In Situ Polymerization of Long Alkyl Chain Functional Groups Enhances the Oil–Water Separation Performance of Porous Organic Polymers
Abstract
1. Introduction
2. Results and Discussion
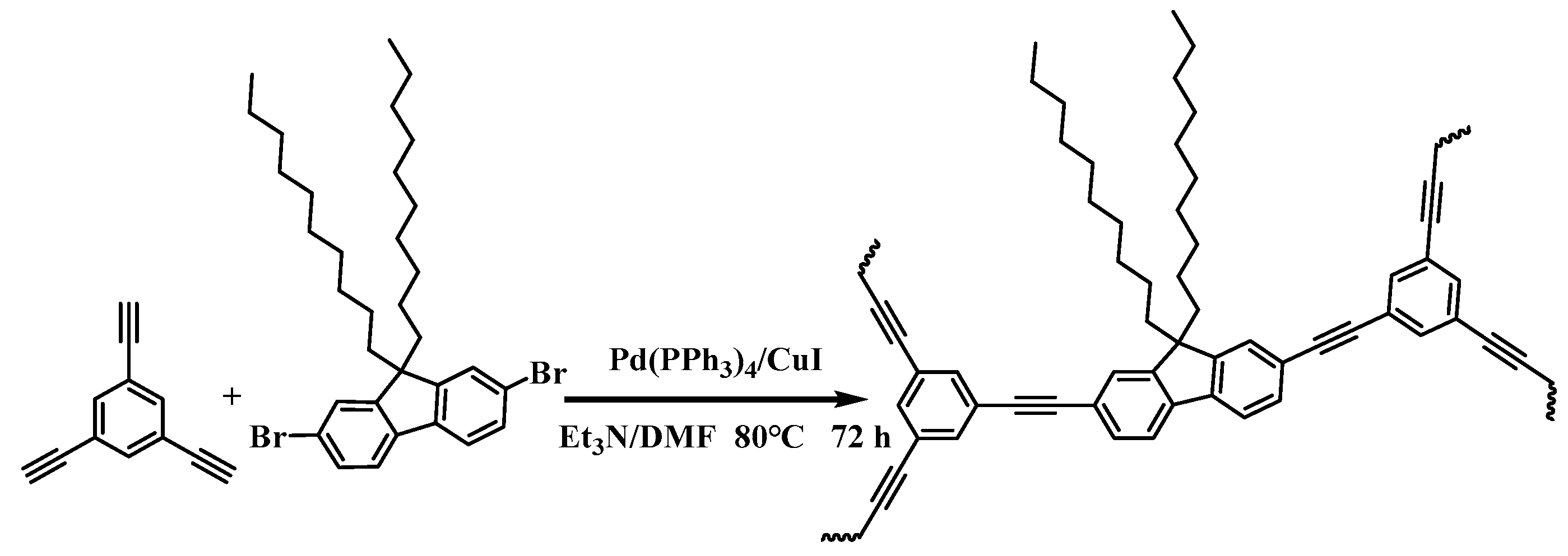
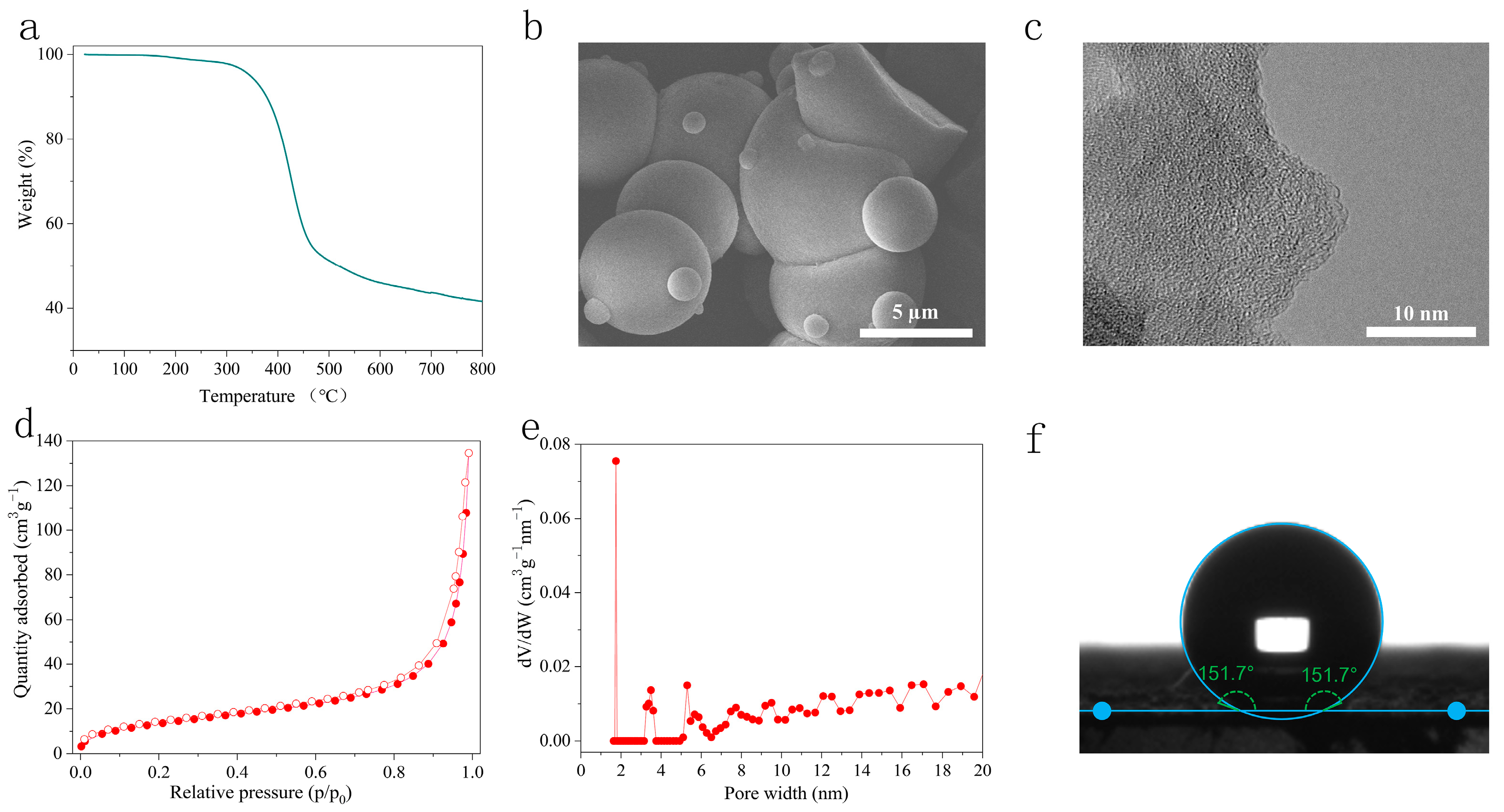
2.1. Structural Characterization of LNU-32
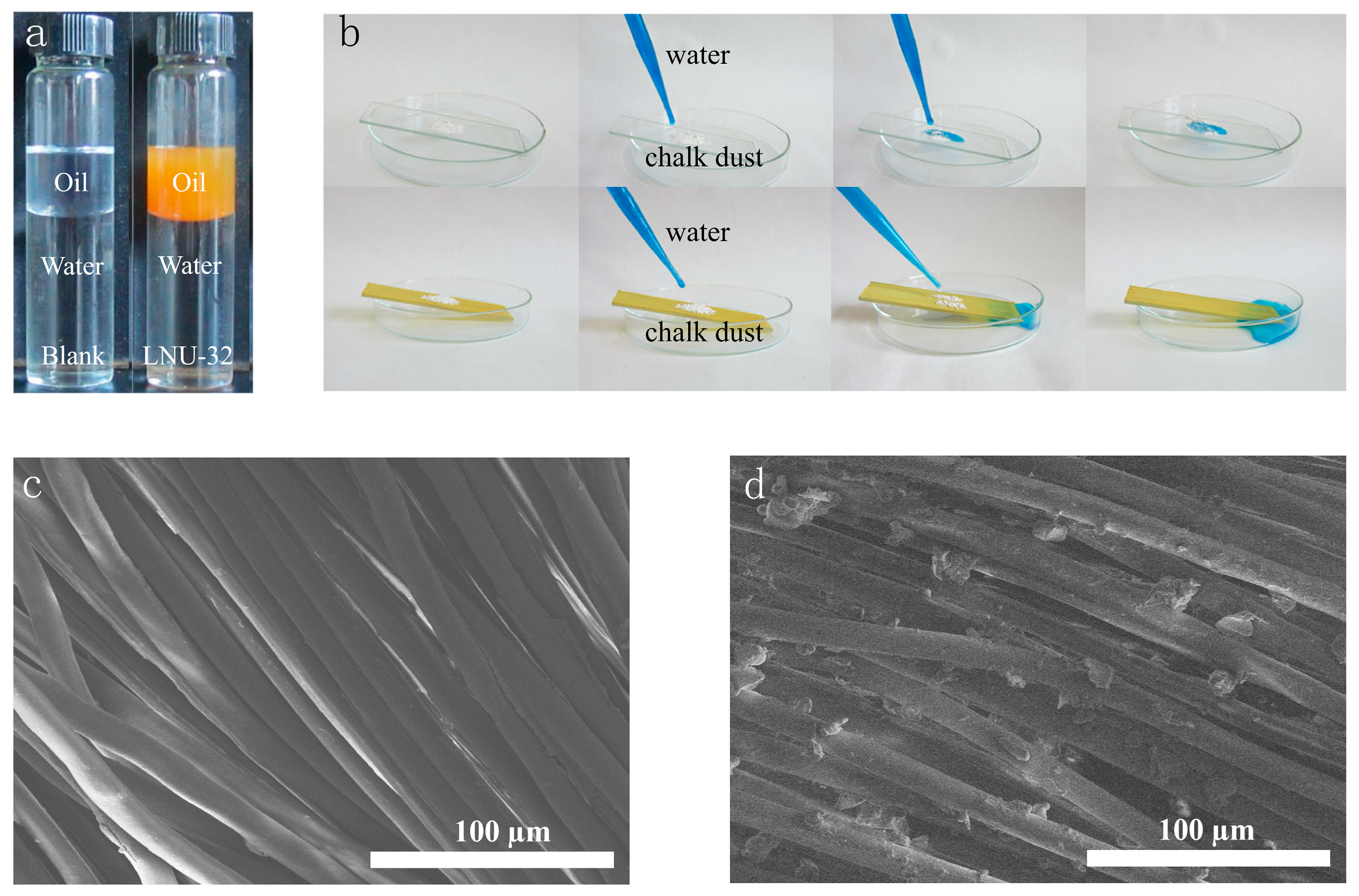
2.2. Superhydrophobicity of LNU-32
2.3. Self-Cleaning Performance of LNU-32
2.4. Performance of LNU-32-Located Superhydrophobic Fabrics
2.5. Oil–Water Separation Properties of Superhydrophobic Polyester Fabrics
2.6. Hydrophobic Properties of Modified Polyurethane Sponges
3. Materials and Methods
3.1. Materials
3.2. Synthesis of LNU-32
3.3. Characterization
3.4. Measurement of Water Contact Angle
3.5. Preparation of the Superhydrophobic Polyester Fabric
3.6. Oil Spill Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.P.; Yang, L.M.; Paul Chen, J.; Zheng, Y.M. Electrospun Spongy Zero-Valent Iron as Excellent Electro-Fenton Catalyst for Enhanced Sulfathiazole Removal by a Combination of Adsorption and Electro-Catalytic Oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Gore, P.M.; Naebe, M.; Wang, X.; Kandasubramanian, B. Silk Fibres Exhibiting Biodegradability & Superhydrophobicity for Recovery of Petroleum Oils from Oily Wastewater. J. Hazard. Mater. 2020, 389, 121823. [Google Scholar]
- Huang, P.; Wu, M.; Pang, Y.; Shen, B.; Wu, F.; Lan, X.; Luo, H.; Zheng, W. Ultrastrong, Flexible and Lightweight Anisotropic Polypropylene Foams with Superior Flame Retardancy. Compos. Part A Appl. Sci. Manuf. 2019, 116, 180–186. [Google Scholar] [CrossRef]
