Abstract
Fluorene has been an extremely valued building block for many chemical compounds for a number of years. As a result, it is possible to design and obtain compounds with precisely defined physicochemical properties adapted to selected applications. An extremely interesting derivative of fluorene, which has been enjoying increasing interest in recent years, is dibenzofulvene (DBF) and its further structural modifications. So far, a number of dibenzofulvene derivatives have been described in the literature. Many of the presented DBFs are extremely structurally complex, which is why the influence of substituents on the physicochemical properties of the final compounds is not easy to determine unequivocally. Therefore, in this article, an attempt was made to explain the influence of N-donor substituents on selected physicochemical properties of dibenzofulvene derivatives (A-1–A-6). Moreover, these properties were compared to the results obtained for unsubstituted fluorene. The studies conducted showed that small modifications of the fluorene structure towards dibenzofulvene derivatives significantly change the absorption and emission properties of the final compounds. Importantly, the abovementioned structural modifications strongly affect the electrochemical properties, significantly reducing the energy gap and causing the oxidation potential to decrease to 0.18–0.42 V. Moreover, the process itself becomes fully reversible. The experimentally determined values coincide with those obtained theoretically via DFT calculations.
1. Introduction
The search for chemical compounds with precisely defined physicochemical properties that allow their use in organic electronics and photovoltaics has been the focus of attention of scientists for a very long time. However, designing and obtaining a chemical compound that will exhibit specific physicochemical properties and simultaneously be processable and cheap to synthesize is not the easiest task. Fluorene has enjoyed great interest for many years, even decades. It is difficult to even list some of the research topics in which fluorene has been studied. However, what distinguishes the aforementioned compound from other aromatic derivatives is the fact that it is an extremely versatile building block. Fluorene can be successfully used to synthesize many extremely useful chemical compounds. Moreover, it can be modified using many chemical reactions, making it possible to obtain new chemical compounds with physicochemical properties tailored to needs. Fluorene reacts very well with chemicals in positions 2, 7, and 9 (Figure 1).

Figure 1.
The most popular numbering of atomic positions in fluorene used in the synthesis of fluorene derivatives.
Positions 2 and 7 are often subjected to halogenation (bromination or iodination), and then to further reactions enabling the formation of the assumed derivatives [1,2,3]. In recent years, coupling reactions such as Suzuki, Sonogashira, and Buchwald–Hartwig have been very often used for this purpose [3,4,5,6,7,8,9]. Many reactions can also modify the 9-positions. In this case, a particularly interesting possibility is using the Knoevenagel condensation. This reaction allows the formation of a double bond at the 9-position of fluorene. Chemical compounds of this type are called dibenzofulvene derivatives (DBF) [10]. The second substrate necessary to carry out the aforementioned condensation is an aldehyde. Most often, aromatic or heteroaromatic aldehydes are used for this purpose, which allows the introduction of an additional substituent to the DBF structure that modifies the physicochemical properties of the final compound [10]. A wide selection of aromatic and heteroaromatic aldehydes available (even commercially) creates the possibility of obtaining an extremely wide group of dibenzofulvene derivatives. Moreover, the mentioned chemical compounds can be modified in positions 2 and 7, similar to fluorene [10]. Dibenzofulvene derivatives are becoming increasingly popular and not only because of their advantageous synthesis. Considering their attractive physicochemical properties, it is easy to indicate specific applications for which they have been studied in recent years. First of all, appropriately structurally modified dibenzofulvene derivatives are being studied for applications in solar cells [10,11,12,13,14,15,16,17,18,19,20,21]. In dye sensitized solar cells (DSSCs), dibenzofulvene derivatives are tested as organic dyes. Cells of this type using DBFs achieve an efficiency of up to 8% [10,11,12,13,21]. The second type of solar cells in which dibenzofulvene derivatives are studied are perovskite solar cells (PSCs) [10,14,15,16,17,18,19,20,21]. DBFs are tested as hole-transporting materials (HTM) in this type of cell. The considered PSC cells achieve efficiencies of up to 19–21% [10,14,15,16,17,18,19,20,21]. Interestingly, dibenzofulvene derivatives have been investigated as hole-transporting materials also in organic light-emitting diodes (OLEDs) [10,22]. Another important property of dibenzofulvene and its derivatives is the conjugated π-type bond system, which implies the ability of such compounds to undergo polymerization reactions under specific conditions. The polymerized DBF derivative is characterized by physicochemical properties, among which we can distinguish high thermal stability, high fluorescence efficiency, and appropriate charge transport [23,24,25,26,27,28,29,30,31]. These features are essential from the perspective of using dibenzofulvene derivatives in polymer light-emitting diodes (PLEDs) [10,23,25,32,33]. In addition, DBFs have been studied as highly efficient electrochromic materials capable of producing a wide range of colors [10,33].
