Conformational Locking of the Geometry in Photoluminescent Cyclometalated N^C^N Ni(II) Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization
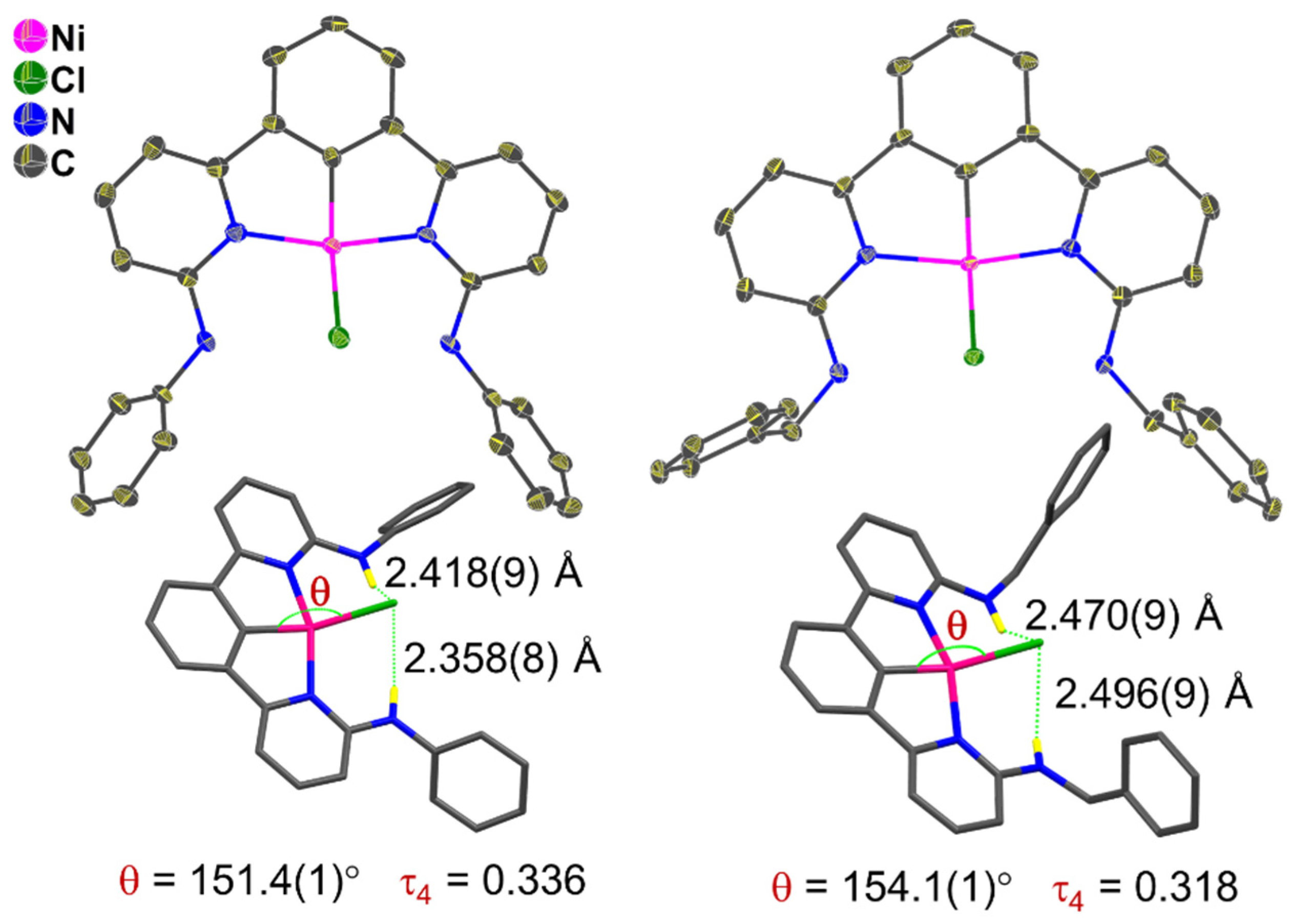
2.2. Experimental Crystal Structures from X-Ray Diffractometry and DFT-Calculated Molecular Structures
2.3. Electrochemistry and DFT-Calculated Frontier Molecular Orbitals
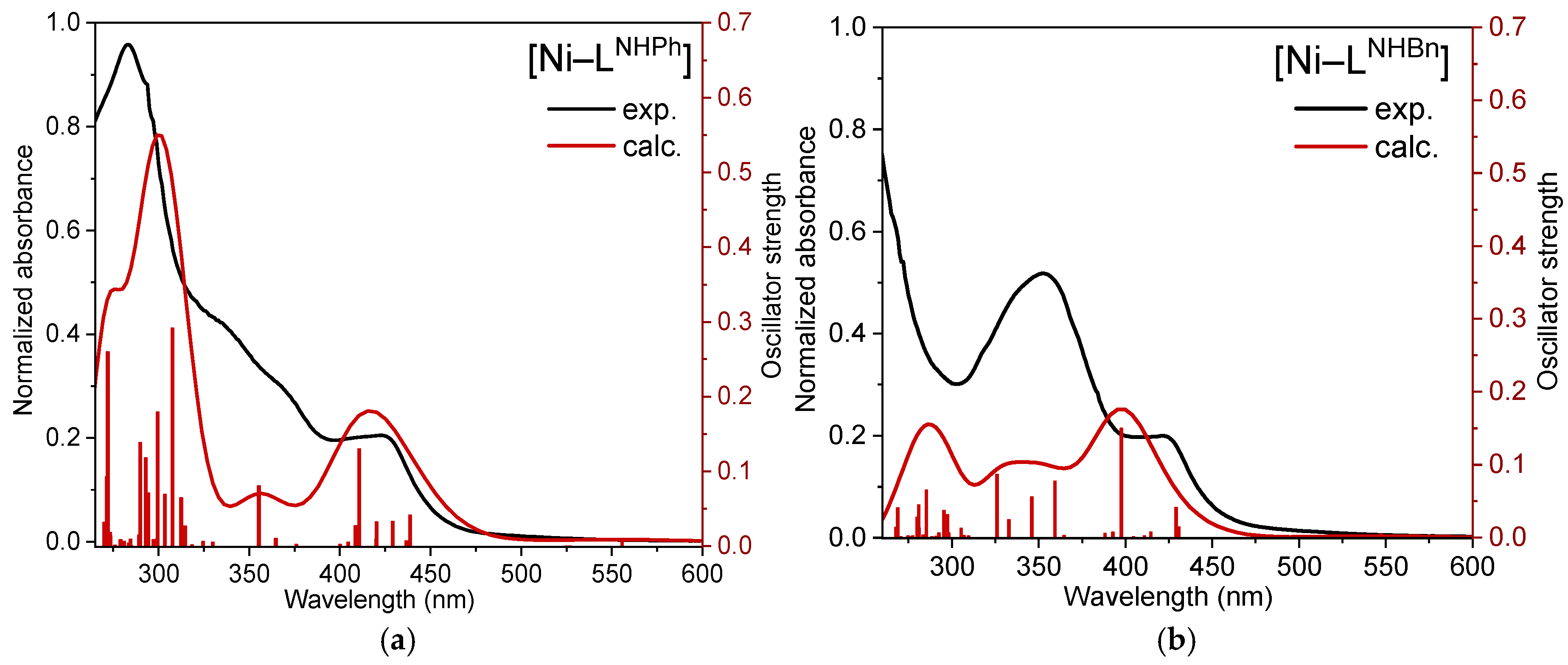
2.4. UV-Vis Absorption Spectra and TD-DFT-Calculated Electronic Transitions
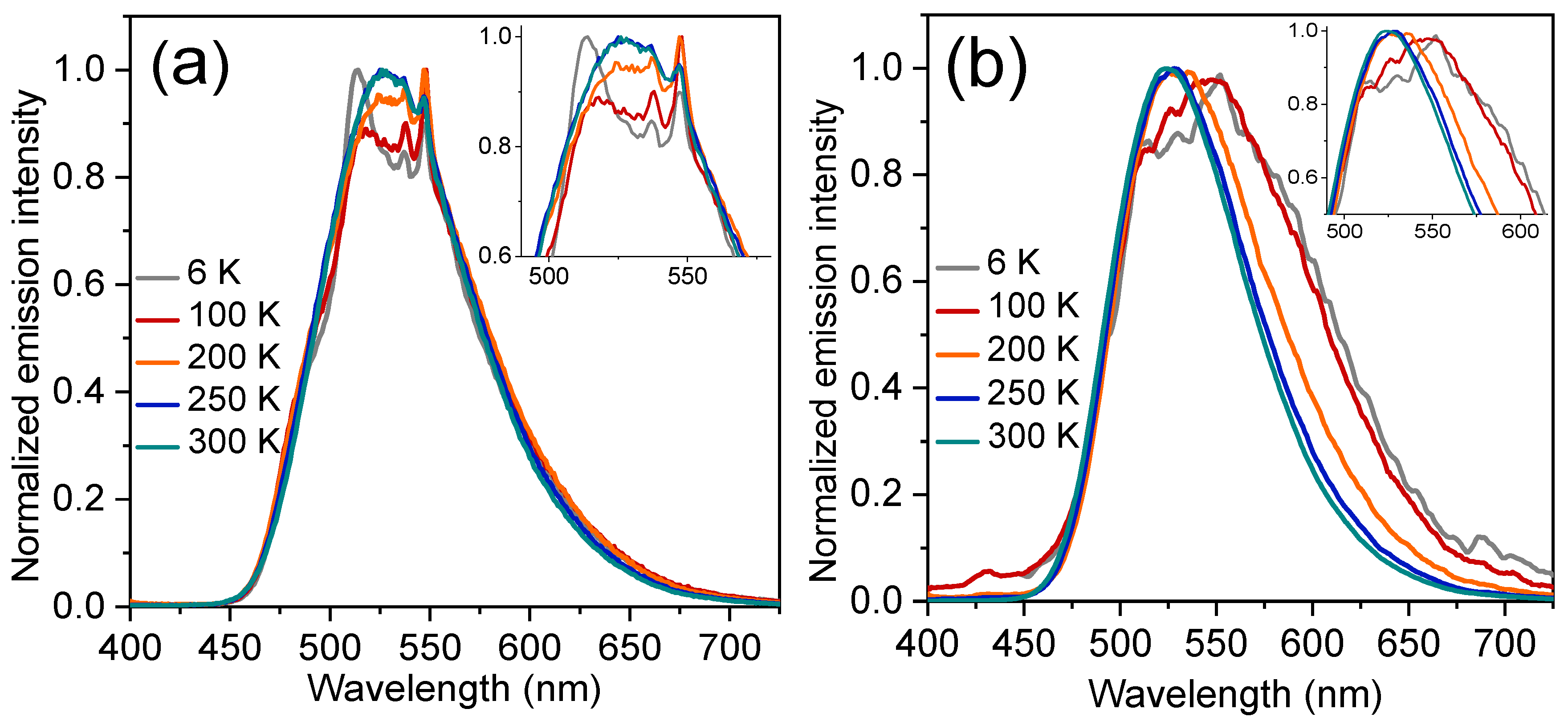
2.5. Photophysical Properties at Low Temperature and in Poly(methyl methacylate) (PMMA) Matrices
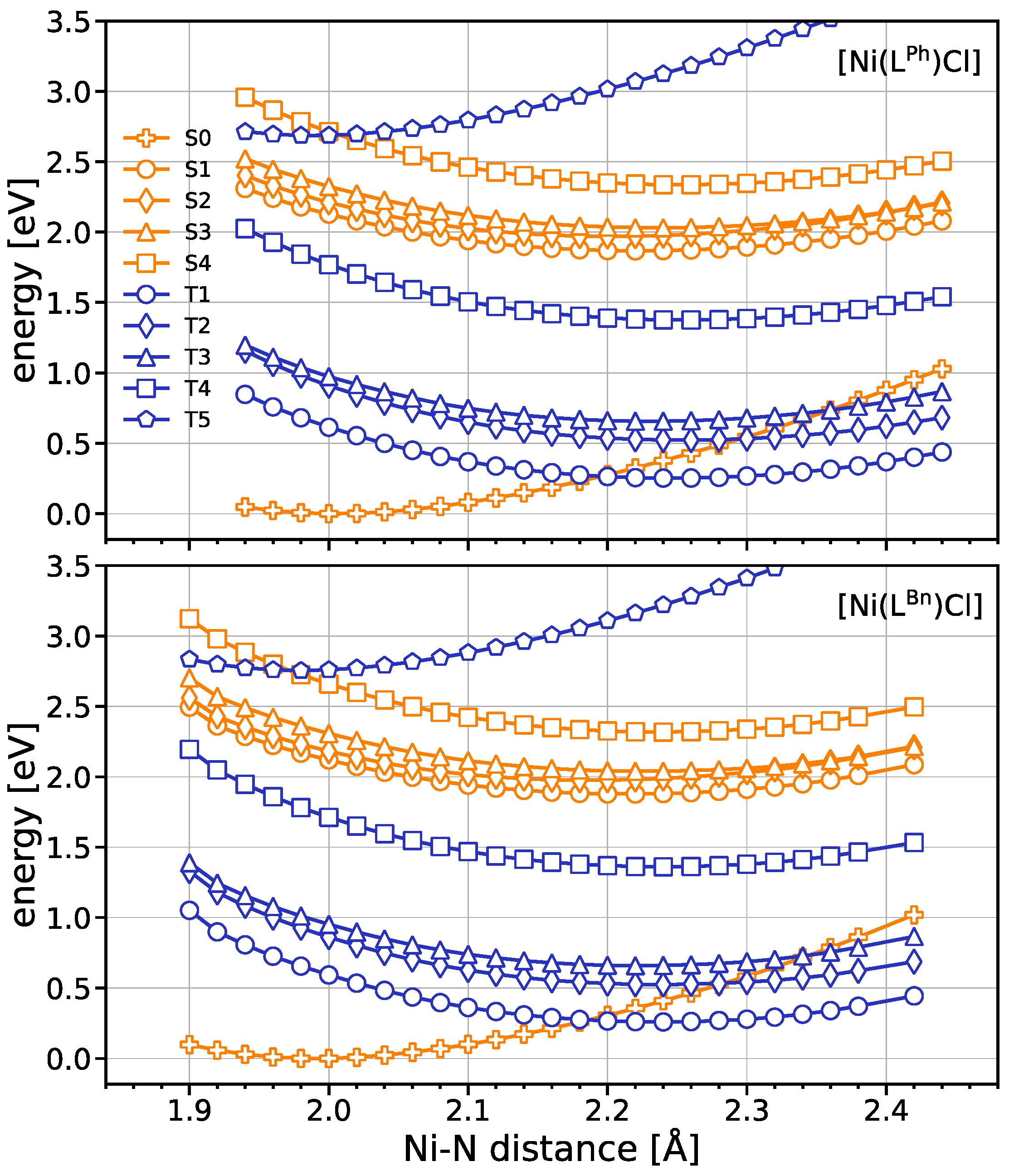
2.6. DFT Calculations
2.7. Femto- and Nanosecond Transient Absorption Spectroscopy (fs-TAS and ns-TAS)
3. Experimental Section
3.1. Materials and Syntheses
3.2. Instrumentation
3.3. Crystal Structure Determination
3.4. Photophysical Measurements
3.5. Quantum Chemical Calculations Using Density Functional Theory (DFT)
3.6. Structural Distortion Calculations Using Time-Dependent Density Functional Theory (TD-DFT) with the Tamm–Dancoff Approximation (TDA)
3.7. Radiative Lifetime Calculations Using TD-DFT with Spin–Orbit Coupling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yam, V.W.-W.; Law, A.S.-Y. Luminescent d8 Metal Complexes of Platinum(II) and Gold(III): From Photophysics to Photofunctional Materials and Probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Parasram, M.; Gevorgyan, V. Visible Light-Induced Transition Metal-Catalyzed Transformations: Beyond Conventional Photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Hussain, F.; Zeng, C.; Wang, B.; Li, Z.; Kozin, I.; Wang, S. Multiresponsive Tetradentate Phosphorescent Metal Complexes as Highly Sensitive and Robust Luminescent Oxygen Sensors: Pd(II) Versus Pt(II) and 1,2,3-Triazolyl Versus 1,2,4-Triazolyl. ACS Appl. Mater. Interfaces 2019, 11, 12666–12674. [Google Scholar] [CrossRef]
- Fleetham, T.; Ji, Y.; Huang, L.; Fleetham, T.S.; Li, J. Efficient and Stable Single-Doped White OLEDs Using a Palladium-Based Phosphorescent Excimer. Chem. Sci. 2017, 8, 7983–7990. [Google Scholar] [CrossRef]
- Hau, F.K.-W.; Lo, H.-S.; Yam, V.W.-W. Synthesis and Photophysical Studies of Calixarene-Based Alkynylplatinum(II) Terpyridine Complexes with Various Receptor Sites for Colorimetric and Luminescence Sensing of Anions. Chem.‒Eur. J. 2016, 22, 3738–3749. [Google Scholar] [CrossRef]
