Surface-Enhanced Raman Spectroscopy on Gold Nanoparticle for Sperm Quality Discrimination
Abstract
1. Introduction
2. Results
2.1. Spermogram Analysis: Conventional Semen Parameters
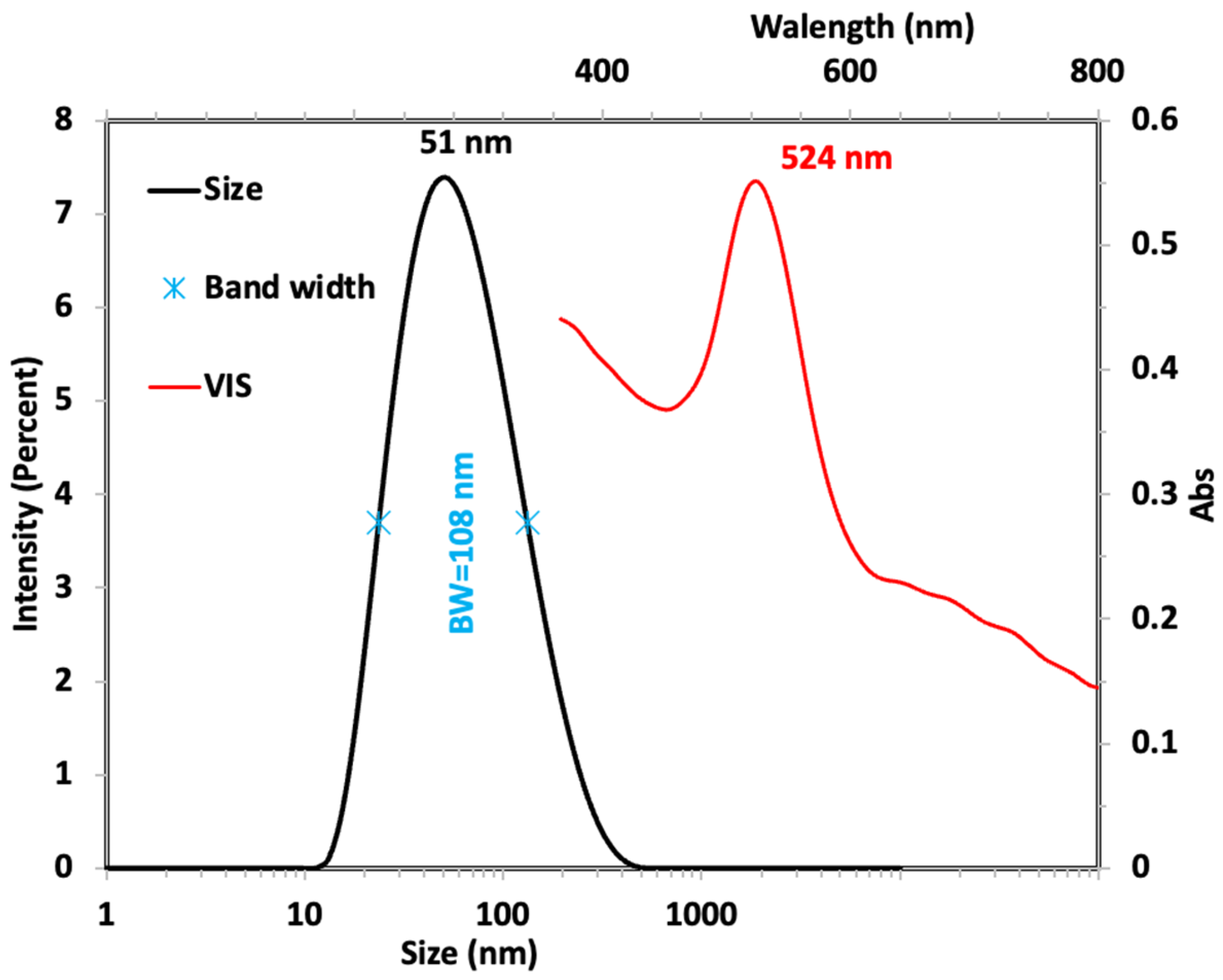
2.2. Characterization and Surface Raman Enhancement Spectroscopy Activity
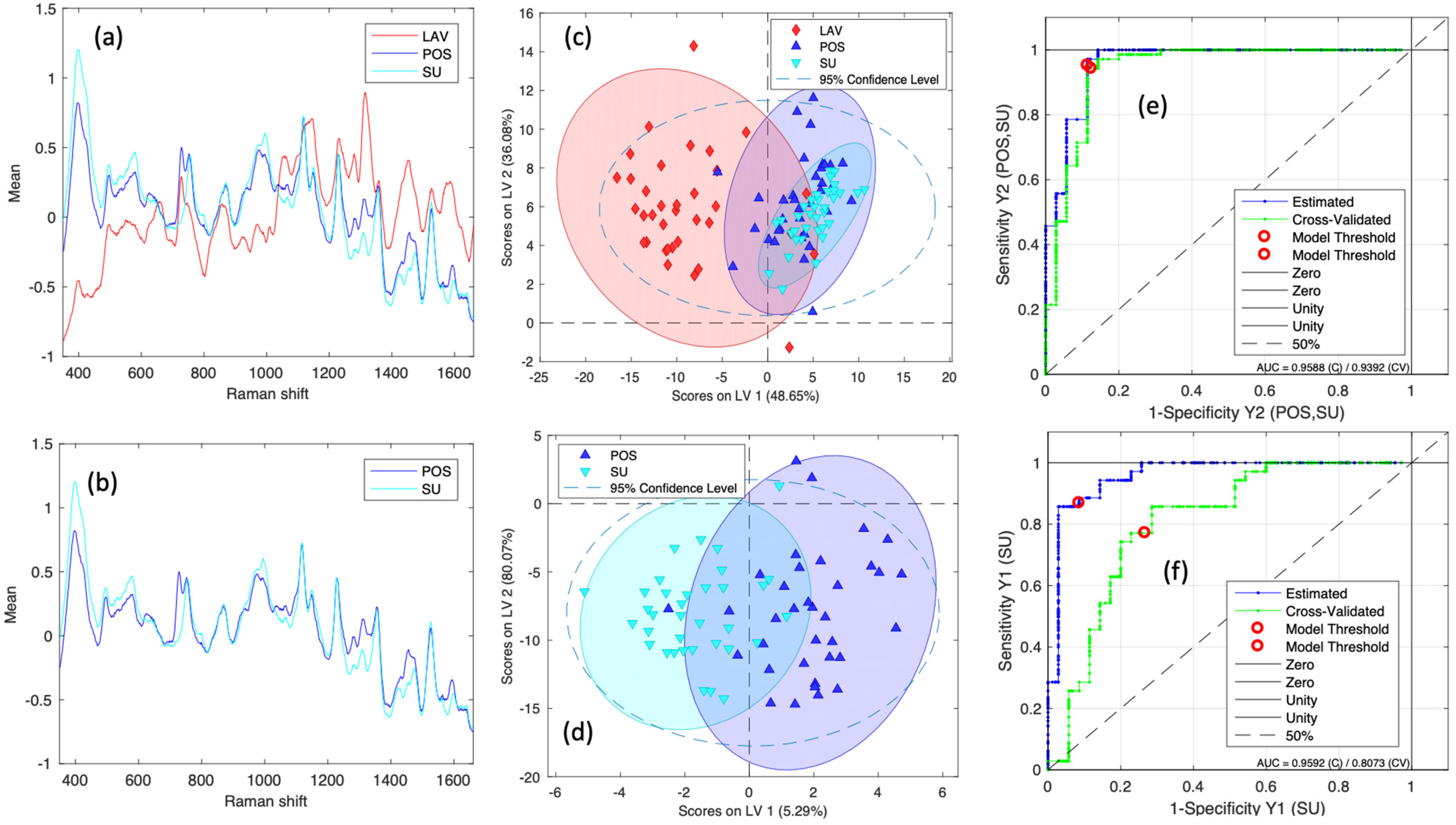
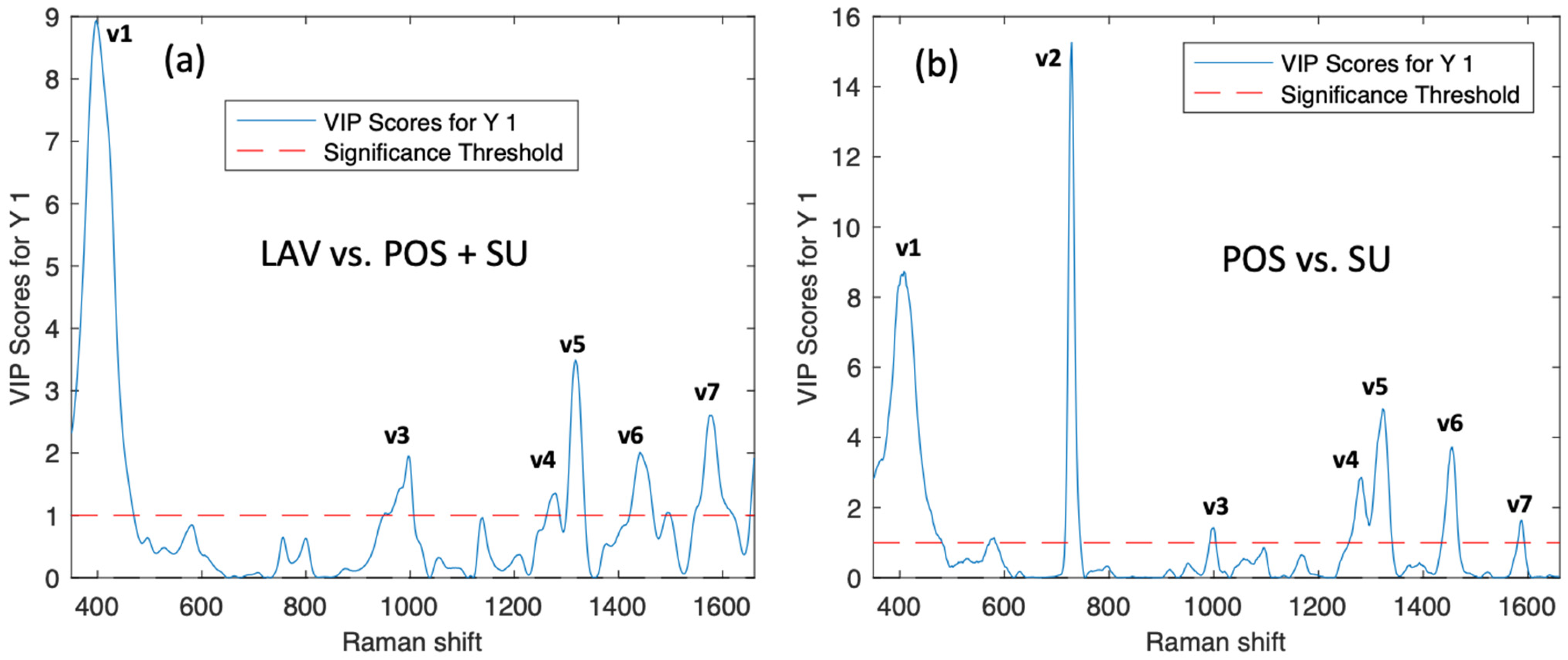
2.3. PLS-DA Classification Model
3. Discussion
4. Materials and Methods
4.1. Semen Collection and Semen Analysis
4.2. Separation of Spermatozoa, Density Gradient Centrifugation, and Swim-Up
4.3. Semen Sample Storage
4.4. Nanoparticle Synthesis and Characterization of Gold Nanoparticles
4.5. Instrumentation and Semen Sample Raman Spectra Acquisition
4.6. Statistical Analysis: Discriminant Analysis (PLS-DA) Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RS | Raman spectroscopy |
| UMI | Dire Unexplained male infertility |
| WHO | World Health Organization |
| ART | Assisted Reproductive Techniques |
| DGC | Gradient Centrifugation |
| SU | Swim-up |
| IUI | intrauterine insemination |
| IVF | in-vitro fertilization |
| PLS-DA | partial least squares discriminant analysis |
| wash | washing |
| POS | post-capacitation |
| AuNPs | Gold nanoparticles |
| SERS | surface Raman enhancement spectroscopy |
| SNV | Standard Normal Variate scaling |
| SEN | sensitivity |
| SPE | specificity |
| ROC | operating characteristic curve |
| AUC | sensing probability |
| VIP | variable importance in projection |
| DLS | Dynamic light scattering |
References