Every year, we find more and more reports in the literature on dibenzofulvene derivatives studied for various applications. The compounds described in the latest publications are often very complex structurally, which makes it difficult to unequivocally assess the effect of individual substituents on the selected physicochemical properties. In this work, we focused on the impact of simple N-donor substituents on the physicochemical properties of dibenzofulvene derivatives. The compounds considered were compared in terms of optical and electrochemical properties. These properties are fundamental from the point of view of the applications for which DBFs are currently being studied. Moreover, the obtained research results were compared with the properties obtained for unsubstituted fluorene. Additionally, the experimental results obtained were compared using DFT calculations.
2. Result and Discussion
2.1. Synthesis
The studies began with the synthesis of the planned compounds. All dibenzofulvene derivatives (A-1–A-6) were obtained by Knoevenagel condensation according to an analogous procedure (Figure 2). The compounds’ synthesis began with preparing fluorene solutions in ethanol. Then, potassium tert-butoxide was added to them. The resulting mixtures were heated in an argon atmosphere for an hour. After this time, a three-fold excess of the appropriate aldehyde was added to each reaction mixture. Using an excess of aldehyde proved extremely beneficial because, in most experiments, the fluorene reacted completely. An excess of fluorene or using the exact proportions of fluorene and aldehyde resulted in difficult-to-separate post-reaction mixtures. After adding the necessary reagents, all reactions were heated at the boiling temperature of the reaction mixture, maintaining an argon atmosphere until the end of the experiments. The optimal time for all syntheses was 24 h. Shortening the reaction time resulted in a decrease in yield. On the other hand, the extension of the synthesis time did not positively affect the final results. The reaction products were isolated from the post-reaction mixtures and purified by extraction and column chromatography.
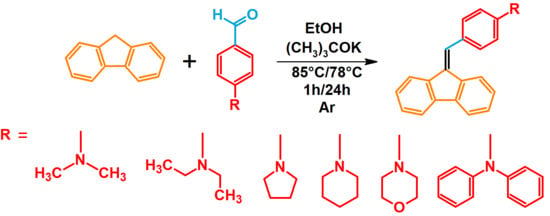
Figure 2.
Synthesis of dibenzofulvene derivatives (A-1–A-6) by Knoevenagel condensation.
The structures of the obtained dibenzofulvene derivatives (A-1–A-6) were confirmed by NMR spectroscopic methods (1H and 13C). The necessary spectra are included in the Supporting Information (Section S3—Figures S1–S12). The structures, yields, and photographs of the obtained compounds are presented in Table 1.

Table 1.
Structures, yields, and photos of derivatives A-1–A-6.
2.2. Optical Properties
Studies of dibenzofulvene derivatives (A-1–A-6) began with recording the UV–Vis spectra. The compounds were studied in several solvents differing in their dielectric constant. This is because the polarity of solvents can affect the energy levels of the ground and/or excited states and consequently shift the emission/absorbance of the compound to a lower/higher wavelength [34]. In addition, the spectra of unsubstituted fluorene (A-0) were recorded. All samples were measured in solutions with a 5 × 10−5 mol/L concentration. The collected data are presented in Figure S18 and Table 2.

Table 2.
Luminescence data of the tested compounds.
The results obtained showed that compound A-0 has two distinct absorption maxima at λ = 289 and 301 nm (Figure S18). These sharp absorption bands correspond to the π–π* transitions for fluorene [11]. Despite using solvents of different polarity, the position of the maximum absorption band (λabs) was essentially the same. This indicates the lack of solvatochromism for this compound. Moreover, it can be concluded that the optical gaps determined in different solvents for A-0 are the same because solvatochromism depends on the degree of charge separation in the molecule’s ground state. The results obtained for derivatives A-1–A-6 were analyzed successively. One broad absorption band in the range λ = 370–414 nm characterized them. The bands in the region of these wavelengths result from the intramolecular charge transfer (ICT) process. These molecules have similar absorption bands in the studied group. They also show similarity in this respect to structurally similar compounds described in the literature [11,34,35,36]. UV–VIS absorption studies of the entire group of derivatives showed that the change in solvent polarity had no significant effect. In turn, we see interesting relationships comparing the A-1–A-6 series with fluorene (A-0) (Figure 3a). Changing the structure of fluorene to dibenzofulvene and introducing an N-donor substituent to the structure of the compound results in a significant redshift of the absorption bands (around 76 nm—Figure S19 and Figure 3a). Moreover, this also changes the nature of the transitions of the last absorption band of these molecules. An important aspect of the research conducted was also the answer to the question of whether changing the nature of the N-donor substituent would affect the absorption bands. The presented derivatives show a wide range of λabs from 370–414 nm. As can be seen, the most red-shifted are A-2 and A-3. The most blue-shifted compound is A-5. It can be concluded that this is due to oxygen in the morpholine ring. This can be clearly seen when comparing A-5 with its analog A-4. The difference in the absorption band shift between these compounds is more than 10 nm. Similarly, when comparing A-1 with A-2, it is visible that the extension of the aliphatic chain causes a shift of the absorption maximum towards the red. Moreover, for compound A-0, emission maxima were determined in the 304–314 nm range. Similar values were obtained for fluorene derivatives [37]. All considered dibenzofulvene derivatives show weak fluorescence in solution relative to A-0. The emission quenching in compounds A-1–A-6 is probably due to the effective involvement of the ICT process [34]. Given the above, the emission of the tested compounds in the solid state was checked. It is clear from the obtained spectra that the modification of fluorene significantly affects the position of the emission bands (maximum shift by more than 100 nm—Figure 3b). The emission spectra in the solid state could not be recorded for compounds with an aliphatic chain.

