The Effect of Grape Seed Extract on Lipid Oxidation, Color Change, and Microbial Growth in a Beef–Pork Sausage Model System
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Antioxidants on pH and Water Activity (aw) of Flat Sausages
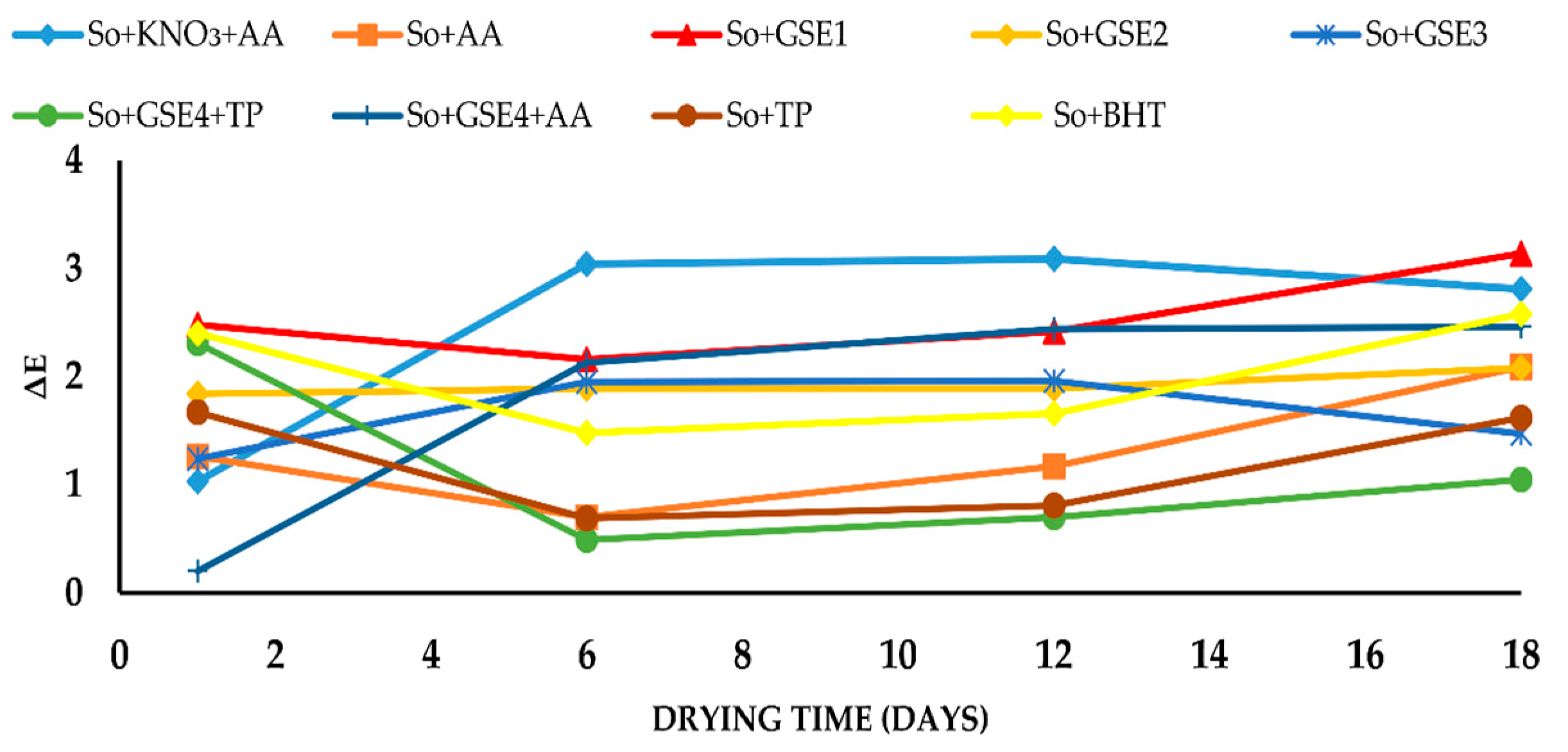
2.2. Effect of Antioxidants on Color Parameters of Flat Sausages
2.3. Effect of Antioxidants on Lipid Oxidation and Antioxidant Capacity of Flat Sausages
2.4. Effect of Antioxidants on the Total Bacterial Count of Flat Sausage Samples During the Drying Process
2.5. Protein and Free Fatty Acid Contents in Flat Sausages
3. Materials and Methods
3.1. Materials
3.2. Preparation of Grape Extract
3.3. Preparation of Flat Sausage Samples
- Without additional additives
- 0.25 g potassium nitrate and 0.125 g ascorbic acid (0.1% KNO3 and 0.05% AA)
- 0.125 g ascorbic acid (0.05%)
- 0.08 g grape seed extract (0.032%)
- 0.125 g grape seed extract (0.05%)
- 0.25 g grape seed extract (0.1%)
- 0.0625 g grape seed extract and 0.0625 g α-tocopherol (0.025% GSE and 0.025% TP)
- 0.0625 g grape seed extract and 0.0625 g ascorbic acid (0.025% GSE and 0.025% AA)
- 0.125 g α-tocopherol (0.05% TP)
- 0.05 g synthetic antioxidant—butylated hydroxytoluene (0.02%)
3.4. Determination of pH
3.5. Determination of Water Activity
3.6. Determination of Color
3.7. Determination of Lipid Oxidation
3.8. Determination of Antioxidant Capacity by DPPH* Assay
3.9. Determination of Total Microbial Count
3.10. Determination of Proteins
3.11. Determination of Free Fatty Acids
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GSE | Grape seed extract |
| AA | Ascorbic acid |
| TF | α-tocopherol |
| GRAS | Generally recognized as safe |
| FDA | Food and Drug Administration |
| BHT | Butylated hydroxytoluene |
| HACCP | Hazard Analysis and Critical Control Points |
| MDA | Malondialdehyde |
| TBA | Thiobarbituric acid |
| TBARS | Thiobarbituric acid reactive substance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TBC | Total bacterial count |
| FFA | Free fatty acid |
References
