Alteration of Sulfur-Bearing Silicate–Phosphate (Agri)Glasses in Soil Environment: Chemical Interactions and Biological Response
Abstract
1. Introduction
2. Results and Discussion
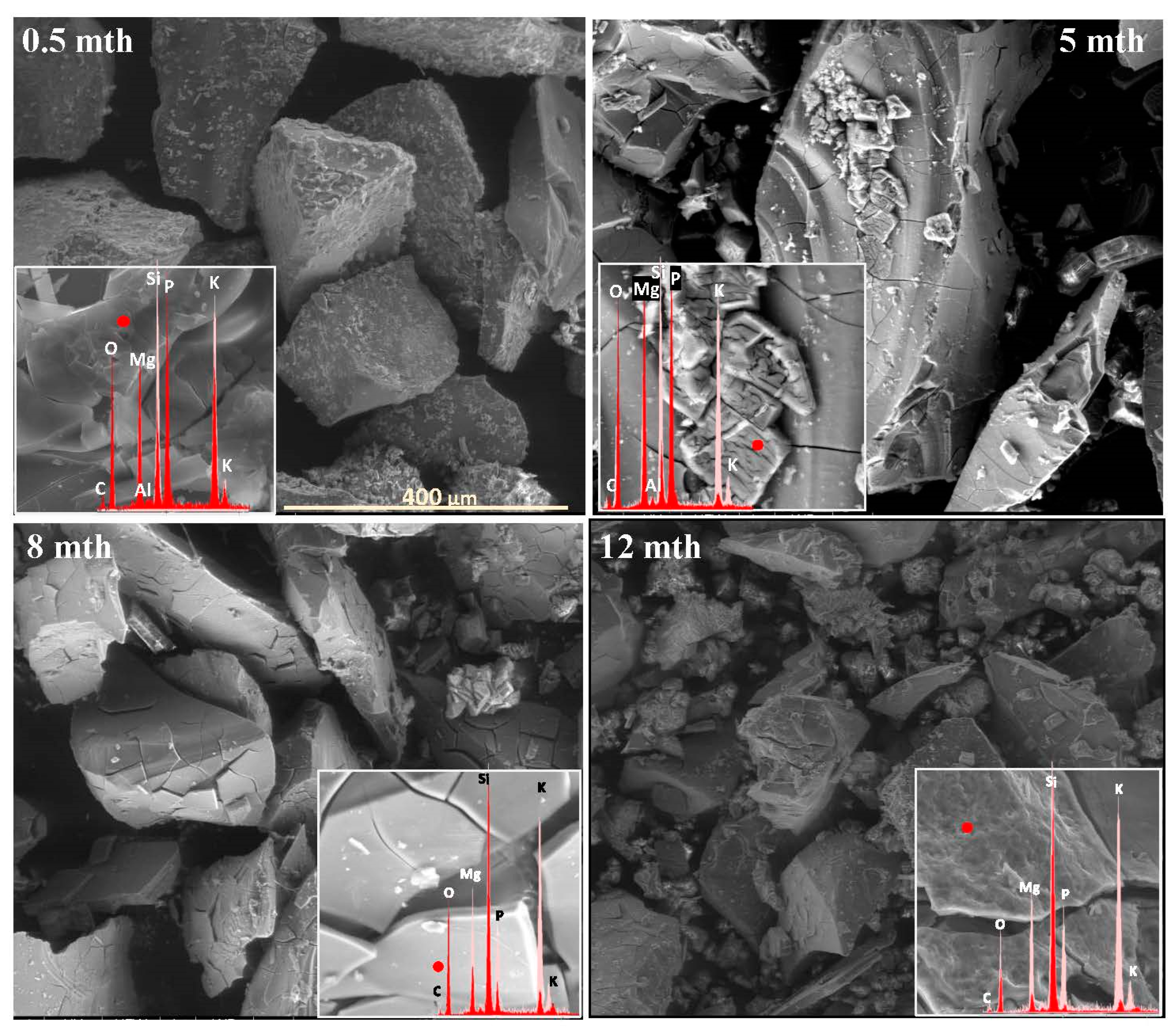
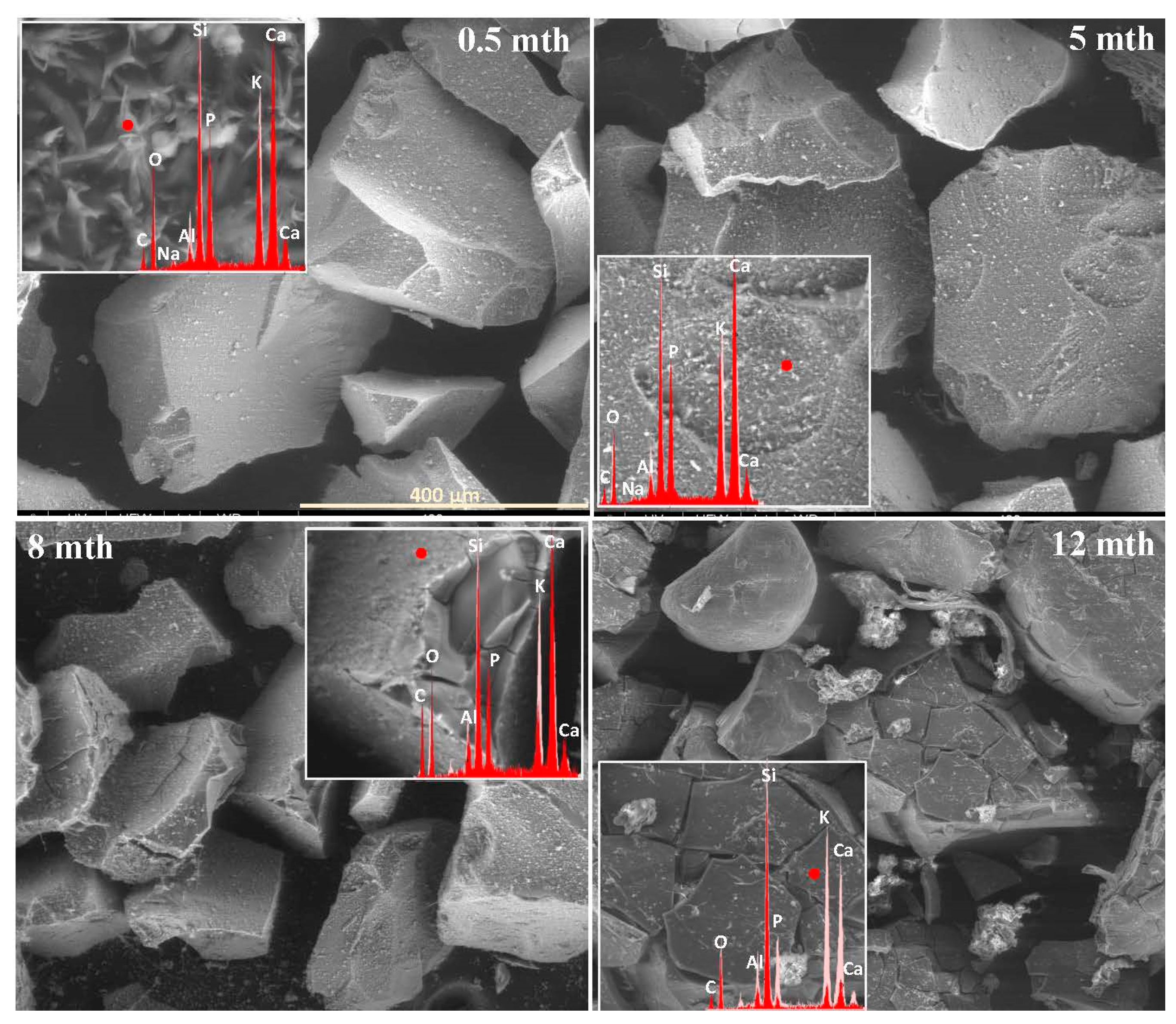
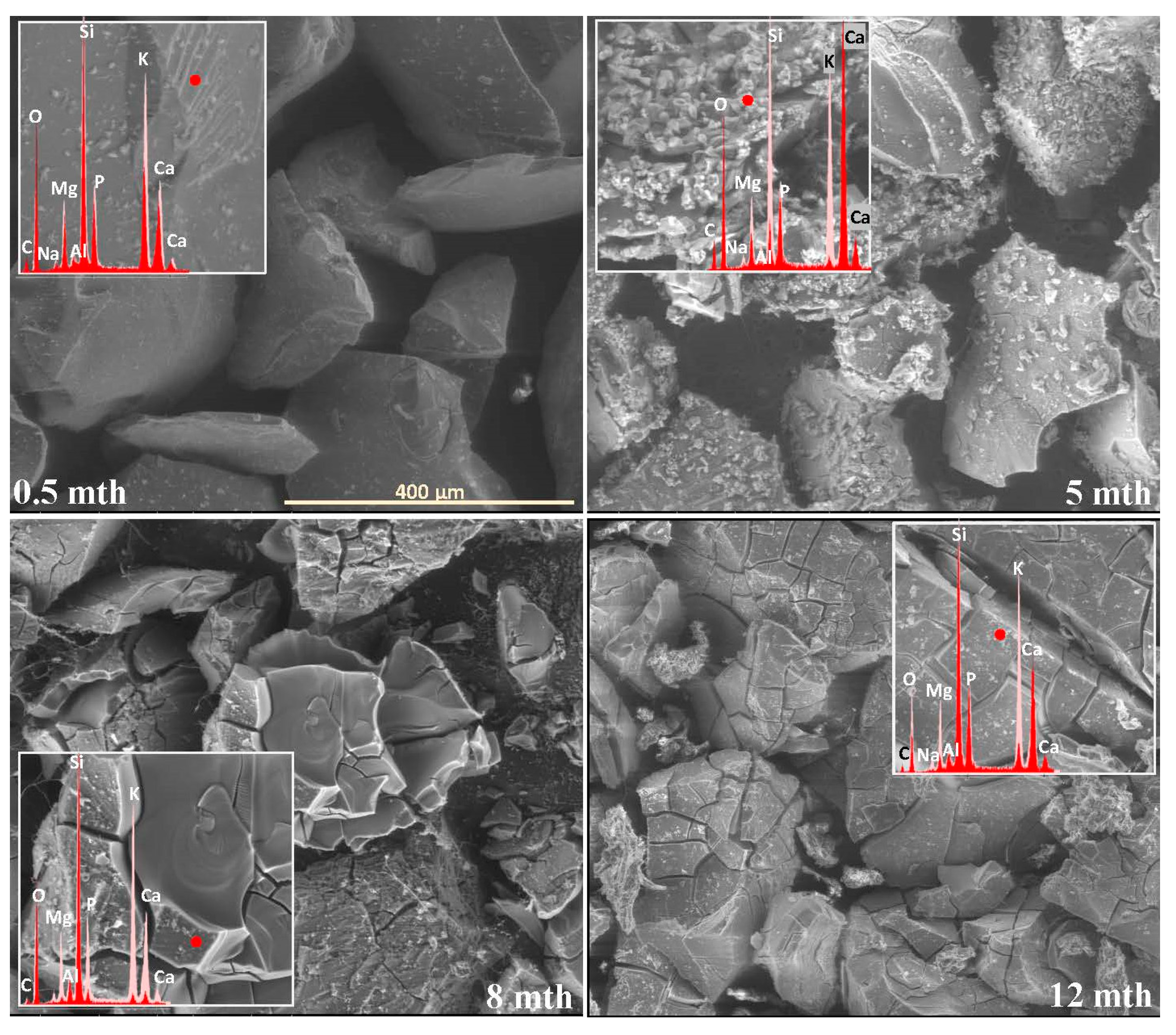
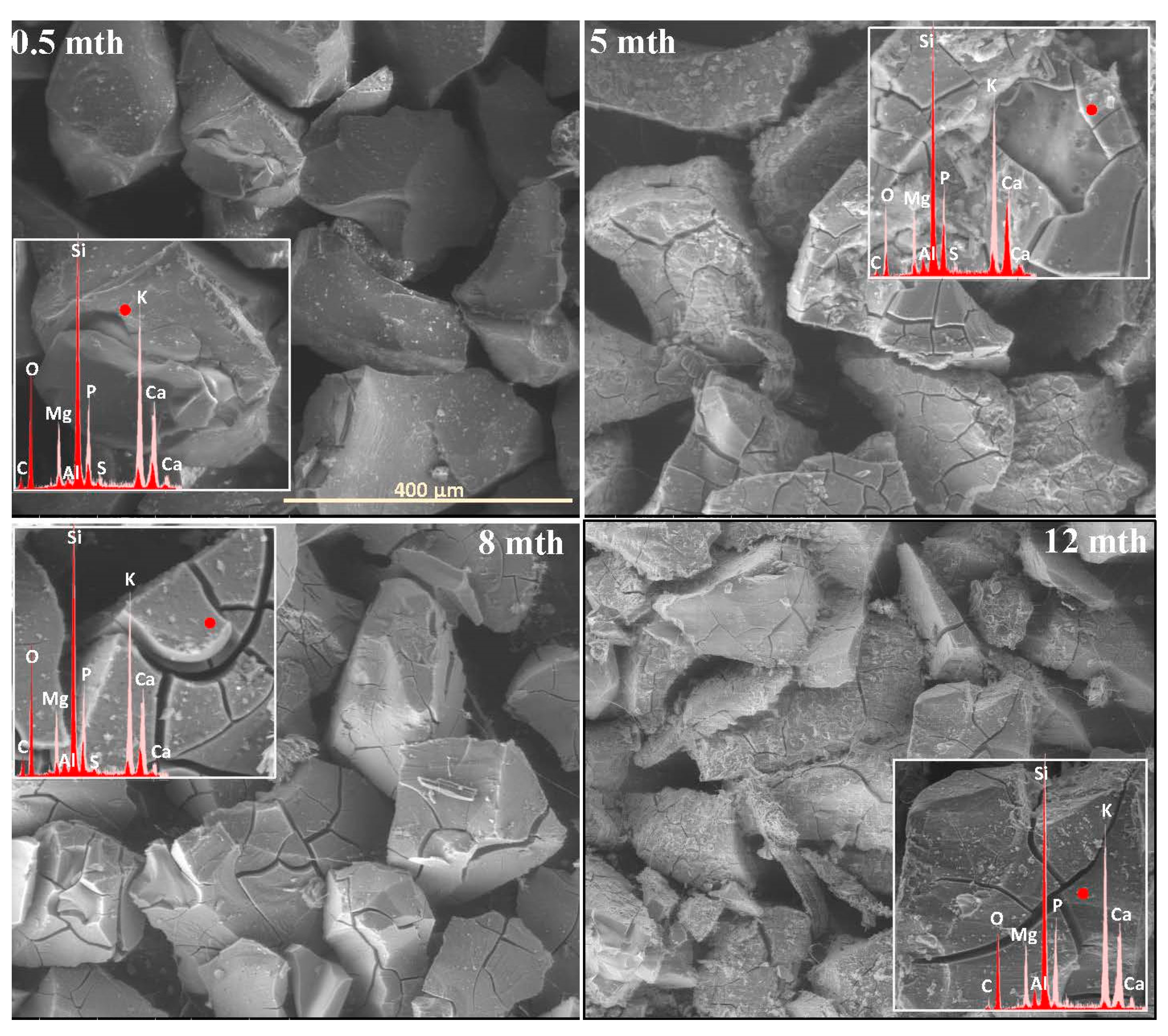
2.1. Surface Alteration Trends Across the Studied Glass Compositions
2.2. Sequence of Glass Component Release into Soil Solution
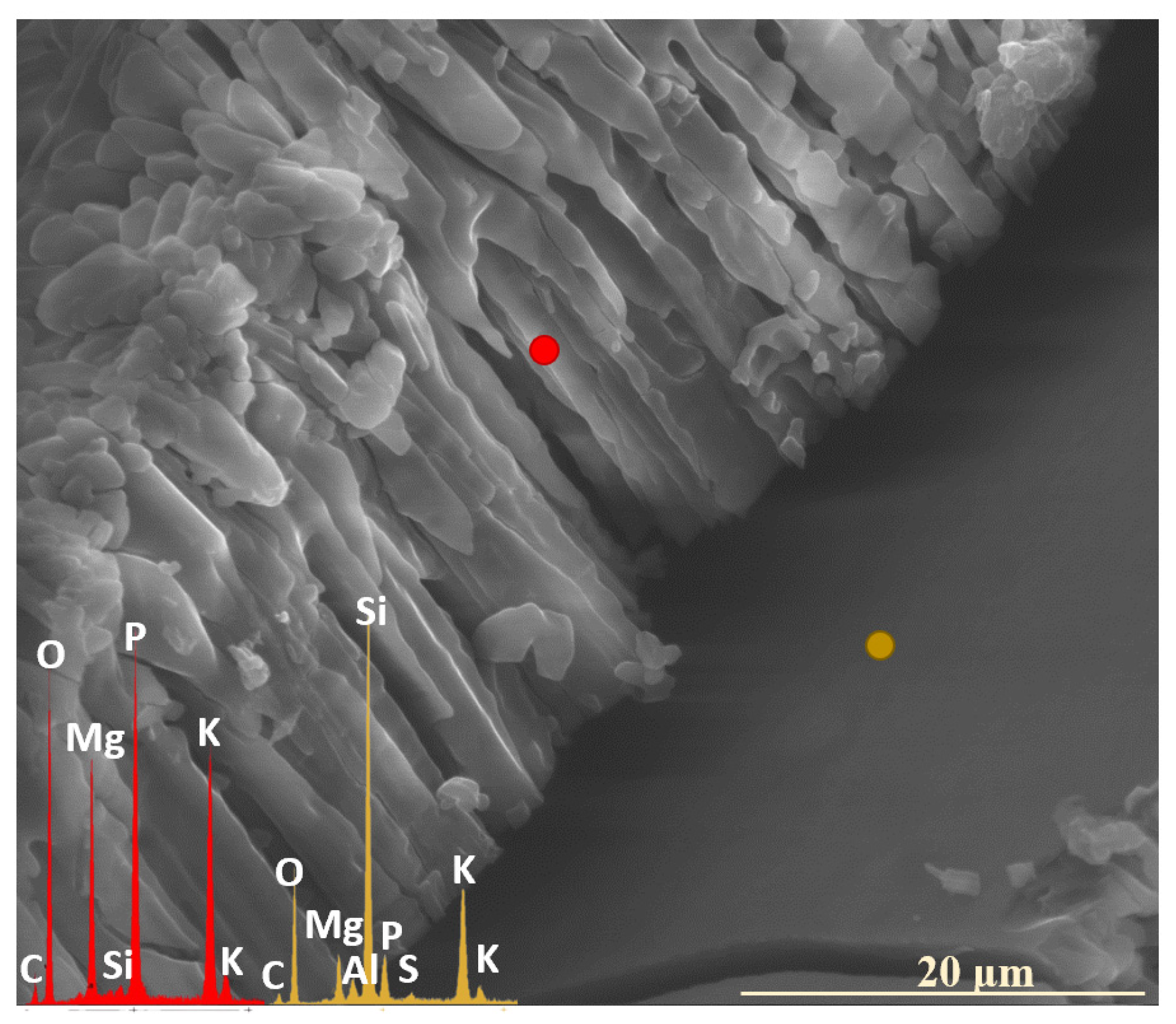
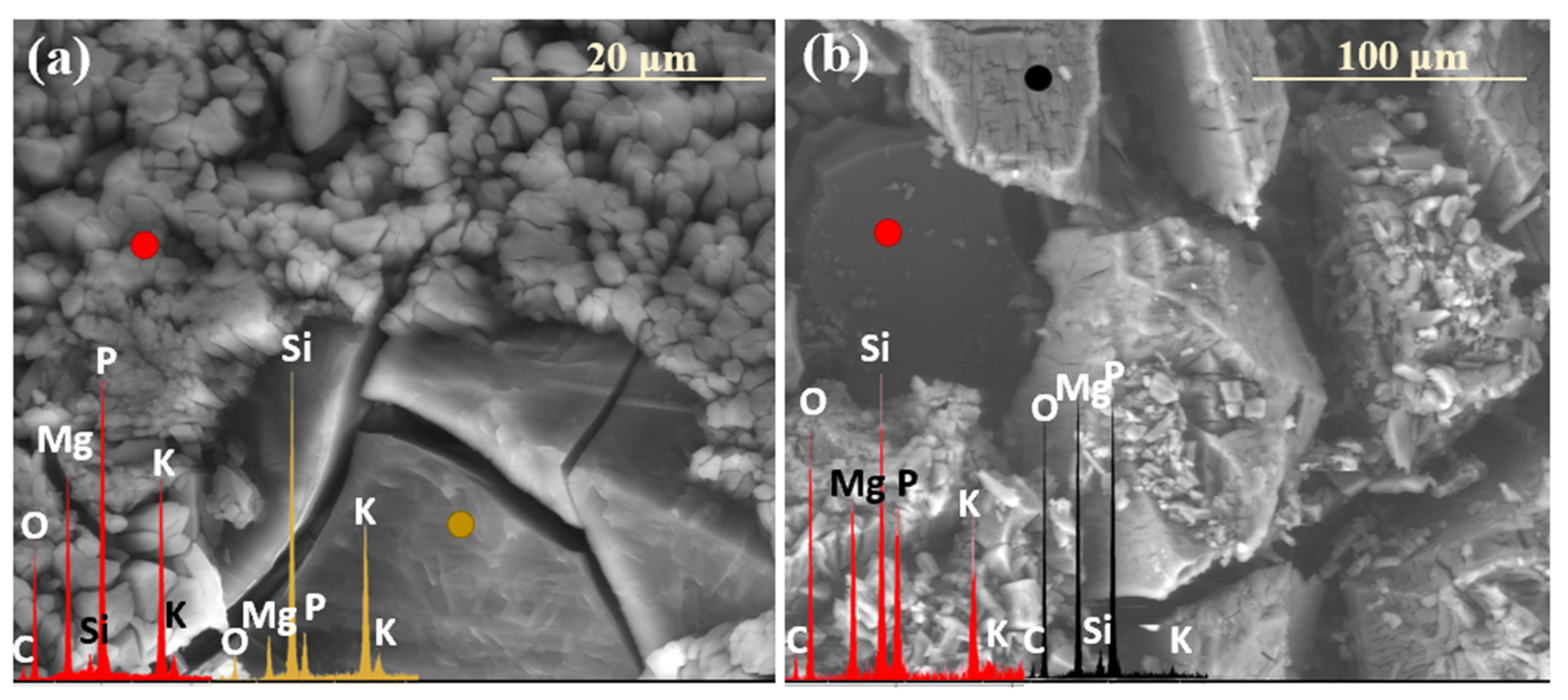
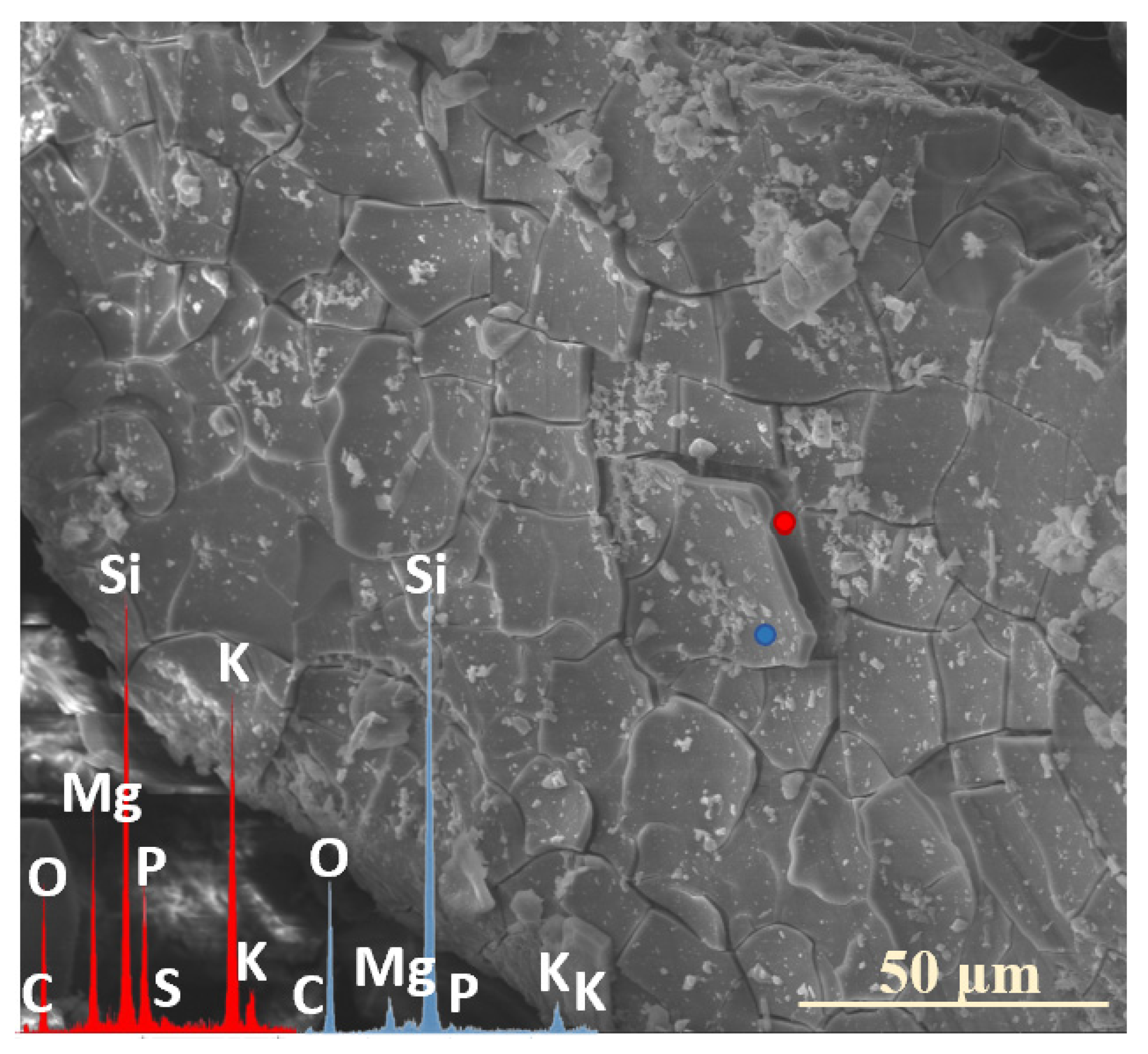
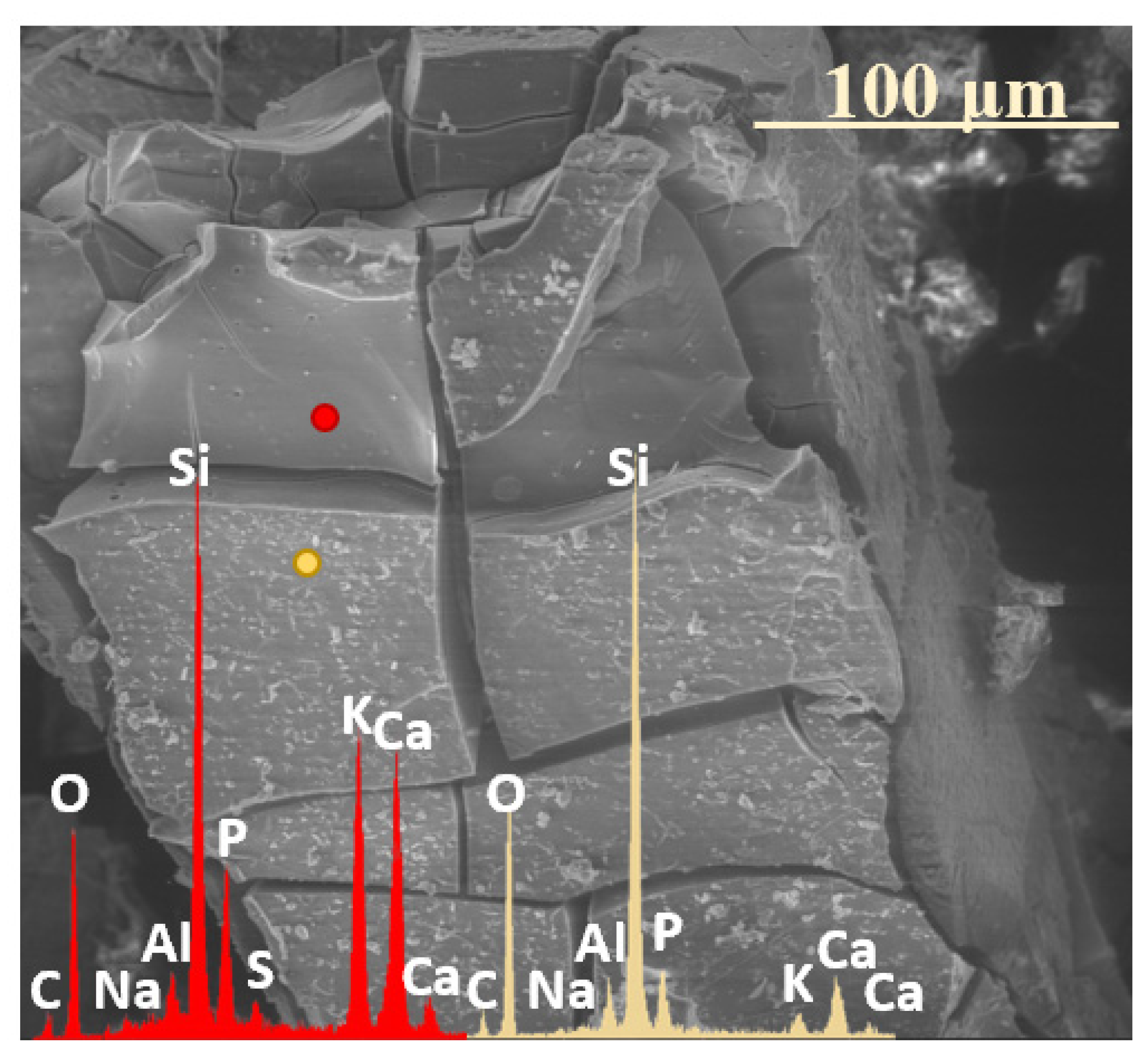
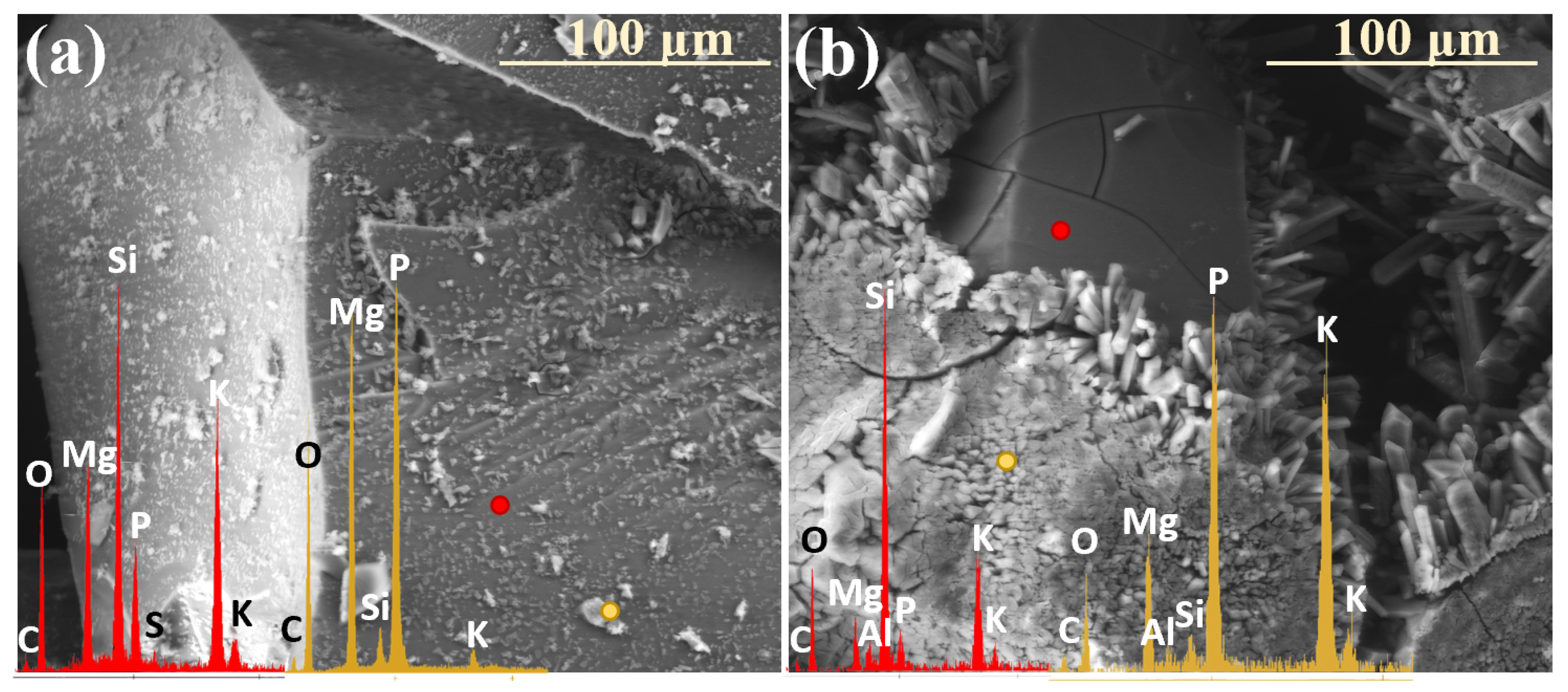
2.3. Characterization of Precipitation Layers
2.4. Mechanism of Glass–Soil Interaction
2.5. Influence of Soil Environment
3. Materials and Methods
3.1. Definitions
- –
- The term ‘in vitro’ refers here to laboratory-based experiments conducted outside the soil system, such as dissolution studies of glass fertilizers in simulated environments (e.g., solutions that mimic soil conditions);
- –
- The term ‘in vivo’ refers here to experiments performed within a biotic soil environment, such as soil incubation studies, where glass fertilizers interact with actual soil conditions to simulate real-world scenarios.
3.2. Synthesis and Compositional Analysis of the Designed Glass Fertilizers
- –
- The XS_6PM system: 41SiO2 · 6P2O5 · 20K2O · 33MgO · XSO3;
- –
- The XS_6PC system: 41SiO2 · 6P2O5 · 20K2O · 33CaO · XSO3;
- –
- The XS_6PMC system: 41SiO2 · 6P2O5 · 20K2O · 16.5MgO · 16.5CaO · XSO3;
- –
- The XS_10PM system: 41SiO2 · 10P2O5 · 20K2O · 29MgO · XSO3.
3.3. Soil Incubation Experiment (‘In Vivo’ Simulation)
4. Conclusions
Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauro, J.C. Decoding the glass genome. Curr. Opin. Solid State Mater. Sci. 2018, 22, 58–64. [Google Scholar] [CrossRef]
- Yanagishima, T.; Russo, J.; Tanaka, H. Common mechanism of thermodynamic and mechanical origin for ageing and crystallization of glasses. Nat. Commun. 2017, 8, 15954. [Google Scholar] [CrossRef] [PubMed]
- Zanini, R.; Franceschin, G.; Cattaruzza, E.; Traviglia, A. A review of glass corrosion: The unique contribution of studying ancient glass to validate glass alteration models. npj Mater. Degrad. 2023, 7, 38. [Google Scholar] [CrossRef]
- Jackson, C.M.; Greenfield, D.; Howie, L.A. An assessment of compositional and morphological changes in model archaeological glasses in an acid burial matrix. Archaeometry 2012, 54, 489–507. [Google Scholar] [CrossRef]
- Gin, S.; Guo, X.; Delaye, J.-M.; Angeli, F.; Damodaran, K.; Testud, V.; Du, J.; Kerisit, S.; Kim, S.H. Insights into the mechanisms controlling the residual corrosion rate of borosilicate glasses. npj Mater. Degrad. 2020, 4, 41. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses—Structure and Properties. Angew. Chem. Int. Ed. Engl. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Hench, L.; Clark, D. Physical chemistry of glass surfaces. J. Non Cryst. Solids 1978, 28, 83–105. [Google Scholar] [CrossRef]
