A Comprehensive Review of the Phytochemistry and Therapeutic Efficacy of Viola yedoensis Makino
Abstract
1. Introduction
2. Botany
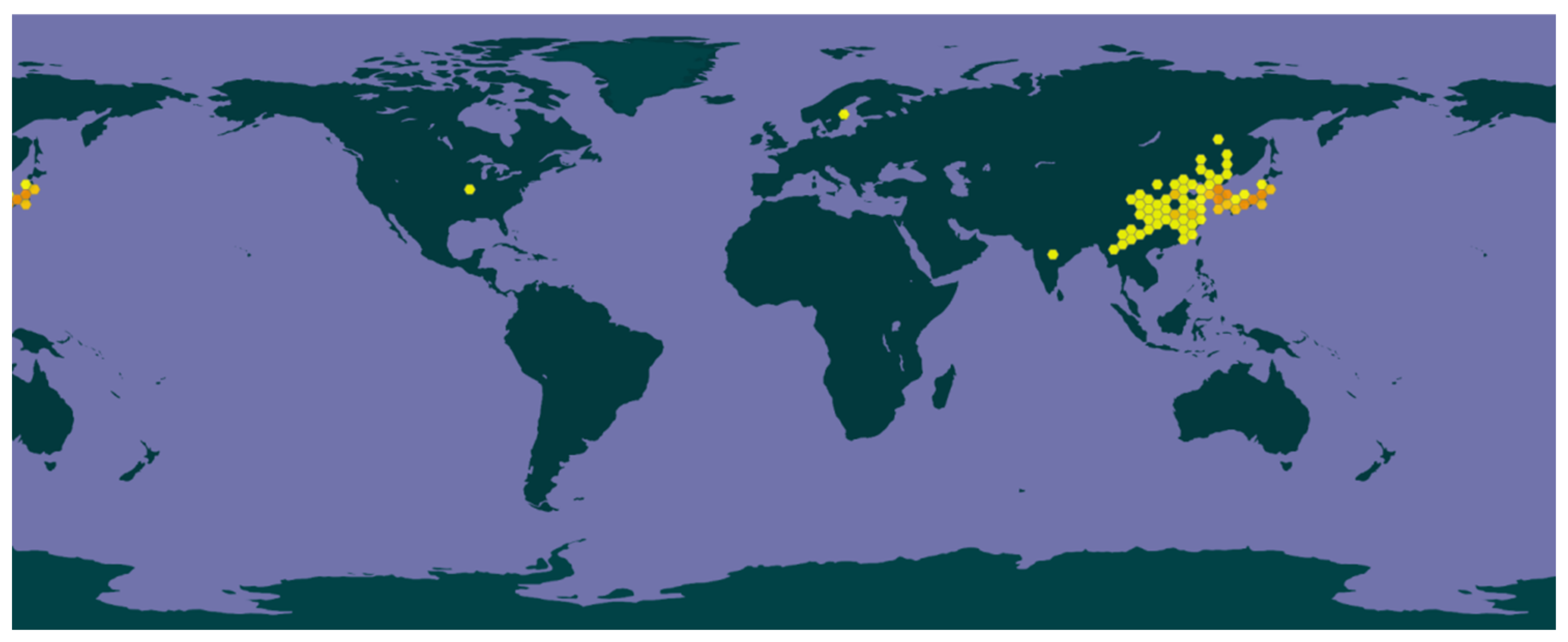
2.1. Plant Sources
2.2. Plant Traits
3. Traditional Uses
4. Phytochemistry
4.1. Flavonoids
4.2. Coumarins
4.3. Terpenoids
4.4. Phenolic Acids
4.5. Alkaloids
4.6. Cyclotides
4.7. Other Compounds
5. Pharmacology
5.1. Anti-Inflammatory Effects
5.2. Anti-Pyretic Effects
5.3. Anti-Virus Effects
5.4. Anti-Cancer Effects
5.5. Anti-Lung Injury Effects
5.6. Anti-Liver Injury Effects
5.7. Anti-Bacterial Effects
5.8. Other Effects
6. Quality Control
7. Potential and Actual Applications
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.Y.; Hong, J.L.; Shu, P.; Ni, Y.J.; Qin, M.J. A new dicoumarin and anticoagulant activity from Viola yedoensis Makino. Fitoterapia 2009, 80, 283–285. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, S.S.; Zhang, N.; Yang, D.W.; Han, H.R.; Wei, K.H.; Li, M.H. Chemical Constituents and Biological Activities of Plants from the Genus Viola. Chem. Biodivers. 2015, 12, 1777–1808. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kokubun, T.; Houghton, P.J.; Simmonds, M.S. Antibacterial activity of the Chinese traditional medicine, Zi Hua Di Ding. Phytother. Res. 2004, 18, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Veitch, N.C.; Houghton, P.J.; Simmonds, M.S. Flavone C-glycosides from Viola yedoensis MAKINO. Chem. Pharm. Bull. 2003, 51, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Narukawa, Y.; Takeda, T.; Kiuchi, F. Collagenase inhibitors from Viola yedoensis. J. Nat. Med. 2013, 67, 240–245. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, L.; Li, M.Y.; Wang, L.W.; Ma, C.M. Lignans, flavonoids and coumarins from Viola philippica and their α-glucosidase and HCV protease inhibitory activities. Nat. Prod. Res. 2019, 33, 1550–1555. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Publisher: Beijing, China, 2020; Volume 1, p. 352.
- Xia, Y.; Luo, X.Z.; Xie, C. Chemical constituents from Viola yedoensis. Chem. Nat. Compd. 2010, 46, 809–810. [Google Scholar] [CrossRef]
- Du, D.; Cheng, Z.; Chen, D. Anti-complement sesquiterpenes from Viola yedoensis. Fitoterapia 2015, 101, 73–79. [Google Scholar] [CrossRef]
- Yu, L.; Pan, H.; Chen, X.; Gong, S.; Zhang, Q.; Zhang, Y.; Zhan, Z. Comprehensive Mapping of Cyclotides from Viola philippica by Using Mass Spectrometry-Based Strategy. Molecules 2024, 29, 4344. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Chen, S. A comprehensive review of phytochemistry, pharmacology and quality control of plants from the genus Viola. J. Pharm. Pharmacol. 2023, 75, 1–32. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Aleem, A.; Khan, H. Ethnomedicinal, phytochemical and pharmacological profile of genus Viola. Phytopharmacology 2012, 3, 214–226. [Google Scholar]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Shin, H.; Lee, K.Y.; Ma, J.Y. Anti-inflammatory effects of Viola yedoensis and the application of cell extraction methods for investigating bioactive constituents in macrophages. BMC Complement. Altern. Med. 2016, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhao, B.; Zhang, D.; Rui, X.; Hou, X.; Chen, X.; Zhang, B.; Yuan, Y.; Deng, H.; Ge, G. Viola yedoensis Makino formula alleviates DNCB-induced atopic dermatitis by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization. Phytomedicine 2022, 103, 154228. [Google Scholar] [CrossRef]
- Chu, H.; Wang, J.; Wang, Q.; Chen, J.; Li, J.; Li, H.; Zhang, L. Protective Effect of n-Butanol Extract from Viola yedoensis on Immunological Liver Injury. Chem. Biodivers. 2021, 18, e2001043. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Colgrave, M.L.; Gustafson, K.R.; Ireland, D.C.; Goransson, U.; Craik, D.J. Anti-HIV cyclotides from the Chinese medicinal herb Viola yedoensis. J. Nat. Prod. 2008, 71, 47–52. [Google Scholar] [CrossRef]
- Ying-Yi, P.A.N.; Song, Z.P.; Zhu, G.F.; Zhu, Y.Z.; Lu, Y.; Li, W.L.; Cheng, Z.H. Antipyretic effects of liposoluble fractions of Viola yedoensis. Chin. Herb. Med. 2015, 7, 80–87. [Google Scholar] [CrossRef]
- Yang, Y.N.; Zhan, J.G.; Cao, Y.; Wu, C.M. From ancient wisdom to modern science: Gut microbiota sheds light on property theory of traditional Chinese medicine. J. Integr. Med. 2024, 22, 413–444. [Google Scholar] [CrossRef]
- Qu, S.; Qiao, M.; Wang, J.; Gao, M.; Chen, D.; Li, S.; Wei, E.; Guo, Y. Network Pharmacology and Data Mining Approach Reveal the Medication Rule of Traditional Chinese Medicine in the Treatment of Premenstrual Syndrome/Premenstrual Dysphoric Disorder. Front. Pharmacol. 2022, 13, 811030. [Google Scholar] [CrossRef]
- Lim, C.; Lim, S.; Lee, B.; Kim, B.; Cho, S. Effect of methanol extract of Salviae miltiorrhizae Radix in high-fat diet-induced hyperlipidemic mice. Chin. Med. 2017, 12, 29. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Lukman, H.Y.; Shaheen, H.M.; Wasef, L.; Hafiz, A.A.; Conte-Junior, C.A.; Lawal, B. A systematic review of phytochemistry, nutritional composition, and pharmacologic application of species of the genus Viola in noncommunicable diseases (NCDs). Evid. Based Complement. Altern. Med. 2023, 1, 5406039. [Google Scholar] [CrossRef]
- Fan, P.; Yang, Y.; Liu, T.; Lu, X.; Huang, H.; Chen, L.; Kuang, Y. Anti-atopic effect of Viola yedoensis ethanol extract against 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin dysfunction. J. Ethnopharmacol. 2021, 280, 114474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Qin, M.J.; Hong, J.L.; Ni, Y.J.; Wu, G. Chemical constituents of Viola yedoensis. Chin. J. Nat. Med. 2009, 7, 290–292. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, Z.F.; Li, Y.; Qi, C.; Wang, Y.; Yao, Q.Q. Analysis of Chemical Constituents in Violae Herba and Investigation of Its Antioxidant Activity in Vitro. Chin. J. Exp. Tradit. Med. Formulae 2021, 24, 117–123. [Google Scholar]
- Liu, H.; Hu, W.; Fang, Y. Chemical constituents in ethyl acetate fraction from Viola yedoensis. Anhui Med. Pharm. J. 2015, 19, 1068–1071. [Google Scholar]
- Xu, J.; Zeng, S.; Qu, H. Chemical constituents from Viola yedoensis. Chin. Trad. Herb. Drugs 2010, 41, 1423–1425. [Google Scholar]
- Cao, J.; Qin, Y.; Yin, C.L.; Cheng, Z.H. Chemical constituents of Viola yedoensis and their antioxidant activity. Chin. J. Exp. Trad. Med. Form 2013, 19, 282–286. [Google Scholar]
- Zeng, H.R.; Wang, B.; Zhao, Z.; Zhang, Q.; Liang, M.Y.; Yao, Y.Q.; Bian, K.; Zhang, W.R. Effects of Viola yedoensis Makino anti-itching compound on degranulation and cytokine generation in RBL-2H3 mast cells. J. Ethnopharmacol. 2016, 189, 132–138. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Xue, Q.; Yu, L.; Zhang, D. Studies on chemical constituents from herbs of Viola yedoensis. Zhongguo Zhong Yao Za Zhi 2009, 34, 1114–1116. [Google Scholar]
- Sun, Y.; Du, L.; Zhou, L.; Zhang, W.; Miao, F.; Yang, X.; Geng, H. Study on antibacterial active components from Viola yedoensis. Zhongguo Zhong Yao Za Zhi 2011, 36, 2666–2671. [Google Scholar]