- Bryant, M.J.; Skelton, J.M.; Hatcher, L.E.; Stubbs, C.; Madrid, E.; Pallipurath, A.R.; Thomas, L.H.; Woodall, C.H.; Christensen, J.; Fuertes, S.; et al. A Rapidly-Reversible Absorptive and Emissive Vapochromic Pt(II) Pincer-Based Chemical Sensor. Nat. Commun. 2017, 8, 1800. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-X.; Mak, E.C.-L.; Lo, K.K.-W. Photofunctional Transition Metal Complexes as Cellular Probes, Bioimaging Reagents and Phototherapeutics. Inorg. Chem. Front. 2021, 8, 4553–4579. [Google Scholar] [CrossRef]
- Lo, K.-W.; Tong, G.S.M.; Cheng, G.; Low, K.-H.; Che, C.-M. Dinuclear PtII Complexes with Strong Blue Phosphorescence for Operationally Stable Organic Light-Emitting Diodes with EQE up to 23% at 1000 cd m−2. Angew. Chem. Int. Ed. 2022, 61, e202115515. [Google Scholar] [CrossRef]
- Ko, C.-C.; Yam, V.W.-W. Coordination Compounds with Photochromic Ligands: Ready Tunability and Visible Light-Sensitized Photochromism. Acc. Chem. Res. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Lai, P.-N.; Brysacz, C.H.; Alam, M.K.; Ayoub, N.A.; Gray, T.G.; Bao, J.; Teets, T.S. Highly Efficient Red-Emitting Bis-Cyclometalated Iridium Complexes. J. Am. Chem. Soc. 2018, 140, 10198–10207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Cheng, C.C.; Chih, Y.R.; Lin, Y.-T.; Chen, H.-Y.; Chen, Y.J.; Endicott, J.F. Low-Temperature Spectra and Density Functional Theory Modeling of Ru(II)-Bipyridine Complexes with Cyclometalated Ancillary Ligands: The Excited State Spin–Orbit Coupling Origin of Variations in Emission Efficiencies. J. Phys. Chem. A 2019, 123, 9431–9449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Song, J.; Qu, J.; Li, B.; Zhu, W.; Wong, W.-Y. Near-Infrared Emitting Materials via Harvesting Triplet Excitons: Molecular Design, Properties, and Application in Organic Light Emitting Diodes. Adv. Opt. Mater. 2018, 6, 1800466. [Google Scholar] [CrossRef]
- Krause, M.; Kourkoulos, D.; González-Abradelo, D.; Meerholz, K.; Strassert, C.A.; Klein, A. Luminescent PtII Complexes of Tridentate Cyclometalating 2,5-Bis(aryl)-pyridine Ligands. Eur. J. Inorg. Chem. 2017, 2017, 5215–5223. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Chelushkin, P.S.; Abakumova, T.O.; Zhemkov, V.A.; Kim, M.; Bezprozvanny, I.; Gurzhiy, V.V.; Melnikov, A.S.; Anufrikov, Y.A.; Koshevoy, I.O.; et al. Reactions of Cyclometalated Platinum(II) [Pt(N^C)(PR3)Cl] Complexes with Imidazole and Imidazole-Containing Biomolecules: Fine-Tuning of Reactivity and Photophysical Properties via Ligand Design. Inorg. Chem. 2019, 58, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Sivchik, V.; Sarker, R.K.; Liu, Z.-Y.; Chung, K.-Y.; Grachova, E.V.; Karttunen, A.J.; Chou, P.