- Christie, J.K.; Ainsworth, R.I.; de Leeuw, N.H. Investigating structural features which control the dissolution of bioactive phosphate glasses: Beyond the network connectivity. J. Non Cryst. Solids 2016, 432, 31–34. [Google Scholar] [CrossRef]
- Tilocca, A.; Cormack, A.N.; de Leeuw, N.H. The Structure of bioactive silicate glasses: New insight from molecular dynamics simulations. Chem. Mater. 2007, 19, 95–103. [Google Scholar] [CrossRef]
- Hill, R.G.; Brauer, D.S. Predicting the bioactivity of glasses using the network connectivity or split network models. J. Non Cryst. Solids 2011, 357, 3884–3887. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Elaboration and Characterization of Vitreous Fertilizers and Study of Their Impact on the Growth, Photosynthesis, and Yield of Wheat (Triticum durum L.). Materials 2021, 14, 1295. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Troni, E.; Gualtieri, S.; Soldati, R.; Famiani, F.; Businelli, D. Agronomic potential of two different glass-based materials as novel inorganic slow-release iron fertilizers. J. Sci. Food Agric. 2021, 102, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Ouis, M.A.; Abd-Eladl, M.; Abou-Baker, N.H. Evaluation of Agriglass as an Environment Friendly Slow Release Fertilizer. Silicon 2016, 10, 293–299. [Google Scholar] [CrossRef]
- Waclawska, I.; Stoch, L. Thermal behaviour of silicate-phosphate glasses in relation to their biochemical activity. J. Therm. Anal. Calorim. 2001, 65, 141–146. [Google Scholar] [CrossRef]
- Waclawska, I.; Szumera, M. Reactivity of silicate–phosphate glasses in soil environment. J. Alloys Compd. 2009, 468, 246–253. [Google Scholar] [CrossRef]
- Stoch, L.; Wacławska, I.; Szumera, M. Phospho-silicate glasses, self-degradable in environment conditions. Adv. Mater. Res. 2008, 39–40, 335–340. [Google Scholar] [CrossRef]
- Conradt, R. Chemical durability of oxide glasses in aqueous solutions: A review. J. Am. Ceram. Soc. 2007, 91, 728–735. [Google Scholar] [CrossRef]
- Gin, S.; Delaye, J.-M.; Angeli, F.; Schuller, S. Aqueous alteration of silicate glass: State of knowledge and perspectives. npj Mater. Degrad. 2021, 5, 42. [Google Scholar] [CrossRef]
- Hellmann, R.; Icenhower, R.J.P. Mechanisms of Glass Corrosion by Aqueous Solutions. Encycl. Glass Sci. Technol. Hist. Cult. 2021, 1, 647–662. [Google Scholar] [CrossRef]
- Rodrigues, A.; Fearn, S.; Palomar, T.; Vilarigues, M. Early stages of surface alteration of soda-rich-silicate glasses in the museum environment. Corros. Sci. 2018, 143, 362–375. [Google Scholar] [CrossRef]
- Thorpe, C.L.; Neeway, J.J.; Pearce, C.I.; Hand, R.J.; Fisher, A.J.; Walling, S.A.; Hyatt, N.C.; Kruger, A.A.; Schweiger, M.; Kosson, D.S.; et al. Forty years of durability assessment of nuclear waste glass by standard methods. npj Mater. Degrad. 2021, 5, 61. [Google Scholar] [CrossRef]
- Gin, S.; Mir, A.H.; Jan, A.; Delaye, J.-M.; Chauvet, E.; De Puydt, Y.; Gourgiotis, A.; Kerisit, S.N. A General Mechanism for Gel Layer Formation on Borosilicate Glass under Aqueous Corrosion. J. Phys. Chem. C 2020, 124, 5132–5144. [Google Scholar] [CrossRef]