- Ianiro, A.; Wu, H.; Van Rijt, M.M.J.; Vena, M.P.; Keizer, A.D.A.; Esteves, A.C.C.; Tuinier, R.; Friedrich, H.; Sommerdijk, N.A.J.M.; Patterson, J.P. Liquid–Liquid Phase Separation during Amphiphilic Self-Assembly. Nat. Chem. 2019, 11, 320–328. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks for Molecular Recognition. ACS Cent. Sci. 2020, 6, 1082–1094. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Ma, X.; Meng, Q.; Wang, L.; Zhao, S.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks and Their Composite Components for Selective Extraction of Uranium Ions. Adv. Mater. 2018, 30, 1706507. [Google Scholar] [CrossRef]
- Xu, C.-L.; Wang, Y.-Z. Self-Assembly of Stearic Acid into Nano Flowers Induces the Tunable Surface Wettability of Polyimide Film. Mater. Des. 2018, 138, 30–38. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y. One-Step Approach to the Growth of ZnO Nano-/Microrods on Cellulose toward Its Durable Superhydrophobicity. Adv. Mater. Interfaces 2017, 4, 1700550. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z.; Liu, W. A Robust Epoxy Resins @ Stearic Acid-Mg(OH)2 Micronanosheet Superhydrophobic Omnipotent Protective Coating for Real-Life Applications. ACS Appl. Mater. Interfaces 2016, 8, 16511–16520. [Google Scholar] [CrossRef]
- Shome, A.; Das, A.; Borbora, A.; Dhar, M.; Manna, U. Role of Chemistry in Bio-Inspired Liquid Wettability. Chem. Soc. Rev. 2022, 51, 5452–5497. [Google Scholar] [CrossRef]
- Han, N.; Zhang, Z.; Gao, H.; Qian, Y.; Tan, L.; Yang, C.; Zhang, H.; Cui, Z.; Li, W.; Zhang, X. Superhydrophobic Covalent Organic Frameworks Prepared via Pore Surface Modifications for Functional Coatings under Harsh Conditions. ACS Appl. Mater. Interfaces 2020, 12, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liang, M.; Yue, X.; Tian, K.; Liu, D.; Qiao, X. Superhydrophobic Conjugated Microporous Polymers Grafted Silica Microspheres for Liquid Chromatographic Separation. J. Chromatogr. A 2020, 1631, 461539. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zheng, X.; You, Y.; Zhang, S.; Zhao, B.; Wang, X.; Huang, G.; Chen, T.; Cao, Y.; He, L.; et al. Comprehensive Chemical Characterization of Gaseous I/SVOC Emissions from Heavy-Duty Diesel Vehicles Using Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry. Environ. Pollut. 2022, 305, 119284. [Google Scholar] [CrossRef]
