Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26
Abstract
1. Introduction
2. Results
2.1. Phenotypic Antimicrobial Resistance Characterization
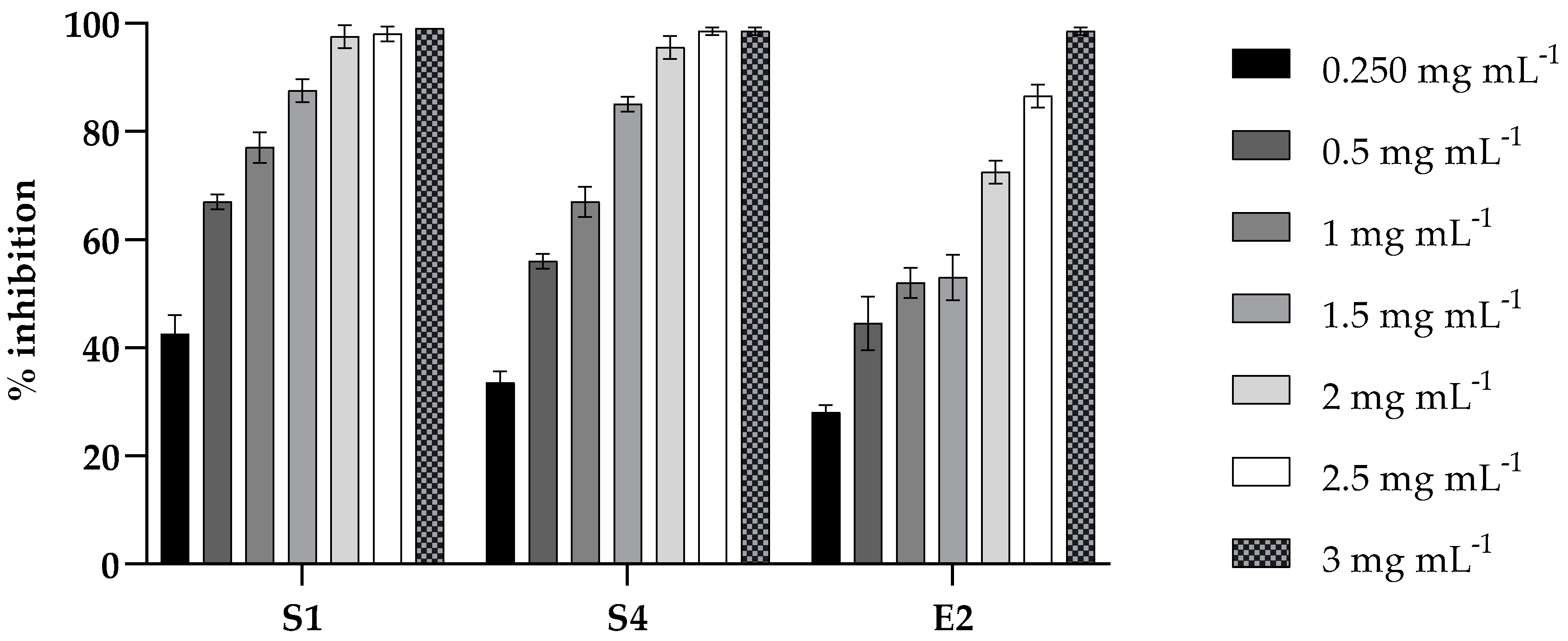
2.2. Antibacterial Activity of PLA
2.3. Challenge Test
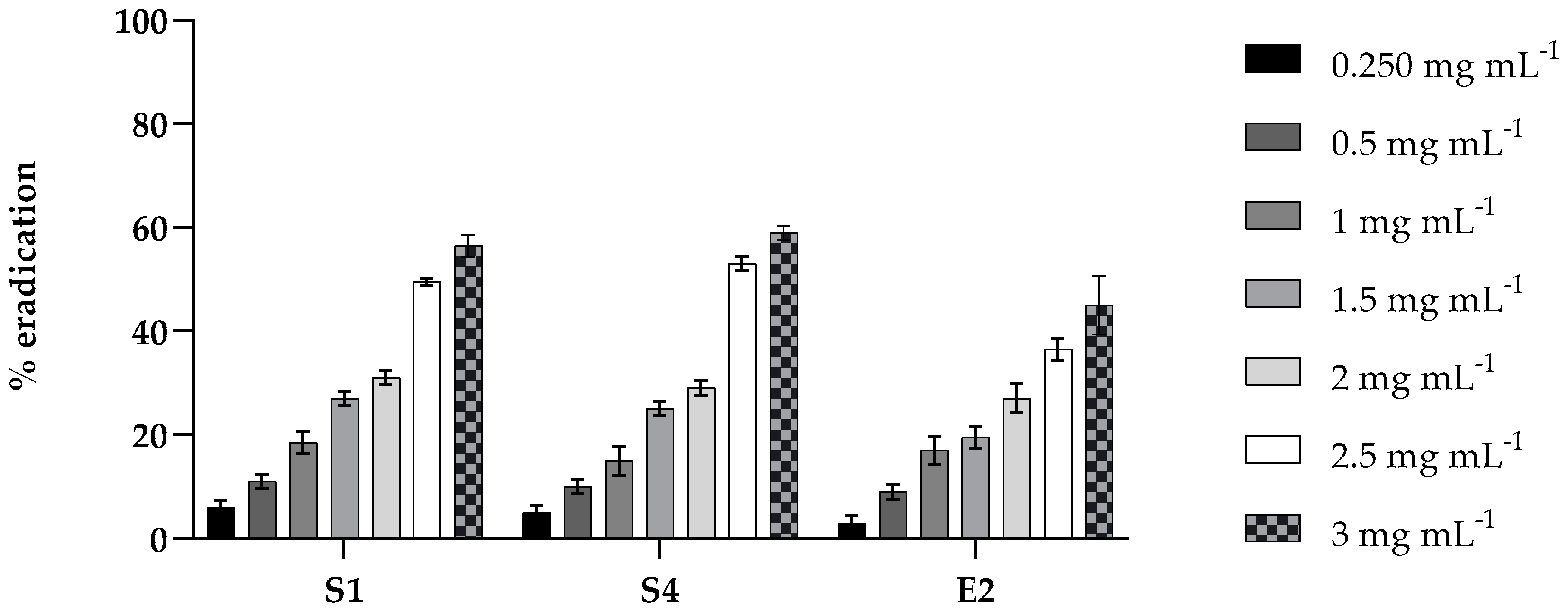
2.4. Antibiofilm Activity of PLA
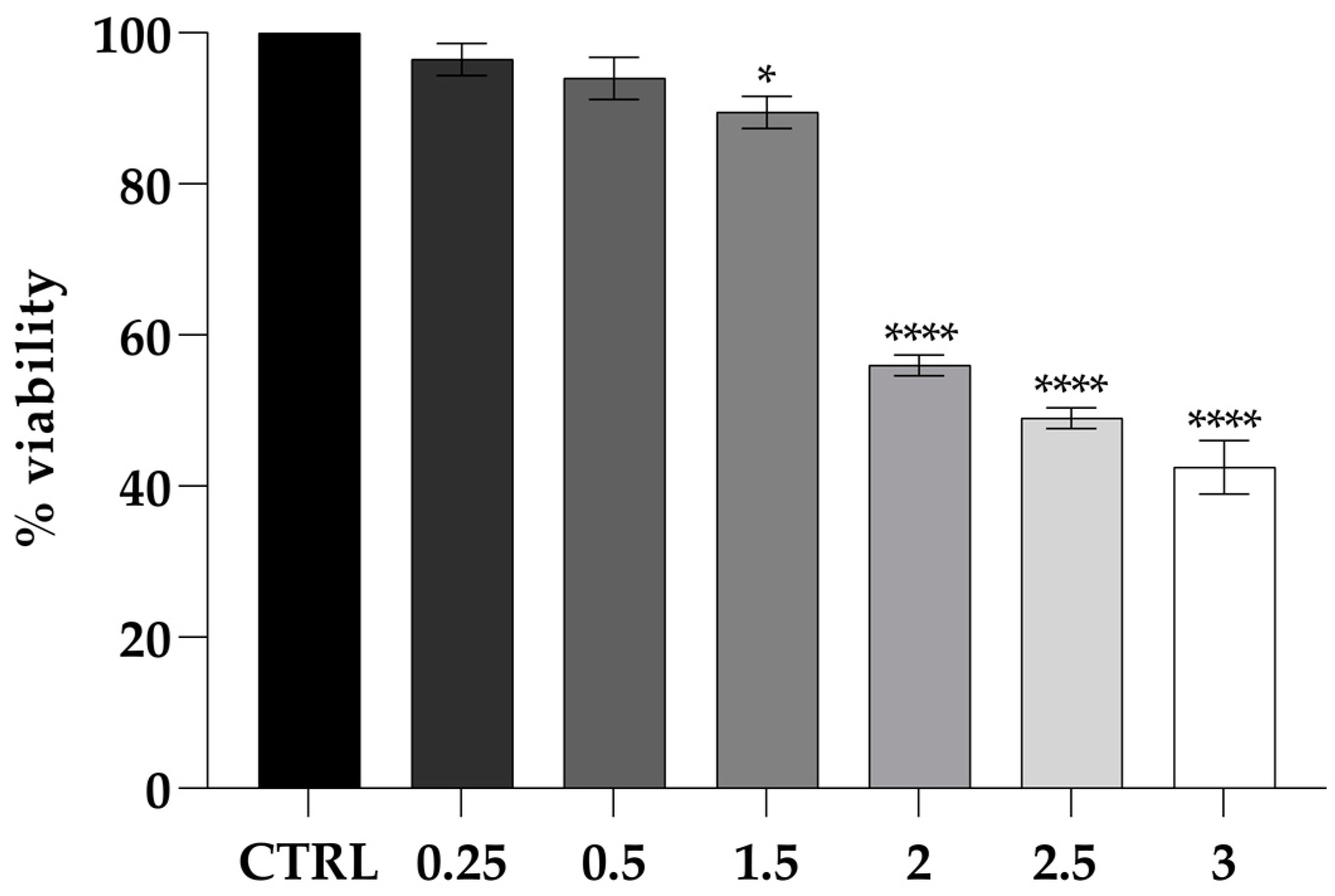
2.5. Cytotoxicity Assays
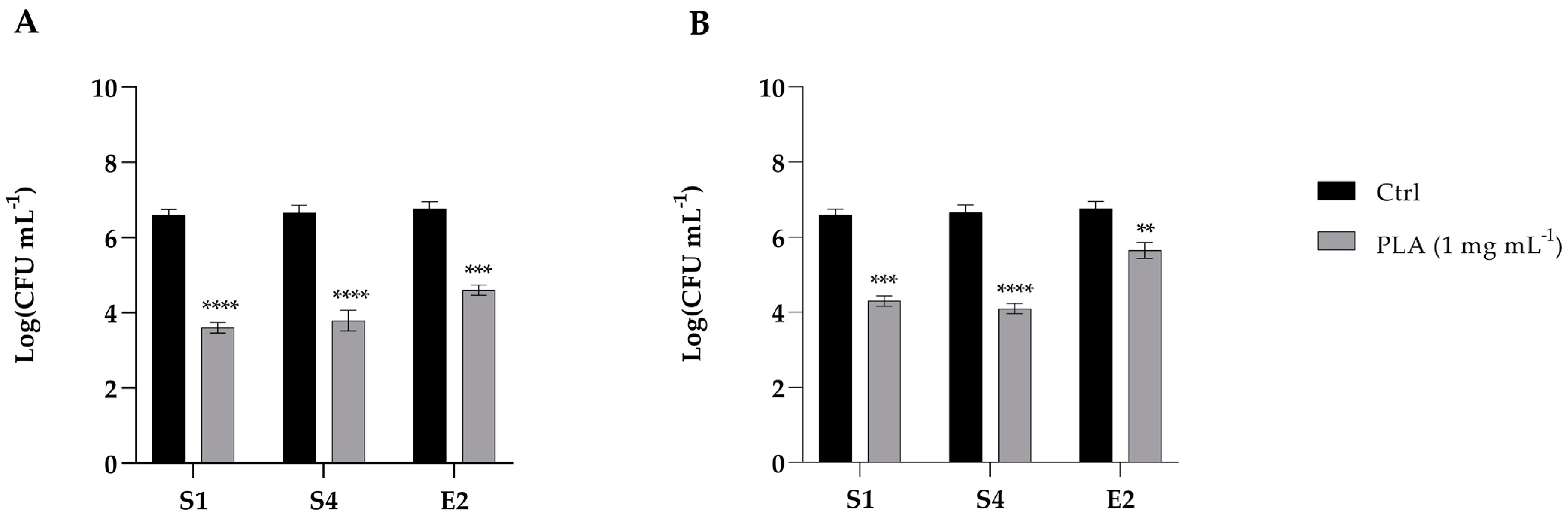
2.6. Adhesion of Enteric Bacteria to Caco-2 Cells with PLA Treatments
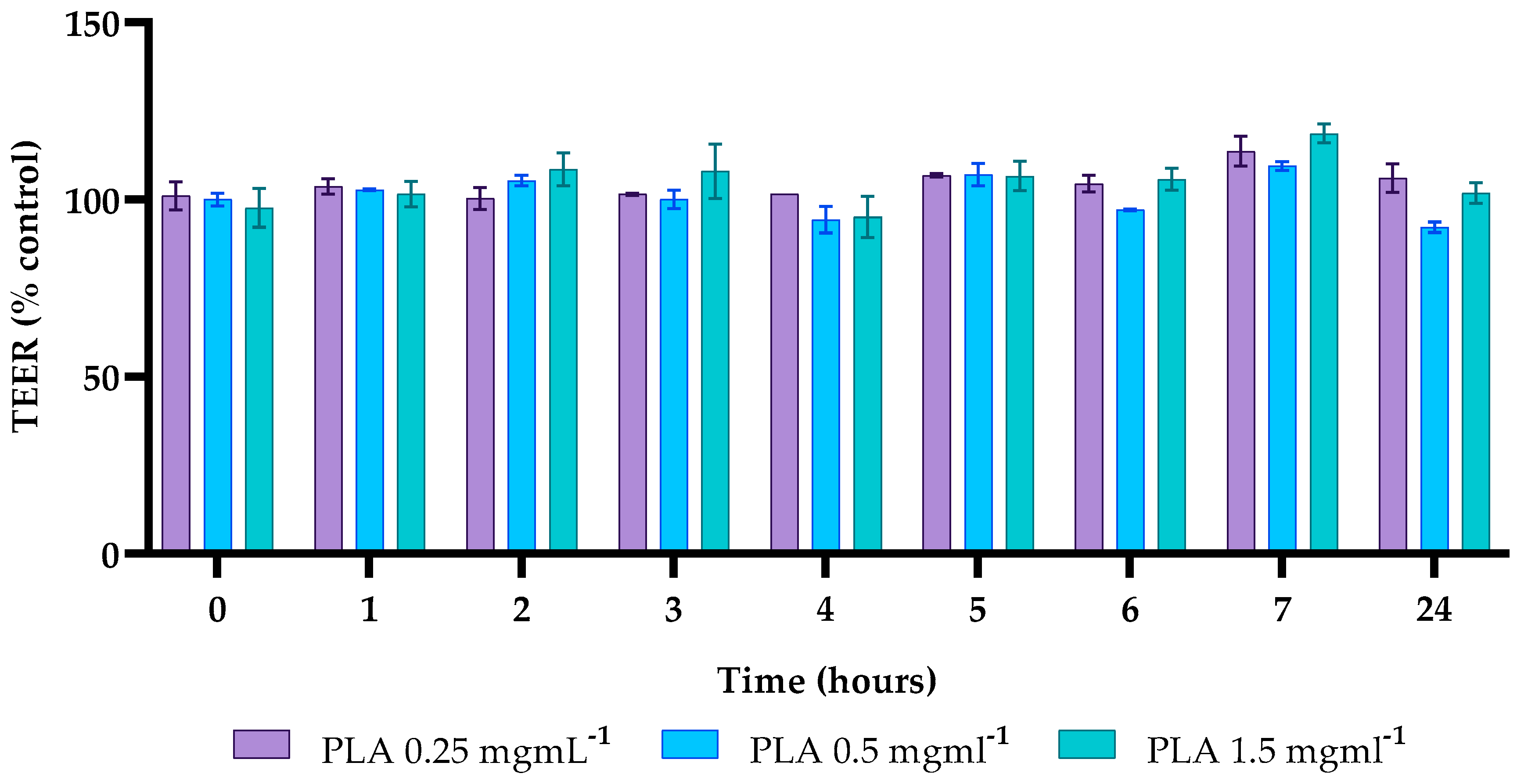
2.7. Effects of PLA on Caco-2 Cell Permeability
3. Discussion
4. Materials and Methods
4.1. Strain and Culture Conditions
4.2. Antibiotic Susceptibility Test
4.3. Determination of the Minimum Inhibitory Concentration (MIC) and of the Minimum Bactericidal Concentration (MBC) of PLA
4.4. Challenge Test
4.5. Biofilm Formation
4.6. Antibiofilm Activity
4.7. Cytotoxicity Assays
4.8. Adhesion of Enteric Bacteria to Caco-2 Cells with PLA Treatments