- Frankel, G.S.; Vienna, J.D.; Lian, J.; Scully, J.R.; Gin, S.; Ryan, J.V.; Wang, J.; Kim, S.H.; Windl, W.; Du, J. A comparative review of the aqueous corrosion of glasses, crystalline ceramics, and metals. npj Mater. Degrad. 2018, 2, 15. [Google Scholar] [CrossRef]
- Bunker, B. Molecular mechanisms for corrosion of silica and silicate glasses. J. Non Cryst. Solids 1994, 179, 300–308. [Google Scholar] [CrossRef]
- Bates, J.K.; Bradley, C.R.; Buck, E.C.; Cunnane, J.C.; Ebert, W.L.; Feng, X.; Mazer, J.J.; Wronkiewicz, D.J. High-Level Waste Boro-silicate Glass, A Compendium of Corrosion Characteristics; United States Department of Energy Office of Waste Management: Washington, DC, USA, 1994. [Google Scholar]
- McNear, D.H. The Rhizosphere—Roots, Soil and Everything In Between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Palomar, T.; Garcia-Heras, M.; Villegas, M.-A. Model historical glasses under simulated burial conditions. Coalition 2012, 23, 2–6. [Google Scholar]
- Cox, G.A.; Ford, B.A. The long-term corrosion of glass by ground-water. J. Mater. Sci. 1993, 28, 5637–5647. [Google Scholar] [CrossRef]
- Zacharias, N.; Palamara, E.; Kordali, R.; Muros, V. Archaeological glass corrosion studies: Composition, environment and content. Sci. Cult. 2020, 6, 53–67. [Google Scholar] [CrossRef]
- Friedrich, K.T.; Degryse, P. Soil vs. glass: An integrated approach towards the characterization of soil as a burial environment for the glassware of Cucagna Castle (Friuli, Italy). STAR: Sci. Technol. Archaeol. Res. 2019, 5, 138–156. [Google Scholar] [CrossRef]
- Palomar, T. Effect of soil pH on the degradation of silicate glasses. Int. J. Appl. Glas. Sci. 2017, 8, 177–187. [Google Scholar] [CrossRef]
- Roemich, H.; Gerlach, S.; Mottner, P.; Mees, F.; Jacobs, P.; van Dyck, D.; Carbó, T.D. Results from burial experiments with simulated medieval glasses. MRS Proc. 2003, 757, 97–108. [Google Scholar] [CrossRef]
- Odegaard, N.; Watkinson, G. Post-Depositional Changes in Archaeological Ceramics and Glass. Handb. Ar Chaeological Sci. 2023, 2, 1103–1116. [Google Scholar] [CrossRef]
- Schalm, O.; Anaf, W. Laminated Altered Layers in Historical Glass: Density Variations of Silica Nanoparticle Random Packings as Explanation for the Observed Lamellae. J Non Cryst Solids 2016, 442, 1–16. [Google Scholar] [CrossRef]
- De Bardi, M.; Hutter, H.; Schreiner, M.; Bertoncello, R. Potash-lime-silica glass: Protection from weathering. Herit. Sci. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. Performance of Wasteform Materials. In An Introduction to Nuclear Waste Immobilisation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 433–461. [Google Scholar] [CrossRef]
- Perera, G.; Doremus, R.H. Dissolution Rates of Commercial Soda–Lime and Pyrex Borosilicate Glasses: Influence of Solution pH. J. Am. Ceram. Soc. 1991, 74, 1554–1558. [Google Scholar] [CrossRef]
- Xing, S.-B.; Muller, I.S.; Pegg, I.L. Non-Linearity in Glass Composition Dependence of Dissolution Rates: Effect of Solution pH. MRS Proc. 1993, 333, 549. [Google Scholar] [CrossRef]
- Cheng, X.; Brow, R.K.; Chen, G. The dissolution behavior in alkaline solutions of a borosilicate glass with and without P2O5. J. Am. Ceram. Soc. 2017, 100, 4519–4532. [Google Scholar] [CrossRef]