- Kumar, N.; Singh, A.K. Trends of Male Factor Infertility, an Important Cause of Infertility: A Review of Literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Bae, J.; Kim, S.; Chen, Z.; Eisenberg, M.L.; Louis, G.M.B. Human Semen Quality and the Secondary Sex Ratio. Asian J. Androl. 2016, 19, 374. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 3 March 2025).
- Sousa, A.P.; Amaral, A.; Baptista, M.; Tavares, R.; Caballero Campo, P.; Caballero Peregrín, P.; Freitas, A.; Paiva, A.; Almeida-Santos, T.; Ramalho-Santos, J. Not All Sperm Are Equal: Functional Mitochondria Characterize a Subpopulation of Human Sperm with Better Fertilization Potential. PLoS ONE 2011, 6, e18112. [Google Scholar] [CrossRef]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Lu, J.-C.; Tang, S.-S. Effects of Six Kinds of Sperm Staining Methods on Human Sperm Size and Evaluation of Their Staining Effects. J. Clin. Lab. Anal. 2022, 36, e24794. [Google Scholar] [CrossRef]
- Raman Spectroscopic Characterization and Differentiation of Seminal Plasma. Available online: https://www.spiedigitallibrary.org/journals/journal-of-biomedical-optics/volume-16/issue-11/110501/Raman-spectroscopic-characterization-and-differentiation-of-seminal-plasma/10.1117/1.3650310.full (accessed on 3 March 2025).
- Rojo-Sánchez, A.; Carmona-Martes, A.; Díaz-Olmos, Y.; Santamaría-Torres, M.; Cala, M.P.; Orozco-Acosta, E.; Aroca-Martínez, G.; Pacheco-Londoño, L.; Navarro-Quiroz, E.; Pacheco-Lugo, L.A. Urinary Metabolomic Profiling of a Cohort of Colombian Patients with Systemic Lupus Erythematosus. Sci. Rep. 2024, 14, 9555. [Google Scholar] [CrossRef]
- Espinosa-Garavito, A.C.; Quiroz, E.N.; Galán-Freyle, N.J.; Aroca-Martinez, G.; Hernández-Rivera, S.P.; Villa-Medina, J.; Méndez-López, M.; Gomez-Escorcia, L.; Acosta-Hoyos, A.; Pacheco-Lugo, L.; et al. Surface-Enhanced Raman Spectroscopy in Urinalysis of Hypertension Patients with Kidney Disease. Sci. Rep. 2024, 14, 3035. [Google Scholar] [CrossRef]