- Verkoyen, P.; Frey, H. Long-Chain Alkyl Epoxides and Glycidyl Ethers: An Underrated Class of Monomers. Macromol. Rapid Commun. 2020, 41, 2000225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, S.; Yang, L.; Zhang, Y.; Dolfing, J.; Sun, Y.; Liu, L.; Li, Q.; Tu, B.; Dai, L.; et al. Preferential Degradation of Long-Chain Alkyl Substituted Hydrocarbons in Heavy Oil under Methanogenic Conditions. Org. Geochem. 2019, 138, 103927. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Shu, X.; Dong, L.; Qiao, M.; Ran, Q. Micelle Conformation of Sodium Alkyl Sulfate Surfactants with Different Hydrophobic Chain Length: A Molecular Dynamics Study. Comput. Mater. Sci. 2023, 229, 112452. [Google Scholar] [CrossRef]
- Naga, N.; Miyanaga, T.; Wang, Y.; Nakano, T. Synthesis and properties of σ-π conjugated porous polymers obtained with Mizoroki–Heck reaction of tetra vinyl cyclic siloxane with dibromo fluorene. J. Polym. Sci. 2020, 58, 2301–2309. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and Superoleophilic Membranes for Oil-Water Separation Application: A Comprehensive Review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Ruan, X.; Yang, Y.; Liu, W.; Ma, X.; Zhang, C.; Meng, Q.; Wang, Z.; Cui, F.; Feng, J.; Cai, F.; et al. Mechanical Bond Approach to Introducing Self-Adaptive Active Sites in Covalent Organic Frameworks for Zinc-Catalyzed Organophosphorus Degradation. ACS Cent. Sci. 2021, 7, 1698–1706. [Google Scholar] [CrossRef]
