Nanoscale Fe3O4 Electrocatalysts for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Experimental
2.1. Materials and Synthesis Methodology
2.2. Characterization
2.3. Electrochemical Measurements
2.4. DFT Calculations
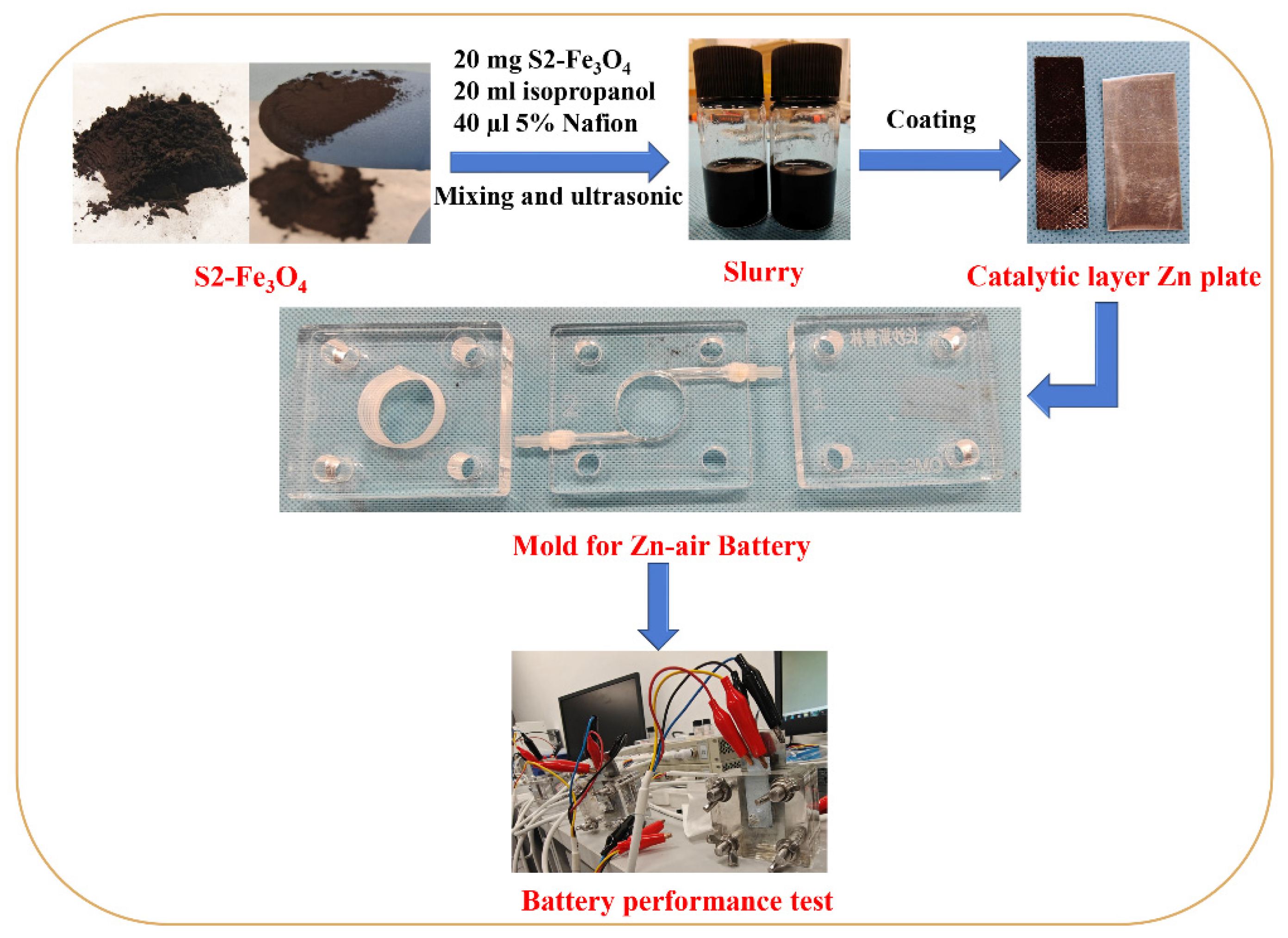
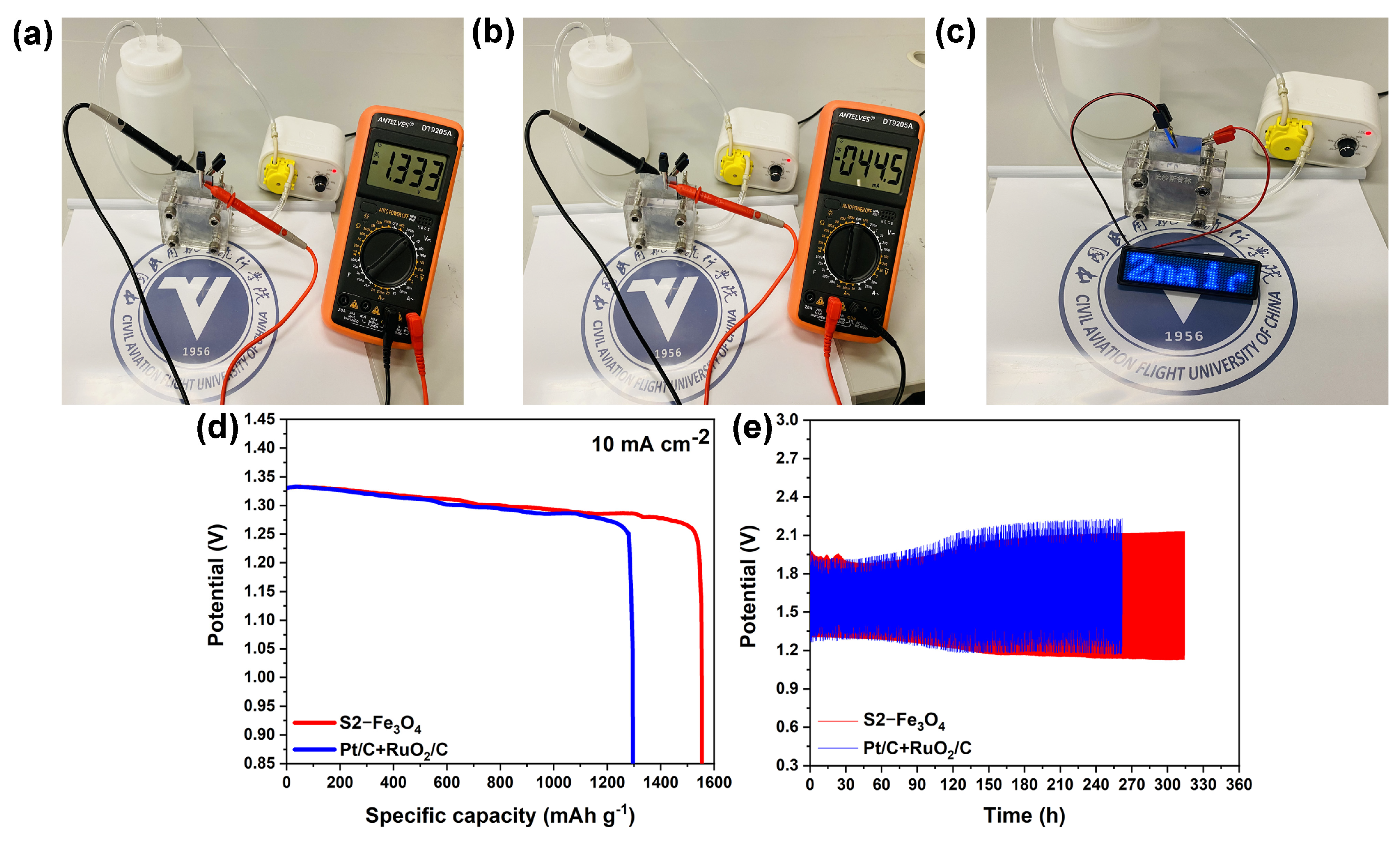
2.5. Assembly of the Zinc–Air Battery
3. Results and Discussions
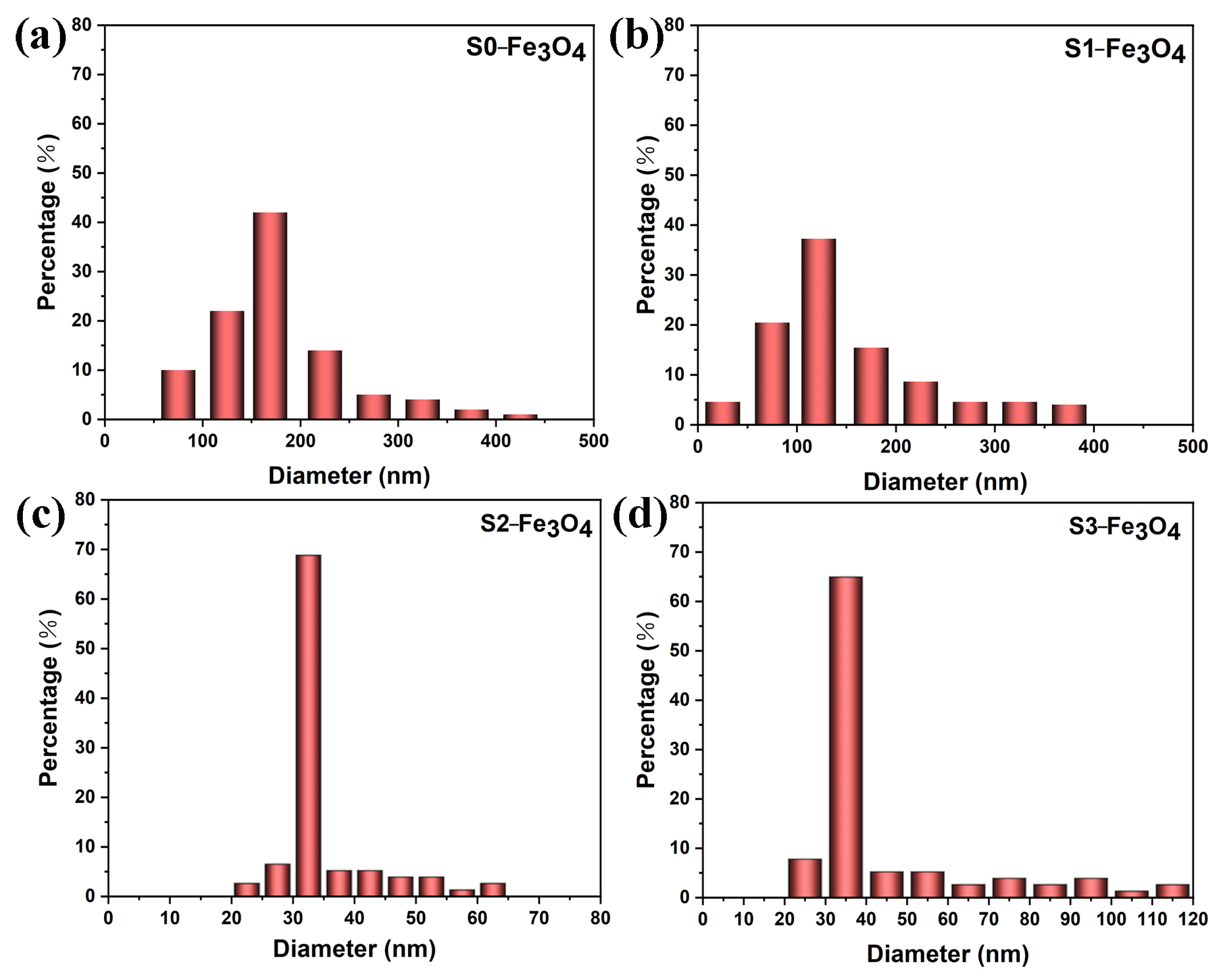
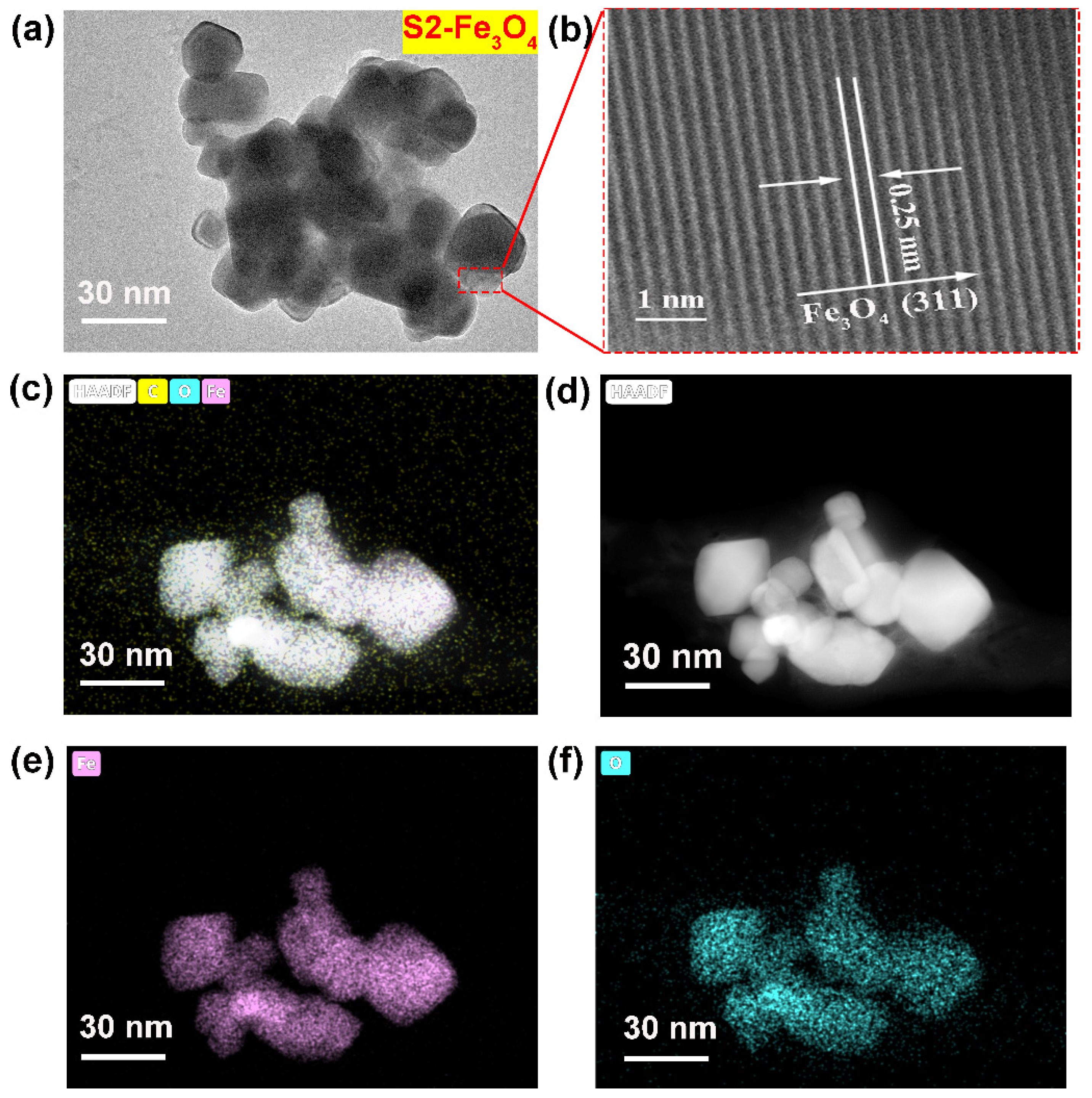
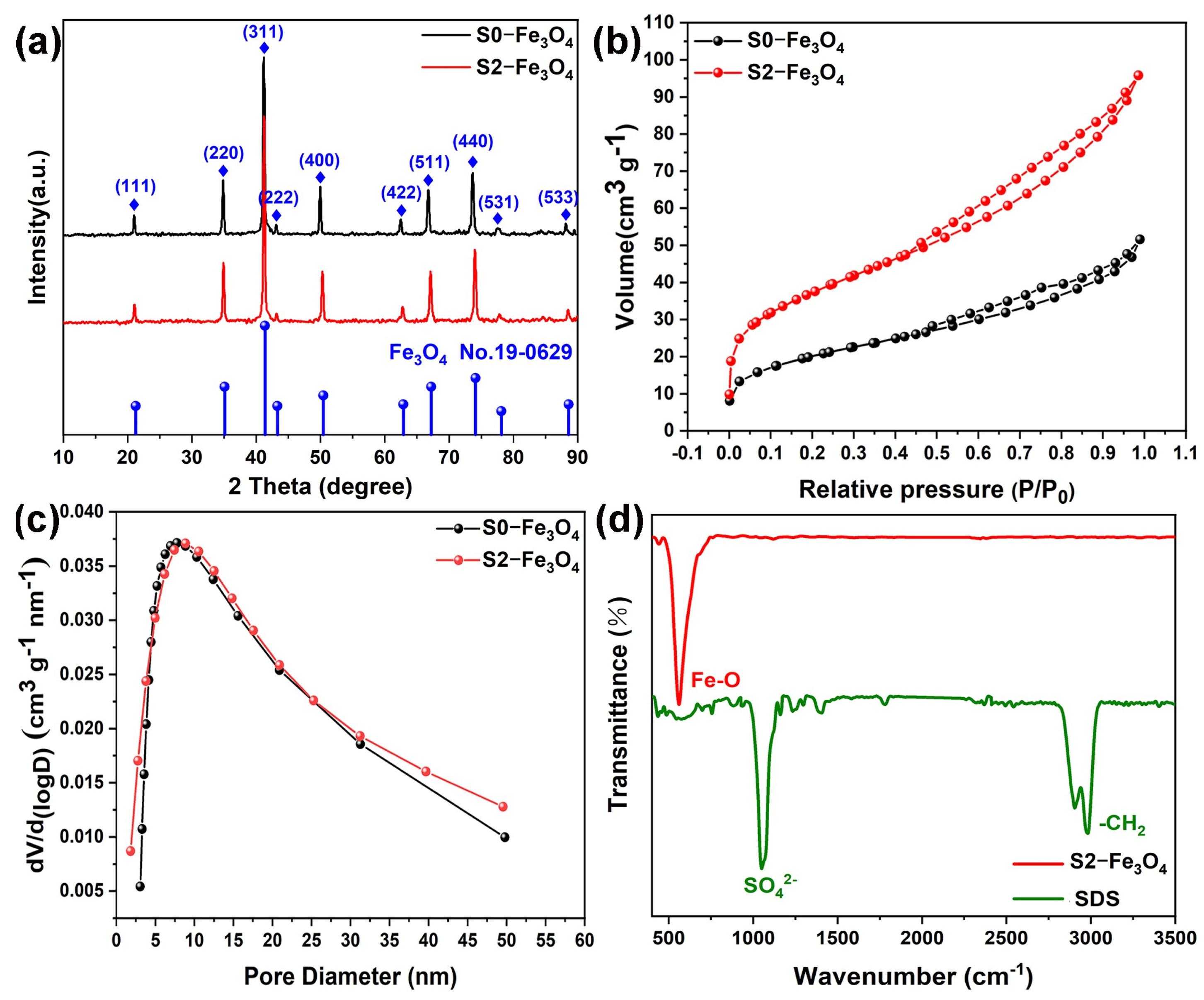
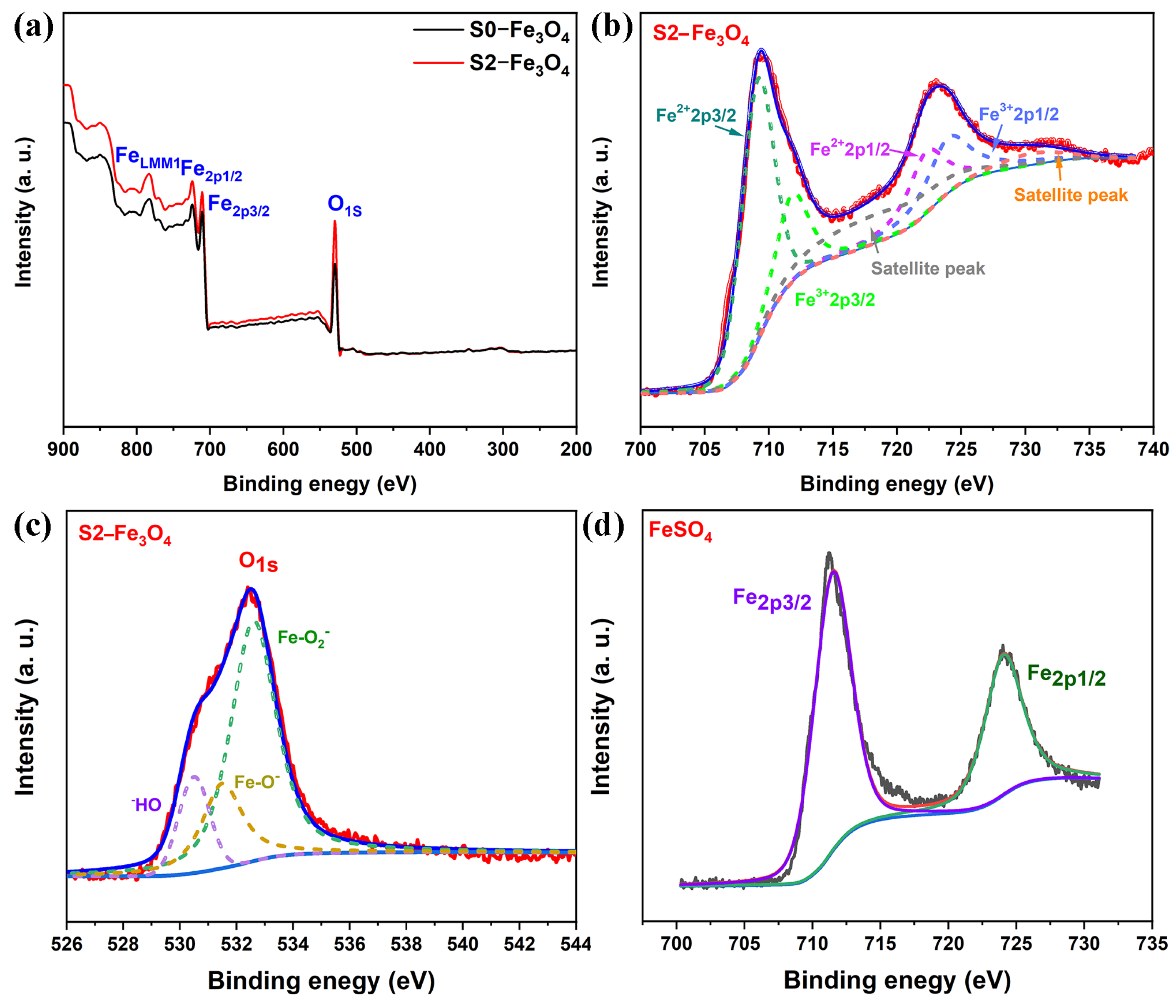
3.1. Structure Analysis
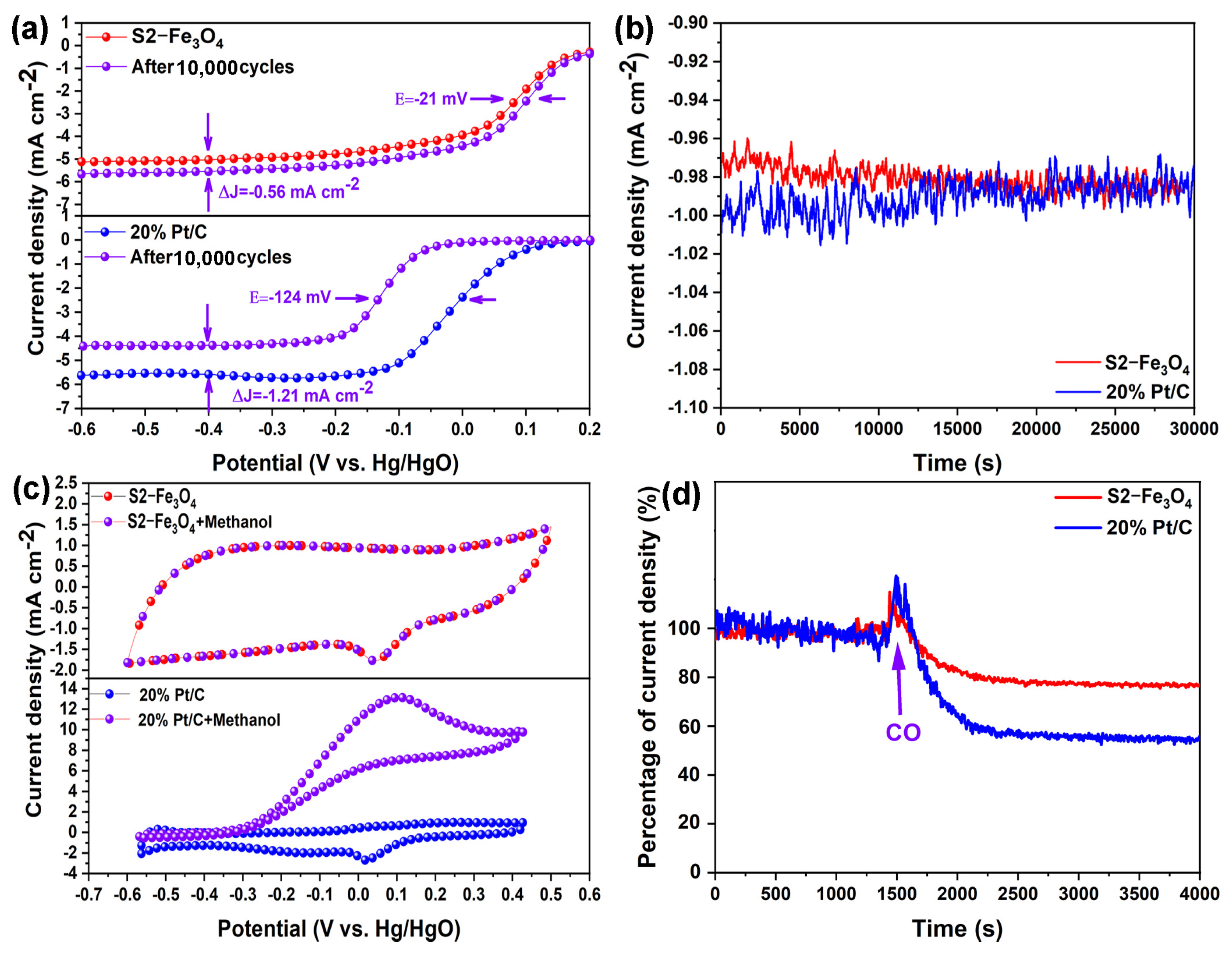
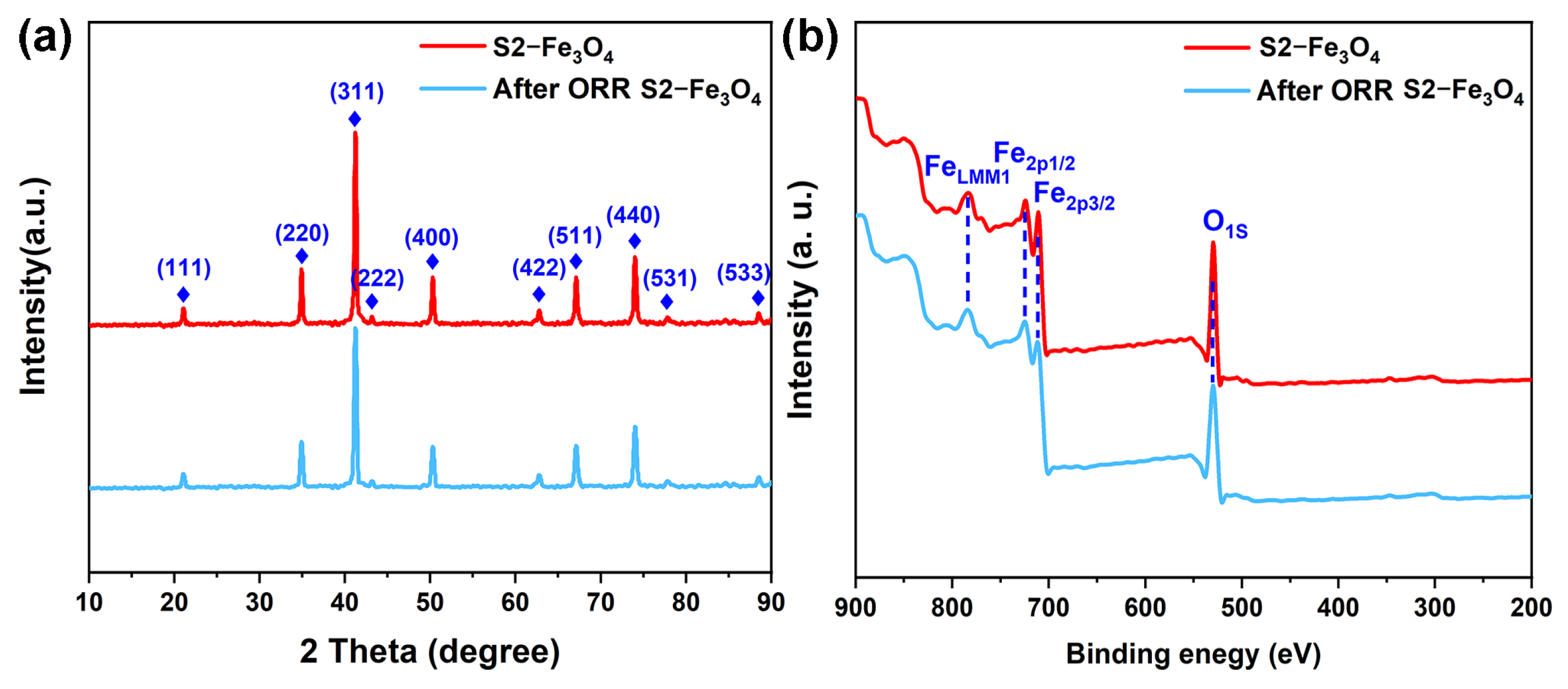
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.L.; Moller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Yang, Z.X.; Lu, Z.S.; Wang, W.C. Bifunctional CoNx embedded graphene electrocatalysts for OER and ORR: A theoretical evaluation. Carbon 2018, 130, 112–119. [Google Scholar] [CrossRef]
- Zhou, X.J.; Qiao, J.L.; Yang, L.; Zhang, J.J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 201301523. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.F.; Wang, W.C.; Cao, D.P. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Duehnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xing, P.; Wei, W.G.; Qiu, C.; Sun, X.L. DFT-guided design and synthesis of sea cucumber-derived N, S dual-doped porous carbon catalyst for enhanced oxygen reduction reaction and Zn-air battery performance. J. Mater. Sci. 2023, 58, 11968–11981. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.L.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Liu, M.L.; Zhao, Z.P.; Duan, X.F.