Figure 3.
(a) Absorption spectrum of A-0–A-6 compounds recorded in ACN and (b) emission spectrum in the solid state.
2.3. DFT Calculations
The density functional theory (DFT) calculations were carried out using the Gaussian16 software package [38] B3LYP/6-311+G** basis set [39,40,41,42,43,44,45,46,47] to understand the structural property of the compounds. On analyzing the optimized structures of compounds A-1–A-6, it could be observed that they exhibited non-planar configurations (Figure S20). The dibenzofulvene fragments and the N-donor substituent are bent/twisted relative to the plane, where the double bond and the phenyl ring are located (Figure S20). The dihedral angles between the substituent and the double bond were 124° (A-1–A-4) and 123° (A-5–A-6), respectively. The conformations adopted by the derivatives constitute a structural compromise that minimizes steric hindrance and the π-coupling path. However, the twisted structure may affect the photoluminescence in the discussed compounds [11]. The molecular orbital distributions of the HOMO and LUMO structures of all molecules are given in Figure S20. The HOMO electron clouds are mainly located on the N-donor substituent due to their ability to donate electrons. On the other hand, the LUMO electron clouds are dominated by the dibenzofulvene fragment (Figure 4). This fact is most visible in the A-6 molecule (Figure S20). Moreover, the ionization potential (IP) and electron affinity (EA) were calculated for the entire presented group (Table 3). The IP energy values in the A-1–A-6 group ranged from −4.72 to −5.22 eV. Comparing the obtained values with A-0 (fluorene), for which this value is −6.22 eV, it can be stated that the modification of fluorene to substituted dibenzofulvene causes significant changes. The same conclusion can be drawn for the EA energy values. For derivatives A-1–A-6, these values range from −2.35 eV to −2.59 eV, while for A-0, these values are −1.32 eV. This directly impacts the energy gap values ranging from 2.13 eV to 2.80 eV for A-1–A-6. For comparison, the energy gap of A-0 is 4.90 eV (Figure 4). Changing fluorene to substituted dibenzofulvene significantly reduces the energy gap, which is promising in terms of the application of these compounds.
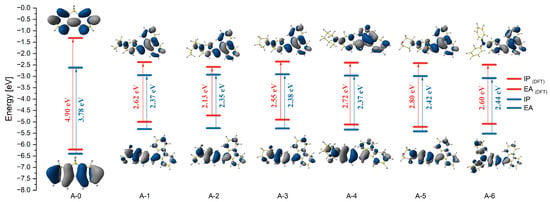
Figure 4.
Comparison of the energy levels of molecular orbitals A-0–A-6 obtained theoretically and experimentally.

Table 3.
IP, EA and energy gap values for A0–6.
In addition, UV–Vis absorption transitions were calculated using the TD-DFT method. These results suggest one major absorption band in the 350–500 nm range for A-1–A-6. The dominant calculated transition for the lowest energy absorption band for all derivatives is the S0 → S1 excitation. The significant increase in the oscillator strengths of this excitation can be attributed to the contribution of intramolecular charge transfer (ICT) transitions originating from charge delocalization from the substituent to the molecule’s core. Moreover, transitions that can be attributed to S0 → S2 excitations taking place within the core of the molecule are observed. The S0 → S2 transitions are characterized by very low oscillator strengths. UV–Vis transitions were simulated for the N-donor substituent group to make a more precise analysis of the absorption profile of the discussed compounds. Then, the absorption bands were assigned by comparing the calculated spectra of A-1–A-6 with those corresponding to their N-donor motifs (Figure S22). The bands appearing in the range of 200–350 nm show spectral features typical of the absorption of π–π* substituents. Compared to the free chromophores, only a small redshift is observed (Figure S22). Therefore, the absorption band in the range of 350–500 nm is caused by charge delocalization between the substituent and the molecule’s core. The simulated absorption spectra of the compounds showed good agreement with the experimental results (Figure S21).