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.d.L.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape byproducts in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Ghouila, Z.; Laurent, S.; Boutry, S.; Vander Elst, L.; Nateche, F.; Muller, R.N.; Baaliouamer, A. Antioxidant, antibacterial and cell toxicity effects of polyphenols Fromahmeur bouamer grape seed extracts. J. Fundam. Appl. Sci. 2017, 9, 392–420. [Google Scholar] [CrossRef]
- Xia, E.O.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Mallekedi, S.; Velmurugan, V.; Kathiravan, M.K. Grape Seed Chemical Composition and Its Activity on Different Leukemia Cell Lines: A Review. JCHR 2023, 13, 2771–2780. [Google Scholar]
- Elejalde, E.; Villarán, M.; ·Esquivel, A.; Alonso, R. Bioaccessibility and Antioxidant Capacity of Grape Seed and Grape Skin Phenolic Compounds After Simulated In Vitro Gastrointestinal Digestion. Plant Foods Hum. Nutr. 2024, 79, 432–439. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds. Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar]
- Machado, A.R.; Atatoprak, T.; Santos, J.; Alexandre, E.M.C.; Pintado, M.E.; Paiva, J.A.P.; Nunes, J. Potentialities of the Extraction Technologies and Use of Bioactive Compounds from Winery By-Products: A Review from a Circular Bioeconomy Perspective. Appl. Sci. 2023, 13, 7754. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rat, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Garcia-Lomillo, J.; Gonzalez-San Jose, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Libera, J.; Latoch, A. Karolina MariaWójciak, Utilization of Grape Seed Extract as a Natural Antioxidant in the Technology of Meat Products Inoculated with a Probiotic Strain of LAB. Foods 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C.d.S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives; European Union: Brussels, Belgium, 2008.
- Commission Regulation (EU) No 1129/2011 of 11 November 2011; European Union: Brussels, Belgium, 2008.
- Commission Regulation (EU) No 601/2014 of 4 June 2014; European Union: Brussels, Belgium, 2008.