- Miranda-Guevara, A.; Muñoz-Acevedo, A.; Fiorillo-Moreno, O.; Acosta-Hoyos, A.; Pacheco-Londoño, L.; Quintana-Sosa, M.; De Moya, Y.; Dias, J.; de Souza, G.S.; Martinez-Lopez, W.; et al. The Dangerous Link between Coal Dust Exposure and DNA Damage: Unraveling the Role of Some of the Chemical Agents and Oxidative Stress. Environ. Geochem. Health 2023, 45, 7081–7097. [Google Scholar] [CrossRef]
- Primera-Pedrozo, O.M.; Jerez-Rozo, J.I.; De La Cruz-Montoya, E.; Luna-Pineda, T.; Pacheco-Londono, L.C.; Hernandez-Rivera, S.P. Nanotechnology-Based Detection of Explosives and Biological Agents Simulants. IEEE Sens. J. 2008, 8, 963–973. [Google Scholar] [CrossRef]
- Luna-Pineda, T.; Soto-Feliciano, K.; Cruz-Montoya, E.D.L.; Londoño, L.C.P.; Ríos-Velázquez, C.; Hernández-Rivera, S.P. Spectroscopic Characterization of Biological Agents Using FTIR, Normal Raman and Surface-Enhanced Raman Spectroscopies. In Proceedings of the Chemical and Biological Sensing VIII; SPIE: Bellingham, WA, USA, 2007; Volume 6554, pp. 154–164. [Google Scholar]
- León-Mejía, G.; Rueda, R.A.; Pérez Pérez, J.; Miranda-Guevara, A.; Moreno, O.F.; Quintana-Sosa, M.; Trindade, C.; De Moya, Y.S.; Ruiz-Benitez, M.; Lemus, Y.B.; et al. Analysis of the Cytotoxic and Genotoxic Effects in a Population Chronically Exposed to Coal Mining Residues. Environ. Sci. Pollut. Res. 2023, 30, 54095–54105. [Google Scholar] [CrossRef]