- Du, D.S.; Cheng, Z.H.; Chen, D.F. Anti-complement alkaloids from whole plants of Viola yedoensis. Zhongguo Zhong Yao Za Zhi 2017, 42, 4794–4800. [Google Scholar]
- Yang, P.P.; Yan, F.L.; Liang, Y.B. Study on chemical constituents of Viola yedoensis. J. Xinxiang Med. Univ. 2008, 25, 185–187. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhu, R.; Han, W.; Han, H.; Ding, L. A multi-wavelength cross-reactive fluorescent sensor ensemble for fingerprinting flavonoids in serum and urine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123893. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhou, D. Synthesis and Antiallergic Activity of Dicoumarin Derivatives. Molecules 2024, 29, 3799. [Google Scholar] [CrossRef]
- Gupta, D.; Guliani, E.; Bajaj, K. Coumarin-Synthetic Methodologies, Pharmacology, and Application as Natural Fluorophore. Top. Curr. Chem. 2024, 382, 16. [Google Scholar] [CrossRef]
- Mitsiou, V.M.; Antonaki, A.N.; Douka, M.D.; Litinas, K.E. An Overview on the Synthesis of Lamellarins and Related Compounds with Biological Interest. Molecules 2024, 29, 4032. [Google Scholar] [CrossRef]
- Bergman, M.E.; Kortbeek, R.W.J.; Gutensohn, M.; Dudareva, N. Plant terpenoid biosynthetic network and its multiple layers of regulation. Prog. Lipid Res. 2024, 95, 101287. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications-A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Zagórska, J.; Michalak-Tomczyk, M.; Karav, S.; Wawruszak, A. Plant Phenolics in the Prevention and Therapy of Acne: A Comprehensive Review. Molecules 2024, 29, 4234. [Google Scholar] [CrossRef]
- Saini, A.; Seni, K.; Chawla, P.A.; Chawla, V.; Ganti, S.S. An insight into recent updates on analytical techniques for bioactive alkaloids. Phytochem. Anal. 2024, 35, 423–444. [Google Scholar] [CrossRef]
- Ain, Q.U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant Alkaloids as Antiplatelet Agent: Drugs of the Future in the Light of Recent Developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Chan, L.Y.; Huang, Y.H.; Nguyen, L.T.T.; Kaas, Q.; Huynh, T.; Craik, D.J. Exploring the Sequence Diversity of Cyclotides from Vietnamese Viola Species. J. Nat. Prod. 2020, 83, 1817–1828. [Google Scholar] [CrossRef]
- Göransson, U.; Svangård, E.; Claeson, P.; Bohlin, L. Novel strategies for isolation and characterization of cyclotides: The discovery of bioactive macrocyclic plant polypeptides in the Violaceae. Curr. Protein Pept. Sci. 2004, 5, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, N.; Huang, Y.H.; Wang, C.K.; Durek, T.; Gilding, E.K.; Craik, D.J. Isolation and Characterization of Insecticidal Cyclotides from Viola communis. J. Nat. Prod. 2024, 88, 24–35. [Google Scholar] [CrossRef]
- He, W.; Chan, L.Y.; Zeng, G.; Daly, N.L.; Craik, D.J.; Tan, N. Isolation and characterization of cytotoxic cyclotides from Viola philippica. Peptides 2011, 32, 1719–1723. [Google Scholar] [CrossRef]
- Liu, M.Z.; Yang, Y.; Zhang, S.X.; Tang, L.; Wang, H.M.; Chen, C.J.; Shen, Z.F.; Cheng, K.D.; Kong, J.Q.; Wang, W. A cyclotide against influenza A H1N1 virus from Viola yedoensis. Yao Xue Xue Bao 2014, 49, 905–912. [Google Scholar]
- Mao, Y.; Kong, X.; Liang, Z.; Yang, C.; Wang, S.; Fan, H.; Ning, C.; Xiao, W.; Wu, Y.; Wu, J.; et al. Viola yedoensis Makino alleviates heat stress-induced inflammation, oxidative stress, and cell apoptosis in the spleen and thymus of broilers. J. Ethnopharmacol. 2024, 319, 117350. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Yang, W.; Huang, C.; Ou, Z.; He, J.; Yang, M.; Wu, J.; Yao, H.; Yang, Y.; et al. Viola yedoensis Makino alleviates lipopolysaccharide-induced intestinal oxidative stress and inflammatory response by regulating the gut microbiota and NF-κB-NLRP3/ Nrf2-MAPK signaling pathway in broiler. Ecotoxicol. Environ. Saf. 2024, 282, 116692. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Z.; Yan, X.L.; Guo, Q.S.; Wang, Y.; Yang, P.F.; Zhao, T.; Liu, L. Effects of light intensity on cleaning away heat property of Viola yedoensis. Zhongguo Zhong Yao Za Zhi 2020, 45, 3812–3818. [Google Scholar]
- Ngan, F.; Chang, R.S.; Tabba, H.D.; Smith, K.M. Isolation, purification and partial characterization of an active anti-HIV compound from the Chinese medicinal herb Viola yedoensis. Antivir. Res. 1988, 10, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Chu, S.C.; Hsieh, Y.H.; Chen, P.N.; Hsieh, Y.S. Viola Yedoensis Suppresses Cell Invasion by Targeting the Protease and NF-κB Activities in A549 and Lewis Lung Carcinoma Cells. Int. J. Med. Sci. 2018, 15, 280–290. [Google Scholar] [CrossRef]
- Li, W.; Xie, J.Y.; Li, H.; Zhang, Y.Y.; Cao, J.; Cheng, Z.H.; Chen, D.F. Viola yedoensis liposoluble fraction ameliorates lipopolysaccharide-induced acute lung injury in mice. Am. J. Chin. Med. 2012, 40, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, T.; Ma, X.; Guo, S.; Zhou, Q.; Zahoor, A.; Deng, G. Recent advances in anti-inflammatory active components and action mechanisms of natural medicines. Inflammopharmacology 2023, 31, 2901–2937. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Vestergaard, C.; Skovsgaard, C.; Johansen, C.; Deleuran, M.; Thyssen, J.P. Treat-to-Target in Atopic Dermatitis. Am. J. Clin. Dermatol. 2024, 25, 91–98. [Google Scholar] [CrossRef]
- Salman, A.; Giménez-Arnau, A.M. Emerging Systemic Treatment Options in Atopic Dermatitis. Balkan Med. J. 2024, 41, 239–247. [Google Scholar] [CrossRef]
- Muluye, R.A.; Bian, Y.; Alemu, P.N. Anti-inflammatory and Antimicrobial Effects of Heat-Clearing Chinese Herbs: A Current Review. J. Tradit. Complement. Med. 2014, 4, 93–98. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Cheng, J.; Wang, H.; Pan, S.; Liu, Y. The Antiviral Potential of Perilla frutescens: Advances and Perspectives. Molecules 2024, 29, 3328. [Google Scholar] [CrossRef] [PubMed]
- Moriel, D.G.; Piccioli, D.; Raso, M.M.; Pizza, M. The overlooked bacterial pandemic. Semin. Immunopathol. 2024, 45, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.M.L.; Komarasamy, T.V.; Adnan, N.A.A.B.; Radhakrishnan, A.K.; Balasubramaniam, V.R.M.T. Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines. Influenza Other Respir. Viruses 2024, 18, e13276. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Yan, H.; Wang, P.Z.; Rong, R.; Zhang, Y.Y.; Zhang, C.B.; Du, R.K.; Rong, L.J. A cell-based high-throughput protocol to screen entry inhibitors of highly pathogenic viruses with Traditional Chinese Medicines. J. Med. Virol. 2017, 89, 908–916. [Google Scholar] [CrossRef]
- Liao, H.F.; Lu, M.C.; Chang, H.C.; Wei, C.C.; Kao, C.H.; Chen, Z.H.; Huang, C.C.; Li, C. Effects of herbal medicinal formulas on suppressing viral replication and modulating immune responses. Am. J. Chin. Med. 2010, 38, 173–190. [Google Scholar] [CrossRef]
- In vitro screening of traditional medicines for anti-HIV activity: Memorandum from a WHO meeting. Bull. World Health Organ. 1989, 67, 613–618.