- Meng, Q.; Qiao, P.; Deng, D.; Zhang, C.; Cui, F.; Ruan, X.; Yang, Y.; Cao, J.; Wang, Z.; Ma, X.; et al. Modulating Hetero-Multimetallic Atoms in Covalent Organic Frameworks for Efficient Oxidization of Olefin Compounds. Chem 2025, 11, 102334. [Google Scholar] [CrossRef]
- Nishijima, A.; Kametani, Y.; Uemura, T. Reciprocal Regulation between MOFs and Polymers. Coord. Chem. Rev. 2022, 466, 214601. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, M.H.; Kim, K.B. Synthesis of Bulk Amorphous Composites with Three Amorphous Phases by Consolidation of Milled Amorphous Powders. Intermetallics 2010, 18, 2019–2023. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Ding, L.; Pan, C.; Xu, Y.; Wang, T.; Wang, J.; Pei, J. Fine-tuning the Backbone Conformation of Conjugated Polymers and the Influence on Solution Aggregation and Optoelectronic Properties. J. Polym. Sci. 2023, 61, 951–958. [Google Scholar] [CrossRef]
- Özlem, S.; Hacaloglu, J. Thermal Degradation of Poly (n-Butyl Methacrylate), Poly (n-Butyl Acrylate) and Poly (t-Butyl Acrylate). J. Anal. Appl. Pyrolysis 2013, 104, 161–169. [Google Scholar] [CrossRef]
- Elzaabalawy, A.; Verberne, P.; Meguid, S.A. Multifunctional Silica–Silicone Nanocomposite with Regenerative Superhydrophobic Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 42827–42837. [Google Scholar] [CrossRef]
- Echavarren, J.; Gall, M.A.Y.; Haertsch, A.; Leigh, D.A.; Spence, J.T.J.; Tetlow, D.J.; Tian, C. Sequence-Selective Decapeptide Synthesis by the Parallel Operation of Two Artificial Molecular Machines. J. Am. Chem. Soc. 2021, 143, 5158–5165. [Google Scholar] [CrossRef]
- Wu, X.; Hong, Y.; Xu, B.; Nishiyama, Y.; Jiang, W.; Zhu, J.; Zhang, G.; Kitagawa, S.; Horike, S. Perfluoroalkyl-Functionalized Covalent Organic Frameworks with Superhydrophobicity for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2020, 142, 14357–14364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Cao, D.; Song, Y.; Zheng, Y.; Cao, J.; Chen, W.; Yuan, Y.; Gao, N.; Yang, Y. Preparation of Meso-Porous Aromatic Frameworks for Rapid Ion Extraction from High Salt and Corrosion Environments. J. Mater. Chem. A 2024, 12, 17270–17276. [Google Scholar] [CrossRef]