- Da Costa, R.; Amaral, S.; Redmann, K.; Kliesch, S.; Schlatt, S. Spectral Features of Nuclear DNA in Human Sperm Assessed by Raman Microspectroscopy: Effects of UV-Irradiation and Hydration. PLoS ONE 2018, 13, e0207786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Di, L.; Osterberg, E.C.; Liu, F.; He, L.; Hu, H.; Huang, Y.; Li, P.S.; Li, Z. Raman Spectroscopy as an Ex Vivo Noninvasive Approach to Distinguish Complete and Incomplete Spermatogenesis within Human Seminiferous Tubules. Fertil. Steril. 2014, 102, 54–60.e2. [Google Scholar] [CrossRef] [PubMed]
- Mallidis, C.; Wistuba, J.; Bleisteiner, B.; Damm, O.S.; Gross, P.; Wübbeling, F.; Fallnich, C.; Burger, M.; Schlatt, S. In Situ Visualization of Damaged DNA in Human Sperm by Raman Microspectroscopy. Hum. Reprod. 2011, 26, 1641–1649. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, Y.; Liu, Y.; Wang, X.; Ping, P.; Zhu, X.; Hu, H.; Li, Z.; He, L. Real-Time Raman Microspectroscopy Scanning of the Single Live Sperm Bound to Human Zona Pellucida. Fertil. Steril. 2013, 99, 684–689.e4. [Google Scholar] [CrossRef]
- Wiwanitkit, V.; Sereemaspun, A.; Rojanathanes, R. Effect of Gold Nanoparticles on Spermatozoa: The First World Report. Fertil. Steril. 2009, 91, e7–e8. [Google Scholar] [CrossRef]
- Pourasil, R.S.M.; Gilany, K. Fast Diagnosis of Men’s Fertility Using Raman Spectroscopy Combined with Chemometric Methods: An Experimental Study. Int. J. Reprod. Biomed. 2021, 19, 121–128. [Google Scholar] [CrossRef]
- Zhang, F.; Tan, Y.; Ding, J.; Cao, D.; Gong, Y.; Zhang, Y.; Yang, J.; Yin, T. Application and Progress of Raman Spectroscopy in Male Reproductive System. Front. Cell Dev. Biol. 2021, 9, 823546. [Google Scholar] [CrossRef]
- De Angelis, A.; Ferrara, M.A.; Coppola, G.; Di Matteo, L.; Siani, L.; Dale, B.; Coppola, G.; De Luca, A.C. Combined Raman and Polarization Sensitive Holographic Imaging for a Multimodal Label-Free Assessment of Human Sperm Function. Sci. Rep. 2019, 9, 4823. Available online: https://www.nature.com/articles/s41598-019-41400-0 (accessed on 3 March 2025). [CrossRef]