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology-A review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Kwak, W.J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.K.; Frimer, A.A.; et al. Lithium-Oxygen Batteries and Related Systems: Potential, Status, and Future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef]
- Hren, M.; Bozic, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Weng, C.C.; Ren, J.T.; Wang, H.Y.; Lv, X.W.; Song, Y.J.; Wang, Y.S.; Chen, L.; Tian, W.W.; Yuan, Z.Y. Triple-phase oxygen electrocatalysis of hollow spherical structures for rechargeable Zn-Air batteries. Appl. Catal. B-Environ. 2022, 307, 121190. [Google Scholar] [CrossRef]
- Park, H.; Oh, S.; Lee, S.; Choi, S.; Oh, M. Cobalt-and nitrogen-codoped porous carbon catalyst made from core-shell type hybrid metal-organic framework (ZIF-L@ZIF-67) and its efficient oxygen reduction reaction (ORR) activity. Appl. Catal. B-Environ. 2019, 246, 322–329. [Google Scholar] [CrossRef]
- Lopez-Chavez, E.; Garcia-Quiroz, A.; Jimenez-Gonzalez, L.J.; Diaz-Gongora, J.A.I.; Pena-Castaneda, Y.A. Quantum chemistry of the oxygen reduction reaction (ORR) on Fe-G iron doped graphene for fuel cells. Int. J. Hydrogen Energy 2019, 44, 12439–12445. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.C.; Wu, Z.P.; Chen, G.Y.; Yang, F.; Zhu, S.Q.; Siddharth, K.; Kong, Z.J.; Lu, A.L.; Li, J.C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.C.; Su, M.; Briega-Martos, V.; Li, L.; Le, J.B.; Radjenovic, P.; Zhou, X.S.; Feliu, J.M.; Tian, Z.Q.; Li, J.F. Direct In Situ Raman Spectroscopic Evidence of Oxygen Reduction Reaction Intermediates at High-Index Pt(hkl) Surfaces. J. Am. Chem. Soc. 2020, 142, 715–719. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Gu, D.G.; Zhou, Y.; Ma, R.G.; Wang, F.F.; Liu, Q.; Wang, J.C. Facile Synthesis of N-Doped Graphene-Like Carbon Nanoflakes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction. Nano-Micro Lett. 2018, 10, 12. [Google Scholar] [CrossRef]
- Shrestha, S.; Asheghi, S.; Timbro, J.; Mustain, W.E. Temperature controlled surface chemistry of nitrogen-doped mesoporous carbon and its influence on Pt ORR activity. Appl. Catal. A Gen. 2013, 464, 233–242. [Google Scholar] [CrossRef]
- Bai, F.; He, Y.X.; Xu, L.C.; Wang, Y.; Wang, Y.; Hao, Z.Z.; Li, F. Improved ORR/OER bifunctional catalytic performance of amorphous manganese oxides prepared by photochemical metal-organic deposition. Rsc Adv. 2022, 12, 2408–2415. [Google Scholar] [CrossRef]
- Yang, G.; Erbay, C.; Yi, S.I.; de Figueiredo, P.; Sadr, R.; Han, A.; Yu, C. Bifunctional nano-sponges serving as non-precious metal catalysts and self-standing cathodes for high performance fuel cell applications. Nano Energy 2016, 22, 607–614. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.Q.; Li, Y.; Yang, H.Y.; Wang, X.R.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Quilez-Bermejo, J.; Morallon, E.; Cazorla-Amoros, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Zhang, J.T.; Qu, L.T.; Shi, G.Q.; Liu, J.Y.; Chen, J.F.; Dai, L.M. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef]
- Liu, M.C.; Guo, X.H.; Hu, L.B.; Yuan, H.F.; Wang, G.; Dai, B.; Zhang, L.L.; Yu, F. Fe3O4/Fe3C@Nitrogen-Doped Carbon for Enhancing Oxygen Reduction Reaction. Chemnanomat 2019, 5, 187–193. [Google Scholar] [CrossRef]