2.4. Electrochemical and Spectroelectrochemical Properties
The electrochemical properties of the obtained compounds were investigated in MeCN solutions using cyclic voltammetry (CV). Using peak onset values determined from CV experiment allows for easy estimation of the molecules’ HOMO and LUMO energies (or, more precisely, the ionization potential (IP) and electron affinity (EA)) as well as the energy gap (Eg) (assuming the IP of ferrocene equals −5.1 eV) [48]. All the electrochemical parameters of the investigated compounds as well as the energy gaps obtained from CV (and DPV) measurements and DFT calculations are summarized in Table 3.
The studied compounds belong to the fluorene derivatives with potential applications in modern technologies, primarily as hole-transporting materials (HTMs). Usually, fluorene derivatives are substituted in phenyl rings or di-substituted in positions 9 and 9’. This means that the linking carbon has sp3 hybridization, and thus, the physicochemical properties of this type of fluorene compound are similar to biphenyl derivatives. However, in the case of the derivatives described in this work, a molecular architecture was used that allows the 9th carbon to obtain the sp2 configuration, thus enabling the planarity of this molecule fragment. As expected, the abovementioned modifications cause significant facilitation of the electrode processes and, therefore, lower the energy band gaps (see Table 3 and Figure 5). Reduction of compounds A-1–A-6 occurs at lower values of external potential (by about 0.4 V) in relation to A-0. Moreover, what is particularly important is that oxidation occurs at a potential between 0.18 (for A-2) and 0.42 (for A-6), i.e., with values similar to those observed for structurally similar dibenzofulvene-triphenylamine derivatives [16,18]. It is also worth emphasizing that in each case, the oxidation process becomes thermodynamically reversible, proving the molecule’s high stability after p-doping—derivatives without stabilizing substituents show low stability after oxidation [10].

Figure 5.
Cyclic voltammograms of the investigated compounds with sweep rate ν = 100 mV/s, 0.1 M Bu4NPF6 in MeCN.
The energy gaps for the entire series of compounds have similar values, and one can also notice the regularity of the influence of amine substituents. Substituents that strongly affect the decrease in the Eox value (i.e., increase the electron density) simultaneously make the reduction more difficult. This behavior can be seen best by comparing compounds A-2 and A-6. The first undergoes oxidation at the lowest potential (0.18 V), while it is reduced at −2.17 V. In turn, A-6 is the opposite, i.e., it is most difficult to oxidize (at 0.42 V), while the reduction occurs the easiest of all the investigated molecules (at −2.02 V). Moreover, since the change of the substituent affects both processes and, thus, indirectly, the energies of the HOMO and LUMO orbitals, it means that both orbitals (at least partially) are overlapping (see also Section 2.3 DFT calculations). Moreover, comparing the energy gaps determined by the electrochemical method with those calculated from the UV–Vis spectrum, it can be seen that the latter is overestimated for the entire series by about 0.3 V (see Table 3). This is a relatively small value (the higher this value would be, the greater the difficulty of charge flow within the molecule).
As mentioned earlier, the obtained derivatives show high stability of the oxidized forms. Therefore, a series of measurements using UV–Vis spectro-electrochemistry was performed (Figure 6 and Figure S23). Derivative A-2 was chosen as a representative example (Figure 6). This compound, in its neutral form, is characterized by one absorption band at the border of the visible range (peak maximum at 412 nm). This peak begins to disappear after exceeding the oxidation potential, while peaks appear and grow in the lower energy part of the spectrum. At a potential of +0.5 V, the highest value is obtained by the peak with a maximum at 492 nm. It is also worth noting the formation of a broad band covering the spectral region from 550 to 950 nm (with three local maxima). Such properties are similar to the properties of conducting polymers and result from the dispersion of positive charge within the conjugated system of double and single bonds. Therefore, this is a highly beneficial property from the point of view of further (potential) applications of the discussed group of compounds.
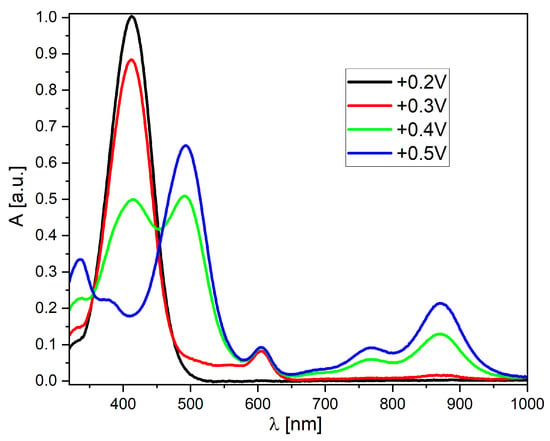
Figure 6.