-T.; Koshevoy, I.O. Improvement of the Photophysical Performance of Platinum-Cyclometalated Complexes in Halogen-Bonded Adducts. Chem. Eur. J. 2018, 24, 11475–11484. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Aleksandrova, I.O.; Karttunen, A.J.; Tunik, S.P.; Koshevoy, I.O. Dibenzothiophene-Platinated Complexes: Probing the Effect of Ancillary Ligands on the Photophysical Performance. Dalton Trans. 2017, 46, 3895–3905. [Google Scholar] [CrossRef]
- Fleetham, T.; Li, G.; Li, J. Phosphorescent Pt(II) and Pd(II) Complexes for Efficient, High-Color-Quality, and Stable OLEDs. Adv. Mater. 2017, 29, 1601861. [Google Scholar] [CrossRef]
- Chow, P.-K.; Cheng, G.; Tong, G.S.M.; Ma, C.; Kwok, W.-M.; Ang, W.-H.; Chung, C.Y.-S.; Yang, C.; Wang, F.; Che, C.-M. Highly luminescent Palladium(II) Complexes with Sub-Millisecond Blue to Green Phosphorescent Excited States. Photocatalysis and Highly Efficient PSF-OLEDs. Chem. Sci. 2016, 7, 6083–6098. [Google Scholar] [CrossRef]
- Chow, P.K.; Ma, C.; To, W.-P.; Tong, G.S.M.; Lai, S.-L.; Kui, S.C.F.; Kwok, W.-M.; Che, C.-M. Strongly Phosphorescent Palladium(II) Complexes of Tetradentate Ligands with Mixed Oxygen, Carbon, and Nitrogen Donor Atoms: Photophysics, Photochemistry, and Applications. Angew. Chem. Int. Ed. 2013, 52, 11775–11779. [Google Scholar] [CrossRef]
- Hung, F.-F.; Wu, S.-X.; To, W.-P.; Kwong, W.-L.; Guan, X.; Lu, W.; Low, K.-H.; Che, C.-M. Palladium(II) Acetylide Complexes with Pincer-Type Ligands: Photophysical Properties, Intermolecular Interactions, and Photo-cytotoxicity. Chem. Asian J. 2017, 12, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kletsch, L.; Jordan, R.; Köcher, A.S.; Buss, S.; Strassert, C.A.; Klein, A. Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules 2021, 26, 5051. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, T.; Buss, S.; Petrovskii, S.K.; Grachova, E.V.; Krause, M.; Klein, A.; Strassert, C.A.; Koshevoy, I.O.; Hirva, P. Photophysics and Excited State Dynamics of Cyclometalated [M(C^N^N)(CN)] (M = Ni, Pd, Pt) Complexes: A Theoretical and Experimental Study. Inorg. Chem. 2021, 60, 8777–8789. [Google Scholar] [CrossRef]
- Mews, N.M.; Reimann, M.; Hörner, G.; Kaupp, M.; Schubert, H.; Berkefeld, A. A four-parameter system for rationalising the electronic properties of transition metal–radical ligand complexes. Dalton Trans. 2020, 49, 9735–9742. [Google Scholar] [CrossRef]
- Poirier, S.; Lynn, H.; Reber, R. Variation of M···H−C Interactions in Square-Planar Complexes of Nickel(II), Palladium(II), and Platinum(II) Probed by Luminescence Spectroscopy and X-ray Diffraction at Variable Pressure. Inorg. Chem. 2018, 57, 7713–7723. [Google Scholar] [CrossRef]