- Yan, Z.; Qiao, Y.; Sun, Q.; Cui, B.; Feng, B.; Bu, N.; Chu, K.; Ruan, X.; Yuan, Y.; Yang, Y.; et al. Introducing Polar Groups in Porous Aromatic Framework for Achieving High Capacity of Organic Molecules and Enhanced Self-Cleaning Applications. Molecules 2022, 27, 6113. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, R.; Gong, J.; Zhang, Z.; Zhang, L.; Qiu, Z.; Alam, P.; Zhang, J.; Liu, Y.; Li, Y.; et al. Unveiling the Role of Alkyl Chain in Boosting Antibacterial Selectivity and Cell Biocompatibility. JACS Au 2025, 5, 675–683. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, H.; Jiang, Z.; Zhao, Y.; Sun, Y.; Zhong, C. Superhydrophobic Conjugated Porous Organic Polymer Coated Polyurethane Sponge for Efficient Oil/Water Separation. J. Porous Mater. 2022, 29, 433–444. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, B.; Zheng, X.; Zheng, J.; Zhu, C.; Wu, M. Hydrophobic and Fire-Resistant Carbon Monolith from Melamine Sponge: A Recyclable Sorbent for Oil–Water Separation. Carbon 2015, 84, 551–559. [Google Scholar] [CrossRef]
- Du, W.; Wang, T.; Xie, Z.; Xia, L.; Lu, J.; Li, P. Superhydrophobic SiO2 Microspheres of a Porous Dehydroabietic-Acid-Based Homopolymer for Oil–Water Separation. Sep. Purif. Technol. 2024, 340, 126838. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, J.; Ren, Y.; Xuan, C.; Liu, C.; Shen, C. Superhydrophobic and Superoleophilic Porous Reduced Graphene Oxide/Polycarbonate Monoliths for High-Efficiency Oil/Water Separation. J. Hazard. Mater. 