Detection of Retinoic Acid in Cosmetics Using Reactive Paper Spray Ionization Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
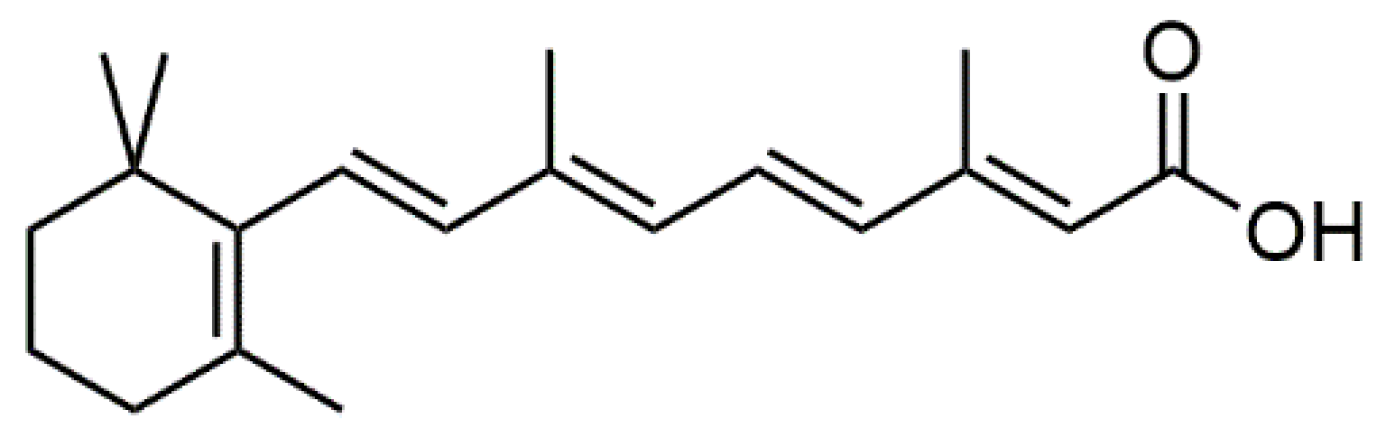
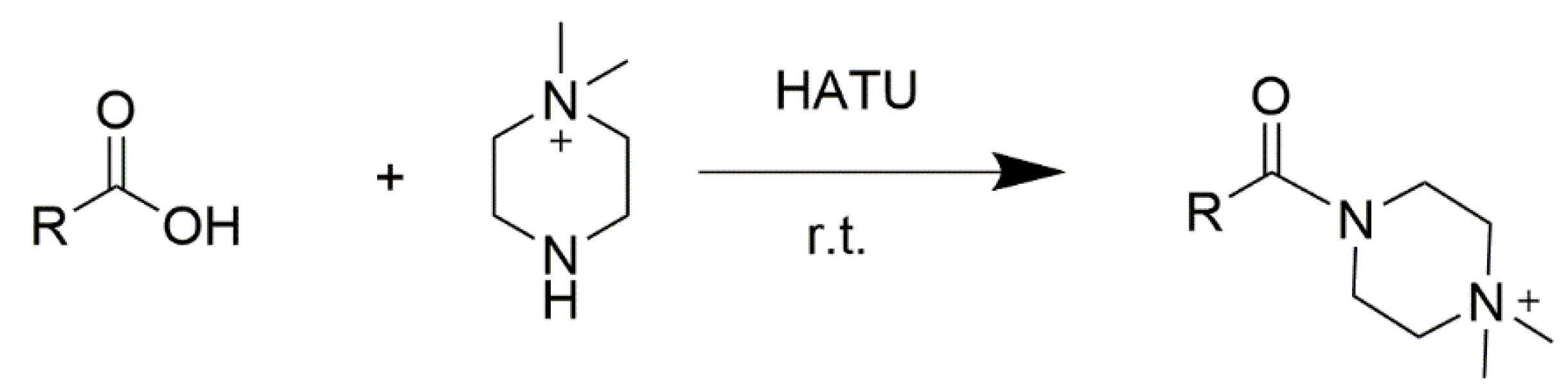
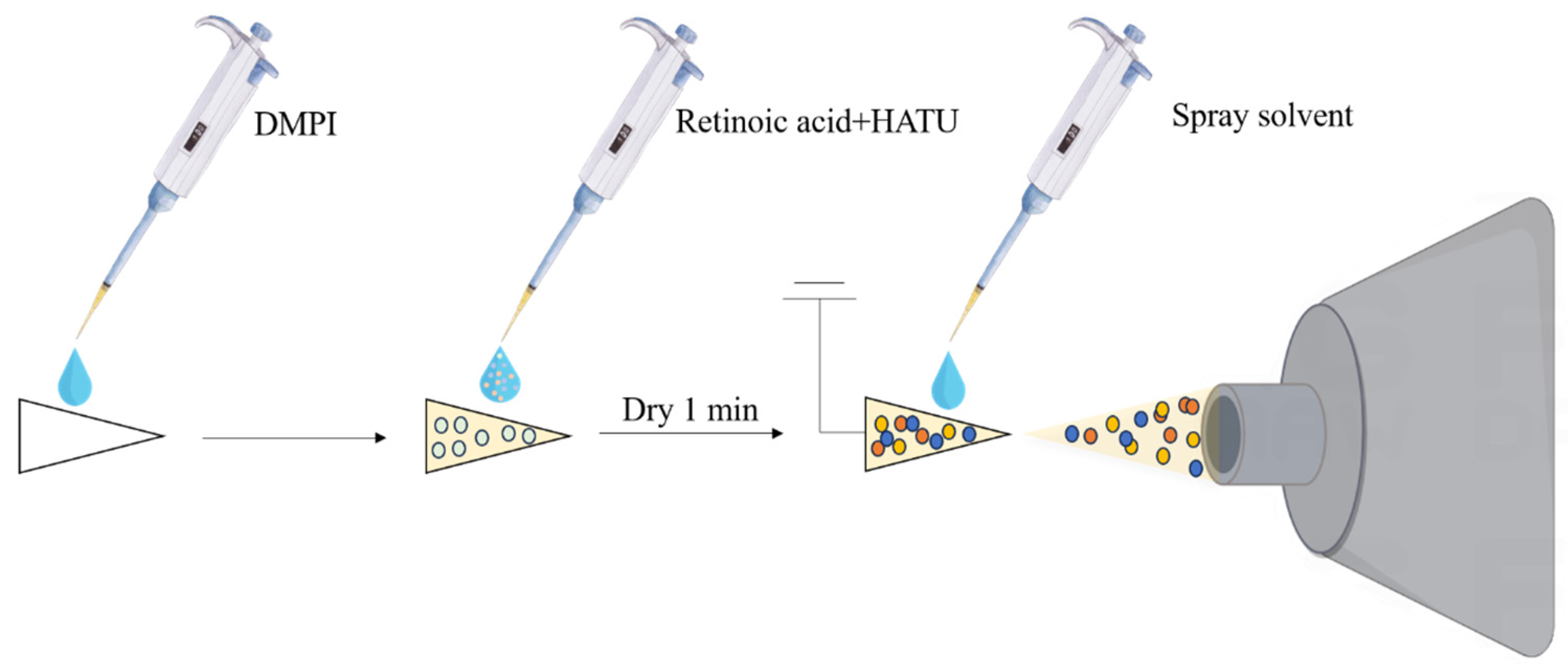
2.1. Online Derivatization Reaction
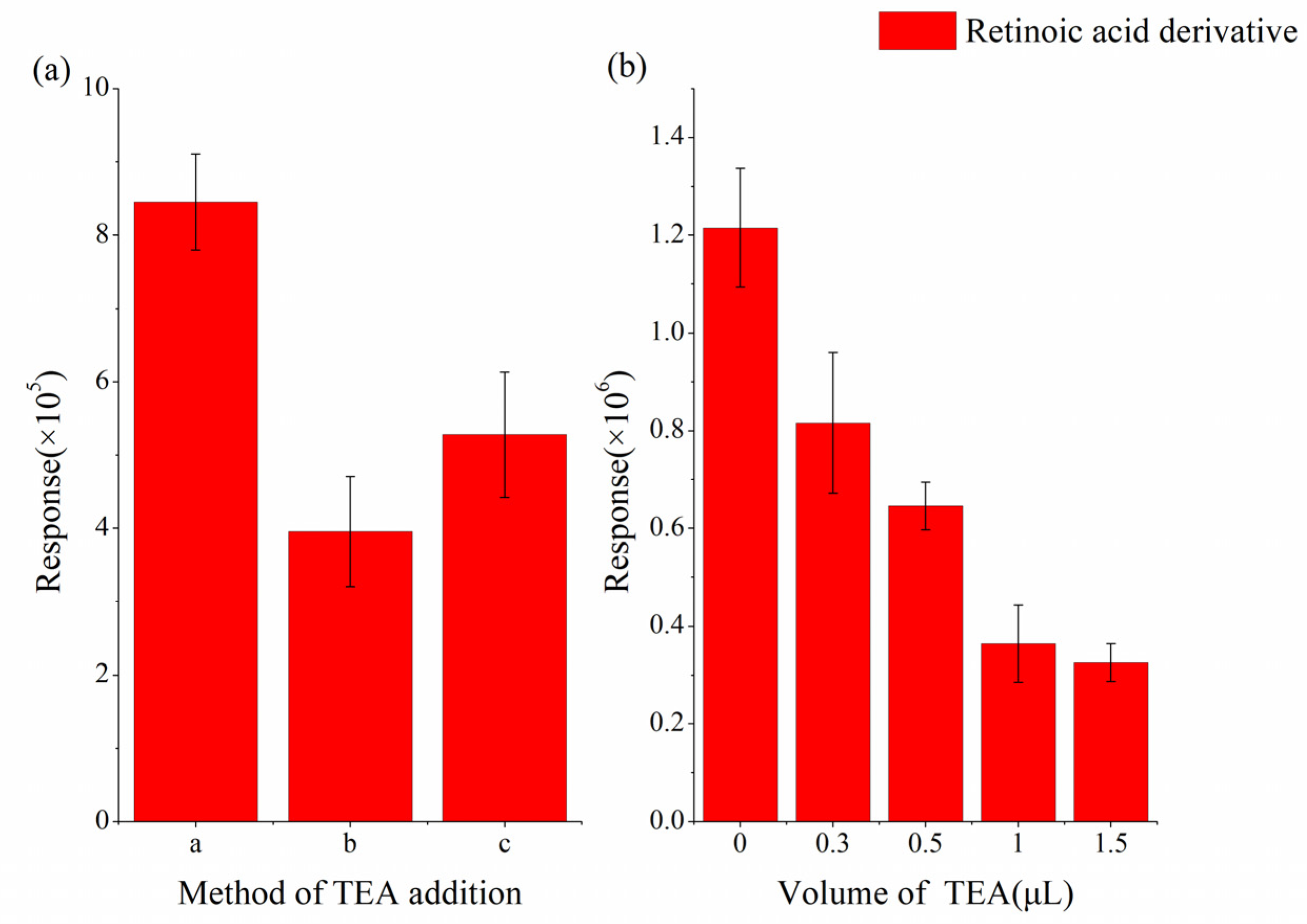
2.2. Optimization of Derivatization Conditions
2.3. Optimization of the Drying Time for Paper Spray MS
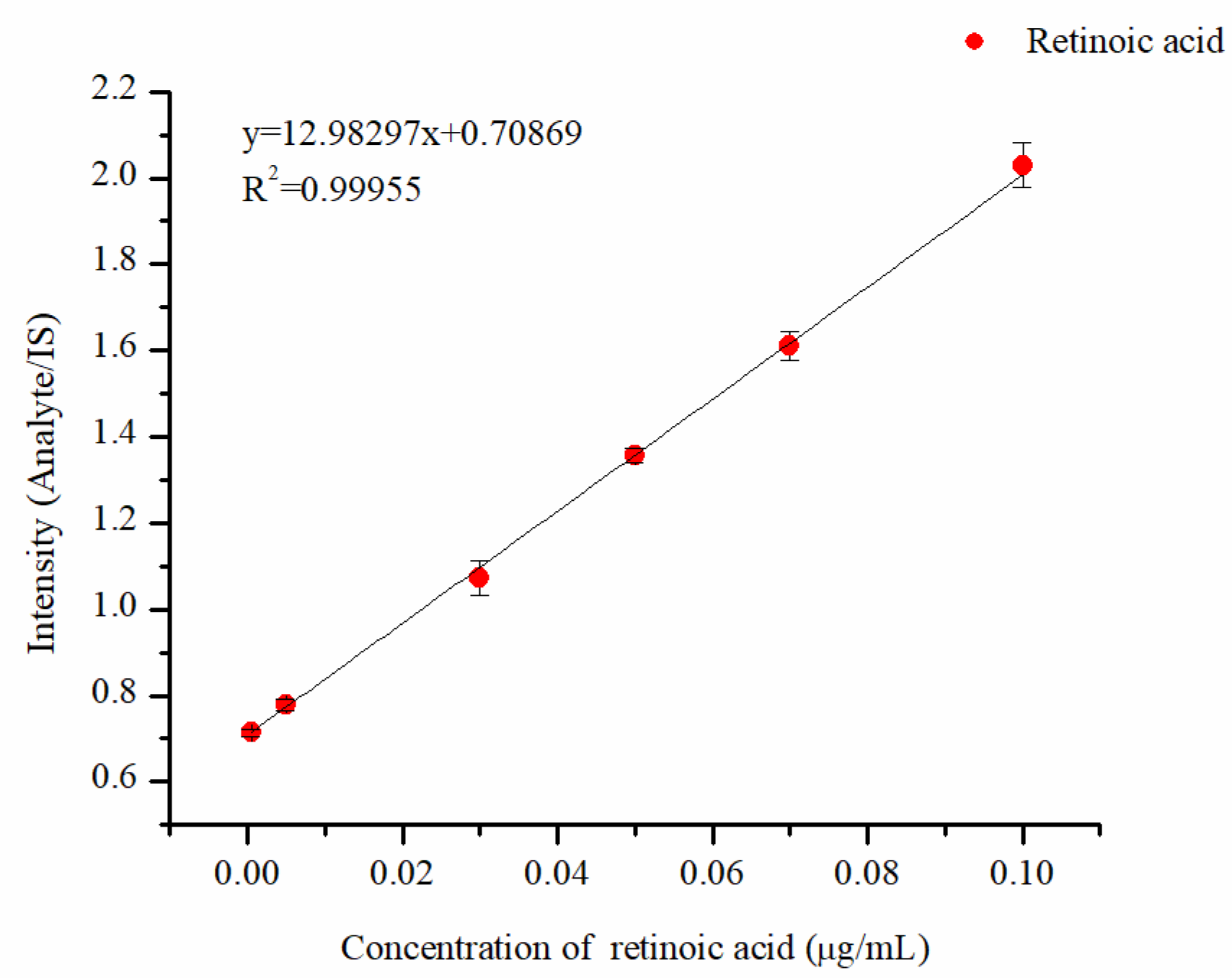
2.4. Sensitivity and Linearity
2.5. Precision of the Experiment
2.6. Sample Recovery Experiment
2.7. Complex Matrix Sample Detection
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instruments
3.3. Sample Preparation
3.4. Derivative Reaction
3.5. MS Conditions
3.6. Derivatization of Retinoic Acid Using Paper Spray MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| TEA | Triethylamine |
| DMPI | N,N′-dimethylpiperazine |
| HATU | 2-(7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| HBTU | O-Benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate |
| PSI-MS | Paper spray ionization mass spectrometry |
| AMS | ambient ionization mass spectrometry |
| RPSI-MS | Reactive Paper Spray Ionization Mass Spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| HPLC | High Performance Liquid Chromatography |
| AJS ESI | Agilent Jet Stream Electrospray Ionization |
| CE | collision energy |
| UHPLC–TOF–MS | Ultra-High Performance Liquid Chromatography—Time of Flight—Mass Spectrometry |
References