- Westervelt, D.M.; Fiore, A.M.; Baublitz, C.B.; Correa, G. Impact of Regional Northern Hemisphere Mid-Latitude Anthropogenic Sulfur Dioxide Emissions on Local and Remote Tropospheric Oxidants. Atmos. Chem. Phys. 2021, 21, 6799–6810. [Google Scholar] [CrossRef]
- Aas, W.; Mortier, A.; Bowersox, V.; Cherian, R.; Faluvegi, G.; Fagerli, H.; Hand, J.; Klimont, Z.; Galy-Lacaux, C.; Lehmann, C.M.B.; et al. Global and Regional Trends of Atmospheric Sulfur. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Rebroš, M.; Jamnický, M.; Lokaj, J.; Kadlečíkova, M. Weathering of the crystal glass in moist and polluted atmospheres. Ceram. Silik. 2006, 50, 44–50. [Google Scholar]
- Papadopoulos, N.; Drosou, C.A. Influence of weather conditions on glass properties. J. Univ. Chem. Technol. Metall. 2012, 47, 429–438. [Google Scholar]
- Gueli, A.M.; Pasquale, S.; Tanasi, D.; Hassam, S.; Lemasson, Q.; Moignard, B.; Pacheco, C.; Pichon, L.; Stella, G.; Politi, G. Weathering and deterioration of archeological glasses from late Roman Sicily. Int. J. Appl. Glas. Sci. 2020, 11, 215–225. [Google Scholar] [CrossRef]
- Kalita, A.; Elayarajan, M.; Janaki, P.; Suganya, S.; Sankari, A.; Parameswari, E. Organo-monomers coated slow-release fertilizers: Current understanding and future prospects. Int. J. Biol. Macromol. 2024, 274, 133320. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997. [Google Scholar]
- Palomar, T.; Redol, P.; Almeida, I.C.; da Silva, E.P.; Vilarigues, M. The influence of environment in the alteration of the stained-glass windows in portuguese monuments. Heritage 2018, 1, 365–376. [Google Scholar] [CrossRef]
- Huisman, D.; Pols, S.; Joosten, I.; van Os, B.; Smit, A. Degradation processes in colourless Roman glass: Cases from the Bocholtz burial. J. Archaeol. Sci. 2008, 35, 398–411. [Google Scholar] [CrossRef]
- Wacławska, I.; Szumera, M. Influence of MgO (CaO) on the structure of silicate-phosphate glasses. J. Therm. Anal. Calorim. 2006, 84, 185–190. [Google Scholar] [CrossRef]
- Chave, T.; Frugier, P.; Gin, S.; Ayral, A. Glass–water interphase reactivity with calcium rich solutions. Geochim. Cosmochim. Acta 2011, 75, 4125–4139. [Google Scholar] [CrossRef]
- Othman, S.M.; Sarsam, S.I.; Ismail, S.A. Gypsum and limestone dissolution within fatha formation (middle miocene) at various ph solutions: A laboratory study. Iraqi Geol. J. 2020, 53, 71–88. [Google Scholar] [CrossRef]
- Kiran-Yildirim, B.; Gencaslan, A.; Titiz-Sargut, S. Thermodynamic Models and Central Composite Design for Calcium Sulfate Dihydrate Solubility. Chem. Eng. Technol. 2020, 43, 1124–1136. [Google Scholar] [CrossRef]
- Türk, F.N.; Eren, M.Ş.A.; Arslanoğlu, H. Obtaining, Characterizing, and Evaluation of Potassium-Struvite (KMgPO4.6H2O) in Phosphorus Removal from Water: Investigation of Low-Cost Mgo Use. Water Conserv. Sci. Eng. 2024, 9, 68. [Google Scholar] [CrossRef]
- Banks, E.; Chianelli, R.; Korenstein, R.J.I.C. Crystal chemistry of struvite analogs of the type MgMPO4. 6H2O (M+= potassium (1+), rubidium (1+), cesium (1+), thallium (1+), ammonium (1+). Inorg. Chem. 1975, 14, 1634–1639. [Google Scholar] [CrossRef]
