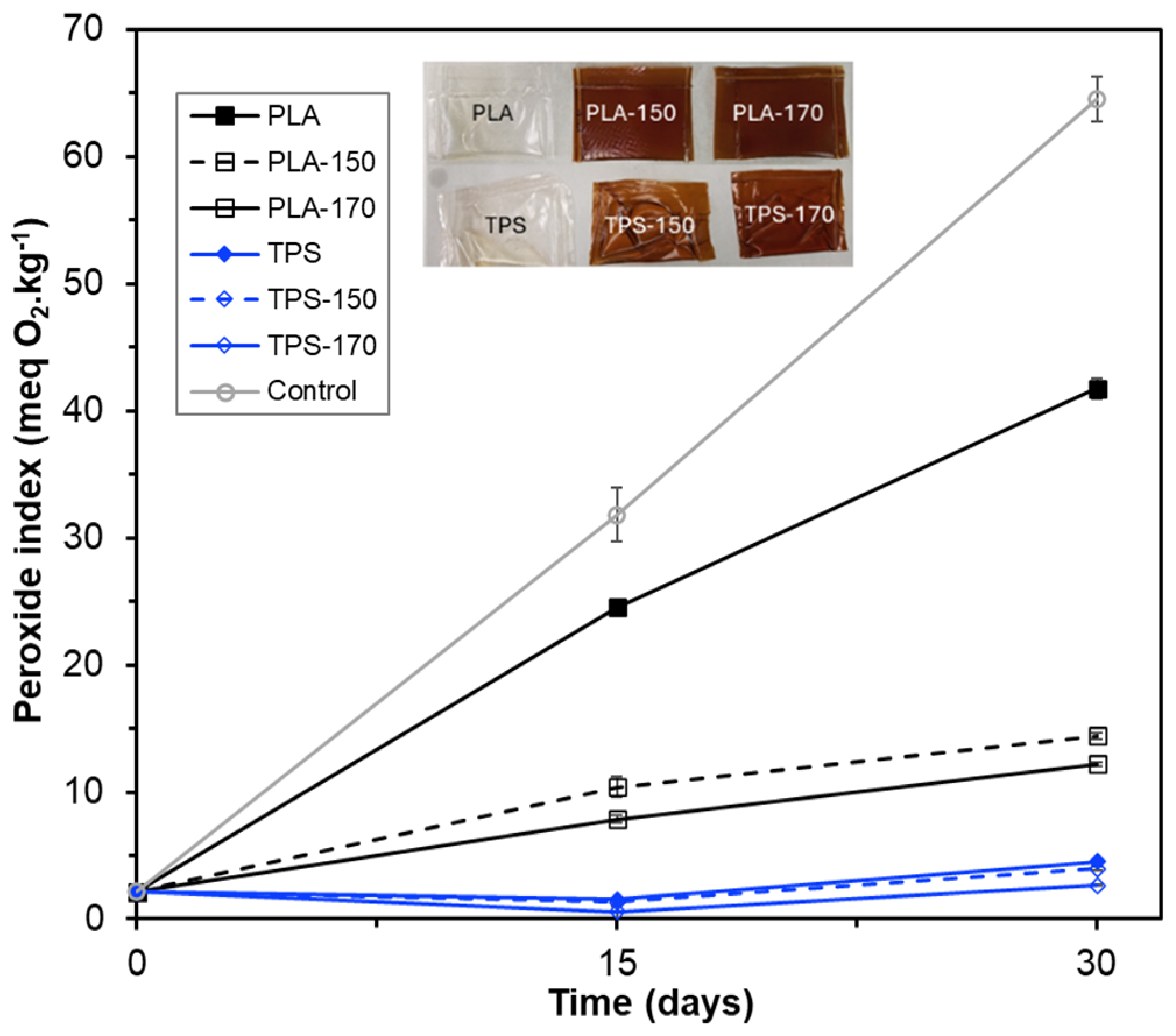
2.4. Antioxidant Capacity of the Films Containing BLS Extracts
3. Materials Methods
3.1. Chemicals
3.2. Plant Preparation
3.3. Subcritical Water Extraction
3.4. Extract Characterization
3.5. Film Preparation
3.5.1. Thermoplastic Starch (TPS) Films
3.5.2. Amorphous PLA Films
3.6. Characterization of the Film Properties
3.6.1. Barrier Properties
3.6.2. Optical Properties
3.6.3. Antioxidant Properties of the Film
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC-Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Rodrigues, P.B.J.; Liberal, A.; Petropoulos, A.S.; Ferreira, C.F.R.I.; Oliveira, P.P.M.B.; Fernandes, A.; Barros, L. Agri-Food Surplus, Waste and Loss as Sustainable Biobased Ingredients: A Review. Molecules 2022, 27, 5200. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Aguero, M.V.; Jagus, R.J. Consumer Acceptability and Impact of Handling Conditions on Sensory, Microbiological and Nutritional Quality of Beet Leaves. Am. J. Food Technol. 2017, 12, 301–310. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Maravić, N.; Teslić, N.; Nikolić, D.; Dimić, I.; Šereš, Z.; Pavlić, B. From agricultural waste to antioxidant-rich extracts: Green techniques in extraction of polyphenols from sugar beet leaves. Sust. Chem. Pharm. 2022, 28, 100728. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.R.; Iglesias, A.H.; Martínez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2021, 67, 102549. [Google Scholar] [CrossRef]
- Chemat, F.; Fabiano-Tixier, A.S.; Vian, M.A.; Allaf, T.; Vorobiev, E. Solvent-free extraction of food and natural products. TrAC Trends Anal. Chem. 2015, 71, 157–168. [Google Scholar] [CrossRef]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Krishna, R.C.; Mohan, M.C. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- Sevenich, R.; Mathys, A. Continuous Versus Discontinuous Ultra-High-Pressure Systems for Food Sterilization with Focus on Ultra-High-Pressure Homogenization and High-Pressure Thermal Sterilization: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.D.A.S.; Martí-Quijal, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative non-thermal technologies for recovery and valorization of value-added products from crustacean processing by-products—An opportunity for a circular economy approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef]
- Lasta, B.H.F.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Perez, L.M.; Guillen, I.G.; González- Martinez, C.; Chiralt, A. Subcritical Water Extraction for Valorization of Almond Skin from Almond Industrial Processing. Foods 2023, 12, 3759. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González- Martínez, C.; Chiralt, A. Using rice straw fractions to develop reinforced, active PLA-starch bilayers for meat preservation. Food Chem. 2023, 405, 134990. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Active poly (lactic acid) Films with rice straw aqueous extracts for meat preservation purposes. Food Bioproc. Tech. 2023, 16, 2635–2650. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Antioxidant starch composite films containing rice straw extract and cellulose fibres. Food Chem. 2023, 400, 134073. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Gil, N.J.B.; González-Martínez, C.; Chiralt, A. Antioxidant poly (lactic acid) films with rice straw extract for food packaging applications. Food Packag. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hui, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymer. Composites 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, T.R. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Goyeneche, R.; Fanovich, A.; Rodrigues, C.R.; Nicolao, M.C.; Di Scala, K. Supercritical CO2 extraction of bioactive compounds from radish leaves: Yield, antioxidant capacity and cytotoxicity. J. Supercrit. Fluids 2018, 135, 78–83. [Google Scholar] [CrossRef]
- Rivas Vela, C.I.; Amaya Llano, S.L.; Castaño Tostado, E.; Castillo-Herrera, G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules 2021, 26, 6655. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef]
- Trigueros, E.; Oliveira, A.P.; Andrade, P.B.; Videira, R.A.; de Pinho, P.G.; Sanz, M.T.; Beltrán, S. Exploring the bioactive potential of algae residue extract via subcritical water extraction: Insights into chemical composition and biological activity. Food Chem. 2024, 458, 140310. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE). Eur. Food Res. Technol. 2014, 238, 85–91. [Google Scholar] [CrossRef]
- Zein, H.; Hashish, A.S.; Ismael, H.H.G. The antioxidant and Anticancer Activities of Swiss Chard and Red Beetroot Leaves. Curr. Sci. Int. 2015, 4, 491–498. [Google Scholar]
- Laqui Vilca, C.; Aguilar Tuesta, S.; Mamani Navarro, W.; Montaño Bustamante, J.; Condezo Hoyos, L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind. Crops Prod. 2018, 111, 606–614. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, N.; Jaime-Fonseca, M.R.; Martin-Martinez, S.E.; Zepeda, L.G. Extraction, Stability and Separation of Betalains from Opuntia joconostle c.v. Using Response Surface Methodology. J. Agric. Food Chem. 2013, 61, 1995–2004. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Wybraniec, S. Thermal Degradation of Major Gomphrenin Pigments in the Fruit Juice of Basella alba L. (Malabar Spinach). J. Agric. Food Chem. 2017, 65, 7500–7508. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; Plaza, M.; Mendiola, J.A.; Ibáñez, E.; Herrero, M. Subcritical Water Extraction and Neoformation of Antioxidants. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; González, H.D., Muñoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–130. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Do, H.N.; Pham, H.H.; Le, M.T.; Lauwaert, J.; Diels, L.; Verberckmoes, A.; Do, N.H.N.; Viet, T.T.; Le, P.K. The novel method to reduce the silica content in lignin recovered from black liquor originating from rice straw. Sci. Rep 2020, 10, 21263. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, Y.; Jiang, L.; Dan, Y. Polylactide with improved optical property by introducing natural functional substance: Aloe-emodin. React. Funct. Polym. 2020, 148, 104486. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Package Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Vilaplana, F. Antioxidant starch films containing sunflower hull extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef]
- Borompichaichartkul, C.; Chinprahast, N.; Devahastin, S.; Wiset, L.; Poomsa-Ad, N.; Ratchapo, T. Multistage heat pump drying of macadamia nut under modified atmosphere. Int. Food Res. J. 2013, 20, 2199–2203. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ASTM D3985-05; Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–7.