- McLafferty, F.W. Tandem mass spectrometry in trace toxicant analysis. Biomed. Mass Spectrom. 1981, 8, 446–448. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.W. Tandem mass spectrometry. Science 1981, 214, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Proctor, B.L. Recent developments in mass spectrometry for the analysis of complex mixtures. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 171–192. [Google Scholar] [CrossRef]
- Bickel, J.; Szewczyk, A.; Aboutara, N.; Jungen, H.; Müller, A.; Ondruschka, B.; Iwersen-Bergmann, S. Chiral analysis of amphetamine, methamphetamine, MDMA and MDA enantiomers in human hair samples. J. Anal. Toxicol. 2024, 48, 226–234. [Google Scholar] [CrossRef]
- Cumin, C.; Gee, L.; Litfin, T.; Muchabaiwa, R.; Martin, G.; Cooper, O.; Heinzelmann-Schwarz, V.; Lange, T.; von Itzstein, M.; Jacob, F.; et al. Highly sensitive spatial glycomics at near-cellular resolution by on-slide derivatization and mass spectrometry imaging. Anal. Chem. 2024, 96, 11163–11171. [Google Scholar] [CrossRef]
- Lopes, M.; Lund, P.J.; Garcia, B.A. An optimized and robust workflow for quantifying the canonical histone ubiquitination marks H2AK119ub and H2BK120ub by LC-MS/MS. bioRxiv 2024, 23, 5405–5420. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Madian, H.; Emad, S.; Hussien, M.; Mazen, D.Z. Utility of green chemistry for sustainable fluorescence derivatization approach for spectrofluorimetric quantification of pemetrexed as antineoplastic drug in pharmaceutical formulation and spiked human plasma. J. Fluoresc. 2024, 1–7. [Google Scholar] [CrossRef]