- Pilia, L.; Espa, D.; Barsella, A.; Fort, A.; Makedonas, C.; Marchiò, L.; Mercuri, M.L.; Serpe, A.; Mitsopoulou, C.A.; Deplano, P. Combined Experimental and Theoretical Study on Redox-Active d8 Metal Dithione Dithiolato Complexes Showing Molecular Second-Order Nonlinear Optical Activity. Inorg. Chem. 2011, 50, 10015–10027. [Google Scholar] [CrossRef]
- Maisuls, I.; Wang, C.; Gutierrez Suburu, M.E.; Wilde, S.; Daniliuc, C.-G.; Brünink, D.; Doltsinis, N.L.; Ostendorp, S.; Wilde, G.; Kösters, J.; et al. Ligand-controlled and nanoconfinement-boosted luminescence employing Pt(II) and Pd(II) complexes: From color-tunable aggregation-enhanced dual emitters towards self-referenced oxygen reporters. Chem. Sci. 2021, 12, 3270–3281. [Google Scholar] [CrossRef]
- You, C.; Xia, F.; Zhao, Y.; Zhang, Y.; Sheng, Y.; Wu, Y.; Hang, X.-C.; Chen, F.; Ma, H.; Shen, K.; et al. Probing Triplet Excited States and Managing Blue Light Emission of Neutral Tetradentate Platinum(II) Complexes. J. Phys. Chem. Lett. 2018, 9, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tong, G.S.M.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly phosphorescent platinum(II) emitters: Photophysics, materials and biological application. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Luminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Goodman, B.A.; Raynor, J.B. Electron Spin Resonance of Transition Metal Complexes. Adv. Inorg. Chem. Radiochem. 1970, 13, 135–362. [Google Scholar] [CrossRef]
- Ogawa, T.; Sinha, N.; Pfund, B.; Prescimone, A.; Wenger, O.S. Molecular Design Principles to Elongate the Metal-to-Ligand Charge Transfer Excited-State Lifetimes of Square-Planar Nickel(II) Complexes. J. Am. Chem. Soc. 2022, 144, 21948–21960. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ran, G.; Hou, C.-L.; Zhang, R.; Mangel, D.N.; Yang, Z.-S.; Zhu, M.; Zhang, W.; Zhang, J.; Sessler, J.L.; et al. Nonaromatic Organonickel(II) Phototheranostics. J. Am. Chem. Soc. 2022, 144, 7346–7356. [Google Scholar] [CrossRef]
- Wegeberg, C.; Wenger, O.S. Luminescent First-Row Transition Metal Complexes. JACS Au 2021, 1, 1860–1876. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Schötz, K.; Papadopoulos, I.; Heinemann, F.W.; Maid, H.; Guldi, D.M.; Köhler, A.; Hörner, G.; Weber, B. A Fluorescence-Detected Coordination-Induced Spin State Switch. J. Am. Chem. Soc. 2021, 143, 3466–3480. [Google Scholar] [CrossRef]