- Amin, R.A.; Edris, S.N. Grape Seed Extract as Natural Antioxidant and Antibacterial in Minced Beef. PSM Biol. Res. 2017, 2, 89–96. [Google Scholar]
- Shimizu, H.; Iwamoto, S. Problems of Lipid Oxidation in Minced Meat Products for a Ready-made Meal during Cooking, Processing, and Storage. Rev. Agric. Sci. 2022, 10, 24–35. [Google Scholar] [CrossRef]
- Ivanov, Y.; Godjevargova, T. The effect of grape seed and skin extracts on oxidative and color stability of minced pork meat. J. Chem. Technol. Met. 2024, 59, 797–803. [Google Scholar] [CrossRef]
- Nafady, N.A.A.; Seleim, M.A.A.; Darwish, S.M.I.; El-Geddawy, M.M.A. Effect of Incorporating Grape Seeds as a Natural Preservative on the Properties of Beef Burgers During Freeze Preservation. Assiut J. Agric. Sci. 2024, 55, 43–60. [Google Scholar] [CrossRef]
- Price, A.; Diaz, P.; Banon, S.; Garrido, M.D. Natural extracts versus sodium ascorbate to extend the shelf life of meat-based ready-to-eat meals. Food Sci. Technol. Int. 2013, 19, 427–438. [Google Scholar] [CrossRef]
- El-Zainy, A.R.M.; Morsy, A.E.; Sedki, A.G.; Mosa, N.M. Polyphenols extracted from grape seeds and its effects as antioxidant and antimicrobial on beef sausage. J. Food Dairy Sci. 2016, 7, 19–25. [Google Scholar] [CrossRef]
- Kulkarni, S.; DeSantos, F.A.; Kattamuri, S.; Rossi, S.J.; Brewer, M.S. Effect of Grape Seed Extract on Oxidative, Color and Sensory Stability of a Pre-Cooked, Frozen, Re-Heated Beef Sausage Model System. Meat Sci. 2011, 88, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Bermúdez, R.; Lorenzo, J.M.; Franco, D. Effect of Addition of Natural Antioxidants on the Shelf-Life of “Chorizo”, a Spanish Dry-Cured Sausage. Antioxidants 2015, 4, 42–67. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Mielnik, M.B.; Lsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Fadhil, Y.S. Effect of grape seed extract on the quality of local meat product (basturma) during storage. Food Sci. Technol. 2023, 43, e4423. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Chen, X.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-fermented bacon. LWT Food Sci. Technol. 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Abidi, S.; Fatima, N.; Mumtaz, T.; Farooq, S.; Naeem, H.; Tariq, M.; Mujahid, S. Exploring the synergistic antibacterial potential of grape seed and cranberry fruit extract combination against methicillin-resistant Staphylococcus Aureus. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 2450–2455. [Google Scholar] [CrossRef]
- El-Beltagi, H.; El-Desouky, W.; Yousef, R. Synergistic Antioxidant Scavenging Activities of Grape Seed and Green Tea Extracts against Oxidative Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 367–374. [Google Scholar] [CrossRef]
- Gupta, R.K.; Chawla, U.P.; Tripathi, M.; Shukla, A.K.; Pandey, A. Synergistic antioxidant activity of tea with ginger, black pepper and tulsi. Int. J. Pharm. Pharm. Sci. 2014, 6, 477–479. [Google Scholar]
- Koç, Z. The Effects of Grape Seed Powder and Extract on Antimicrobial of Fermented Turkish Sausage. IJOEAR 2017, 3, 79–85. [Google Scholar]
- Li, L.; Shao, J.; Zhu, X.; Zhou, G.; Xu, X. Effect of Plant Polyphenols and Ascorbic Acid on Lipid Oxidation, Residual Nitrite and N-Nitrosamines Formation in Dry-Cured Sausage. Int. J. Food Sci. Technol. 2013, 48, 1157–1164. [Google Scholar] [CrossRef]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of Identification and Quantification of Polyphenolic Compounds in Skins and Seeds of Four Grape Varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Gómez, M.; Fonseca, S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control 2014, 46, 382–389. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Franco, D. Fat effect on physico-chemical, microbial and textural changes through the manufactured of dry-cured foal sausage. Lipolysis, proteolysis and sensory properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Temperán, S.; Bermúdez, R.; Cobas, N.; Purriños, L. Changes in physico-chemical, microbiological, textural and sensory attributes during ripening of dry-cured foal salchichón. Meat Sci. 2012, 90, 194–198. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural Antioxidants Used in Meat Products: A Brief Review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Barretto, T.L.; Rodriguez, J.M.L.; Bis-Souza, C.V.; Barba, F.J.; da Barretto, A.C.S. Natural Colorants Improved the Physicochemical and Sensorial Properties of Frozen Brazilian Sausage (Linguiça) with Reduced Nitrite. Sci. Agric. 2021, 78, e20190211. [Google Scholar] [CrossRef]
- Font, R.M.; Mateo, N.M.; Gomez, S.V.; Gomez, L.F.; Catalán, E.B.; Bruna, N.M.; López, D.S.; Parreno, P.M.; Molina, V.M.; Roche, M.V.; et al. Synergistic Combination of Flavonoids and Vitamin C 2013. Spain Patent ES242 0080A1.