- De Martin, H.; Miranda, E.P.; Cocuzza, M.S.; Monteleone, P.A.A. Density Gradient Centrifugation and Swim-up for ICSI: Useful, Unsafe, or Just Unsuitable? J. Assist. Reprod. Genet. 2019, 36, 2421–2423. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm Selection with Density Gradient Centrifugation and Swim up: Effect on DNA Fragmentation in Viable Spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Volpes, A.; Sammartano, F.; Rizzari, S.; Gullo, S.; Marino, A.; Allegra, A. The Pellet Swim-up Is the Best Technique for Sperm Preparation during in Vitro Fertilization Procedures. J. Assist. Reprod. Genet. 2016, 33, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Cho, C.-L.; Agarwal, A. Sperm Assessment: Traditional Approaches and Their Indicative Value. In In Vitro Fertilization: A Textbook of Current and Emerging Methods and Devices; Nagy, Z.P., Varghese, A.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 249–263. ISBN 978-3-319-43011-9. [Google Scholar]
- Surmacki, J.M.; Woodhams, B.J.; Haslehurst, A.; Ponder, B.A.J.; Bohndiek, S.E. Raman Micro-Spectroscopy for Accurate Identification of Primary Human Bronchial Epithelial Cells. Sci. Rep. 2018, 8, 12604. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Ghosh, S.; Dutta, M.; Subramani, E.; Khalpada, J.; Roychoudhury, S.; Chakravarty, B.; Chaudhury, K. Identification of Key Contributory Factors Responsible for Vascular Dysfunction in Idiopathic Recurrent Spontaneous Miscarriage. PLoS ONE 2013, 8, e80940. [Google Scholar] [CrossRef]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Oldenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S.; Mallidis, C. Oxidative DNA Damage in Human Sperm Can Be Detected by Raman Microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129.e3. [Google Scholar] [CrossRef]