UV–Vis spectro-electrochemistry of the A-2 derivative in MeCN solution (c = 1 × 10−5 mol/L, as an inset on each graph, all potentials vs. Fc/Fc+ redox couple).
3. Experimental Data—Materials, Methods, and Other Research Results
All experimental results in the form of spectra, thermograms, graphs, and photos confirming the research described in the article are documented in the Supporting Information (SI). In addition, the materials, methods, and experimental procedures used in the research are described in detail there [49]. The following solvent abbreviations are used in the article: Tol—toluene, CHCl3—chloroform, DCM—dichloromethane, MeOH—methanol, MeCN—acetonitrile.
4. Conclusions
As a result of the conducted studies, six dibenzofulvene derivatives containing selected N-donor substituents in their structure (A-1–A-6) were obtained. All compounds were synthesized via the simple Knoevenagel condensation. The considered derivatives were purified by column chromatography. The compounds tested in the A-1–A-6 group showed one distinct absorption band in the 299–414 nm range. It was shown that the change of the substituent in position 9 affects the position of the absorption band in the entire group of compounds. Compound A-5 was the most blue-shifted. It can be concluded that this is due to oxygen in the morpholine ring. Compounds A-2 and A-3 are the most red-shifted. Comparing the obtained group of dibenzofulvene derivatives with fluorene (A-0), it can be seen that the change in the structure of the compounds significantly affects the bathochromic shift of the absorption bands (approximately 76 nm). Moreover, A-0–A-6 did not show solvatochromism during the studies that were conducted. Moreover, dibenzofulvene derivatives did not show emission in solution, unlike A-0. Electrochemical tests showed significant facilitation of the electrode processes in the A-1–A-6 group, compared to A-0. The oxidation process for the entire group was thermodynamically reversible, which proves the high stability of the molecule after p-doping. The easiest to oxidize was A-2 (0.18 V) and the most difficult was A-6 (0.42 V). In the case of the reduction process, an opposite trend was observed. Substituents influencing the facilitation of the oxidation process hindered the reduction process. Experimental studies were enriched with theoretical results. As shown, the group of A-1–A-6 derivatives are characterized by a non-planar configuration of structures. On analyzing the distribution of frontier orbitals, we observe that the HOMO electron clouds are located mainly on the N-donor substituent. On the other hand, the LUMO electron clouds are dominated by the dibenzofulvene fragment. Modifying fluorene to substituted dibenzofulvene significantly influenced the obtained IP and EA values. The obtained energy gap values for A-1–A-6 (from 2.13 eV to 2.80 eV) were lower than for A-0 (4.90 eV). This makes the A-1–A-6 molecules promising in terms of application. Interestingly, the A-2 derivative was characterized by the smallest energy gap, and the A-5 compound by the largest one.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30091924/s1, Figure S1: 1H NMR of A-1; Figure S2: 13C NMR of A-1; Figure S3: 1H NMR of A-2; Figure S4: 13C NMR of A-2; Figure S5: 1H NMR of A-3; Figure S6: 13C NMR of A-3; Figure S7: 1H NMR of A-4; Figure S8: 13C NMR of A-4; Figure S9: 1H NMR of A-5; Figure S10: 13C NMR of A-5; Figure S11: 1H NMR of A-6; Figure S12: 13C NMR of A-6; Figure S13: DSC thermograms of A-1; Figure S14: DSC thermograms of A-3; Figure S15: DSC thermograms of A-4; Figure S16: DSC thermograms of A-5; Figure S17: DSC thermograms of A-6; Figure S18: Comparison of the absorption behavior of dibenzofulvene derivatives in different solvents; Figure S19: Comparison of the absorption properties of compounds A-1–A-6 with fluorene A-0; Figure S20: HOMO, LUMO and angles plots; Figure S21: Electronic spectra of A-1–A-6 in dichloromethane (pink line) alongside with national transition orbitals (black sticks) calculated for vertical excitations, which were assigned to the lowest energy absorption band; Figure S22: Comparison of simulated UV–Vis spectra of A-1–A-6 with their N-donor substituents; Figure S23: UV–Vis spectro-electrochemistry of the A-3 derivative in MeCN solution (c = 1 × 10−5 mol/L, as an inset on each graph, all potentials vs. Fc/Fc+ redox couple.