- Mathew, M.; Schroeder, L.W. Crystal structure of a struvite analogue, MgKPO4.6H2O. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 11–13. [Google Scholar] [CrossRef]
- Prywer, J.; Torzewska, A.; Płociński, T. Unique surface and internal structure of struvite crystals formed by Proteus mirabilis. Urol. Res. 2012, 40, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Szterner, P.; Antosik, A.; Pagacz, J.; Tymowicz-Grzyb, P. Morphology Control of Hydroxyapatite as a Potential Reinforcement for Orthopedic Biomaterials: The Hydrothermal Process. Crystals 2023, 13, 793. [Google Scholar] [CrossRef]
- McDonogh, D.P.; Kirupananthan, P.; Gebauer, D. Counterintuitive Crystallization: Rate Effects in Calcium Phosphate Nucleation at Near-Physiological pH. Cryst. Growth Des. 2023, 23, 7037–7043. [Google Scholar] [CrossRef]
- Mekmene, O.; Rouillon, T.; Quillard, S.; Pilet, P.; Bouler, J.-M.; Pezennec, S.; Gaucheron, F. Effects of citrate and NaCl on size, morphology, crystallinity and microstructure of calcium phosphates obtained from aqueous solutions at acidic or near-neutral pH. J. Dairy Res. 2012, 79, 238–248. [Google Scholar] [CrossRef]
- Putri, P.Y. Effect of temperature and solution PH on calcium carbonate precipitation as bio-based repair material in concrete. Int. J. Geomate 2024, 26, 101–108. [Google Scholar] [CrossRef]
- Chen, W.C.; Ju, C.P.; Cheng, W.H.; Chernlin, J.H. Green synthesis of calcium and phosphate compounds by varying ph value and ca/p atomic ratio using aqueous precipitations. Ceram. Silik. 2013, 57, 14–21. [Google Scholar]
- Gin, S.; Jollivet, P.; Fournier, M.; Angeli, F.; Frugier, P.; Charpentier, T. Origin and consequences of silicate glass passivation by surface layers. Nat. Commun. 2015, 6, 6360. [Google Scholar] [CrossRef]
- Cailleteau, C.; Angeli, F.; Devreux, F.; Gin, S.; Jestin, J.; Jollivet, P.; Spalla, O. Insight into silicate-glass corrosion mechanisms. Nat. Mater. 2008, 7, 978–983. [Google Scholar] [CrossRef]
- Verney-Carron, A.; Sessegolo, L.; Chabas, A.; Lombardo, T.; Rossano, S.; Perez, A.; Valbi, V.; Boutillez, C.; Muller, C.; Vaulot, C.; et al. Alteration of medieval stained glass windows in atmospheric medium: Review and simplified alteration model. npj Mater. Degrad. 2023, 7, 49. [Google Scholar] [CrossRef]
- Abdelouas, A.; Neeway, J.; Grambow, B. Chemical Durability of Glasses. In Springer Handbook of Glass. Springer Handbooks; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer: Cham, Switzerland, 2019; ISBN 9783319937281. [Google Scholar]
- Hellmann, R.; Cotte, S.; Cadel, E.; Malladi, S.; Karlsson, L.S.; Lozano-Perez, S.; Cabié, M.; Seyeux, A. Nanometre-Scale Evidence for Interfacial Dissolution–Reprecipitation Control of Silicate Glass Corrosion. Nat. Mater. 2015, 14, 307–311. [Google Scholar] [CrossRef]
- Sterpenich, J.; Libourel, G. Water Diffusion in Silicate Glasses under Natural Weathering Conditions: Evidence from Buried Medieval Stained Glasses. J Non Cryst Solids 2006, 352, 5446–5451. [Google Scholar] [CrossRef]