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar]
- Zhou, F.; Zhu, F.; Zhu, T.; Zhao, Z.; Li, L.; Lin, S.; Zhao, H.; Yang, L.; Zhao, C.; Wang, L.; et al. AT7519 against lung cancer via the IL6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2022, 609, 31–38. [Google Scholar] [CrossRef]
- Wu, S.; Sun, Z.; Guo, Z.; Li, P.; Mao, Q.; Tang, Y.; Chen, H.; Peng, H.; Wang, S.; Cao, Y. The effectiveness of blood-activating and stasis-transforming traditional Chinese medicines (BAST) in lung cancer progression-a comprehensive review. J. Ethnopharmacol. 2023, 314, 116565. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, Y.; Chen, S.; Sun, J.; Wu, J.; Meng, X. Therapeutic potential of Scutellaria baicalensis Georgi in lung cancer therapy. Phytomedicine 2022, 95, 153727. [Google Scholar] [CrossRef] [PubMed]
- Vishnupriya, S.; Priya Dharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020, 260, 118308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Ma, S.; Cheng, L.; Yu, G. Repair and regeneration of the alveolar epithelium in lung injury. FASEB J. 2024, 38, e23612. [Google Scholar] [CrossRef]
- Jing, W.; Qin, F.; Guo, X.; Sun, Y.; Yan, C.; Qiu, C.; Tanaka, M.; Shi, B.; Zhao, Y. G-CSF mediates lung injury in mice with adenine-induced acute kidney injury. Int. Immunopharmacol. 2018, 63, 1–8. [Google Scholar] [CrossRef]
- Liu, Q.H.; Zhang, K.; Feng, S.S.; Zhang, L.J.; Li, S.Y.; Wang, H.Y.; Wang, J.H. Rosavin Alleviates LPS-Induced Acute Lung Injure by Modulating the TLR-4/NF-κB/MAPK Singnaling Pathways. Int. J. Mol. Sci. 2024, 25, 1875. [Google Scholar] [CrossRef]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Beigoli, S.; Khazdair, M.R.; Amin, F.; Boskabady, M.H. Experimental and Clinical Studies on the Effects of Natural Products on Noxious Agents-Induced Lung Disorders, a Review. Front. Nutr. 2022, 9, 867914. [Google Scholar] [CrossRef]
- Villegas, W.; Vaughan, J. Trends in Reduction of Mortality in Liver Trauma. Crit. Care Nurs. Clin. N. Am. 2022, 34, 351–359. [Google Scholar] [CrossRef]
- Ding, K.; Li, X.; Ren, X.; Ding, N.; Tao, L.; Dong, X.; Chen, Z. GBP5 promotes liver injury and inflammation by inducing hepatocyte apoptosis. FASEB J. 2022, 36, e22119. [Google Scholar] [CrossRef]
- Lu, G.; Qiao, J.; Wang, L.; Liu, H.; Wu, G.; Zhu, Y.; Zhao, Y.; Xie, G.; Qin, M. An integrated study of Violae Herba (Viola philippica) and five adulterants by morphology, chemical compositions and chloroplast genomes: Insights into its certified plant origin. Chin. Med. 2022, 17, 32. [Google Scholar] [CrossRef]
- Rizwan, K.; Khan, S.A.; Ahmad, I.; Rasool, N.; Ibrahim, M.; Zubair, M.; Jaafar, H.Z.; Manea, R. A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits. Molecules 2019, 24, 3138. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.L.; Zhang, X.J.; Xie, S.Q.; Fan, S.J.; Qu, X.J. Application of chloroplast genome in the identification of Traditional Chinese Medicine Viola philippica. BMC Genom. 2022, 23, 540. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L.; Sheng, K.; Zou, L.; Ye, B. Highly sensitive determination of esculetin on TiO2-NPs-coated poly(diallyldimethylammonium chloride)-functionalized graphene modified electrode. Talanta 2016, 161, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Li, C.; Cai, R.; Chen, B.; Jiang, G.; Chen, X. Identification and Semi-quantification of 36 Compounds from Violae Herba (Zihuadiding) via UHPLC-Q-Orbitrap-MS/MS as well as Proposal of Anti-counterfeiting Quality-marker for Pharmacopeia. Chromatographia 2024, 87, 597–608. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the study of flavone di-C-glycosides by high performance liquid chromatography-tandem ion trap mass spectrometry and its application to characterization of flavonoid composition in Viola yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.S.; Gao, J.M.; Xu, Y.J.; Li, L.F.; Li, C.H. Rapid separation and identification of anthocyanins from flowers of Viola yedoensis and V. prionantha by high-performance liquid chromatography-photodiode array detection-electrospray ionisation mass spectrometry. Phytochem. Anal. 2012, 23, 16–22. [Google Scholar] [CrossRef]
- Hong, J.L.; Zhou, H.Y.; Zhu, J.; Li, L.; Shu, P.; Qin, X.Y.; Qin, M.J. Comparative analysis of major constituents in Viola yedoensis Makino and different species from the Genus Viola by high-performance liquid chromatography with chemometrics methods. J. Med. Plants Res. 2011, 5, 5230–5239. [Google Scholar]
- Koike, A.; Barreira, J.C.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.; Ferreira, I.C. Edible flowers of Viola tricolor L. as a new functional food: Antioxidant activity, individual phenolics and effects of gamma and electron-beam irradiation. Food Chem. 2015, 179, 6–14. [Google Scholar] [CrossRef]