- Jiang, L.T.; Chen, J.F.; An, Y.; Han, D.Q.; Chang, S.; Liu, Y.; Yang, R.N. Enhanced electrochemical performance by nickel-iron layered double hydroxides (LDH) coated on Fe3O4 as a cathode catalyst for single-chamber microbial fuel cells. Sci. Total. Environ. 2020, 745, 141163. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Yan, W.M.; Chiang, W.H.; Thangavel, S.; Wang, C.H.; Wang, C.T. Innovative multi-processed N-doped carbon and Fe3O4 cathode for enhanced bioelectro-Fenton microbial fuel cell performance. Int. J. Energy Res. 2019, 43, 7594–7603. [Google Scholar]
- Gao, S.Y.; Fan, B.F.; Feng, R.; Ye, C.L.; Wei, X.J.; Liu, J.; Bu, X.H. N-doped-carbon-coated Fe3O4 from metal-organic framework as efficient electrocatalyst for ORR. Nano Energy 2017, 40, 462–470. [Google Scholar] [CrossRef]
- Chen, Z.K.; Lin, F.; He, D.D.; Jiang, H.Q.; Zhang, J.J.; Wang, X.; Huang, M.H. A hybrid composite catalyst of Fe3O4 nanoparticles-based carbon for electrochemical reduction of oxygen. New J. Chem. 2017, 41, 4959–4965. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gan, R.H.; Liu, H.; Dirican, M.; Wei, C.B.; Ma, C.; Shi, J.L.; Zhang, X.W. Fe3O4/Fe2O3/Fe nanoparticles anchored on N-doped hierarchically porous carbon nanospheres as a high-efficiency ORR electrocatalyst for rechargeable Zn-air batteries. J. Mater. Chem. A 2021, 9, 2764–2774. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Zhang, B.S.; Wang, D.W.; Su, D.S. Order of Activity of Nitrogen, Iron Oxide, and FeNx Complexes towards Oxygen Reduction in Alkaline Medium. Chemsuschem 2015, 8, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Chouchaine, A.; Kouass, S.; Touati, F.; Amdouni, N.; Dhaouadi, H. Fe3O4 nanomaterials: Synthesis, optical and electrochemical properties. J. Aust. Ceram. Soc. 2021, 57, 469–477. [Google Scholar] [CrossRef]