2018, 344, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, J.; Wang, J.; Zhang, X.; Wei, L.; Du, Y.; Yu, B.; Ye, S. Superhydrophobic Metal Organic Framework Doped Polycarbonate Porous Monolith for Efficient Selective Removal Oil from Water. Chemosphere 2020, 260, 127583. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Guo, X.; Zhong, C. Superhydrophobic Ether-Based Porous Organic Polymer-Coated Polyurethane Sponge for Highly Efficient Oil–Water Separation. Ind. Eng. Chem. Res. 2020, 59, 13228–13238. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Geyer, F.; Petr, M.; Zboril, R.; Vollmer, D.; Fischer, R.A. Shape Controlled Hierarchical Porous Hydrophobic/Oleophilic Metal-Organic Nanofibrous Gel Composites for Oil Adsorption. Adv. Mater. 2017, 29, 1605307. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Datta, K.K.R.; Rösler, C.; Petr, M.; Otyepka, M.; Zboril, R.; Fischer, R.A. Biomimetic Superhydrophobic/Superoleophilic Highly Fluorinated Graphene Oxide and ZIF-8 Composites for Oil–Water Separation. Angew. Chem. Int. Ed. 2016, 55, 1178–1182. [Google Scholar] [CrossRef]
- Gong, L.; Wu, W.; Lin, D.; Yang, K. A Superhydrophobic and Porous Polymer Adsorbent with Large Surface Area. J. Mater. Chem. A 2021, 9, 254–258. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, D.-H.; Kim, S.; Kim, H.-J.; Park, T.J.; Kim, K.B.; Park, Y.M. Synergistic Enhancement of Hydrogel Adhesion via Tough Chemical Bonding and Physical Entanglements. Polym. Test. 2022, 107, 107482. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, T.; Cui, Z.; Kang, L.; Li, J.; Cai, Y.; Tian, D. Facile Fabrication of Hydrophobic Covalent Organic Framework Decorated Melamine Sponge for Efficient Separation of Immiscible Oil-Water Mixture and Water-in-Oil Emulsion. J. Environ. Chem. Eng. 2023, 11, 110337. [Google Scholar] [CrossRef]

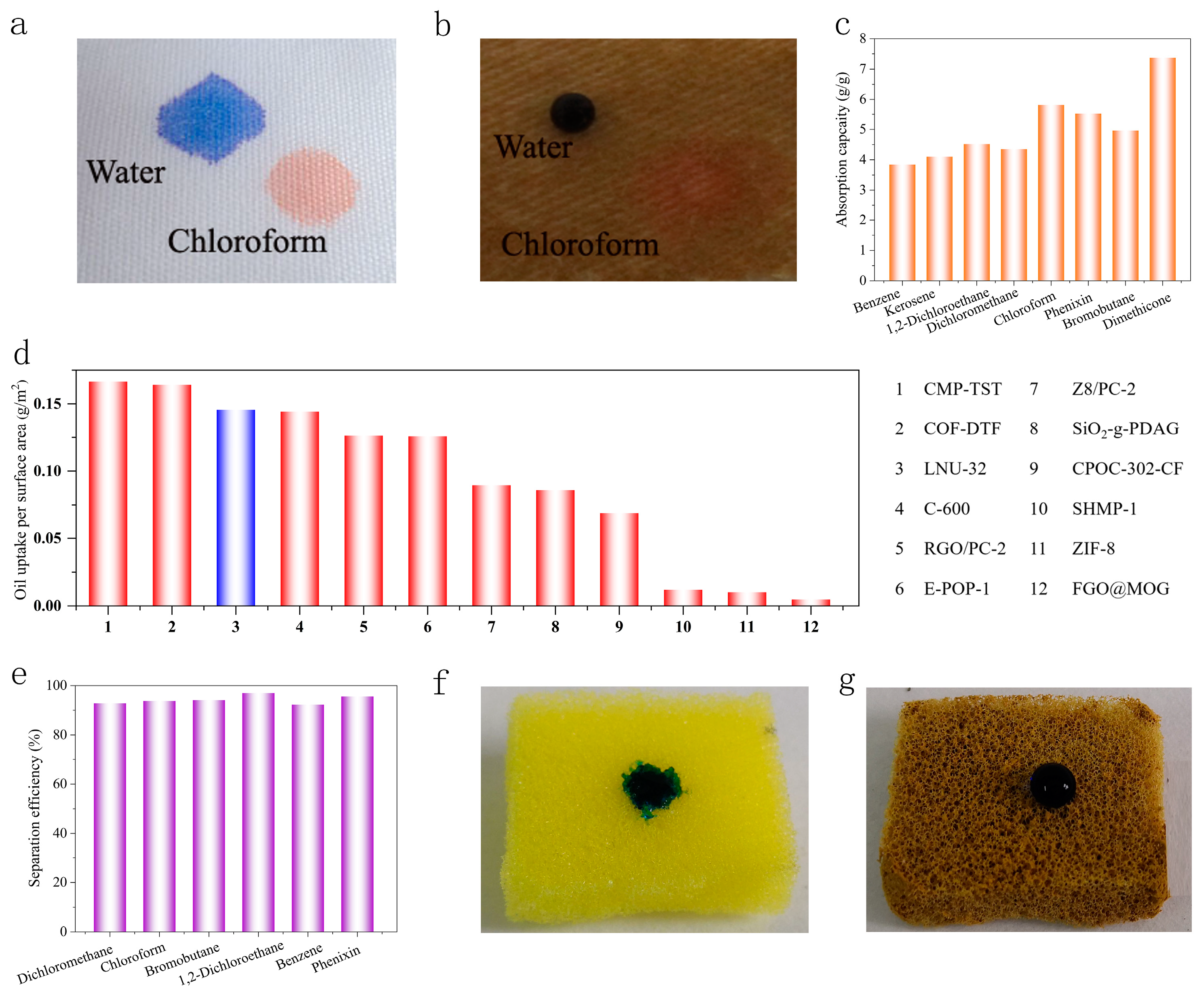
| The Name of the Material | Specific Surface Area (m2/g) | Oil Uptake per Surface Area (g/m2) | Structure |