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman Micro-Spectroscopy Analysis of Different Sperm Regions: A Species Comparison. Mol. Hum. Reprod. 2018, 24, 185–202. [Google Scholar] [CrossRef]
- Li, M.; Ji, Y.; Wang, D.; Zhang, Y.; Zhang, H.; Tang, Y.; Lin, G.; Hu, L. Evaluation of Laser Confocal Raman Spectroscopy as a Non-Invasive Method for Detecting Sperm DNA Contents. Front. Physiol. 2022, 13, 827941. [Google Scholar] [CrossRef]
- Fikiet, M.A.; Lednev, I.K. Raman Spectroscopic Method for Semen Identification: Azoospermia. Talanta 2019, 194, 385–389. [Google Scholar] [CrossRef]
- Virkler, K.; Lednev, I.K. Raman Spectroscopic Signature of Semen and Its Potential Application to Forensic Body Fluid Identification. Forensic Sci. Int. 2009, 193, 56–62. [Google Scholar] [CrossRef]
- Sakkas, D.; Manicardi, G.C.; Tomlinson, M.; Mandrioli, M.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. The Use of Two Density Gradient Centrifugation Techniques and the Swim-up Method to Separate Spermatozoa with Chromatin and Nuclear DNA Anomalies. Hum. Reprod. 2000, 15, 1112–1116. [Google Scholar] [CrossRef]
- Lestari, S.W.; Lestari, S.H.; Pujianto, D.A. Sperm Quality After Swim Up and Density Gradient Centrifugation Sperm Preparation with Supplementation of Alpha Lipoic Acid (ALA): A Preliminary Study. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; Available online: https://pubs.aip.org/aip/acp/article/1933/1/030015/791481/Sperm-quality-after-swim-up-and-density-gradient (accessed on 3 March 2025).