- Zeng, G.; Li, X.; Zhao, C.; Pang, Y.; Luo, X.; Tang, Z. Proximate Composition and In Vitro Bioactive Properties of Leaf Extracts from Seven Viola Species. Foods 2025, 14, 302. [Google Scholar] [CrossRef]
- Thonthula, S.; Sousa, S.; Dubuis, A.; Boudah, S.; Mehta, R.; Singh, A.; Eilstein, J.; Tabet, J.C.; John, S.; Roy, D.; et al. Improved Skin Barrier Function Along with Hydration Benefits of Viola yedoensis Extract, Aesculin, and Schaftoside and LC-HRMS/MS Dereplication of Its Bio-Active Components. Int. J. Mol. Sci. 2024, 25, 12770. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.K.; Sachan, A.; Singh, B.; Kaur, R.; Pal, B.; Yadav, A.K.; Rao, M.M. A Review on Herbal Plants used in Skin and Hair Treatment. Res. J. Top. Cosmet. Sci. 2010, 1, 13–17. [Google Scholar]
- Gao, J.; Luo, M.; Zhu, Y.; He, Y.; Wang, Q.; Zhang, C. Transcriptome sequencing and differential gene expression analysis in Viola yedoensis Makino (Fam. Violaceae) responsive to cadmium (Cd) pollution. Biochem. Biophys. Res. Commun. 2015, 459, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Gao, J.; Zhang, C.; Ou, L.L.; Luo, M. Novel SSR and SNP markers in Viola yedoensis makino resistant to cadmium stress. ScienceAsia 2020, 46, 280–287. [Google Scholar] [CrossRef]

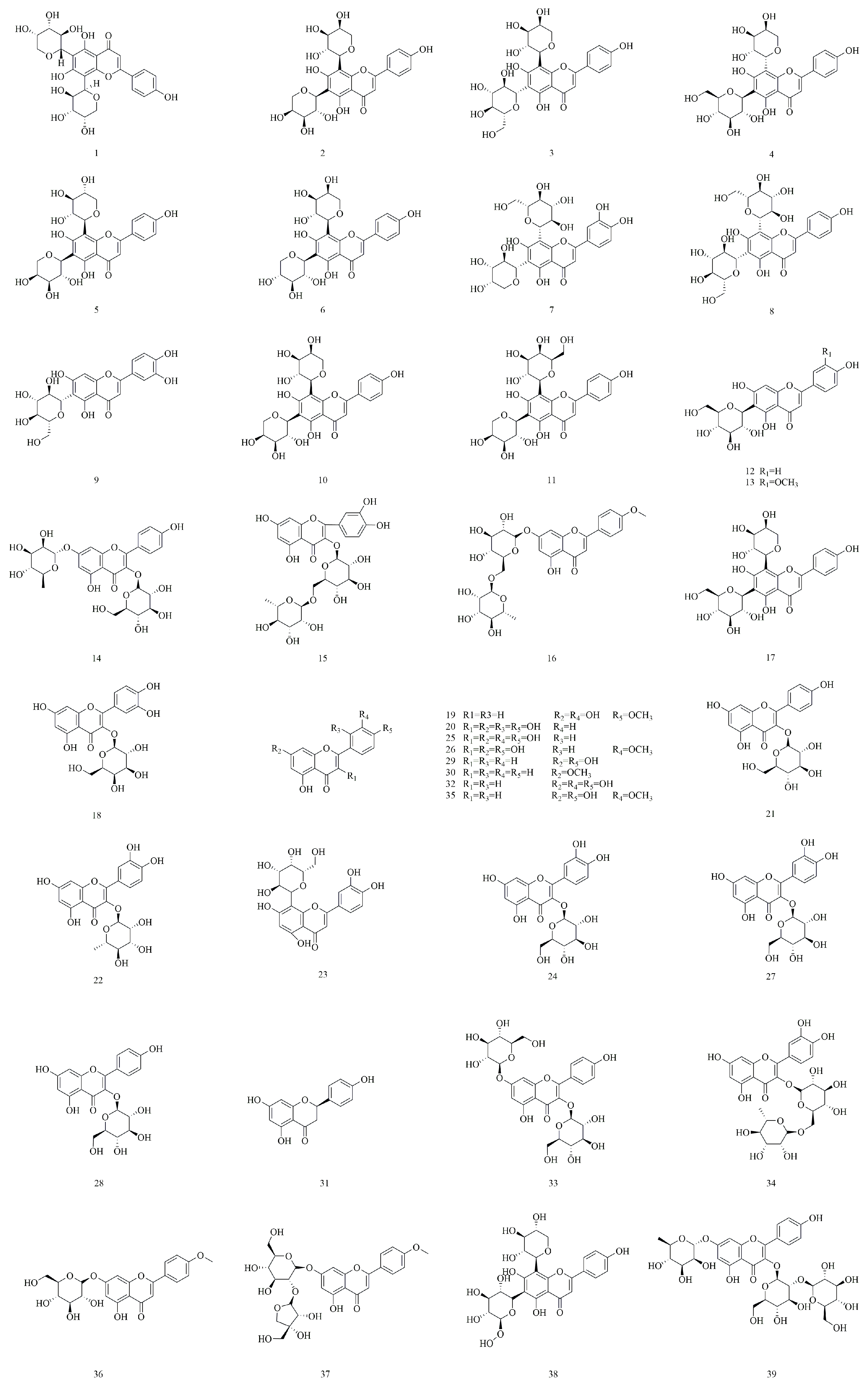
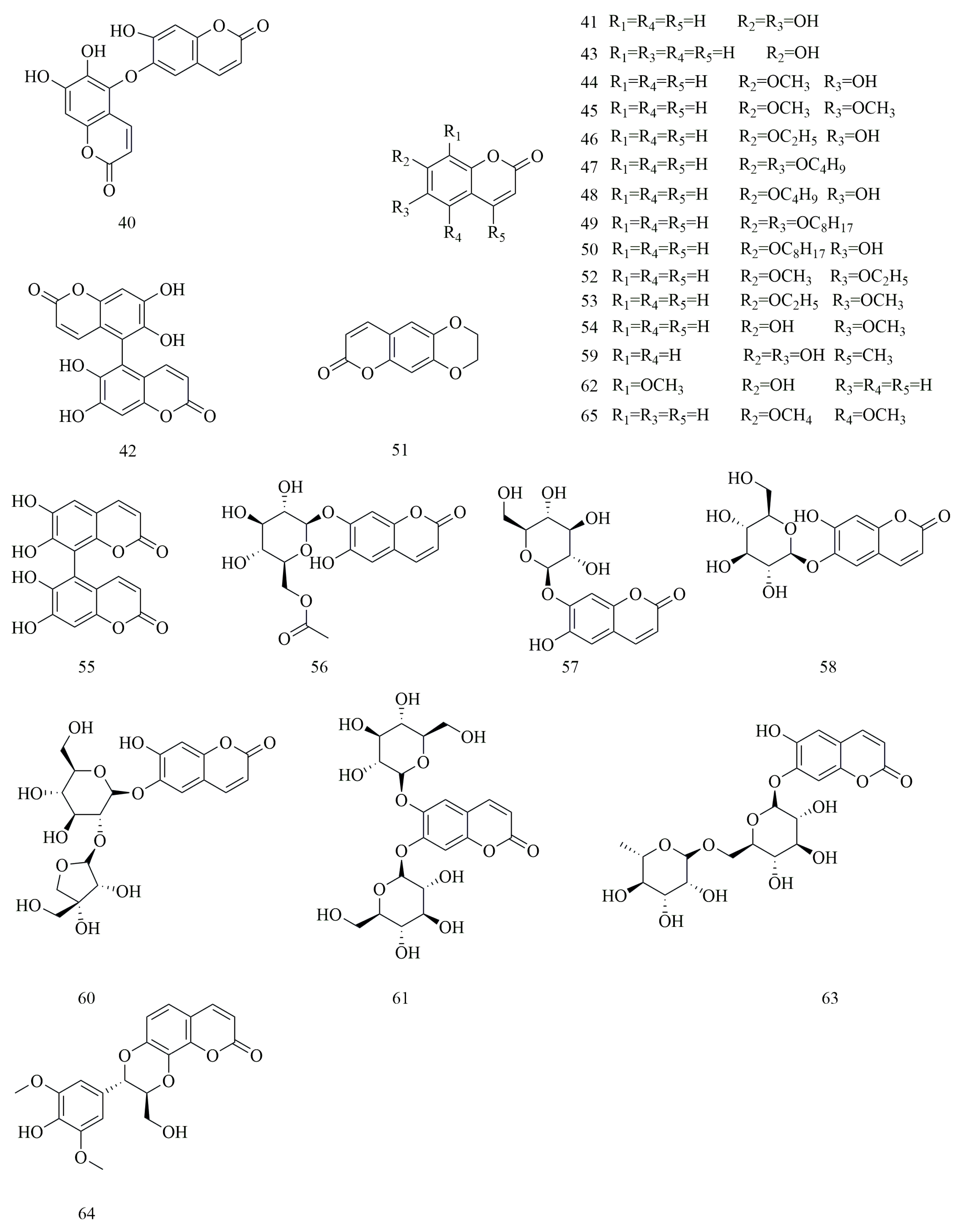
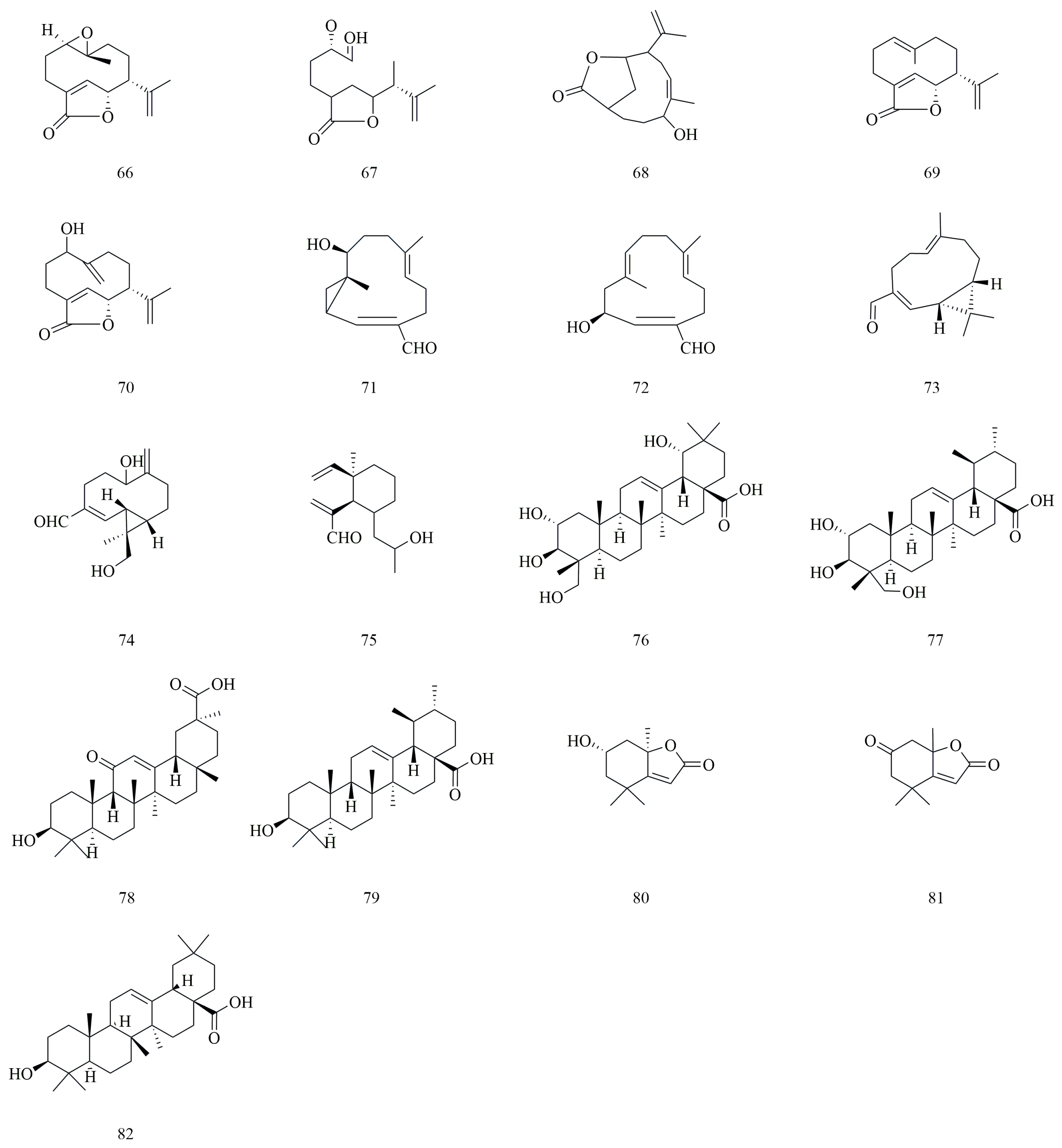
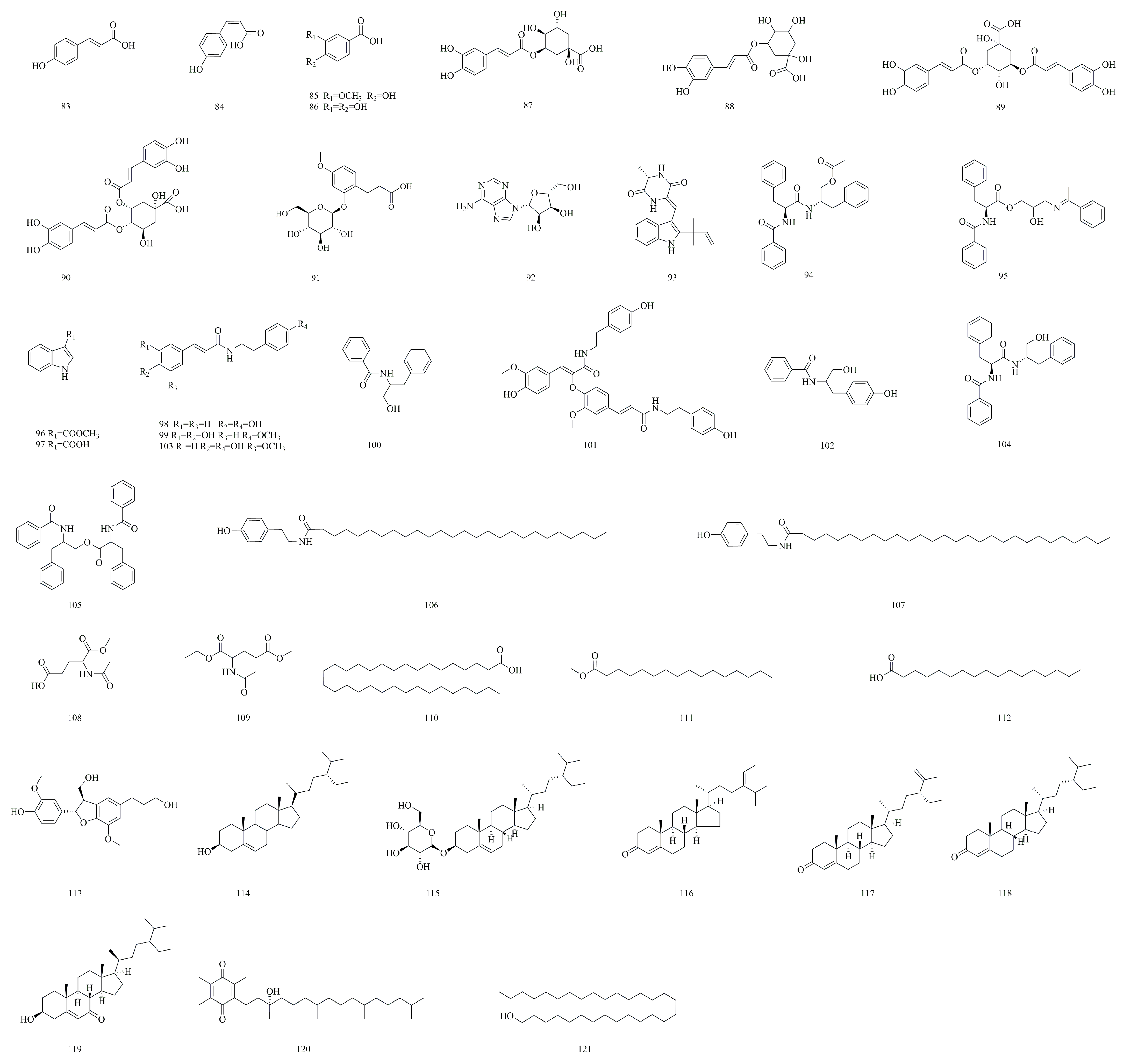

| No. | Compounds | Molecular Formula | Identification Method | Extraction Method | Extraction Solvent | Isolation and Purification Procedure | References |
|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||
| 1 | Apigenin-6-C-α-l-arabinopyranosyl-8-C-β-l-arabinopyranoside | C25H26O13 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 2 | Apigenin 6,8-di-C-α-l-arabinopyranoside | C25H26O13 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 3 | Schaftoside | C26H28O14 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 4 | Neoschaftoside | C26H28O14 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 5 | Apigenin-6-C-α-l-arabinopyranosyl-8-C-β-d-xylopyranoside | C25H26O13 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 6 | Apigenin-6-C-β-d-xylopyranosyl-8-C-α-l-arabinopyranoside | C25H26O13 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 7 | Isocarlinoside | C26H28O15 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4] |
| 8 | Vicenin-2 | C27H30O15 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4,5] |
| 9 | Isoorientin | C21H20O11 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4,5] |
| 10 | Apigenin-6,8-di-C-α-l-arabinopyranoside | C25H26O13 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4,13] |
| 11 | Isoschaftoside | C26H28O14 | NMR, DQF-COSY, HSQC, HMBC | Sequential solvent extraction | Methanol | Amberlite XAD2 column chromatography, Sephadex LH-20 column chromatography, Semi-preparative HPLC | [4,13] |
| 12 | Isovitexin | C21H20O10 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 13 | Isoscoparin | C22H22O11 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 14 | Kaempferol-3-O-β-d-Glucosyl-7-O-α-l-rhamnosylkaempferol | C27H34O17 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 15 | Rutin | C27H30O16 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5,22] |
| 16 | 5-Hydroxy-4′-methoxyflavone-7-O-rutinoside | C28H32O14 | NMR, MS, CD spectroscopic data | N/A | N/A | Silica gel column chromatography, Thin-layer chromatography, Recrystallization | [6] |
| 17 | Apigenin-6-C-β-d-glucopyranosyl-8-C-α-l-arabinopyranoside | C26H28O14 | NMR, MS, CD spectroscopic data | N/A | N/A | Silica gel column chromatography, Thin-layer chromatography, Recrystallization | [6] |
| 18 | Quercetin-3-O-galactoside | C21H20O12 | NMR, MS | Ultrasonic extraction | 60% Ethanol | Silica gel column chromatography, Recrystallization | [15] |
| 19 | Diosmetin | C16H12O6 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 20 | Morin | C15H10O7 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 21 | Astragalin | C21H20O11 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 22 | Quercitrin | C21H20O11 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 23 | 1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-d-galactitol | C21H20O11 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 24 | Quercetin-3-β-d-glucoside | C21H20O12 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 25 | Quercetin | C15H10O7 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 26 | Isorhamnetin | C16H12O7 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 27 | Quercetin-3-O-β-d-glucoside | C21H20O12 | NMR, ESI-MS | Solvent extraction method | 75% Ethanol | Silica gel column chromatography | [23] |
| 28 | Kaempferol-3-O-β-d-glucoside | C21H20O9 | NMR, ESI-MS | Solvent extraction method | 75% Ethanol | Silica gel column chromatography | [23] |
| 29 | Apigenin | C15H10O5 | NMR, ESI-MS | Solvent extraction method | 75% Ethanol | Silica gel column chromatography | [23] |