4.9. Transepithelial Electrical Resistance (TEER)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Estimating the Burden of Foodborne Diseases: A Practical Handbook for Countries; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- European Centre for Disease Prevention and Control. Salmonellosis. In ECDC. Annual Epidemiological Report for 2022; ECDC: Solna, Sweden, 2024. [Google Scholar]
- European Centre for Disease Prevention and Control. Chlamydia infection. In ECDC. Annual Epidemiological Report for 2021; ECDC: Solna, Sweden, 2022. [Google Scholar]
- Zavišić, G.; Ristić, S.; Petričević, S.; Janković, D.; Petković, B. Microbial Contamination of Food: Probiotics and Postbiotics as Potential Biopreservatives. Foods 2024, 13, 2487. [Google Scholar] [CrossRef] [PubMed]
- Alba, P.; Carfora, V.; Feltrin, F.; Diaconu, E.L.; Sorbara, L.; Dell’Aira, E.; Cerci, T.; Ianzano, A.; Donati, V.; Franco, A.; et al. Evidence of structural rearrangements in ESBL-positive pESI(like) megaplasmids of S.Infantis. FEMS Microbiol. Lett. 2023, 370, fnad014. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Willson, N.L. Nontyphoidal Salmonella infections acquired from poultry. Curr. Opin. Infect. Dis. 2022, 35, 431–435. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Mindlin, M.J.; Lang, N.; Maguire, H.; Walsh, B.; Verlander, N.Q.; Lane, C.; Taylor, C.; Bishop, L.A.; Crook, P.D. Outbreak investigation and case-control study: Penta-resistant Salmonella Typhimurium DT104 associated with biltong in London in 2008. Epidemiol. Infect. 2013, 141, 1920–1927. [Google Scholar] [CrossRef]
- Gieraltowski, L.; Higa, J.; Peralta, V.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H.; et al. National Outbreak of Multidrug Resistant Salmonella Heidelberg Infections Linked to a Single Poultry Company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.; Rodrigues da Costa, M.; Nesbakken, T.; Meemken, D.; on behalf of the RIBMINS Cost Action. Assessment of the Effectiveness of Pre-harvest Meat Safety Interventions to Control Foodborne Pathogens in Broilers: A Systematic Review. Curr. Clin. Microbiol. Rep. 2021, 8, 21–30. [Google Scholar] [CrossRef]
- Salines, M.; Lazou, T.; Gomez-Luengo, J.; Holthe, J.; Nastasijevic, I.; Bouwknegt, M.; Dadios, N.; Houf, K.; Blagojevic, B.; Antic, D. Risk categorisation of abattoirs in Europe: Current state of play. Food Control 2023, 152, 109863. [Google Scholar] [CrossRef]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2020, 10, 624622. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in Food Processing Environments: Challenges and Opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, P.K.; Rath, D. A critical review on food adulteration and its risk on health. IJNRD-Int. J. Nov. Res. Dev. 2022, 7, 353–356. [Google Scholar]
- Debonne, E.; Vermeulen, A.; Bouboutiefski, N.; Ruyssen, T.; Van Bockstaele, F.; Eeckhout, M.; Devlieghere, F. Modelling and validation of the antifungal activity of DL-3-phenyllactic acid and acetic acid on bread spoilage moulds. Food Microbiol. 2020, 88, 103407. [Google Scholar] [CrossRef]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Shao, L.; Xi, Y.; Weng, Y. Recent Advances in PLA-Based Antibacterial Food Packaging and Its Applications. Molecules 2022, 27, 5953. [Google Scholar] [CrossRef]
- Ponzio, A.; Rebecchi, A.; Zivoli, R.; Morelli, L. Reuterin, Phenyllactic Acid, and Exopolysaccharides as Main Antifungal Molecules Produced by Lactic Acid Bacteria: A Scoping Review. Foods 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Tremonte, P.; Succi, M.; Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Sturchio, M.; Coppola, R. Detection of Antilisterial Activity of 3-Phenyllactic Acid Using Listeria innocua as a Model. Front. Microbiol. 2018, 9, 1373. [Google Scholar] [CrossRef]
- Ji, Q.Y.; Wang, W.; Yan, H.; Qu, H.; Liu, Y.; Qian, Y.; Gu, R. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods 2023, 12, 3011. [Google Scholar] [CrossRef]
- Industry Guideline for Minimizing the Risk of Shiga Toxin-Producing Escherichia coli (STEC) in Beef (Including Veal) Slaughter Operations 2021 Guideline. 2021. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-08/FSIS-GD-2021-0008.pdf (accessed on 10 September 2024).
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Maqoud, F.; Orlando, A.; Tricarico, D.; Antonacci, M.; Di Turi, A.; Giannelli, G.; Russo, F. Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells. Int. J. Mol. Sci. 2024, 25, 3802. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Alharbi, M.G.; Al-Hindi, R.R.; Esmael, A.; Alotibi, I.A.; Azhari, S.A.; Alseghayer, M.S.; Teklemariam, A.D. The “Big Six”: Hidden Emerging Foodborne Bacterial Pathogens. Trop. Med. Infect. Dis. 2022, 7, 356. [Google Scholar] [CrossRef]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Inoue, Y.; Sugiyama, M. 3-Phenyllactic acid generated in medicinal plant extracts fermented with plant-derived lactic acid bacteria inhibits the biofilm synthesis of Aggregatibacter actinomycetemcomitans. Front. Microbiol. 2022, 13, 991144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, C.; Yu, T.; Jiang, X.; Kang, R.; Ren, S.; Chen, H.; Zhang, Y.; Li, Y.; Meng, H.; et al. Phenyllactic acid application to control Listeria monocytogenes biofilms and its growth in milk and spiced beef. Int. J. Food Microbiol. 2022, 381, 109910. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Liu, F.; Du, L.; Zhao, T.; Zhao, P.; Doyle, M.P. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control 2017, 78, 72–78. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; p. 338. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobal Susceptibility Testing. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2022.