- Elgayar, I.; Hill, R.; Chen, X.; Bubb, N.; Wood, D. Dielectric spectroscopy and dissolution studies of bioactive glasses. Int. J. Appl. Glas. Sci. 2017, 8, 418–427. [Google Scholar] [CrossRef]
- Tilocca, A. Sodium migration pathways in multicomponent silicate glasses: Car–Parrinello molecular dynamics simulations. J. Chem. Phys. 2010, 133, 014701. [Google Scholar] [CrossRef] [PubMed]
- Bouabdalli, E.M.; El Jouad, M.; Touhtouh, S.; Hajjaji, A. First investigation of the effect of strontium oxide on the structure of phosphate glasses using molecular dynamics simulations. Comput. Mater. Sci. 2023, 220, 112068. [Google Scholar] [CrossRef]
- Meyer, K.; Barz, A.; Stachel, D. Effects of atmospheric humidity on the infrared reflectivity of vitreous P2O5 and ultraphosphate glasses. J. Non Cryst. Solids 1995, 191, 71–78. [Google Scholar] [CrossRef]
- Stachel, D.; Barz, A. Structure investigations of ultraphosphate glasses with some water content. J. Phys. Chem. Solids 2007, 68, 1021–1023. [Google Scholar] [CrossRef]
- Wacławska, I.; Szumera, M.; Stoch, P.; Sitarz, M. Structural role of Fe in the soil active glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 728–732. [Google Scholar] [CrossRef]
- Wacławska, I.; Szumera, M. Thermal analysis of glasses for proecological applications. J. Therm. Anal. Calorim. 2003, 72, 1065–1072. [Google Scholar] [CrossRef]
- Goj, P.; Stoch, P. Molecular dynamics simulations of P2O5-Fe2O3-FeO-Na2O glasses. J. Non Cryst. Solids 2018, 500, 70–77. [Google Scholar] [CrossRef]
- Stoch, P.; Goj, P.; Ciecińska, M.; Jeleń, P.; Błachowski, A.; Stoch, A.; Krakowiak, I. Influence of aluminum on structural properties of iron-polyphosphate glasses. Ceram. Int. 2020, 46, 19146–19157. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-based composites for slow-release fertilization: A case study in sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Toprak, G.; Ozdemir, E. Stability of CaCO3 in Ca(OH)2 solution. Int. J. Miner. Process. 2016, 147, 1–9. [Google Scholar] [CrossRef]
- Weaver, J.L.; DePriest, P.T.; Plymale, A.E.; Pearce, C.I.; Arey, B.; Koestler, R.J. Microbial interactions with silicate glasses. npj Mater. Degrad. 2021, 5, 11. [Google Scholar] [CrossRef]
- Berezicka, A.; Sułowska, J.; Jeleń, P.; Komarek, S.; Szumera, M. Effect of sulfur addition on glass-forming ability, structure and thermal stability of multicomponent silicate-phosphate glasses. J. Therm. Anal. Calorim. 2024, 149, 10405–10427. [Google Scholar] [CrossRef]
- Berezicka, A.; Sułowska, J.; Szumera, M. Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies. Molecules 2025, 30, 1684. [Google Scholar] [CrossRef]
- Ulén, B.; Pietrzak, S.; Ramnerö, B.; Strand, L. Agricultural soil acidity and phosphorus leaching risk at farm level in two focus areas. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 359–368. [Google Scholar] [CrossRef]