| 30 | Techtochrysin | C16H12O4 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 31 | Naringenin | C15H12O5 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [25] |
| 32 | Luteolin | C15H10O6 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [25] |
| 33 | Keampferol-3-O-β-d-glucosyl-(1-2)-O-α-l-rhamnoside | C27H30O15 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [25] |
| 34 | Quercetin-3-O-β-d-glucosyl-(1-4)-O-α-l-rhamnoside | C27H30O16 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [25] |
| 35 | Chrysoeriol | C16H12O6 | HPLC, MS, NMR | Solvent extraction method | 95% Ethanol | Macroporous resin adsorption | [26] |
| 36 | Acacetin-7-O-β-d-glucoside | C22H22O10 | HPLC, MS, NMR | Solvent extraction method | 95% Ethanol | Macroporous resin adsorption | [26] |
| 37 | Acacetin-7-O-β-d-apiosyl-(1-2)-β-d-glucoside | C27H30O14 | HPLC, MS, NMR | Solvent extraction method | 95% Ethanol | Macroporous resin adsorption | [26] |
| 38 | Vicenin-3 | C26H28O14 | NMR, MS | Solvent extraction method | N/A | N/A | [27] |
| 39 | Kaempferol-3-O-β-d-sophorose-7-O-α-l-rhamnoside | C33H40O20 | NMR, MS | Solvent extraction method | N/A | N/A | [27] |
| Coumarins | |||||||
| 40 | Dimeresculetin | C18H10O8 | NMR, HSQC, HMBC, ROEST, UV, IR | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [1] |
| 41 | Esculetin | C9H6O4 | NMR, HSQC, HMBC, ROEST, UV, IR | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [1,5,22,28] |
| 42 | Euphorbetin | C18H10O8 | NMR, HSQC, HMBC, ROEST, UV, IR | Reflux extraction method | Ethyl acetate | Silica gel column chromatography | [1,28] |
| 43 | 7-Hydroxycoumarine | C9H6O3 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 44 | 6-Hydroxy-7-methoxycoumarin | C10H8O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 45 | 6,7-Dimethoxycoumarin | C11H10O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 46 | 6-Hydroxy-7-ethoxycoumarin | C11H10O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 47 | 6,7-Dibutoxycoumarin | C17H22O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 48 | 6-Hydroxy-7-butoxycoumarin | C13H14O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 49 | 6,7-Dioctyloxycoumarin | C25H38O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 50 | 6-Hydroxy-7-octyloxycoumarin | C17H22O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 51 | 2,3-Dihydro-7H-pyrano[2,3-g]-1,4-benzodioxin-7-one | C11H8O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 52 | 6-Ethoxy-7-methoxycoumarin | C12H12O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 53 | 7-Ethoxy-6-methoxycoumarin | C12H12O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 54 | Scopoletin | C10H8O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5,22] |
| 55 | 6,6’,7,7’-Tetrahydroxy-5,8’-biscoumarin | C18H10O8 | NMR, MS, CD spectroscopic data | N/A | N/A | Silica gel column chromatography, Thin-layer chromatography, Recrystallization | [6] |
| 56 | Prionanthoside | C17H18O10 | NMR, MS | Ultrasonic extraction | 60% Ethanol | Silica gel column chromatography, Recrystallization | [15] |
| 57 | Cichoriin | C15H16O9 | NMR, MS | Ultrasonic extraction | 60% Ethanol | Silica gel column chromatography, Recrystallization | [15] |
| 58 | Esculin | C15H16O9 | NMR, UHPLC-MS | Ultrasonic extraction | 60% Ethanol | Silica gel column chromatography, Recrystallization | [15,22] |
| 59 | 6,7-Dihydroxy-4-methylcoumarin | C10H8O4 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 60 | Aesculetin-6-O-β-d-apiofuranosyl-(1-2)-β-Dglucopyranoside | C20H24O13 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 61 | 6,7-Di-O-β-d-Glucopyranosylesculetin | C21H26O14 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography, Gel column chromatography, Polyamide column chromatography | [29] |
| 62 | 7-Hydroxy-8-methoxycoumarin | C10H8O4 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography, Gel column chromatography, Polyamide column chromatography | [29] |
| 63 | 6-Hydroxy-coumarin-7-O-α-l-rhamnosyl-(1-6)-O-β-d-glucoside | C21H28O13 | NMR, MS | Reflux extraction method | Ethyl acetate | Silica gel column chromatography, Gel column chromatography, Polyamide column chromatography | [29] |
| 64 | Daphneticin | C20H18O8 | NMR, MS | Ultrasonic extraction | N/A | Liquid-liquid extraction, Silica gel column chromatography, Sephadex LH-20 column chromatography | [30] |
| 65 | 5-Methoxy-7-hydroxymethyl coumarin | C11H10O4 | NMR, MS | Ultrasonic extraction | N/A | Liquid-liquid extraction, Silica gel column chromatography, Sephadex LH-20 column chromatography | [30] |
| Terpenoids | |||||||