- Serio, A.; Chiarini, M.; Tettamanti, E.; Paparella, A. Electronic paramagnetic resonance investigation of the activity of Origanum vulgare L. essential oil on the Listeria monocytogenes membrane. Lett. Appl. Microbiol. 2010, 51, 149–157. [Google Scholar] [CrossRef]
- Maione, A.; Buonanno, A.; Galdiero, M.; de Alteriis, E.; Petrillo, F.; Reibaldi, M.; Guida, M.; Galdiero, E. A Re-Purposing Strategy: Sub-Lethal Concentrations of an Eicosanoid Derived from the Omega-3-Polyunsaturated Fatty Acid Resolvin D1 Affect Dual Species Biofilms. Int. J. Mol. Sci. 2023, 24, 12876. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; La Pietra, A.; de Alteriis, E.; Mileo, A.; De Falco, M.; Guida, M.; Galdiero, E. Effect of Myrtenol and Its Synergistic Interactions with Antimicrobial Drugs in the Inhibition of Single and Mixed Biofilms of Candida auris and Klebsiella pneumoniae. Microorganisms 2022, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; Imparato, M.; Buonanno, A.; Galdiero, M.; de Alteriis, E.; Guida, M.; Galdiero, E. Protective and immunomodulatory effects of the novel probiotic yeast Pichia kudriavzevii isolated from a home-made kefir during infection in human colon epithelial cells: An exploratory study. J. Funct. Foods 2025, 125, 106666. [Google Scholar] [CrossRef]

| Strain | Antibiotic | Cut-Off R (EUCAST) | MIC | R/S |
|---|---|---|---|---|
| (µg mL−1) | ||||
| S1 | AMP | R > 8 | >512 | R |
| KANA | R > 16 | 4 | S | |
| CHL | R > 8 | - | S | |
| TET | R > 8 | 4 | S | |
| S4 | AMP | R > 8 | 128 | R |
| KANA | R > 16 | 4 | S | |
| CHL | R > 8 | 4 | S | |
| TET | R > 8 | 4 | S | |
| E2 | AMP | R > 8 | 16 | R |
| KANA | R > 16 | S ≥ 512 | R | |
| CHL | R > 8 | S ≥ 512 | R | |
| TET | R > 8 | 16 | R | |
| MIC (µg mL−1) | MBC (µg mL−1) | MBC/MIC Ratio | ||
|---|---|---|---|---|
| S1 | 2.0 ± 0.5 | 5.0 ± 0.5 | 2.5 | bactericide |
| S4 | 2.5 ± 0.2 | 5.0 ± 0.5 | 2.0 | bactericide |
| E2 | 2.75 ± 0.35 | 5.5 ± 0.25 | 1.81 | bactericide |
| Means (OD570) ± SD | Classification | |
|---|---|---|
| S1 | 0.243 ± 0.011 | Moderately forming biofilm |
| S4 | 1.904 ± 0.136 | Strongly forming biofilm |
| E2 | 1.561 ± 0.282 | Strongly forming biofilm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maione, A.; Buonanno, A.; Imparato, M.; Maglione, G.; Rossetti, C.; Montone, A.M.I.; Guida, M.; Galdiero, E.; Zinno, P. Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26. Molecules 2025, 30, 1738. https://doi.org/10.3390/molecules30081738
Maione A, Buonanno A, Imparato M, Maglione G, Rossetti C, Montone AMI, Guida M, Galdiero E, Zinno P. Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26. Molecules. 2025; 30(8):1738. https://doi.org/10.3390/molecules30081738
Chicago/Turabian StyleMaione, Angela, Annalisa Buonanno, Marianna Imparato, Giuseppe Maglione, Cristina Rossetti, Angela Michela Immacolata Montone, Marco Guida, Emilia Galdiero, and Paola Zinno. 2025. "Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26" Molecules 30, no. 8: 1738. https://doi.org/10.3390/molecules30081738
APA StyleMaione, A., Buonanno, A., Imparato, M., Maglione, G., Rossetti, C., Montone, A. M. I., Guida, M., Galdiero, E., & Zinno, P. (2025). Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26. Molecules, 30(8), 1738. https://doi.org/10.3390/molecules30081738