- ASTM E96/E96M; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2005; pp. 1–11.
- Mchugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]


| Extract | TPC (mg GAE·g−1 Extract) | EC50 (mg Extract·mg−1 DPPH) | Betalains | |
|---|---|---|---|---|
| BX (µg·g−1 Extract) | BC (µg·g−1 Extract) | |||
| E-150 | 51.3 ± 1.8 b | 30.1 ± 0.2 a | 752 ± 16 b | 396 ± 15 b |
| E-170 | 73.2 ± 1.9 a | 22.2 ± 0.2 b | 900 ± 30 a | 456 ± 17 a |
| Formulation | Visual Appearance | L* | Cab* | hab* | ∆E* |
|---|---|---|---|---|---|
| PLA |  | 75.6 ± 0.1 a | 4.2 ± 0.1 c | 15.3 ± 0.6 b | - |
| PLA-150 |  | 32.0 ± 0.4 b | 19.2 ± 0.3 b | 58.2 ± 0.6 a | 33.2 ± 0.4 b |
| PLA-170 |  | 36.0 ± 6.9 b | 25.4 ± 9.1 a | 60.8 ± 2.7 a | 37.2 ± 0.4 a |
| TPS |  | 69.7 ± 1.4 a | 8.2 ± 0.1 c | 60.1 ± 1.2 b | - |
| TPS-150 |  | 39.7 ± 0.6 b | 22.4 ± 0.4 a | 63.9 ± 0.3 a | 46.5 ± 0.3 a |
| TPS-170 |  | 33.6 ± 0.4 c | 17.1 ± 0.3 b | 55.0 ± 0.3 c | 46.3 ± 1.4 a |
| Formulation | OP ×1014 (cm3·m−1·s−1·Pa−1) | WVP ×1011 (g·Pa−1·s−1·m−1) |
|---|---|---|
| PLA | 188.0 ± 2.0 a | 10.0 ± 2.0 a |
| PLA-150 | 162.3 ± 1.2 c | 5.5 ± 1.2 b |
| PLA-170 | 182.0 ± 3.0 b | 6.6 ± 2.0 b |
| TPS | 6.5 ± 0.1 b | 248 ± 40 b |
| TPS-150 | 9.1 ± 1.6 a | 351 ± 40 a |
| TPS-170 | 8.6 ± 0.1 a | 401 ± 13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, M.C.; Freitas, P.A.V.; Jagus, R.J.; Agüero, M.V.; Chiralt, A. Valorization of Beetroot Waste via Subcritical Water Extraction for Developing Active Food Packaging Materials. Molecules 2025, 30, 1928. https://doi.org/10.3390/molecules30091928
de Carvalho MC, Freitas PAV, Jagus RJ, Agüero MV, Chiralt A. Valorization of Beetroot Waste via Subcritical Water Extraction for Developing Active Food Packaging Materials. Molecules. 2025; 30(9):1928. https://doi.org/10.3390/molecules30091928
Chicago/Turabian Stylede Carvalho, Márcia Correa, Pedro A. V. Freitas, Rosa J. Jagus, María V. Agüero, and Amparo Chiralt. 2025. "Valorization of Beetroot Waste via Subcritical Water Extraction for Developing Active Food Packaging Materials" Molecules 30, no. 9: 1928. https://doi.org/10.3390/molecules30091928
APA Stylede Carvalho, M. C., Freitas, P. A. V., Jagus, R. J., Agüero, M. V., & Chiralt, A. (2025). Valorization of Beetroot Waste via Subcritical Water Extraction for Developing Active Food Packaging Materials. Molecules, 30(9), 1928. https://doi.org/10.3390/molecules30091928