| 66 | Yedoensins A | C15H20O3 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 67 | Yedoensins B | C14H20O4 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 68 | Versicolactone B | C15H22O3 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 69 | Aristolactone | C15H20O2 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 70 | Madolin U | C15H20O3 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 71 | Madolin W | C15H22O2 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 72 | Aristoyunnolin E | C15H22O2 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 73 | Isobicyclogermacrenal | C15H22O | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 74 | Madolin Y | C15H22O3 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 75 | Madolin R | C15H24O2 | HSQC, HMBC, NOESY, HR-ESI-MS | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Semi-preparative HPLC | [9] |
| 76 | Arjungenin | C30H48O6 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 77 | Asiatic acid | C30H48O5 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 78 | 18-β-Glycyrrhetinic acid | C30H46O4 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 79 | Ursolic acid | C30H48O3 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 80 | Loliolide | C11H16O3 | NMR, ESI-MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, Gel column chromatography, Polyamide column chromatography | [29] |
| 81 | Dehydrololiolide | C11H14O3 | NMR, ESI-MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, Gel column chromatography, Polyamide column chromatography | [29] |
| 82 | Oleanolic acid | C30H48O3 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| phenolic acid | |||||||
| 83 | Trans-p-coumaric acid | C9H8O3 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 84 | Cis-p-coumaric acid | C9H8O3 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 85 | Vanillic acid | C8H8O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 86 | Protocatechuic acid | C7H6O4 | NMR, EI-MS | Solvent extraction method | 95% Ethanol | N/A | [8] |
| 87 | Neochlorogenic acid | C16H18O9 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 88 | Chlorogenic acid | C16H18O9 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 89 | 3,5-Dicaffeoylquinic acid | C25H24O12 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 90 | 4,5-Dicaffeoylquinic acid | C25H24O12 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| 91 | 3-[2-(β-d-Glucopyranosyloxy)-4-methoxyphenyl]propanoic acid | C16H22O9 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 75% Ethanol | N/A | [22] |
| Alkaloids | |||||||
| 92 | Adenosine | C10H13N5O4 | NMR, HPLC, MALDI-TOF MS | Sequential solvent extraction | 50% Ethanol | Silica gel column chromatography, Activated carbon column chromatography, Diaion HP-20 column chromatography | [5] |
| 93 | Neoechinulin A | C19H21N3O2 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 94 | Aurantiamide acetate | C27H28N2O4 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 95 | Trichosanatine | C27H28N2O4 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 96 | Methyl indole-3-carboxylate | C10H9NO2 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 97 | Indole-3-carboxylic acid | C9H7NO2 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 98 | N-p-trans-Coumaroyltyramine | C17H17NO3 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 99 | 7’-(3’,4’-Dihydroxyphenyl)-N-[(4-methoxyphenyl) ethyl] propenamide | C18H19NO4 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 100 | N-benzoyl-l-phenylalaninol | C16H17NO2 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 101 | Cannabisin F | C36H36N2O8 | ESI-MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 102 | N-benzoyl-l-p-hydroxy-phenylalaninol | C16H17NO3 | MS, NMR, HPLC | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 103 | N-trans-feruloyl-tyramine | C18H19NO4 | MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 104 | Aurantiamide | C25H26N2O3 | MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 105 | Anabellamide | C32H30N2O4 | MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 106 | N-(4-hydroxyphenethyl) hexacosanamide | C34H61NO2 | MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 107 | N-(4-hydroxyphenethyl) octacosanamide | C36H65NO2 | MS, NMR | Solvent extraction method | 95% Ethanol | Silica gel column chromatography, Sephadex LH-20 gel column chromatography | [31] |
| 108 | N-acetyl-1-ethyl ester glutamic acid | C8H13NO5 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 109 | N-acetyl glutamic acid-1-ethyl-5-methyl ester | C10H17NO5 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| Others | |||||||
| 110 | Lacceroic acid | C32H64O2 | NMR, EI-MS | Solvent extraction method | 95% Ethanol | N/A | [8] |
| 111 | Methyl palmitate | C17H34O2 | MS, NMR | Solvent extraction method | N/A | N/A | [32] |
| 112 | Stearic acid | C18H36O2 | MS, NMR | Solvent extraction method | N/A | N/A | [32] |
| 113 | Rel-(2α,3β)-7-O-methylcedrusin | C20H24O6 | UHPLC-Q-Orbitrap-MS | Reflux extraction method | 95% Ethanol | N/A | [22] |