- Lauenstein, R.; Mader, S.L.; Derondeau, H.; Esezobor, O.Z.; Block, M.; Römer, A.J.; Jandl, C.; Riedle, E.; Kaila, V.R.I.; Hauer, J.; et al. The central role of the metal ion for photoactivity: Zn– vs. Ni–Mabiq. Chem. Sci. 2021, 12, 7521–7532. [Google Scholar] [CrossRef]
- Ting, S.I.; Garakyaraghi, S.; Taliaferro, C.M.; Shields, B.J.; Scholes, G.D.; Castellano, F.N.; Doyle, A.G. 3d-d Excited States of Ni(II) Complexes Relevant to Photoredox Catalysis: Spectroscopic Identification and Mechanistic Implications. J. Am. Chem. Soc. 2020, 142, 5800–5810. [Google Scholar] [CrossRef]
- Wong, Y.-S.; Tang, M.-C.; Ng, M.; Yam, V.W.-W. Toward the Design of Phosphorescent Emitters of Cyclometalated Earth-Abundant Nickel(II) and Their Supramolecular Study. J. Am. Chem. Soc. 2020, 142, 7638–7646. [Google Scholar] [CrossRef]
- Cope, J.D.; Denny, J.A.; Lamb, R.W.; McNamara, L.E.; Hammer, N.I.; Webster, C.E.; Hollis, T.K. Synthesis, Characterization, Photophysics, and a Ligand Rearrangement of CCC-NHC Pincer Nickel Complexes: Colors, Polymorphs, Emission, and Raman Spectra. J. Organomet. Chem. 2017, 845, 258–265. [Google Scholar] [CrossRef]
- Gao, M.; To, W.-P.; Tong, G.S.M.; Du, L.; Low, K.-H.; Tang, Z.; Lu, W.; Che, C.-M. Dinuclear Cyclometalated Pincer Nickel(II) Complexes with Metal-Metal-to-Ligand Charge Transfer Excited States and Near-Infrared Emission. Angew. Chem. Int. Ed. 2024, 64, e202414411. [Google Scholar] [CrossRef]
- Klein, A.; Hurkes, N.; Kaiser, A.; Wielandt, W. π-Stacking Modulates the Luminescence of [(dppz)Ni(Mes)Br] (dppz = dipyrido[3,2-a:2’,3’-c]phenazine, Mes = 2,4,6-trimethylphenyl). Z. Anorg. Allg. Chem. 2007, 633, 1659–1665. [Google Scholar] [CrossRef]
- Ogawa, T.; Wenger, O.S. Nickel(II) Analogues of Phosphorescent Platinum(II) Complexes with Picosecond Excited-State Decay. Angew. Chem. Int. Ed. 2023, 62, e202312851. [Google Scholar] [CrossRef]
- Moreth, D.; Cappellari, M.V.; Müller, A.; Oster, A.; Schwab, D.; Doltsinis, N.L.; Strassert, C.A.; Schatzschneider, U. Luminescent N^C^N Pincer Ni(II), Pd(II), and Pt(II) Complexes with a Pendant Coumarin Group: The Role of Auxiliary Ligands and Environments. Inorg. Chem. 2025, 64, 4223–4235. [Google Scholar] [CrossRef]
- Niazi, M.; Maisuls, I.; Strassert, C.A.; Klein, A. Molecular Rigidification of Cyclometalated N^C^N Pt(II)- and Pd(II)-Based Triplet Emitters. Organometallics 2024, 43, 1547–1556. [Google Scholar] [CrossRef]
- Kletsch, L.; Hörner, G.; Klein, A. Cyclometalated Ni(II) Complexes [Ni(N∧C∧N)X] of the Tridentate 2,6-di(2-pyridyl)phen-ide Ligand. Organometallics 2020, 39, 2820–2829. [Google Scholar] [CrossRef]