- Zhong, Z.J.; Ling, J.; Yao, Z.P.; Liu, L.F.; Zheng, J.Y.; Xin, G.Z. Targeted quantification of glutathione/arginine redox metabolism based on a novel paired mass spectrometry probe approach for the functional assessment of redox status. Anal. Chem. 2024, 96, 9885–9893. [Google Scholar] [CrossRef] [PubMed]
- Ljoncheva, M.; Heath, E.; Heath, D.; Džeroski, S.; Kosjek, T. Contaminants of emerging concern: Silylation procedures, evaluation of the stability of silyl derivatives and associated measurement uncertainty. Sci. Total Environ. 2023, 899, 165669. [Google Scholar] [CrossRef]
- Oflu, S.; Erarpat, S.; Zaman, B.T.; Eroğlu, K.; Günkara, Ö.T.; Bakırdere, S.; Turak, F. Quantification of trace fenuron in waste water samples by matrix matching calibration strategy and gas chromatography-mass spectrometry after simultaneous derivatization and preconcentration. Environ. Monit. Assess. 2023, 195, 1063. [Google Scholar] [CrossRef]
- von Törne, W.J.; Klyk-Seitz, U.A.; Piechotta, C. Developing a GC-EI-MS/MS method for quantifying warfarin and five hydroxylated metabolites generated by the Fenton reaction. Environ. Sci. Pollut. 2024, 31, 16986–16994. [Google Scholar] [CrossRef]
- Nguyen, L.A.M.; Pham, T.H.; Ganeshalingam, M.; Thomas, R. A multimodal analytical approach is important in accurately assessing terpene composition in edible essential oils. Food Chem. 2024, 454, 139792. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.; Horinouchi, M.; Yoshiba, Y.; Sakamoto, T.; Sugasawa, H.; Fukushima, T. Determination of imidazole dipeptides and related amino acids in natural seafoods by liquid chromatography-tandem mass spectrometry using a pre-column derivatization reagent. Foods 2024, 13, 1951. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, Y.; Ma, L. Determination of o-quinones in foods by a derivative strategy combined with UHPLC-MS/MS. Food Chem. 2024, 453, 139638. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Hendricks, P.I.; Reynolds, J.C.; Cooks, R.G. Biogenic aldehyde determination by reactive paper spray ionization mass spectrometry. Anal. Chim. Acta 2015, 860, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Borden, S.A.; Saatchi, A.; Palaty, J.; Gill, C.G. A direct mass spectrometry method for cannabinoid quantitation in urine and oral fluid utilizing reactive paper spray ionization. Analyst 2022, 147, 3109–3117. [Google Scholar] [CrossRef]
- Bouza, M.; Foest, D.; Brandt, S.; García-Reyes, J.F.; Franzke, J. Enhanced compound analysis using reactive paper spray mass spectrometry: Leveraging Schiff base reaction for amino acid detection. Anal. Chem. 2024, 96, 5289–5297. [Google Scholar] [CrossRef]
- Song, D.; Liu, J.; Liu, Y. Reactive paper spray ionization mass spectrometry for rapid detection of estrogens in cosmetics. Molecules 2023, 28, 5675. [Google Scholar] [CrossRef]
- de Paula, C.C.A.; Binatti, I.; Coelho Pimenta, J.V.; Augusti, R. Accelerated synthesis of phthalimide derivatives: Intrinsic reactivity of diamines towards phthalic anhydride evaluated by paper spray ionization mass spectrometry. Rapid Commun. Mass. Spectrom. 2022, 36, e9407. [Google Scholar] [CrossRef]
- Sarih, N.M.; Romero-Perez, D.; Bastani, B.; Rauytanapanit, M.; Boisdon, C.; Praneenararat, T.; Tajuddin, H.A.; Abdullah, Z.; Badu-Tawiah, A.K.; Maher, S. Accelerated nucleophilic substitution reactions of dansyl chloride with aniline under ambient conditions via dual-tip reactive paper spray. Sci. Rep. 2020, 10, 21504. [Google Scholar] [CrossRef]