| 114 | β-Sitosterol | C29H50O | NMR, ESI-MS | Solvent extraction method | 75% Ethanol | Silica gel column chromatography | [23] |
| 115 | Daucosterol | C35H60O6 | NMR, ESI-MS | Solvent extraction method | 75% Ethanol | Silica gel column chromatography | [23] |
| 116 | Stigmasta-4,24(28)-dien-3-one | C29H46O | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 117 | Stigmasta-4,25-dien-3-one | C29H46O | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 118 | β-Sitostenone | C29H48O | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 119 | (24R)-3β-Hydroxy-ethylcholest-5-en-7-one | C29H48O2 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 120 | α-Tocophe rol-quinone | C29H50O3 | HPLC, NMR, MS | Reflux extraction method | 95% Ethanol | Silica gel column chromatography, ODS column chromatography, Sephadex LH-20 gel column chromatography, Preparative high-performance liquid chromatography | [24] |
| 121 | Triacontanol | C30H62O | MS, NMR | Solvent extraction method | N/A | N/A | [32] |
| No. | Compounds | Amino Acid Sequence of Cyclotides | References |
|---|---|---|---|
| 122 | Viphi I | Cyclo-(VPCGDPSPTCVNTCNTPGCSCSWPVCTR) | [10] |
| 123 | Viphi J | Cyclo-(XGPVCADTCTXGTCYTAGCSCSWPVCTR) | [10] |
| 124 | Viphi K | Cyclo-(XGPVCGETCTXGTCYTAGCSCSWPVCTR) | [10] |
| 125 | Viphi L | Cyclo-(NGXPVCGETCVCYSSDPGCTCSWPVCTR) | [10] |
| 126 | Viphi M | Cyclo-(VPCGETCVAVGGTCNTPGCTCSWPVCTR) | [10] |
| 127 | Viphi N | Cyclo-(DGXPXCGETCVGGTCNTPGCSCSWPVCTR) | [10] |
| 128 | Viphi O | Cyclo-(DGXPVCGETCVGGTCNTPGCSCSWPVCTR) | [10] |
| 129 | Viphi P | Cyclo-(NGXPXCGETCVGGTCNTPGCVCSWPVCTR) | [10] |
| 130 | Viphi Q | Cyclo-(DGXPVCGETCTXGTCYTAGCSCSWPVCTR) | [10] |
| 131 | Viphi R | Cyclo-(NGXPXCGETCVGGTCDTPGCTCSWPVCTR) | [10] |
| 132 | Viphi S | Cyclo-(NGXPXCGETCVGDSDPTPGCTCXCPVCTR) | [10] |
| 133 | Viphi T | Cyclo-(DGXPVCGETCVGGTCNTPGCACSWPVCTR) | [10] |
| 134 | Viphi U | Cyclo-(NGXPVCEGTCVGGTCNYGGCSCSWPVCTR) | [10] |
| 135 | Viphi V | Cyclo-(VPCGETCVGGAVCQSNTPGCTCSWPVCTR) | [10] |
| 136 | Viphi W | Cyclo-(NGXPVCADTCVGGTCNTPGCACYNPVCTR) | [10] |
| 137 | Viphi X | Cyclo-(NGXPXCADTCVGGTCNTPGCSCSMAPVCTR) | [10] |
| 138 | Viphi Y | Cyclo-(VCYNGXTMCSSCVWXPCTVTAXVGCSCSDK) | [10] |
| 139 | Viphi Z | Cyclo-(NGXPXCEGTCVGGTCNTPGCSCSMAPVCTR) | [10] |
| 140 | Cycloviolacin Y1 | Cyclo-(GGTIFDCGETCFLGTCYGCSCGNYGFCYGTN) | [16] |
| 141 | Cycloviolacin Y2 | Cyclo-(GGTIFDCGESCFLGTCYTAGCSCGNWGLCYGTN) | [16] |
| 142 | Cycloviolacin Y3 | Cyclo-(GGTIFDCGETCFLGTCYTAGCSCGNWGLCYGTN) | [16] |
| 143 | Cycloviolacin Y4 | Cyclo-(GVPCGESCVFIPCITGVIGCSCSSNVCYLN) | [16] |
| 144 | Cycloviolacin Y5 | Cyclo-(GIPCAESCVWIPCTVTALVGCSCSDKVCYN) | [16] |
| 145 | Kalata B1 | Cyclo-(GLPVCGETCVGGTCNTPGCTCSWPVCTRN) | [16] |
| 146 | Varv A | Cyclo-GETCVGGTCNTPGCSCSWPVCTRNGLPVC | [16] |
| 147 | Varv E | Cyclo-(GETCVGGTCNTPGCSCSWPVCTRNGLPIC) | [16] |
| 148 | Viphi A | Cyclo-(CGESCVFIPCISSVIGCACKSKVCYKNGSIP) | [49] |
| 149 | Viphi B | Cyclo-(CGETCTIGTCYTAGCTCSWPICTRNGLPV) | [49] |
| 150 | Viphi C | Cyclo-(CGESCVYIPCITSVIGCSCSSKVCYINGVP) | [49] |
| 151 | Viphi D | Cyclo-(CGESCVFPCISSVIGCSCSSKVCYRNGIP) | [49] |
| 152 | Viphi E | Cyclo-(CGESCVFPCISAVIGCSCSNKVCYKNGSIP) | [49] |
| 153 | Viphi F | Cyclo-(CGESCVFIPCISAIIGCSCSSKVCYKNGSIP) | [49] |
| 154 | Viphi G | Cyclo-(CGESCVFIPCISAIIGCSCSNKVCYKNGSIP) | [49] |
| 155 | Viphi H | Cyclo-(CAESCVWIPCTVTAIVGCSCSWGVCYNGIP) | [49] |
| 156 | Viba 17 | Cyclo-(CGETCVGGTCNTPGCGCSWPVCTRNGLPV) | [49] |
| 157 | Mram 8 | Cyclo-(CGESCVFIPCLTSAIGCSCKSKVCYRNGIP) | [49] |
| 158 | Viba 15 | Cyclo-(CGETCVGGTCNTPGCACSWPVCTRNGLPV) | [10,49] |
| 159 | Cycloviolacin O2 | Cyclo-(CGESCVWIPCISSAIGCSCKSKVCYRNGIP) | [49] |
| 160 | Cycloviolacin O12 | N/A | [49] |
| 161 | Viba 11 | Cyclo-(CGESCVWIPCISGAIGCSCKSKVCYRNGIP) | [49] |
| 162 | Cycloviolacin VY1 | Cyclo-(CGESCVFIPCITTVLGCSCSIKVCYKNGSIP) | [50] |
| Pharmacological Activities | Component/Compound | Study Design | Models | Results/Mechanisms | Dosages | Reference |
|---|---|---|---|---|---|---|
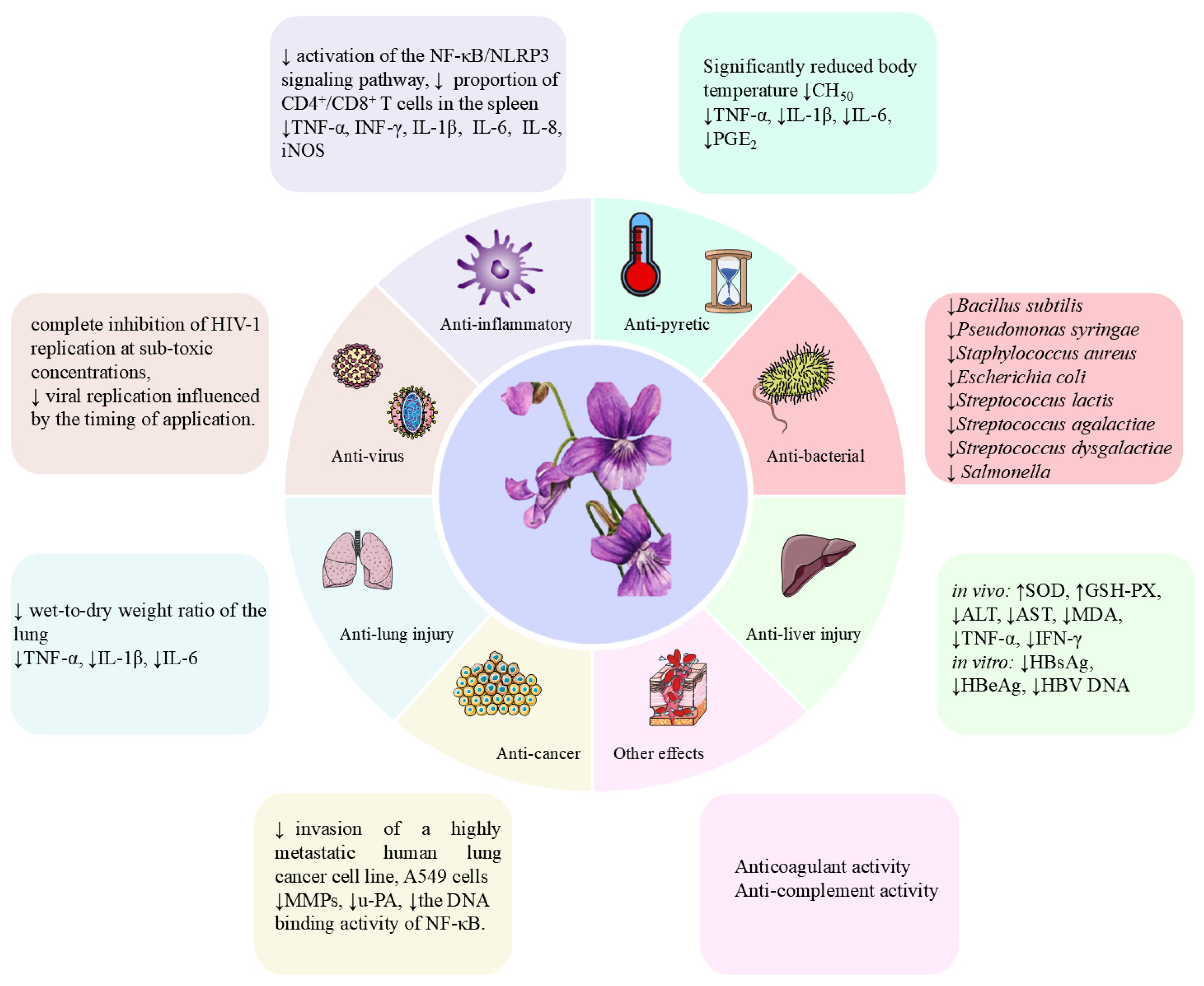
| Anti-inflammatory effects | Aqueous extract of V. yedoensis | in vivo | Heat stress-induced broiler chickens | ↓the inflammatory damage of heat stress on the spleen and thymus of broilers. ↑IgA, ↑IgG, ↑IgM, ↑ND, ↑IBD, ↓IL-1β, ↓INF-γ. | 1.5%, 4.5% | [51] |
| Aqueous extract of V. yedoensis | in vivo | LPS-induced broiler chickens | ↓the activation of the NF-κB/NLRP3 signaling pathway ↑the abundance of protective bacteria ↓the number of pathogenic bacteria ↓TNF-α, ↓IL-1β, ↓IL-8, ↓NLRP3, ↓Caspase-1, ↓MyD88, ↓TLR4 | 0.5%, 1.5%, and 4.5% | [52] | |
| Anti-itching Compound of V. yedoensis | in vitro | RBL-2H3 mast cells | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓iNOS | 100, 200, and 400 μg/mL | [28] | |
| Ethanol extract of V. yedoensis | in vivo | DNCB-induced atopic dermatitis-like mice | ↓DNCB-stimulated AD-like lesion symptoms. ↓the ratio of CD4+/CD8+ T lymphocyte in the spleen and the number of activated macrophages stimulated by DNCB. ↓TNF-α, ↓IL-1β, ↓IL-6, ↓iNOS, ↓COX-2 | 5 mg/day, 10 mg/day | [22] | |