- Kitao, S.; Teramoto, M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Stabilizing Effect of Grape Seed Extract on Ascorbic Acid. Food Sci. Technol. Res. 2006, 12, 15–21. [Google Scholar] [CrossRef]
- Agregán, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.; Silva Barretto, A.C.; Lorenzo, J.M. Fucus vesiculosus Extracts as Natural Antioxidants for Improvement of Physicochemical Properties and Shelf Life of Pork Patties Formulated with Oleogels. J. Sci. Food Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Costa-Lima, B.R.C.; Guedes-Oliveira, J.M.; Alves, V.H.M.; Almeida, A.L.; Tonon, R.V.; Rosenthal, A.; Conte-Junior, C.A. Effect of Microencapsulated Extract of Pitaya (Hylocereus costaricensis) Peel on Color, Texture and Oxidative Stability of Refrigerated Ground Pork Patties Submitted to High Pressure Processing. Innov. Food Sci. Emerg. Technol. 2018, 49, 136–145. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries Extracts as Natural Antioxidants in Meat Products: A Review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Hodgson, J.; Croft, K.D.; Burke, V.; Beilin, L.J.; Puddey, I.B. The combination of vitamin C and grape-seed polyphenols increases blood pressure: A randomized, double-blind, placebo-controlled trial. J. Hypertens. 2005, 23, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Zanardi, E.; Dorigoni, V.; Badiani, A.; Chizzolini, R. Lipid and colour stability of Milano type sausages: Effect of packing conditions. Meat Sci. 2002, 61, 7–14. [Google Scholar] [CrossRef]
- ISO 2917; Meat and Meat Products—Measurement of pH—Reference Method. ISO: Geneva, Switzerland, 1999.
- Hassan, H.E.; Rahman Abdel, A.A.; El-Din Diaa, M.; El-Salam Abd, Z.A.; Shehata, F.M. Impact of ultraviolet irradiation processing on quality of fresh beef meat during cold storage. CIGR J. 2015, 17, 3, 130–139. [Google Scholar]
- Lorenzo, J.M.; Gonzalez-Rodriquez, R.M.; Sanchez, M.; Amado, I.R.; Franco, D. Efects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylated hydroxytoluene, BHT) on physical, chemical, microbiological and sensory characteristic of dry cured sausage “chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Sofi, F.R.; Raju, S.V.; Lakshmisha, L.P.; Ratankumar, S.R. Antioxidant and antimicrobial properties of grape and papaya seed extracts and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. J. Food Sci. Technol. 2016, 53, 104–117. [Google Scholar] [CrossRef]