- Sandleben, A.; Vogt, N.; Hörner, G.; Klein, A. Redox Series of Cyclometalated Nickel Complexes [Ni((R)Ph(R′)bpy)Br]+/0/–/2– (H–(R)Ph(R′)bpy = Substituted 6-Phenyl-2,2′-bipyridine). Organometallics 2018, 37, 3332–3341. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheung, T.-C.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Luminescent Mononuclear and Binuclear Cyclometalated Palladium(II) Complexes of 6-Phenyl-2,2′-bipyridines: Spectroscopic and Structural Comparisons with Platinum(II) Analogues. Inorg. Chem. 2000, 39, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.-C.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Photoluminescent cyclometallated diplatinum(II,II) complexes: Photophysical properties and crystal structures of [PtL(PPh3)]ClO4, and [Pt2L2(µ-dppm)][ClO4] (HL = 6-phenyl-2,2′-bipyridine, dppm = Ph2PCH2PPh2). J. Chem. Soc. Dalton Trans. 1996, 8, 1645–1651. [Google Scholar] [CrossRef]
- Karlen, T.; Ludi, A.; Güdel, H.U. One-Dimensional Migration of 3MLCT Excitation Energy in PdII(phbpy)Cl (phbpy = 6-Phenyl-2,2′-bipyridine). Inorg. Chem. 1991, 10, 2249–2250. [Google Scholar] [CrossRef]
- Zheng, Q.; Borsley, S.; Nichol, G.S.; Duarte, F.; Cockroft, S.L. The Energetic Significance of Metallophilic Interactions. Angew. Chem. Int. Ed. 2019, 58, 12617–12623. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Jordan, R.; Schäfer, S.A.; Sander, N.; Maisuls, I.; Hamacher, C.; Friedel, J.; Strassert, C.A.; Klein, A. Assessing the Character of the C6F5 Ligand from the Electrochemical and Photophysical Properties of [Ni(C6F5)2(N^N)] Complexes. Inorg. Chem. 2024, 63, 11079–11091. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternative Route to Highly Luminescent Platinum(II) Complexes: Cyclometalation with N^C^N-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef]
- Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Facile Synthesis and Characterization of Phosphorescent Pt(N∧C∧N)X Complexes. Inorg. Chem. 2010, 49, 11276–11286. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, A.; Konno, Y.; Shinozaki, K. Determination of excimer emission quantum yield of Pt(dpb)Cl (dpbH = 1,3-di(2-pyridyl)benzene and its analogues in solution. J. Lumin. 2019, 207, 482–490. [Google Scholar] [CrossRef]
- Tarran, W.A.; Freeman, G.R.; Murphy, L.; Benham, A.M.; Kataky, R.; Williams, J.A.G. Platinum(II) Complexes of N^C^N-Coordinating 1,3-Bis(2-pyridyl)benzene Ligands: Thiolate Coligands Lead to Strong Red Luminescence from Charge-Transfer States. Inorg. Chem. 2014, 53, 5738–5749. [Google Scholar] [CrossRef]