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-12-370501-3. [Google Scholar]
- Sutriyo; Mutalib, A.; Ristaniah; Anwar, E.; Radji, M.; Pujiyanto, A.; Purnamasari, P.; Joshita, D.; Adang, H.G. Synthesis of Gold Nanoparticles with Polyamidoamine (Pamam) Generation 4 Dendrimer as Stabilizing Agent for CT Scan Contrast Agent. In Sutriyo-2015-Macromolecula Symposia; Wiley Online Library: Hoboken, NJ, USA, 2015; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/masy.201550312 (accessed on 3 March 2025).
- Kaman, P.; Dutta, P.; Bhattacharyya, A. Synthesis of Gold Nanoparticles from Metarhizium Anisopliae for Management of Blast Disease of Rice and Its Effect on Soil Biological Index and Physicochemical Properties. 2022. [Google Scholar]
- Grisanti, E.; Totska, M.; Huber, S.; Krick Calderon, C.; Hohmann, M.; Lingenfelser, D.; Otto, M. Dynamic Localized SNV, Peak SNV, and Partial Peak SNV: Novel Standardization Methods for Preprocessing of Spectroscopic Data Used in Predictive Modeling. J. Spectrosc. 2018, 2018, 5037572. [Google Scholar] [CrossRef]
- Pacheco-Londoño, L.C.; Aparicio-Bolaño, J.A.; Galán-Freyle, N.J.; Román-Ospino, A.D.; Ruiz-Caballero, J.L.; Hernández-Rivera, S.P. Classical Least Squares-Assisted Mid-Infrared (MIR) Laser Spectroscopy Detection of High Explosives on Fabrics. Appl. Spectrosc. 2019, 73, 17–29. [Google Scholar] [PubMed]
- An Introduction to ROC Analysis-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S016786550500303X (accessed on 6 November 2021).
| Fresh Sperm | ||||
|---|---|---|---|---|
| Parameter | Mean ± SD | Median | Minimum | Maximum |
| Age | 37.4 ± 5.9 | 38 | 26 | 50 |
| Volume (mL) | 2.85 ± 1.41 | 3 | 1 | 8 |
| Motile Spermatozoa (1 × 106/mL) | 39.1 ± 33.8 | 26 | 1.6 | 124 |
| Total Concentration (1 × 106/mL) | 63.1 ± 47.6 | 47.6 | 2.9 | 185 |
| Motility A % | 2.1 ± 5.6 | 0 | 0 | 20 |
| Motility B % | 54.4 ± 8.6 | 53 | 36 | 73 |
| Motility C % | 12.1 ± 6.3 | 12 | 2 | 25 |
| Motility D % | 31.3 ± 10.8 | 31 | 13 | 55 |
| Normal morphology % | 2.69 ± 1.68 | 2 | 0 | 9 |
| DFI (Morphology %) | 2.01 ± 0.19 | 2.01 | 1.64 | 2.47 |
| Post-capacitation Sperm | ||||
| Volume | 0.96 ± 0.14 | 1 | 0.8 | 1.4 |
| Motile Spermatozoa (1 × 106/mL) | 34.8 ± 27.5 | 30 | 2 | 120 |
| Total Concentration (1 × 106/mL) | 53.1 ± 38.2 | 43 | 4.8 | 150 |
| Motility A % | 12 ± 5.9 | 10 | 3 | 25 |
| Motility B % | 55 ± 10.2 | 58 | 24 | 68 |
| Motility C % | 14.9 ± 9.9 | 15 | 0 | 54 |
| Motility D % | 18.2 ± 7.6 | 18 | 0 | 33 |
| Post-Normal morphology % | 5.49 ± 3.12 | 5 | 1 | 14 |
| Post-DFI (% Morphology) | 1.82 ± 0.17 | 1.8 | 1.45 | 2.39 |