- ISO 21528-2; Microbiology of the Food Chain, Horizontal. Method for the Detection and Enumeration of Enterobacteriaceae, Part 2: Colony—Count Technique. ISO: Geneva, Switzerland, 2017.
- Montegiove, N.; Pellegrino, R.M.; Emiliani, C.; Pellegrino, A.; Leonardi, L. An Alternative Approach to Evaluate the Quality of Protein-Based Raw Materials for Dry Pet Food. Animals 2021, 11, 458. [Google Scholar] [CrossRef]
- Uçar, B.; Gholami, Z.; Svobodová, K.; Hradecká, I.; Hönig, V. A Comprehensive Study for Determination of Free Fatty Acids in Selected Biological Materials: A Review. Foods 2024, 13, 1891. [Google Scholar] [CrossRef]
| Samples | pH | |||
| Dryingtime (days) | ||||
| 1 | 6 | 12 | 18 | |
| 1. So | 5.93 ± 0.01 c,B | 5.81 ± 0.01 c,C | 6.05 ± 0.01 c,B | 6.15 ± 0.01 c,A |
| 2. So+KNO3+AA | 6.56 ± 0.01 a,B | 6.53 ± 0.01 a,C | 6.59 ± 0.01 a,B | 6.62 ± 0.01 a,A |
| 3. So+AA | 6.03 ± 0.01 b,B | 6.00 ± 0.01 b,C | 6.04 ± 0.01 b,B | 6.14 ± 0.01 b,A |
| 4. So+GSE1 | 5.82 ± 0.01 e,B | 5.73 ± 0.01 e,C | 5.77 ± 0.00 e,B | 5.81 ± 0.01 e,A |
| 5. So+GSE2 | 5.49 ± 0.01 g,B | 5.41 ± 0.01 g,C | 5.25 ± 0.18 g,B | 5.41 ± 0.01 g,A |
| 6. So+GSE3 | 5.39 ± 0.01 f,B | 5.36 ± 0.01 f,C | 5.73 ± 0.02 f,B | 5.80 ± 0.01 f,A |
| 7. So+GSE4+TP | 6.08 ± 0.00 d,B | 5.95 ± 0.01 d,C | 5.78 ± 0.04 d,B | 5.86 ± 0.01 d,A |
| 8. So+GSE4+AA | 6.01 ± 0.01 c,B | 5.95 ± 0.00 c,C | 6.03 ± 0.02 c,B | 6.05 ± 0.01 c,A |
| 9. So+TP | 6.13 ± 0.01 b,B | 6.09 ± 0.01 b,C | 5.99 ± 0.01 b,B | 6.02 ± 0.00 b,A |
| 10. So+BHT | 5.66 ± 0.01 e,B | 5.47 ± 0.01 e,C | 5.97 ± 0.00 e,B | 6.01 ± 0.01 e,A |
| Water activity (aw) | ||||
| Drying time (days) | ||||
| 1 | 6 | 12 | 18 | |
| 1. So | 0.90 ± 0.01 e,A | 0.86 ± 0.01 e,B | 0.83 ± 0.01 e,C | 0.83 ± 0.01 e,C |
| 2. So+KNO3+AA | 0.87 ± 0.00 g,A | 0.85 ± 0.01 g,B | 0.80 ± 0.01 g,C | 0.80 ± 0.01 g,C |
| 3. So+AA | 0.88 ± 0.01 f,A | 0.85 ± 0.01 f,B | 0.82 ± 0.01 f,C | 0.82 ± 0.01 f,C |
| 4. So+GSE1 | 0.94 ± 0.01 bc,A | 0.87 ± 0.01 bc,B | 0.84 ± 0.01 bc,C | 0.83 ± 0.01 bc,C |
| 5. So+GSE2 | 0.95 ± 0.01 cd,A | 0.88 ± 0.00 cd,B | 0.82 ± 0.01 cd,C | 0.80 ± 0.00 cd,C |
| 6. So+GSE3 | 0.93 ± 0.00 de,A | 0.87 ± 0.01 de,B | 0.81 ± 0.01 de,C | 0.81 ± 0.01 de,C |
| 7. So+GSE4+TP | 0.91 ± 0.01 e,A | 0.88 ± 0.01 e,B | 0.81 ± 0.01 e,C | 0.82 ± 0.01 e,C |
| 8. So+GSE4+AA | 0.91 ± 0.00 e,A | 0.87 ± 0.01 e,B | 0.81 ± 0.00 e,C | 0.81 ± 0.01 e,C |
| 9. So+TP | 0.95 ± 0.01 b,A | 0.89 ± 0.01 b,B | 0.83 ± 0.01 b,C | 0.83 ± 0.01 b,C |
| 10. So+BHT | 0.95 ± 0.01 a,A | 0.88 ± 0.00 a,B | 0.85 ± 0.01 a,C | 0.86 ± 0.01 a,C |