- Shen, W.; Shi, M.M.; Wang, M.; Chen, H.Z. A simple synthesis of Fe3O4 nanoclusters and their electromagnetic nanocomposites with polyaniline. Mater. Chem. Phys. 2010, 122, 588–594. [Google Scholar] [CrossRef]
- Shen, N.L.; Chen, N.N.; Lai, Q.X.; Pang, Y.S.; Sheng, Y.; Chen, H.; Zhang, W.Y.; Zheng, J.; Liang, Y.Y. Efficient K-Storage of Fe-Coupled Organic Molecule Anode in Ether-Based Electrolytes. Chem.-A Eur. J. 2023, 29, e202300424. [Google Scholar] [CrossRef]
- Huang, N.B.; Zhang, J.J.; Sun, Y.; Sun, X.N.; Qiu, Z.Y.; Ge, X.W. A non-traditional biomass-derived N, and S ternary self-doped 3D multichannel carbon ORR electrocatalyst. New J. Chem. 2020, 44, 14604–14614. [Google Scholar] [CrossRef]
- Kumeda, T.; Laverdure, L.; Honkala, K.; Melander, M.M.; Sakaushi, K. Cations Determine the Mechanism and Selectivity of Alkaline Oxygen Reduction Reaction on Pt(111). Angew. Chem. Int. Ed. Engl. 2023, 62, e202312841. [Google Scholar] [CrossRef]
- Brege, J.J.; Hamilton, C.E.; Crouse, C.A.; Barron, A.R. Ultrasmall Copper Nanoparticles from a Hydrophobically Immobilized Surfactant Template. Nano Lett. 2009, 9, 2239–2242. [Google Scholar] [CrossRef]
- Li, S.S.; Fang, J.H.; Li, L.; Zhu, M.; Zhang, F.; Zhang, B.Y.; Jiang, T.J.; Zhang, Y.X. An ultra-sensitive electrochemical sensor of Ni/Fe-LDH toward nitrobenzene with the assistance of surface functionalization engineering. Talanta 2021, 225, 122087. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Wu, C.; Wang, W.; Pan, L.; Zou, J.J.; Wang, L.; Zhang, X.W.; Li, G.Z. Tailoring the hetero-structure of iron oxides in the framework of nitrogen doped carbon for the oxygen reduction reaction and zinc-air batteries. J. Mater. Chem. A 2020, 8, 25791–25804. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.W.; Huang, C.; Zhong, L.S.; Fang, K.G. Carbon-encapsulated highly dispersed FeMn nanoparticles for Fischer-Tropsch synthesis to light olefins. New J. Chem. 2018, 42, 2413–2421. [Google Scholar] [CrossRef]
- Guo, L.K.; Zhang, J.J.; Sun, Y.; Liu, S.; Liu, W.; Dong, W.J.; Huang, N.B.; Sun, X.N. High Dispersion FeFe2O4 nanoparticles synthesis and its Oxygen Reduction Reaction catalytic performance. Chemistryselect 2022, 7, e202203544. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yu, D.S.; Dai, L.M.; Chang, D.W.; Baek, J.B. Polyelectrolyte-Functionalized Graphene as Metal-Free Electrocatalysts for Oxygen Reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Du, X.M.; Zhang, J.J.; Huang, N.B.; Yang, L.; Sun, X.N. Microwave-assisted preparation and improvement mechanism of carbon nanotube@NiMn2O4 core-shell nanocomposite for high performance asymmetric supercapacitors. J. Power Sources 2020, 473, 44. [Google Scholar] [CrossRef]
- Amornwutiroj, S.; Manpetch, P.; Singhapong, W.; Srinophakun, P.; Jaroenworaluck, A. Controllable synthesis of mesoporous magnetite/activated carbon composites as efficient adsorbents for hexavalent chromium removal. J. Dispers. Sci. Technol. 2020, 41, 1427–1444. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, S.M.; Liu, J.H.; Yu, M. Preparation and Anti-mildew Properties of TPN-SDS-layered Double Hydroxide Nanohybrids. J. Inorg. Mater. 2014, 29, 515–522. [Google Scholar]