| Ethanol extract of V. yedoensis formula | in vivo | DNCB-induced atopic dermatitis-like mice | ↓macrophage infiltration and promoted M2 macrophage polarization. ↓TNF-α, ↓IL-1β, ↓IL-18, ↑IL-10, ↑JAK2/STAT3 signaling pathway | 150, 300 and 600 mg/kg | [14] | |
| Anti-pyretic effects | Aqueous and ethanolic extracts of V. yedoensis | in vivo | LPS-induced rabbits | ↓body temperature, ↓CH50 | 10, 30, 50 mg/kg | [17] |
| Aqueous extract of V. yedoensis | in vivo | Carrageenan-induced acute inflammation ICR mice | ↓body temperature ↓TNF-α, ↓IL-1β, ↓IL-6, ↓PGE2 | 5%, 35%, 50%, 80%, 100% full sunlight | [53] | |
| Anti-virus effects | Dimethyl sulfoxide extract of V. yedoensis | in vitro | HIV-1, H9 cells | Does not induce interferons, does not inactivate extracellular HIV or herpes simplex virus, inhibitory to HIV | 100 μg/mL | [54] |
| Cycloviolacin Y5 | in vitro | XTT, HIV | EC50: 0.04 µM, IC50: 1.8 µM | N/A | [16] | |
| Cycloviolacin VY1 | in vitro | H1N1 | IC50: 2.27 ± 0.20 μg/mL | 4.00–0.25 μg/mL | [50] | |
| Anti-cancer effects | Aqueous extract of V. yedoensis | in vitro | Human lung adenocarcinoma A549 cells, mouse Lewis lung cancer cells | ↓the invasion of a highly metastatic human lung cancer cell line, A549 cells. ↓MMPs, ↓u-PA, ↓the DNA-binding activity of NF-κB. | 10, 25, 50, 75, and 100 µg/mL | [55] |
| Anti-lung injury effects | Petroleum ether extract of V. yedoensis | in vivo | LPS-induced ALI mouse model | ↓the wet-to-dry weight ratio of the lung, total cells, red blood cells, protein concentration, and myeloperoxidase activity in bronchoalveolar lavage fluid. ↓TNF-α, ↓IL-1β, ↓IL-6 | 2, 4 and 8 mg/kg | [56] |
| Anti-liver injury effects | Esculetin, prionanthoside, cichoriin, esculin, and quercetin-3-O-galactoside | in vivo and in vitro | in vivo: ConA-induced ILI mouse model in vitro: HepG2.2.15 cells | in vivo: ↑SOD, ↑GSH-PX, ↓ALT, ↓AST, ↓MDA, ↓TNF-α, ↓IFN-γ in vitro: ↓HBsAg, ↓HBeAg, ↓HBV DNA | in vivo: 3.00, 6.00, 12.00 g/kg/d in vitro: 0.5, 1 and 2 mM | [15] |
| Anti-bacterial effects | Petroleum ether and ethyl acetate extract of V. yedoensis | in vitro | Bacillus subtilis and Pseudomonas syringae | Petroleum ether and ethyl acetate extracts exhibited inhibitory effects against Bacillus subtilis and Pseudomonas syringae | 6.25 µg/mL | [3] |
| Aesculetin, 6,7-dimethoxycoumarin, scopoletin, 5-methoxy-7-hydroxymethylcoumarin | in vitro | A range of animal pathogens | ↓Staphylococcus aureus, ↓Escherichia coli, ↓Streptococcus lactis, ↓Streptococcus agalactiae, ↓Streptococcus dysgalactiae, ↓ Salmonella | 0.031–0.313 g/L, 0.313 -0.625 g/L | [32] | |
| Other effects | ||||||
| Anti-coagulant | Dimeresculetin, euphorbetin, esculetin | in vitro | Activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT) | Esculetin and dimeresculetin had similar effects on PT and TT, while euphorbetin had more significant anticoagulant activities (except TT at a concentration of 100 µg/mL). | 25, 60, and 100 µg/mL | [1] |
| Anti-complement | yedoensins A, yedoensins B, versicolactone B, madolin W, aristoyunnolin E, madolin Y | in vitro | Classical pathway (CP), alternative pathway (AP) | yedoensins A and versicolactone B act on C1q, C3, and C9, while madolin W, aristoyunnolin E, madolin Y interact with C1q, C3, C5, and C9 | CH50: 0.14 to 0.37 mg/mL AP50: 0.32 to 0.54 mg/mL | [9] |
| Alkaloid compounds of V. yedoensis | in vitro | Classical pathway (CP), alternative pathway (AP) | These alkaloid compounds have effects on components of the complement system, such as C1q, C2, C3, C4, C5, and C9. Different compounds in Viola yedoensis exert their anti-complement effects by inhibiting various targets or multiple targets of the complement system. | CH50: 0.12 to 0.33 g/L AP50: 0.22 to 0.50 g/L | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Shen, C.; Zhang, S.; Di, H.; Wang, Y.; Guan, F. A Comprehensive Review of the Phytochemistry and Therapeutic Efficacy of Viola yedoensis Makino. Molecules 2025, 30, 1922. https://doi.org/10.3390/molecules30091922
Wang S, Shen C, Zhang S, Di H, Wang Y, Guan F. A Comprehensive Review of the Phytochemistry and Therapeutic Efficacy of Viola yedoensis Makino. Molecules. 2025; 30(9):1922. https://doi.org/10.3390/molecules30091922
Chicago/Turabian StyleWang, Shuang, Congcong Shen, Shengyu Zhang, Han Di, Yanhong Wang, and Feng Guan. 2025. "A Comprehensive Review of the Phytochemistry and Therapeutic Efficacy of Viola yedoensis Makino" Molecules 30, no. 9: 1922. https://doi.org/10.3390/molecules30091922
APA StyleWang, S., Shen, C., Zhang, S., Di, H., Wang, Y., & Guan, F. (2025). A Comprehensive Review of the Phytochemistry and Therapeutic Efficacy of Viola yedoensis Makino. Molecules, 30(9), 1922. https://doi.org/10.3390/molecules30091922