| Samples | Drying Time (Days) | |||
|---|---|---|---|---|
| 1 | 6 | 12 | 18 | |
| Lightness (L* value) | ||||
| 1. So | 26.61 ± 0.03 b,A | 26.76 ± 0.06 b,A | 26.66 ± 0.08 b,B | 27.59 ± 0.01 b,B |
| 2. So+KNO3+AA | 26.82 ± 0.25 c,A | 26.52 ± 0.02 c,A | 26.63 ± 0.13 c,B | 26.56 ± 0.06 c,B |
| 3. So+AA | 26.06 ± 0.08 e,A | 26.20 ± 0.01 e,A | 25.60 ± 0.01 e,B | 25.50 ± 0.07 e,B |
| 4. So+GSE1 | 25.18 ± 0.03 g,A | 25.05 ± 0.07 g,A | 24.97 ± 0.05 g,B | 24.70 ± 0.28 g,B |
| 5. So+GSE2 | 26.53 ± 0.39 d,A | 26.28 ± 0.10 d,A | 26.06 ± 0.08 d,B | 26.03 ± 0.04 d,B |
| 6. So+GSE3 | 27.05 ± 0.07 a,A | 27.16 ± 0.20 a,A | 26.96 ± 0.06 a,B | 27.02 ± 0.13 a,B |
| 7. So+GSE4+TP | 27.03 ± 0.12 c,A | 26.52 ± 0.02 c,A | 26.68 ± 0.06 c,B | 26.58 ± 0.04 c,B |
| 8. So+GSE4+AA | 26.76 ± 0.06 c,A | 26.71 ± 0.01 c,A | 26.74 ± 0.13 c,B | 26.18 ± 0.03 c,B |
| 9. So+TP | 26.17 ± 0.23 d,A | 26.13 ± 0.18 d,A | 26.08 ± 0.11 d,B | 26.18 ± 0.03 d,B |
| 10. So+BHT | 25.23 ± 0.32 f,A | 25.33 ± 0.04 f,A | 25.09 ± 0.10 f,B | 25.03 ± 0.04 f,B |
| Redness (a* value) | ||||
| 1. So | 8.97 ± 0.05 f,A | 4.48 ± 0.03 f,B | 4.17 ± 0.04 f,C | 4.66 ± 0.04 f,C |
| 2. So+KNO3+AA | 8.00 ± 0.01 a,A | 7.50 ± 0.14 a,B | 7.26 ± 0.01 a,C | 7.27 ± 0.05 a,C |
| 3. So+AA | 7.85 ± 0.22 f,A | 4.67 ± 0.04 f,B | 4.66 ± 0.06 f,C | 4.62 ± 0.02 f,C |
| 4. So+GSE1 | 7.03 ± 0.04 e,A | 5.77 ± 0.24 e,B | 5.89 ± 0.10 e,C | 5.89 ± 0.01 e,C |
| 5. So+GSE2 | 7.23 ± 0.04 d,A | 6.26 ± 0.07 d,B | 5.96 ± 0.06 d,C | 6.03 ± 0.10 d,C |
| 6. So+GSE3 | 7.98 ± 0.04 c,A | 6.39 ± 0.06 c,B | 6.10 ± 0.15 c,C | 6.01 ± 0.03 c,C |
| 7. So+GSE4+TP | 6.71 ± 0.13 i,A | 4.53 ± 0.13 i,B | 4.47 ± 0.05 i,C | 4.46 ± 0.06 i,C |
| 8. So+GSE4+AA | 8.99 ± 0.02 b,A | 6.58 ± 0.04 b,B | 6.51 ± 0.01 b,C | 6.55 ± 0.08 b,C |
| 9. So+TP | 7.52 ± 0.003 h,A | 4.51 ± 0.01 h,B | 4.38 ± 0.04 h,C | 4.32 ± 0.04 h,C |
| 10. So+BHT | 7.13 ± 0.18 g,A | 4.87 ± 0.04 g,B | 4.66 ± 0.08 g,C | 4.66 ± 0.08 g,C |
| Yellowness (b* value) | ||||
| 1. So | 6.86 ± 0.08 ab,A | 6.08 ± 0.68 ab,B | 6.30 ± 0.00 ab,B | 6.31 ± 0.01 ab,B |
| 2. So+KNO3+AA | 6.57 ± 0.05 a,A | 6.38 ± 0.04 a,B | 6.39 ± 0.07 a,B | 6.36 ± 0.04 a,B |
| 3. So+AA | 6.67 ± 0.06 a,A | 6.46 ± 0.08 a,B | 6.29 ± 0.02 a,B | 6.29 ± 0.01 a,B |
| 4. So+GSE1 | 6.27 ± 0.66 bcd,A | 6.32 ± 0.03 bcd,B | 6.10 ± 0.01 bcd,B | 6.30 ± 0.03 bcd,B |
| 5. So+GSE2 | 6.28 ± 0.39 abc,A | 6.49 ± 0.02 abc,B | 6.27 ± 0.05 abc,B | 6.29 ± 0.12 abc,B |