- Bertolucci, E.; Galletti, A.M.R.; Antonetti, C.; Piccinelli, F.; Marracci, M.; Tellini, B.; Visone, C. Chemical and magnetic properties characterization of magnetic nanoparticles. In Proceedings of the 32nd Annual IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Pisa, Italy, 11–14 May 2015; pp. 1492–1496. [Google Scholar]
- Hssain, A.H.; Gündüz, B.; Majid, A.; Bulut, N. NTCDA compounds of optoelectronic interest: Theoretical insights and experimental investigation. Chem. Phys. Lett. 2021, 780, 138918. [Google Scholar] [CrossRef]
- Qiao, M.T.; Lei, X.F.; Ma, Y.; Tian, L.D.; He, X.W.; Su, K.H.; Zhang, Q.Y. Application of yolk-shell Fe3O4@N-doped carbon nanochains as highly effective microwave-absorption material. Nano Res. 2018, 11, 1500–1519. [Google Scholar] [CrossRef]
- Ai, Q.; Yuan, Z.W.; Huang, R.Z.; Yang, C.X.; Jiang, G.D.; Xiong, J.; Huang, Z.; Yuan, S.D. One-pot co-precipitation synthesis of Fe3O4 nanoparticles embedded in 3D carbonaceous matrix as anode for lithium ion batteries. J. Mater. Sci. 2019, 54, 4212–4224. [Google Scholar] [CrossRef]
- Zhang, H.J.; Guan, D.Q.; Gu, Y.X.; Xu, H.Y.; Wang, C.C.; Shao, Z.P.; Guo, Y.M. Tuning synergy between nickel and iron in Ruddlesden-Popper perovskites through controllable crystal dimensionalities towards enhanced oxygen-evolving activity and stability. Carbon Energy 2024, 6, e465. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ho, M.H.V.; Vo, T.K.; Dinh, Q.K.; Nguyen, C.V. Three-Dimensional Hierarchical Flower-Like Fe3O4 Porous Microstructure Decorated with Au Nanoparticles: Synthesis and Application for Sensitive Electrochemical Detection of Chloramphenicol. J. Electron. Mater. 2022, 51, 5795–5807. [Google Scholar]