- Zamanian, K.; Taghizadeh-Mehrjardi, R.; Tao, J.; Fan, L.; Raza, S.; Guggenberger, G.; Kuzyakov, Y. Acidification of European croplands by nitrogen fertilization: Consequences for carbonate losses, and soil health. Sci. Total. Environ. 2024, 924, 171631. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Bednarek, W.; Dresler, S.; Krzyszczak, J.; Baranowski, P.; Sławiński, C. Relationship between assimilable-nutrient content and physicochemical properties of topsoil. Int. Agrophysics 2017, 31, 551–562. [Google Scholar] [CrossRef]
- Stoch, L. Flexibility of structure as a criterion of glass formation and stability. Opt. Appl. 2000, 30, 647–655. [Google Scholar]
- Stoch, L. Flexibility of Structure and Glass-Forming Ability: A Chemical Approach. Glas. Phys. Chem. 2001, 27, 167–174. [Google Scholar] [CrossRef]
- Berezicka, A.; Szumera, M.; Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Stępień, J.; Zając, M.; Pollastri, S.; Olivi, L. Unraveling the nature of sulfur-bearing silicate-phosphate glasses: Insights from multi-spectroscopic (Raman, MIR, 29Si, 31P MAS-NMR, XAS, XANES) investigation. Ceram. Int. 2022, 48, 4238–4254. [Google Scholar] [CrossRef]



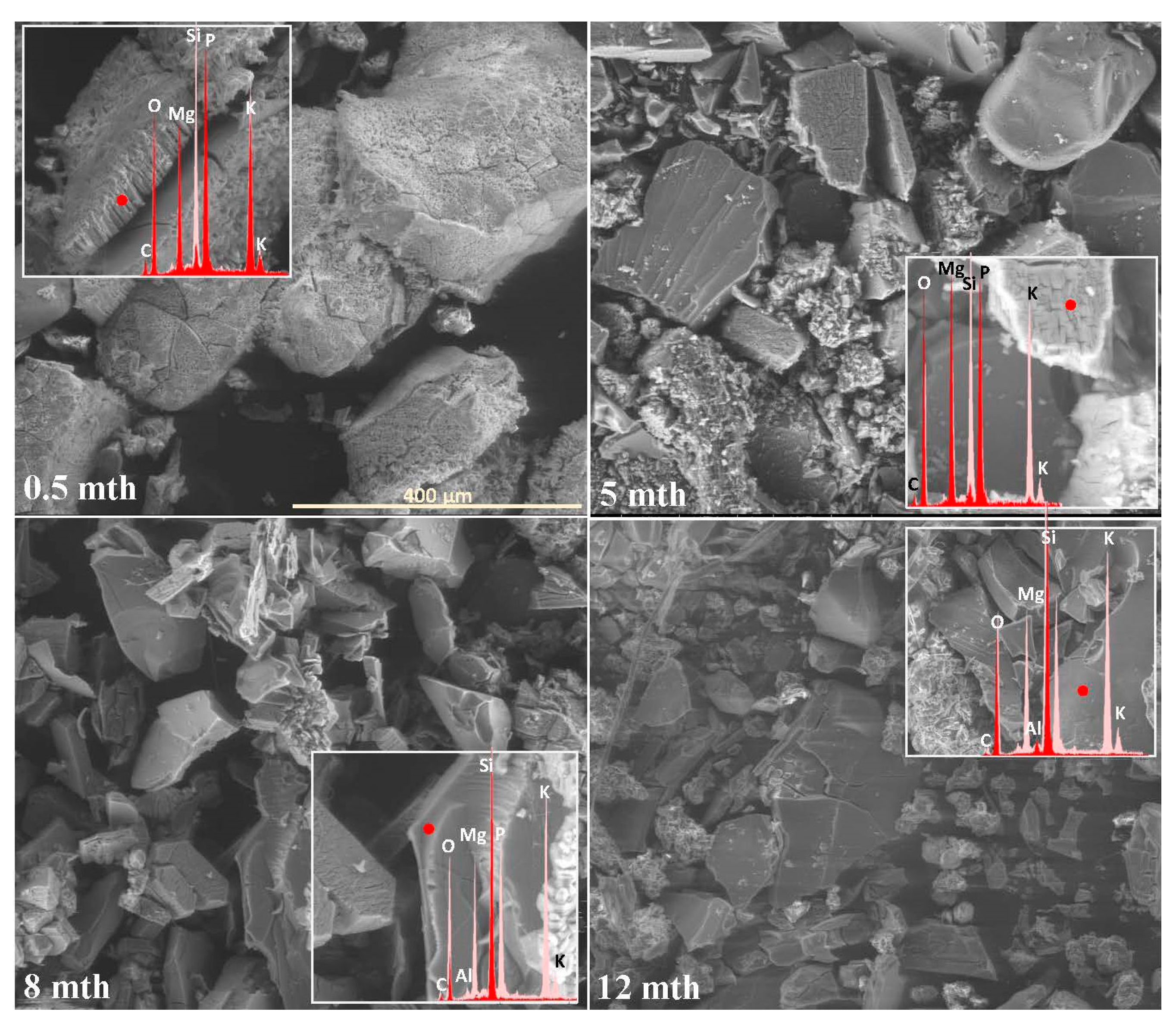
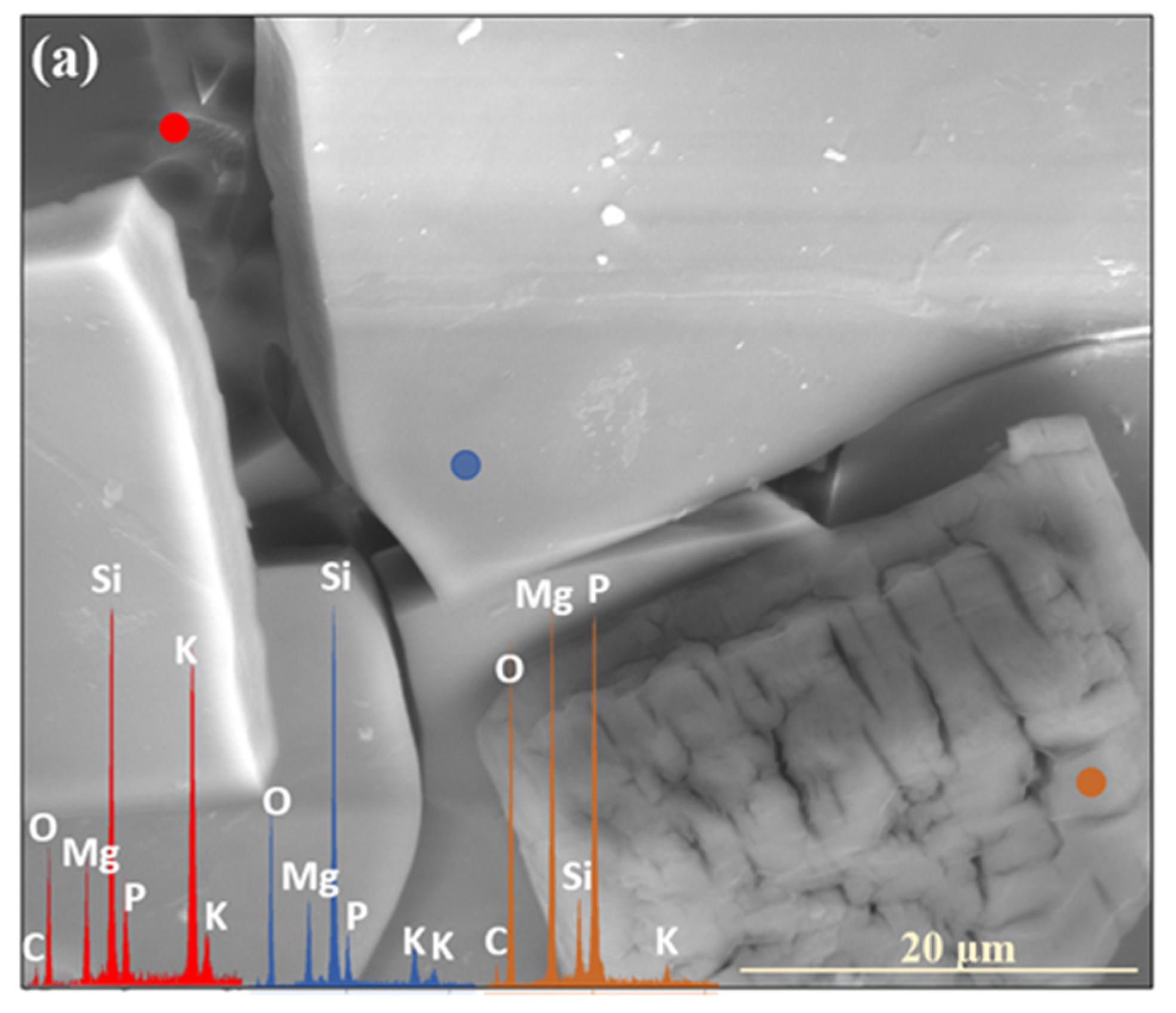

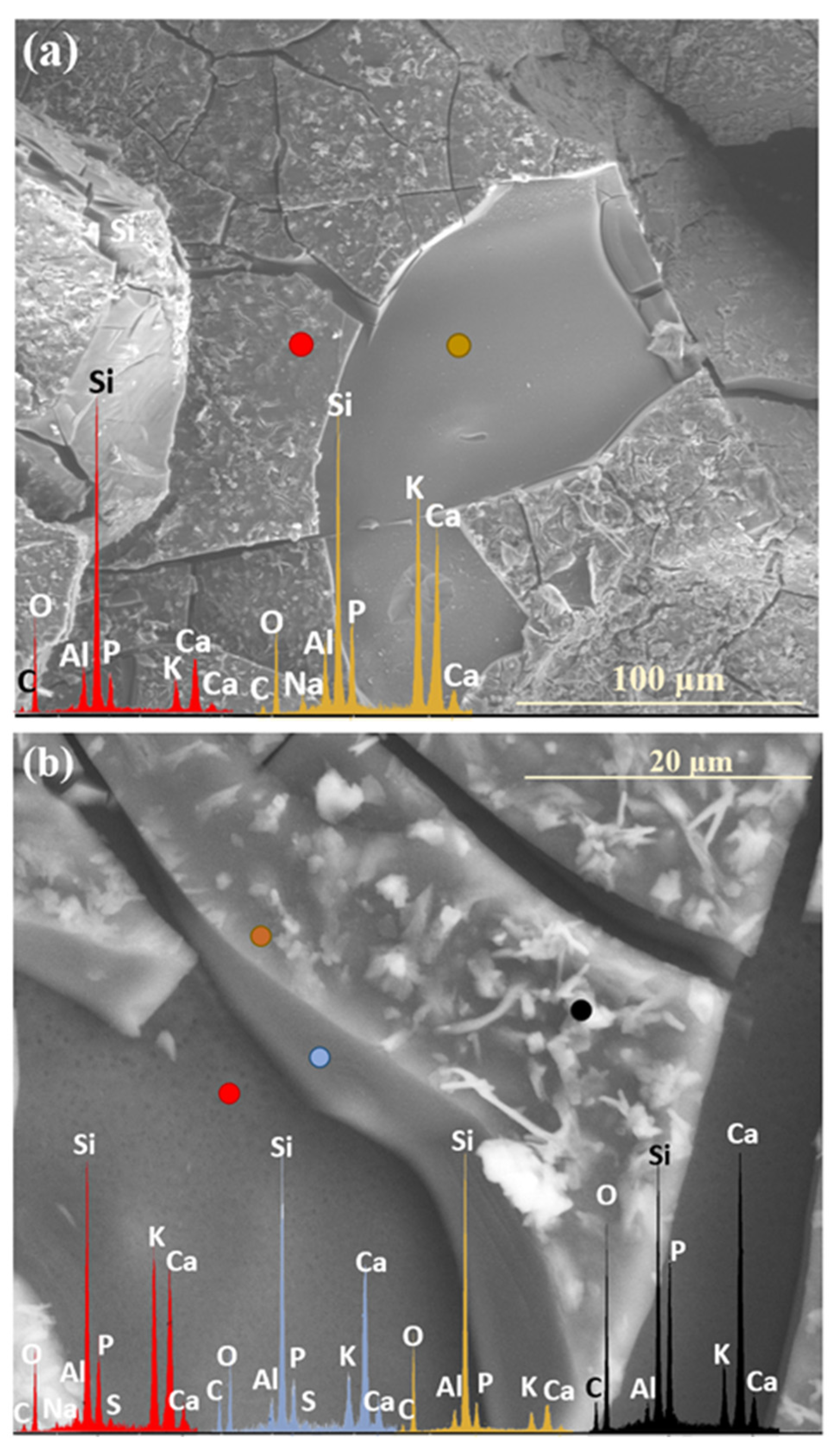
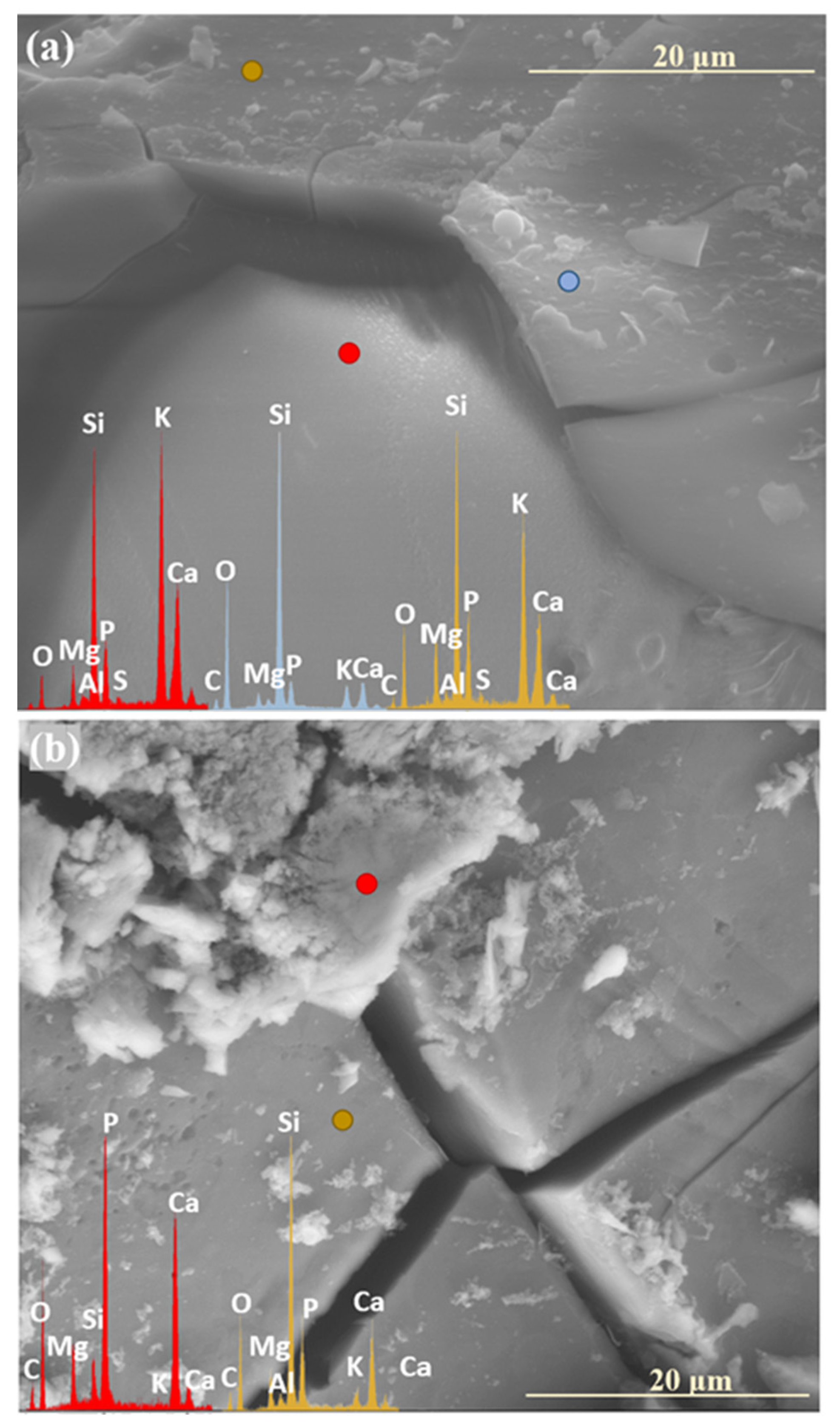




| Month | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0S_ 6PM | M | I | I | N | M | H | E | H | E | H | E | H | H |
| MP | KMP | MP | MP | MP | MP | MP | MP | MP | MP | MP | MP | MP | |
| Localized leaching | Intense leaching | ||||||||||||
| 3S_ 6PM | I | N | I | M | M | M | M | E | E | E | H | H | E |
| - | KMP | MP | MP | MP | MP | MP | MP | MP | MP | MP | MP | MP | |
| Localized leaching | Intense leaching | ||||||||||||
| 0S_ 6PC | N | I | I | I | M | N | M | M | E | M | H | H | H |
| CP small precipitate deposit | Thinning of the CP deposit | ||||||||||||
| Localized leaching | Intense leaching | ||||||||||||
| 3S_ 6PC | M | I | M | E | H | E | E | E | H | H | E | E | E |
| CaP small precipitate deposit | Thinning of the CaP deposit | ||||||||||||
| Localized leaching | Intense leaching | ||||||||||||
| 0S_ 6PMC | N | M | N | M | M | M | H | H | E | E | H | H | E |
| - | CP | MP | MP CC | CP CC | CC | CP CC | CP | CP | CP | CP | CC | CP | |
| Localized leaching | Intense leaching | ||||||||||||
| 3S_6PMC | I | I | I | * | * | E | E | E | E | E | E | E | E |
| - | CC | MP CC | KMP CC | KMP CC | KMP CC | CP | CP | CP | CP | - | - | - | |
| Localized leaching | Intense leaching | ||||||||||||
| 0S_10PM | E | E | E | E | E | E | C | C | M | H | H | C | M |
| KMP | KMP | KMP | KMP | KMP | KMP | - | - | - | - | - | - | - | |
| Localized leaching | Intense leaching | ||||||||||||
| 3S_10PM | E | E | E | E | E | C | C | C | C | C | C | C | C |
| KMP | KMP MP | KMP | KMP | KMP | KMP MP | MP | MP | MP | MP | MP | MP | MP | |
| Localized leaching | Intense leaching | ||||||||||||
| Soil Composition, mol.% | |||
|---|---|---|---|
| SiO2 | 38.64 | P2O5 | 0.88 |
| CaO | 27.72 | Na2O | 0.62 |
| SO3 | 8.94 | MnO | 0.21 |
| Fe2O3 | 6.28 | Br | 0.20 |
| Al2O3 | 5.02 | ZnO | 0.20 |
| Cl | 3.80 | PbO | 0.19 |
| MgO | 3.09 | SrO | 0.13 |
| K2O | 2.68 | ZrO2 | 0.10 |
| TiO2 | 1.30 | ||
| No. | SiO2 | P2O5 | K2O | MgO | CaO | SO3 |
|---|---|---|---|---|---|---|