Author Contributions
Conceptualization, P.K., M.F. and S.K.; methodology, P.K., M.F., A.S.-K. and S.K.; validation, P.K.; formal analysis, P.K., M.F., A.S.-K. and S.K.; investigation, P.K., M.F., A.S.-K. and S.K.; resources, M.F. and S.K.; data curation, P.K.; writing—original draft preparation, P.K., M.F., A.S.-K. and S.K.; writing—review and editing, M.F. and S.K.; visualization, P.K., M.F., A.S.-K. and S.K.; supervision, M.F. and S.K.; project administration, M.F. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the Article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Calculations were carried out using resources provided by Wroclaw Centre for Networking and Supercomputing (https://wcss.pl) (accessed on 8 April 2025), grant No 18.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zhang, D.; Liu, Y.; Gao, H.; Chang, Q.; Cheng, X. α-Cyanostilbene and Fluorene Based Bolaamphiphiles: Synthesis, Self-Assembly, and AIEE Properties with Potential as White-Light Emissive Materials and Light-Emitting Liquid Crystal Displays. J. Mater. Chem. C 2020, 8, 17474–17481. [Google Scholar] [CrossRef]
- Islam, S.N.; Sil, A.; Patra, S.K. Achieving Yellow Emission by Varying the Donor/Acceptor Units in Rod-Shaped Fluorenyl-Alkynyl Based π-Conjugated Oligomers and Their Binuclear Gold (I) Alkynyl Complexes. Dalton Trans. 2017, 46, 5918–5929. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, H.; Ma, L.; Wu, W.; Liu, Y.; Zhao, J. Tuning the Photophysical Properties of N^N Pt(Ii) Bisacetylide Complexes with Fluorene Moiety and Its Applications for Triplet–Triplet-Annihilation Based Upconversion. J. Mater. Chem. 2012, 22, 5319. [Google Scholar] [CrossRef]
- Rouxel, C.; Charlot, M.; Mongin, O.; Krishna, T.R.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. From Graftable Biphotonic Chromophores to Water-Soluble Organic Nanodots for Biophotonics: The Importance of Environmental Effects. Chem. A Eur. J. 2012, 18, 16450–16462. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, B.; Zhang, S.; Jiang, C.; Zou, Y.; Li, S.; Yang, Y.; Yao, Z.; Wan, X.; Chen, Y. Side Chain Isomerization Enables High Efficiency and Thickness Tolerant Organic Solar Cells. J. Mater. Chem. A 2023, 11, 700–707. [Google Scholar] [CrossRef]
- Zhang, D.; Wu‡, T.; Xu, P.; Ou, Y.; Sun, A.; Ma, H.; Cui, B.; Sun, H.; Ding, L.; Hua, Y. Importance of Terminated Groups in 9,9-Bis(4-Methoxyphenyl)-Substituted Fluorene-Based Hole Transport Materials for Highly Efficient Organic–Inorganic Hybrid and All-Inorganic Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 10319–10324. [Google Scholar] [CrossRef]
- Jegorovė, A.; Daškevičienė, M.; Kantminienė, K.; Jankauskas, V.; Čepas, R.J.; Gruodis, A.; Getautis, V.; Genevičius, K. New Fluorene-Based Bipolar Charge Transporting Materials. RSC Adv. 2024, 14, 2975–2982. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Q.; Chen, Q.; Wang, Y.; Zhou, Y.; Song, B.; Yuan, N.; Ding, J.; Li, Y. High Efficiency Planar P-i-n Perovskite Solar Cells Using Low-Cost Fluorene-Based Hole Transporting Material. Adv. Funct. Mater. 2019, 29, 1900484. [Google Scholar] [CrossRef]
- Pasini, M.; Destri, S.; Porzio, W.; Botta, C.; Giovanella, U. Electroluminescent Poly(Fluorene-Co-Thiophene-S,S-Dioxide): Synthesis, Characterisation and Structure-Property Relationships. J. Mater. Chem. 2003, 13, 807–813. [Google Scholar] [CrossRef]
- Szlapa-Kula, A.; Ledwon, P.; Krawiec, A.; Kula, S. Dibenzofulvene Derivatives as Promising Materials for Photovoltaic and Organic Electronics. Energies 2023, 16, 8027. [Google Scholar] [CrossRef]