- Yan, X.; Augusti, R.; Li, X.; Cooks, R.G. Chemical reactivity assessment using reactive paper spray ionization mass spectrometry: The Katritzky reaction. ChemPlusChem 2013, 78, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hwang, J.S.; Kim, H.S. N-Nicotinoyl dopamine inhibits skin pigmentation by suppressing of melanosome transfer. Eur. J. Pharmacol. 2015, 769, 250–256. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, A.; Zhou, S.; Ruan, J.; Qian, C.; Wu, C.; Ye, L. Preparation of VC nanoliposomes by high pressure homogenization: Process optimization and evaluation of efficacy, transdermal absorption, and stability. Heliyon 2024, 10, e29516. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiatica in dermatology: An overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.; Del Rosso, J. Going beyond ceramides in moisturizers: The role of natural moisturizing factors. J. Drugs Dermatol. 2024, 23, 466–471. [Google Scholar] [CrossRef]
- Zhen, A.X.; Kang, K.A.; Piao, M.J.; Madushan Fernando, P.D.S.; Lakmini Herath, H.M.U.; Hyun, J.W. Protective effects of astaxanthin on particulate matter 2.5-induced senescence in HaCaT keratinocytes via maintenance of redox homeostasis. Exp. Ther. Med. 2024, 28, 275. [Google Scholar] [CrossRef]
- Mellody, K.T.; Bradley, E.J.; Mambwe, B.; Cotterell, L.F.; Kiss, O.; Halai, P.; Loftus, Z.; Bell, M.; Griffiths, T.W.; Griffiths, C.E.M.; et al. Multifaceted amelioration of cutaneous photoageing by (0.3%) retinol. Int. J. Cosmet. Sci. 2022, 44, 625–635. [Google Scholar] [CrossRef]