| Model 1 Confusion Table (CV) | # | LV | SEN | SPE | Accuracy | ||
|---|---|---|---|---|---|---|---|
| POS, SU | WASH | 4 | 0.94 | 0.86 | 0.91 | ||
| Predicted as POS, SU | 66 | 5 | 70 | ||||
| Predicted as Wash | 4 | 30 | 35 | ||||
| Model 2 Confusion Table (CV) | # | SEN | SPE | Accuracy | |||
| POS | SU | 6 | 0.71 | 0.77 | 0.74 | ||
| Predicted as POS | 25 | 8 | 35 | ||||
| Predicted as SU | 10 | 27 | 35 | ||||
| Assignments of Raman Peaks Sperm Spectra | ||||
|---|---|---|---|---|
| Signals | Sperm Peaks (cm−1) | VIP Wash vs. POS + SU | VIP POS vs. SU | Assignments |
| 1 | 408 | 9 | 8.5 | S–S stretch, symmetric skeletal vibration Lysozyme |
| 2 | 728 | <1 | 13.5 | deoxyadenine |
| 3 | 1000 | 2.5 | 1.3 | Phosphate backbone CH2/NH3 rocking (Protein) |
| 4 | 1280 | 1.3 | 3.5 | DNA PO43− asymmetric stretching |
| 5 | 1319 | 3.6 | 5 | Guanine, CH3, CH2 wagging nucleic acids. (Protein) |
| 6 | 1455 | 2.4 | 4 | CH2, CH3, bending mode. Tryptophane |
| 7 | 1575 | 2.8 | 2 | DNA/RNA Guanine, adenine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Lora, Y.P.; Galán-Freyle, N.J.; Vidal-Figueroa, N.; Cardozo-Puello, A.A.; Acosta-Hoyos, A.J.; Parra-Anaya, G.; Lebrón-Ramírez, E.S.; Espitia-Almeida, F.; Hernández-Rivera, S.P.; Méndez-López, M.; et al. Surface-Enhanced Raman Spectroscopy on Gold Nanoparticle for Sperm Quality Discrimination. Molecules 2025, 30, 1876. https://doi.org/10.3390/molecules30091876
Lopez-Lora YP, Galán-Freyle NJ, Vidal-Figueroa N, Cardozo-Puello AA, Acosta-Hoyos AJ, Parra-Anaya G, Lebrón-Ramírez ES, Espitia-Almeida F, Hernández-Rivera SP, Méndez-López M, et al. Surface-Enhanced Raman Spectroscopy on Gold Nanoparticle for Sperm Quality Discrimination. Molecules. 2025; 30(9):1876. https://doi.org/10.3390/molecules30091876
Chicago/Turabian StyleLopez-Lora, Yeira P., Nataly J. Galán-Freyle, Natally Vidal-Figueroa, Antony A. Cardozo-Puello, Antonio J. Acosta-Hoyos, Guido Parra-Anaya, Elvin S. Lebrón-Ramírez, Fabián Espitia-Almeida, Samuel P. Hernández-Rivera, Maximiliano Méndez-López, and et al. 2025. "Surface-Enhanced Raman Spectroscopy on Gold Nanoparticle for Sperm Quality Discrimination" Molecules 30, no. 9: 1876. https://doi.org/10.3390/molecules30091876
APA StyleLopez-Lora, Y. P., Galán-Freyle, N. J., Vidal-Figueroa, N., Cardozo-Puello, A. A., Acosta-Hoyos, A. J., Parra-Anaya, G., Lebrón-Ramírez, E. S., Espitia-Almeida, F., Hernández-Rivera, S. P., Méndez-López, M., Fiorillo-Moreno, O., Rondon-Payare, K., & Pacheco-Londoño, L. C. (2025). Surface-Enhanced Raman Spectroscopy on Gold Nanoparticle for Sperm Quality Discrimination. Molecules, 30(9), 1876. https://doi.org/10.3390/molecules30091876