- Tigreros, A.; Rivera-Nazario, D.M.; Ortiz, A.; Martin, N.; Insuasty, B.; Echegoyen, L.A. Fluoren-9-Ylidene-Based Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2015, 2015, 5537–5545. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Giannuzzi, R.; Corrente, G.A.; De Marco, L.; Fabiano, E.; Cardone, A.; Gigli, G.; Ciccarella, G. Synthesis and Photovoltaic Performance of Dibenzofulvene-Based Organic Sensitizers for DSSC. Tetrahedron 2016, 72, 5788–5797. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, G.-W.; Chang, Y.-J.; Wen, J.-J. Branched Dibenzofulvene-Based Organic Dyes for Dye-Sensitized Solar Cells under One Sun and Dim Light. J. Mater. Sci: Mater. Electron. 2019, 30, 12981–12991. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Li, Y.-H.; Chung, C.-L.; Hsu, H.-L.; Chen, C.-P. Triphenylamine Dibenzofulvene–Derived Dopant-Free Hole Transporting Layer Induces Micrometer-Sized Perovskite Grains for Highly Efficient near 20% for p-i-n Perovskite Solar Cells. Prog. Photovolt. Res. Appl. 2020, 28, 49–59. [Google Scholar] [CrossRef]
- Lin, H.-C.; Chen, L.-Y.; Lu, C.-C.; Lai, J.-Y.; Chen, Y.-C.; Hung, Y.-J. Ambipolar Carrier Transport Properties of Triphenylamine/Dibenzofulvene Derivative and Its Application for Efficient n-i-p Perovskite Solar Cells. Org. Electron. 2021, 95, 106200. [Google Scholar] [CrossRef]
- Lin, S.-C.; Cheng, T.-H.; Chen, C.-P.; Chen, Y.-C. Structural Effect on Triphenylamine Dibenzofulvene Based Interfacial Hole Transporting Materials for High-Performance Inverted Perovskite Solar Cells. Mater. Chem. Phys. 2022, 288, 126385. [Google Scholar] [CrossRef]
- Mandati, S.; Juneja, N.; Katerski, A.; Jegorovė, A.; Grzibovskis, R.; Vembris, A.; Dedova, T.; Spalatu, N.; Magomedov, A.; Karazhanov, S.; et al. 4.9% Efficient Sb2S3 Solar Cells from Semitransparent Absorbers with Fluorene-Based Thiophene-Terminated Hole Conductors. ACS Appl. Energy Mater. 2023, 6, 3822–3833. [Google Scholar] [CrossRef]
- Sholihah, N.; Cheng, H.-C.; Wang, J.-C.; Ni, J.-S.; Yu, Y.-Y.; Chen, C.-P.; Chen, Y.-C. Passivation of Inverted Perovskite Solar Cells by Trifluoromethyl-Group-Modified Triphenylamine Dibenzofulvene Hole Transporting Interfacial Layers. J. Phys. Chem. C 2023, 127, 6167–6178. [Google Scholar] [CrossRef]
- Kotowicz, S.; Tavgeniene, D.; Beresneviciute, R.; Zaleckas, E.; Krucaite, G.; Katarzyna Pająk, A.; Korzec, M.; Grzegorz Małecki, J.; Lipiński, M.; Grigalevicius, S.; et al. Effect of Substituent Structure in Fluorene Based Compounds: Experimental and Theoretical Study. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2023, 300, 122832. [Google Scholar] [CrossRef]
- Sánchez, J.G.; Aktas, E.; Martínez-Ferrero, E.; Capodilupo, A.L.; Corrente, G.A.; Beneduci, A.; Palomares, E. Increasing the Stability of Perovskite Solar Cells with Dibenzofulvene-Based Hole Transporting Materials. Electrochim. Acta 2022, 432, 141190. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Liu, X.; Wang, Y.; Han, M.; Ghadari, R.; Wu, Y.; Ding, Y.; Cai, M.; Dai, S. Efficient Furan-Bridged Dibenzofulvene-Triphenylamine Hole Transporting Materials for Perovskite Solar Cells. Mater. Adv. 2023, 4, 515–522. [Google Scholar] [CrossRef]
- Nagar, M.R.; Choudhury, A.; Tavgeniene, D.; Beresneviciute, R.; Blazevicius, D.; Jankauskas, V.; Kumar, K.; Banik, S.; Ghosh, S.; Grigalevicius, S.; et al. Solution-Processable Phenothiazine and Phenoxazine Substituted Fluorene Cored Nanotextured Hole Transporting Materials for Achieving High-Efficiency OLEDs. J. Mater. Chem. C 2022, 10, 3593–3608. [Google Scholar] [CrossRef]
- Lu, B.; Chen, S.; Wang, J.; Xu, J.; Duan, X.; Pei, M. Soluble and Green-Light-Emitting Oligo(9-Fluorenylideneacetic Acid): Electrosynthesis and Characterization. Chin. J. Chem. 2012, 30, 1177–1184. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Gao, X.; Zhang, L.; Zhang, Q.; Chen, J. Highly Efficient Green PLED Based on Triphenlyaminesilole-Carbazole-Fluorene Copolymers with TPBI as the Hole Blocking Layer. Dye. Pigment. 2016, 127, 155–160. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Das, D.; Adil, L.R.; Iyer, P.K. Saturated and Stable White Electroluminescence from Linear Single Polymer Systems Based on Polyfluorene and Mono-Substituted Dibenzofulvene Derivatives. J. Phys. Chem. C 2017, 121, 18137–18143. [Google Scholar] [CrossRef]