|---|---|---|---|
| CMP-TST | 423.3 | 0.1665 | 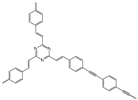 |
| COF-DTF | 1056.26 | 0.164 | 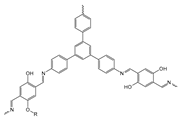 |
| LNU-32 | 50.6208 | 0.1456 | 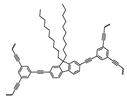 |
| C-600 | 686 | 0.1443 | – |
| RGO/PC-2 | 137.19 | 0.1262 | – |
| E-POP-1 | 652.3 | 0.1257 |  |
| Z8/PC-2 | 146.84 | 0.0895 | 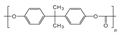 |
| SiO2-g-PDAG | 70.9 | 0.0859 |  |
| CPOC-302-CF | 1782 | 0.069 |  |
| SHMP-1 | 2100 | 0.0119 |  |
| ZIF-8 | 590 | 0.0102 |  |
| FGO@MOG | 1036 | 0.0048 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Cai, S.; Hua, R.; Li, C.; Xia, C.; Cui, B.; Shao, H.; Bu, N.; Yuan, Y. In Situ Polymerization of Long Alkyl Chain Functional Groups Enhances the Oil–Water Separation Performance of Porous Organic Polymers. Molecules 2025, 30, 1925. https://doi.org/10.3390/molecules30091925
Zhao H, Cai S, Hua R, Li C, Xia C, Cui B, Shao H, Bu N, Yuan Y. In Situ Polymerization of Long Alkyl Chain Functional Groups Enhances the Oil–Water Separation Performance of Porous Organic Polymers. Molecules. 2025; 30(9):1925. https://doi.org/10.3390/molecules30091925
Chicago/Turabian StyleZhao, Hongbo, Shijie Cai, Ruoting Hua, Cong Li, Chunlong Xia, Bo Cui, Huimin Shao, Naishun Bu, and Ye Yuan. 2025. "In Situ Polymerization of Long Alkyl Chain Functional Groups Enhances the Oil–Water Separation Performance of Porous Organic Polymers" Molecules 30, no. 9: 1925. https://doi.org/10.3390/molecules30091925
APA StyleZhao, H., Cai, S., Hua, R., Li, C., Xia, C., Cui, B., Shao, H., Bu, N., & Yuan, Y. (2025). In Situ Polymerization of Long Alkyl Chain Functional Groups Enhances the Oil–Water Separation Performance of Porous Organic Polymers. Molecules, 30(9), 1925. https://doi.org/10.3390/molecules30091925