| 6. So+GSE3 | 6.26 ± 0.00 cd,A | 6.16 ± 0.06 cd,B | 6.17 ± 0.04 cd,B | 6.23±0.04 cd,B |
| 7. So+GSE4+TP | 6.63 ± 0.04 ef,A | 5.66 ± 0.06 ef,B | 5.67 ± 0.04 ef,B | 6.12 ± 0.02 ef,B |
| 8. So+GSE4+AA | 6.73 ± 0.04 fg,A | 5.70 ± 0.04 fg,B | 5.61 ± 0.01 fg,B | 5.61 ± 0.02 fg,B |
| 9. So+TP | 6.16 ± 0.07 g,A | 5.80 ± 0.04 g,B | 5.77 ± 0.06 g,B | 5.59 ± 0.01 g,B |
| 10. So+BHT | 6.17 ± 0.04 de,A | 6.10 ± 0.16 de,B | 6.10 ± 0.03 de,B | 6.03 ± 0.04 de,B |
| Samples | MDA, mg/kg | AO Capacity, % | |||||
|---|---|---|---|---|---|---|---|
| Drying Time (Days) | Drying Time (Days) | ||||||
| 1 | 6 | 12 | 18 | 2 | 9 | 18 | |
| 1. So | 0.49 ± 0.01 a,A | 0.81 ± 0.00 a,A | 0.81 ± 0.01 a,A | 0.97 ± 0.00 a,A | 29.03 ± 0.04 j,A | 24.64 ± 0.53 j,B | 18.86 ± 0.06 j,C |
| 2.So+KNO3+AA | 0.28 ± 0.00 ab,A | 0.29 ± 0.01 ab,A | 0.35 ± 0.00 ab,A | 0.44 ± 0.00 ab,A | 45.85 ± 0.23 d,A | 42.55 ± 0.13 d,B | 33.07 ± 0.05 d,C |
| 3. So+AA | 0.31 ± 0.00 bc,A | 0.41 ± 0.00 bc,A | 0.51 ± 0.00 bc,A | 0.65 ± 0.00 bc,A | 38.73 ± 0.04 g,A | 35.19 ± 0.06 g,B | 28.53 ± 0.15 g,C |
| 4. So+GSE1 | 0.24 ± 0.00 bc,A | 0.39 ± 0.00 bc,A | 0.49 ± 0.01 bc,A | 0.53 ± 0.00 bc,A | 39.89 ± 1.92 e,A | 37.04 ± 0.05 e,B | 33.38 ± 0.08 e,C |
| 5. So+GSE2 | 0.22 ± 0.00 c,A | 0.39 ± 0.02 c,A | 0.41 ± 0.00 c,A | 0.43 ± 0.00 bc,A | 44.93 ± 0.12 c,A | 42.16 ± 0.01 c,B | 38.69 ± 0.05 c,C |
| 6. So+GSE3 | 0.22 ± 0.00 c,A | 0.39 ± 0.00 c,A | 0.42 ± 0.00 c,A | 0.44 ± 0.00 c,A | 45.32 ± 0.04 b,A | 43.67 ± 0.02 b,B | 38.25 ± 0.14 b,C |
| 7. So+GSE4+TP | 0.23 ± 0.00 bc,A | 0.49 ± 0.01 bc,A | 0.54 ± 0.00 bc,A | 0.71 ± 0.00 bc,A | 35.41 ± 0.01 h,A | 32.56 ± 0.11 h,B | 29.03 ± 0.04 h,C |
| 8. So+GSE4+AA | 0.22 ± 0.00 c,A | 0.37 ± 0.00 c,A | 0.41 ± 0.00 c,A | 0.41 ± 0.00 c,A | 48.47 ± 0.09 a,A | 44.93 ± 0.12 a,B | 39.87 ± 0.08 a,C |
| 9. So+TP | 0.39 ± 0.01 abc,A | 0.50 ± 0.00 abc,A | 0.63 ± 0.00 abc,A | 0.73 ± 0.00 abc,A | 30.59 ± 0.01 i,A | 28.64 ± 0.11 i,B | 23.12 ± 0.05 i,C |
| 10. So+BHT | 0.26 ± 0.00 c,A | 0.29 ± 0.00 c,A | 0.32 ± 0.00 c,A | 0.45 ± 0.00 c,A | 35.53 ± 0.04 f,A | 35.12 ± 0.02 f,B | 33.18 ± 0.05 f,C |
| Samples | Total Bacterial Count of Sausage Samples | |
|---|---|---|
| Drying Time (2 Days) | Drying Time (18 Days) | |
| cfu/g (1 × 103) | cfu/g (1 × 103) | |
| 1. So | 277.00 ± 8.49 a,A | 162.50 ± 14.85 a,B |
| 2. So+KNO3+AA | 107.50 ± 7.78 h,A | 66.50 ± 4.95 h,B |
| 3. So+AA | 227.50 ± 9.19 c,A | 116.00 ± 5.66 c,B |
| 4. So+GSE1 | 212.50 ± 3.54 d,A | 108.50 ± 4.95 d,B |