- Giangregorio, M.M.; Gambino, S.; Fabiano, E.; Leoncini, M.; Cardone, A.; Corrente, G.A.; Beneduci, A.; Accorsi, G.; Gigli, G.; Losurdo, M.; et al. Synthesis and Investigation of Electro-Optical Properties of H-Shape Dibenzofulvene Derivatives. Molecules 2022, 27, 1091. [Google Scholar] [CrossRef]
- Wong, M.Y.; Leung, L.M. A Comprehensive Study of Substituent Effects on Poly(Dibenzofulvene)s. New J. Chem. 2017, 41, 512–520. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, L.; Lu, B.; Xu, J. Low-Potential Electrosynthesis of Novel Electroactive Poly(9-Fluorenemethanol) and Its Electrochromic and Blue-Light-Emitting Properties. Electrochim. Acta 2013, 90, 452–460. [Google Scholar] [CrossRef]
- He, Y.; Guo, W.; Pei, M.; Zhang, G. Electrosynthesis and Characterization of Poly(N-(9-Fluorenylmethoxycarbony)-Glycine). Chin. J. Chem. 2012, 30, 985–991. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T.; Fukuda, Y.; Yamaguchi, T.; Okumura, S. Free-Radical Polymerization of Dibenzofulvene Leading to a π-Stacked Polymer: Structure and Properties of the Polymer and Proposed Reaction Mechanism. Macromolecules 2005, 38, 8140–8148. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T. Synthesis, Structure, and Photophysical and Electrochemical Properties of a π-Stacked Polymer. J. Am. Chem. Soc. 2003, 125, 15474–15484. [Google Scholar] [CrossRef]
- Asiri, A.M.; Ahmed, S.A.; El-Daly, S.A.; Hussein, M.A.; Al-Soliemy, A.M.; Osman, O.I.; Shaaban, M.R.; Althagafi, I.I. Synthesis, Spectral Characteristics and DFT Studies of the New Dye 2,7-Diacetyl-9-((Dimethylamino)Methylene)-9H-Fluorene (DMMF) in Different Solvents. J. Fluoresc. 2015, 25, 1303–1314. [Google Scholar] [CrossRef]
- Corrente, G.A.; Fabiano, E.; Manni, F.; Chidichimo, G.; Gigli, G.; Beneduci, A.; Capodilupo, A.-L. Colorless to All-Black Full-NIR High-Contrast Switching in Solid Electrochromic Films Prepared with Organic Mixed Valence Systems Based on Dibenzofulvene Derivatives. Chem. Mater. 2018, 30, 5610–5620. [Google Scholar] [CrossRef]
- Majeed, S.; Waseem, M.T.; Junaid, H.M.; Khan, G.S.; Nawazish, S.; Mahmood, T.; Khan, A.M.; Shahzad, S.A. Aggregation Induced Emission Based Fluorenes as Dual-Channel Fluorescent Probes for Rapid Detection of Cyanide: Applications of Smartphones and Logic Gates. RSC Adv. 2022, 12, 18897–18910. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Iyer, P.K. Monosubstituted Dibenzofulvene-Based Luminogens: Aggregation-Induced Emission Enhancement and Dual-State Emission. J. Phys. Chem. C 2016, 120, 26556–26568. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Aldred, M.P.; Chen, Z.-Q.; Chen, T.; Meng, X.; Zhu, M.-Q. Efficient Green-Red Piezofluorochromism of Bisanthracene-Modified Dibenzofulvene. RSC Adv. 2015, 5, 1079–1082. [Google Scholar] [CrossRef]
- Sicard, L.; Quinton, C.; Peltier, J.-D.; Tondelier, D.; Geffroy, B.; Biapo, U.; Métivier, R.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. Spirobifluorene Regioisomerism: A Structure–Property Relationship Study. Chem. A Eur. J. 2017, 23, 7719–7727. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Ab Initio Study of Ionic Solutions by a Polarizable Continuum Dielectric Model. Chem. Phys. Lett. 1998, 286, 253–260. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum Solvation Models: A New Approach to the Problem of Solute’s Charge Distribution and Cavity Boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Feller, D. The Role of Databases in Support of Computational Chemistry Calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for Electronics and Spintronics. Chem. Soc. Rev. 2013, 42, 8895. [Google Scholar] [CrossRef]
- Majeed, S.; Junaid, H.M.; Waseem, M.T.; Khan, Z.A.; Khan, A.M.; Shahzad, S.A. Mechanochromic and AIE Active Fluorescent Probes for Solution and Vapor Phase Detection of Picric Acid: Application of Logic Gate. J. Photochem. Photobiol. A Chem. 2022, 432, 114057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).