- Tong, G.S.-M.; Che, C.-M. Emissive or Nonemissive? A Theoretical Analysis of the Phosphorescence Efficiencies of Cyclometalated Platinum(II) Complexes. Chem.–Eur. J. 2009, 15, 7225–7237. [Google Scholar] [CrossRef]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Hofbeck, T.; Czerwieniec, R.; Suleymanova, A.F.; Kozhevnikov, D.N.; Yersin, H. Brightly Luminescent Pt(II) Pincer Complexes with a Sterically Demanding Carboranyl-Phenylpyridine Ligand: A New Material Class for Diverse Optoelectronic Applications. J. Am. Chem. Soc. 2014, 136, 9637–9642. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-dependent Density Functional Theory within the Tamm–Dancoff Approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dunlop, D.; Ludvíková, L.; Banerjee, A.; Ottosson, H.; Slanina, T. Excited-state (anti)aromaticity explains why azulene disobeys Kasha’s rule. J. Am. Chem. Soc. 2023, 145, 21569–21575. [Google Scholar] [CrossRef] [PubMed]
- Röhrs, M.; Escudero, D. Multiple anti-Kasha emissions in transition-metal complexes. J. Phys. Chem. Lett. 2019, 10, 5798–5804. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Chakrabarti, S.; Ruud, K. Anomalous phosphorescence from an organometallic white-light phosphor. J. Phys. Chem. Lett. 2017, 8, 4893–4897. [Google Scholar] [CrossRef]
- APEX3—Software Suite for Crystallographic Programs; Bruker AXS, Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr., Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Van Stokkum, I.H.M.; Larsen, D.S.; Van Grondelle, R. Global and Target Analysis of Time-resolved Spectra. Biochim. Biophys. Acta. 2004, 1657, 82–104. [Google Scholar] [CrossRef]
- Snellenburg, J.J.; Laptenok, S.; Seger, R.; Mullen, K.M.; Van Stokkum, I.H.M. Glotaran: A Java-Based Graphical User Interface for the R Package TIMP. J. Stat. Soft. 2012, 49, 1–22. [Google Scholar] [CrossRef]
- Mullen, K.M.; Van Stokkum, I.H.M. TIMP: An R Package for Modeling Multi-way Spectroscopic Measurements. J. Stat. Soft. 2007, 18, 1–46. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative Assessment of a New Nonempirical Density Functional: Molecules and Hydrogen-bonded Complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef]
- Rohlfing, C.M.; Hay, P.J.; Martin, R.L. An Effective Core Potential Investigation of Ni, Pd, and Pt and their Monohydrides. J. Chem. Phys. 1986, 85, 1447–1455. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- De Souza, B.; Farias, G.; Neese, F.; Izsák, R. Predicting Phosphorescence Rates of Light Organic Molecules Using Time-Dependent Density Functional Theory and the Path Integral Approach to Dynamics. J. Chem. Theory Comput. 