| 0S_6PM | 41.42 (±0.40) | 6.75 (±0.09) | 20.42 (±0.00) | 30.17 (±0.45) | - | - |
| 1S_6PM | 37.39 (±0.41) | 5.96 (±0.09) | 19.53 (±0.00) | 31.72 (±46) | - | 0.84 (±0.12) |
| 3S_6PM | 40.00 (±0.41) | 6.92 (±0.09) | 20.63 (±0.00) | 32.67 (±0.46) | - | 1.84 (±0.14) |
| 5S_6PM | 41.60 (±0.40) | 6.49 (±0.40) | 17.69 (±0.00) | 33.44 (±0.40) | - | 1.59 (±0.40) |
| 0S_6PC | 39.11 (±0.44) | 6.29 (±0.09) | 18.88 (±0.00) | - | 33.63 (±0.71) | - |
| 1S_6PC | 38.28 (±0.45) | 6.14 (±0.10) | 17.21 (±0.00) | - | 35.79 (±0.73) | 1.00 (±0.16) |
| 3S_6PC | 37.90 (±0.45) | 6.46 (±0.09) | 18.40 (±0.00) | - | 35.12 (±0.72) | 2.05 (±0.13) |
| 5S_6PC | 43.25 (±0.45) | 6.10 (±0.01) | 15.03 (±0.00) | - | 37.42 (±0.72) | 1.39 (±0.15) |
| 0S_6PMC | 39.91 (±0.41) | 6.33 (±0.09) | 18.40 (±0.00) | 15.46 (±0.34) | 18.16 (±0.44) | - |
| 1S_6PMC | 38.20 (±0.42) | 6.33 (±0.09) | 19.59 (±0.00) | 15.53 (±0.33) | 18.09 (±0.56) | 0.95 (±0.12) |
| 3S_6PMC | 38.08 (±0.09) | 6.64 (±0.09) | 18.92 (±0.00) | 16.57 (±0.34) | 20.15 (±0.45) | 1.76 (±0.15) |
| 5S_6PMC | 41.70 (±0.42) | 6.46 (±0.09) | 16.41 (±0.00) | 16.32 (±0.35) | 20.89 (±0.56) | 1.40 (±0.14) |
| 0S_10PM | 38.11 (±0.40) | 11.01 (±0.08) | 21.56 (±0.00) | 29.12 (±0.44) | - | - |
| 1S_10PM | 39.00 (±0.42) | 10.89 (±0.09) | 19.95 (±0.00) | 28.98 (±0.50) | - | 0.25 (±0.05) |
| 3S_10PM | 39.37 (±0.42) | 11.02 (±0.09) | 21.14 (±0.00) | 29.35 (±0.47) | - | 0.83 (±0.10) |
| 5S_10PM | 42.29 (±0.41) | 11.48 (±0.08) | 22.29 (±0.00) | 24.71 (±0.46) | - | 0.48 (±0.07) |
| Simulated Seasons | Spring (Mar–May) | Summer (Jun–Aug) | Autumn (Sep–Nov) | Winter (Dec–Feb) |
|---|---|---|---|---|
| Day/night average temperature (°C) | 13.5/3.8 | 23.1/13.8 | 13.2/5.5 | 1.8/−4.2 |
| Average humidity (%) | 71 | 71 | 79 | - * |
| Data Origin | SiO2 | P2O5 | K2O | MgO | SO3 |
|---|---|---|---|---|---|
| XRF | 40.00 (±0.40) | 6.92 (±0.08) | 20.63 (±0.00) | 32.67 (±0.59) | 1.84 (±0.14) |
| SEM-EDS | 44.64 (±0.34) | 7.70 (±0.23) | 19.15 (±1.96) | 27.02 (±2.17) | 1.49 (±0.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezicka, A.; Wojteczko, A.; Sułowska, J.; Szumera, M. Alteration of Sulfur-Bearing Silicate–Phosphate (Agri)Glasses in Soil Environment: Chemical Interactions and Biological Response. Molecules 2025, 30, 1790. https://doi.org/10.3390/molecules30081790
Berezicka A, Wojteczko A, Sułowska J, Szumera M. Alteration of Sulfur-Bearing Silicate–Phosphate (Agri)Glasses in Soil Environment: Chemical Interactions and Biological Response. Molecules. 2025; 30(8):1790. https://doi.org/10.3390/molecules30081790
Chicago/Turabian StyleBerezicka, Anna, Agnieszka Wojteczko, Justyna Sułowska, and Magdalena Szumera. 2025. "Alteration of Sulfur-Bearing Silicate–Phosphate (Agri)Glasses in Soil Environment: Chemical Interactions and Biological Response" Molecules 30, no. 8: 1790. https://doi.org/10.3390/molecules30081790
APA StyleBerezicka, A., Wojteczko, A., Sułowska, J., & Szumera, M. (2025). Alteration of Sulfur-Bearing Silicate–Phosphate (Agri)Glasses in Soil Environment: Chemical Interactions and Biological Response. Molecules, 30(8), 1790. https://doi.org/10.3390/molecules30081790