| 5. So+GSE2 | 170.50 ± 3.54 f,A | 89.00 ± 1.41 f,B |
| 6. So+GSE3 | 178.00 ± 8.49 f,A | 92.00 ± 4.24 f,B |
| 7. So+GSE4+TP | 189.50 ± 6.36 e,A | 100.00 ± 2.83 e,B |
| 8. So+GSE4+AA | 116.00 ± 5.66 g,A | 78.50 ± 4.95 g,B |
| 9. So+TP | 238.50 ± 4.95 b,A | 126.50 ± 4.95 b,B |
| 10. So+BHT | 163.50 ± 2.12 f,A | 95.50 ± 7.78 f,B |
| Samples | Protein Content, % | Free Fatty Acids, % | ||
|---|---|---|---|---|
| Drying Time (Days) | Drying Time (Days) | |||
| 2 | 15 | 2 | 15 | |
| 1. So | 18.63 ± 0.04 f,B | 22.33 ± 0.04 f,A | 19.01 ± 0.01 a,B | 27.03 ± 0.04 a,A |
| 2. So+KNO3+AA | 18.29 ± 0.01 c,B | 23.20 ± 0.02 c,A | 12.49 ± 0.06 h,B | 19.76 ± 0.16 h,A |
| 3. So+AA | 18.48 ± 0.00 f,B | 22.45 ± 0.01 f,A | 15.42 ± 0.02 d,B | 22.69 ± 0.46 d,A |
| 4. So+GSE1 | 18.42 ± 0.01 f,B | 22.48 ± 0.00 f,A | 13.32 ± 0.03 g,B | 20.80 ± 0.06 g,A |
| 5. So+GSE2 | 18.31 ± 0.03 b,B | 23.57 ± 0.02 b,A | 13.42 ± 0.00 g,B | 20.41 ± 0.01 g,A |
| 6. So+GSE3 | 18.41 ± 0.01 d,B | 22.99 ± 0.02 d,A | 14.41 ± 0.01 f,B | 21.44 ± 0.01 f,A |
| 7. So+GSE4+TP | 18.59 ± 0.02 g,B | 22.03 ± 0.04 g,A | 16.95 ± 0.08 c,B | 23.58 ± 0.46 c,A |
| 8. So+GSE4+AA | 18.30 ± 0.01 a,B | 23.86 ± 0.06 a,A | 13.45 ± 0.01 g,B | 20.39 ± 0.06 g,A |
| 9. So+TP | 18.54 ± 0.02 g,B | 22.04 ± 0.06 g,A | 14.53 ± 0.04 e,B | 21.97 ± 0.12 e,A |
| 10. So+BHT | 18.28 ± 0.04 e,B | 22.96 ± 0.06 e,A | 17.58 ± 0.09 b,B | 26.66 ± 0.15 b,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.; Godjevargova, T.; Atanasova, M.; Nakov, G. The Effect of Grape Seed Extract on Lipid Oxidation, Color Change, and Microbial Growth in a Beef–Pork Sausage Model System. Molecules 2025, 30, 1739. https://doi.org/10.3390/molecules30081739
Ivanov Y, Godjevargova T, Atanasova M, Nakov G. The Effect of Grape Seed Extract on Lipid Oxidation, Color Change, and Microbial Growth in a Beef–Pork Sausage Model System. Molecules. 2025; 30(8):1739. https://doi.org/10.3390/molecules30081739
Chicago/Turabian StyleIvanov, Yavor, Tzonka Godjevargova, Milka Atanasova, and Gjore Nakov. 2025. "The Effect of Grape Seed Extract on Lipid Oxidation, Color Change, and Microbial Growth in a Beef–Pork Sausage Model System" Molecules 30, no. 8: 1739. https://doi.org/10.3390/molecules30081739
APA StyleIvanov, Y., Godjevargova, T., Atanasova, M., & Nakov, G. (2025). The Effect of Grape Seed Extract on Lipid Oxidation, Color Change, and Microbial Growth in a Beef–Pork Sausage Model System. Molecules, 30(8), 1739. https://doi.org/10.3390/molecules30081739