2019, 15, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- van Lenthe, E.; Snijders, E.G.; Baerends, E.J. The zero-order regular approximation for relativistic effects: The effect of spin-orbit coupling in closed shell molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.; Sandleben, A.; Kletsch, L.; Schäfer, S.; Chin, M.T.; Vicic, D.A.; Hörner, G.; Klein, A. Role of the X Coligands in Cyclometalated [Ni(Phbpy)X] Complexes (HPhbpy = 6-Phenyl-2,2′-bipyridine). Organometallics 2021, 40, 1776–1785. [Google Scholar] [CrossRef]



| [Ni(LNHPh)Cl] | [Ni(LNHBn)Cl] | ||
|---|---|---|---|
| λem, max (nm) | 510 | 504 | |
| 2MeTHF (77 K) | ΦPL ± 0.05 | 0.30 | 0.40 |
| τav_amp (ns) τav_amp (ms) | 3.34 ± 0.01 648 ± 3 | 3.63 ± 0.02 180 ± 10 | |
| PMMA (6 K) | τav_amp (ns) τav_amp (ms) | 1.91 ± 0.04 476 ± 6 | 2.73 ± 0.02 93 ± 3 |
| PMMA (100 K) | τav_amp (ns) τav_amp (ms) | 1.80 ± 0.05 464 ± 6 | 2.69 ± 0.03 74 ± 3 |
| PMMA (200 K) | τav_amp (ns) τav_amp (ms) | 1.69 ± 0.04 161 ± 5 | 2.46 ± 0.05 16 ± 1 |
| PMMA (250 K) | τav_amp (ns) τav_amp (ms) | 1.51 ± 0.03 163 ± 4 | 2.24 ± 0.04 10.4 ± 0.2 |
| PMMA (300 K) | τav_amp (ns) τav_amp (ms) | 1.41 ± 0.04 143 ± 5 | 2.18 ± 0.02 1.05 ± 0.01 |
| ΦPL ± 0.05 | 0.03 ± 0.02 | 0.04 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, M.; Maisuls, I.; Mai, L.A.; Schäfer, S.A.; Oster, A.; Diaz, L.S.; Guldi, D.M.; Doltsinis, N.L.; Strassert, C.A.; Klein, A. Conformational Locking of the Geometry in Photoluminescent Cyclometalated N^C^N Ni(II) Complexes. Molecules 2025, 30, 1901. https://doi.org/10.3390/molecules30091901
Niazi M, Maisuls I, Mai LA, Schäfer SA, Oster A, Diaz LS, Guldi DM, Doltsinis NL, Strassert CA, Klein A. Conformational Locking of the Geometry in Photoluminescent Cyclometalated N^C^N Ni(II) Complexes. Molecules. 2025; 30(9):1901. https://doi.org/10.3390/molecules30091901
Chicago/Turabian StyleNiazi, Maryam, Iván Maisuls, Lukas A. Mai, Sascha A. Schäfer, Alex Oster, Lukas Santiago Diaz, Dirk M. Guldi, Nikos L. Doltsinis, Cristian A. Strassert, and Axel Klein. 2025. "Conformational Locking of the Geometry in Photoluminescent Cyclometalated N^C^N Ni(II) Complexes" Molecules 30, no. 9: 1901. https://doi.org/10.3390/molecules30091901
APA StyleNiazi, M., Maisuls, I., Mai, L. A., Schäfer, S. A., Oster, A., Diaz, L. S., Guldi, D. M., Doltsinis, N. L., Strassert, C. A., & Klein, A. (2025). Conformational Locking of the Geometry in Photoluminescent Cyclometalated N^C^N Ni(II) Complexes. Molecules, 30(9), 1901. https://doi.org/10.3390/molecules30091901