- Fan, R.; Chen, X.H.; Gui, Z.; Liu, L.; Chen, Z.Y. A new simple hydrothermal preparation of nanocrystalline magnetite Fe3O4. Mater. Res. Bull. 2001, 36, 497–502. [Google Scholar] [CrossRef]
- Liu, R.L.; Wu, D.Q.; Feng, X.L.; Mullen, K. Nitrogen-Doped Ordered Mesoporous Graphitic Arrays with High Electrocatalytic Activity for Oxygen Reduction. Angew. Chem.-Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef]
- Guan, D.Q.; Xu, H.Y.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.M.; Lin, Z.Z.; Tang, J.Y.; Wang, Z.H.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2024, 37, e2413073. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Fu, Y.; Peng, X.; Xia, M.; Peng, W.; Liang, Y.; Wei, W. Nanoscale Fe3O4 Electrocatalysts for Oxygen Reduction Reaction. Molecules 2025, 30, 1753. https://doi.org/10.3390/molecules30081753
Zhang J, Wang J, Fu Y, Peng X, Xia M, Peng W, Liang Y, Wei W. Nanoscale Fe3O4 Electrocatalysts for Oxygen Reduction Reaction. Molecules. 2025; 30(8):1753. https://doi.org/10.3390/molecules30081753
Chicago/Turabian StyleZhang, Junjie, Jilong Wang, Yaoming Fu, Xing Peng, Maosong Xia, Weidong Peng, Yaowei Liang, and Wuguo Wei. 2025. "Nanoscale Fe3O4 Electrocatalysts for Oxygen Reduction Reaction" Molecules 30, no. 8: 1753. https://doi.org/10.3390/molecules30081753
APA StyleZhang, J., Wang, J., Fu, Y., Peng, X., Xia, M., Peng, W., Liang, Y., & Wei, W. (2025). Nanoscale Fe3O4 Electrocatalysts for Oxygen Reduction Reaction. Molecules, 30(8), 1753. https://doi.org/10.3390/molecules30081753