- Hakak, H.; Neimann, K.; Toledano, O.; Erlich, M. Individual sol-gel microencapsulation of benzoyl peroxide and tretinoin enables controlled release onto the skin. Heliyon 2024, 10, e32275. [Google Scholar] [CrossRef]
- Kakpovbia, E.E.; Young, T.; Milam, E.C.; Qian, Y.; Yassin, S.; Nicholson, J.; Hu, J.; Troxel, A.B.; Nagler, A.R. Efficacy of topical treatments for mild-to-moderate acne: A systematic review and meta-analysis of randomized control trials. J. Eur. Acad. Dermatol. Venereol. 2024, 39, 775–784. [Google Scholar] [CrossRef]
- Podwojniak, A.; Tan, I.J.; Sauer, J.; Parikh, A.; Cohen, B.A.; Heath, C. Updates on topical dyad and triple combination therapies approved for acne vulgaris. Cureus 2024, 16, e61413. [Google Scholar] [CrossRef] [PubMed]
- Bubna, A.K. Comparison of the clinical efficacy of topical tretinoin 0.05% cream and tacrolimus 0.1% ointment plus iontophoresis in the management of palmoplantar psoriasis. Clin. Exp. Dermatol. 2024, 49, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, M.; Pang, X.; Du, M.; Chen, H.; Li, Z. Effects of secukinumab combined with tretinoin on metabolism, liver enzymes, and inflammatory factors in patients with moderate to severe psoriasis vulgaris. Postepy Dermatol. Alergol. 2024, 41, 113–120. [Google Scholar] [CrossRef]
- Technical Specifications for Cosmetic Safety; China Standard Publishing House: Beijing, China, 2016.
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; The European Parliament and of the Council: Strasbourg, France, 2009; pp. 59–209.
- Desmedt, B.; Rogiers, V.; Courselle, P.; De Beer, J.O.; De Paepe, K.; Deconinck, E. Development and validation of a fast chromatographic method for screening and quantification of legal and illegal skin whitening agents. J. Pharm. Biomed. Anal. 2013, 83, 82–88. [Google Scholar] [CrossRef]
- Desmedt, B.; Van Hoeck, E.; Rogiers, V.; Courselle, P.; De Beer, J.O.; De Paepe, K.; Deconinck, E. Characterization of suspected illegal skin whitening cosmetics. J. Pharm. Biomed. Anal. 2014, 90, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.; Nam, H.G.; Zare, R.N. Microdroplet fusion mass spectrometry for fast reaction kinetics. Proc. Natl. Acad. Sci. USA 2015, 112, 3898–3903. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Chen, H.; Zare, R.N. Scale-up of microdroplet reactions by heated ultrasonic nebulization. Chem. Sci. 2019, 10, 9367–9373. [Google Scholar] [CrossRef]
- Müller, T.; Badu-Tawiah, A.; Cooks, R.G. Accelerated carbon-carbon bond-forming reactions in preparative electrospray. Angew. Chem. Int. Ed. Engl. 2012, 51, 11832–11835. [Google Scholar] [CrossRef]
- Nie, H.; Wei, Z.; Qiu, L.; Chen, X.; Holden, D.T.; Cooks, R.G. High-yield gram-scale organic synthesis using accelerated microdroplet/thin film reactions with solvent recycling. Chem. Sci. 2020, 11, 2356–2361. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, X.; Wang, J.; Zhang, S.; Zhang, X.; Cooks, R.G. High yield accelerated reactions in nonvolatile microthin films: Chemical derivatization for analysis of single-cell intracellular fluid. Chem. Sci. 2018, 9, 7779–7786. [Google Scholar] [CrossRef]
- Yan, X. Emerging microdroplet chemistry for synthesis and analysis. Int. J. Mass. Spectrom. 2021, 468, 116639. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Cooks, R.G. The role of the interface in thin film and droplet accelerated reactions studied by competitive substituent effects. Angew. Chem. Int. Ed. Engl. 2016, 55, 3433–3437. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bain, R.M.; Cooks, R.G. Organic reactions in microdroplets: Reaction acceleration revealed by mass spectrometry. Angew. Chem. Int. Ed. Engl. 2016, 55, 12960–12972. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, Y.J.; Zhang, J.; Sun, T.Q.; Guo, Y.L. Derivatization strategy for simultaneous molecular imaging of phospholipids and low-abundance free fatty acids in thyroid cancer tissue sections. Anal. Chem. 2019, 91, 4070–4076. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, N.; Hu, X.; Di, B.; Liu, Y.; Sun, H. Chemical derivatization and paper spray ionization mass spectrometry for fast screening of retinoic acid in cosmetics. Molecules 2024, 29, 4491. [Google Scholar] [CrossRef]


| Chemical Compound | Added (μg·mL−1) | Found (μg·mL−1) | Recovery Rate (%) | Average Recovery Rate (%) | RSD (%) (N = 3) |
|---|---|---|---|---|---|
| Retinoic acid | 0.001 | 0.0009789 | 97.89 | 96.29 | 1.77 |
| 0.0009449 | 94.49 | ||||
| 0.0009649 | 96.49 | ||||
| 0.01 | 0.01039 | 103.9 | 100.9 | 4.25 | |
| 0.009623 | 96.23 | ||||
| 0.01025 | 102.5 | ||||
| 0.1 | 0.09847 | 98.47 | 98.78 | 1.51 | |
| 0.09746 | 97.46 | ||||
| 0.1004 | 100.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Wang, C.; Zhang, N.; Li, J.; Yuan, S.; Yu, L.; Di, B.; Liu, Y. Detection of Retinoic Acid in Cosmetics Using Reactive Paper Spray Ionization Mass Spectrometry. Molecules 2025, 30, 1906. https://doi.org/10.3390/molecules30091906
Bao Y, Wang C, Zhang N, Li J, Yuan S, Yu L, Di B, Liu Y. Detection of Retinoic Acid in Cosmetics Using Reactive Paper Spray Ionization Mass Spectrometry. Molecules. 2025; 30(9):1906. https://doi.org/10.3390/molecules30091906
Chicago/Turabian StyleBao, Yuzhang, Chenyu Wang, Na Zhang, Jie Li, Song Yuan, Liju Yu, Bin Di, and Yang Liu. 2025. "Detection of Retinoic Acid in Cosmetics Using Reactive Paper Spray Ionization Mass Spectrometry" Molecules 30, no. 9: 1906. https://doi.org/10.3390/molecules30091906
APA StyleBao, Y., Wang, C., Zhang, N., Li, J., Yuan, S., Yu, L., Di, B., & Liu, Y. (2025). Detection of Retinoic Acid in Cosmetics Using Reactive Paper Spray Ionization Mass Spectrometry. Molecules, 30(9), 1906. https://doi.org/10.3390/molecules30091906







