Abstract
Ammonia is one of the most widely produced inorganic chemicals, with extensive applications in the military, agricultural, and industrial sectors. However, its strong stimulation and corrosive properties pose significant health risks, as long-term exposure to ammonia environments can lead to respiratory tract damage, loss of consciousness, and even cardiopulmonary dysfunction. Over the years, researchers have focused on exploring suitable materials for ammonia adsorption fields such as activated carbon and zeolites. Porous framework materials (PFMs), including metal–organic frameworks, covalent organic frameworks, and hydrogen-bonded organic frameworks, have emerged as possible ammonia adsorption materials due to their high specific surface area, pore size, and structural adjustability. This review focuses on the research and application of materials with excellent adsorption based on PFMs for ammonia adsorption, highlighting their potential applications and providing insights into future developments in this field.
1. Introduction
Since the industrial adoption of the Haber–Bosch process in 1908, ammonia has become one of the most widely used inorganic chemicals, with its annual production exceeding 200 million tons. Ammonia is essential in industries such as petrochemicals, metal manufacturing, paper production, textiles, and agriculture [1,2,3]. However, despite its economic benefits, ammonia emissions pose significant environmental and health risks. NH3 is considered an important contributor to the formation of particulate matter 2.5 (PM2.5). Through various physicochemical reactions, particles such as ammonium sulfide and ammonium nitrate form, accounting for approximately 30 wt% of the atmospheric PM2.5 content, with peak levels reaching up to 60 wt% [4,5]. In addition, airborne NH3 concentrations above 0.02 vol% can lead to chronic poisoning. Short-term exposure (less than 8 h) to an ammonia atmosphere can irritate the respiratory tract, eyes, and skin, while long-term exposure to NH3 concentrations above 300 × 10−6 vol% can cause serious illness and even death [6]. Currently, while ammonia adsorption technology at high concentrations is well developed, capturing ammonia molecules at low concentrations remains a critical issue. This is particularly pressing due to the rapid development of the semiconductor industry, where NH3 concentrations are strictly limited to 7–345 ppb in semiconductor clean rooms, with less than 2 ppb being permitted in layer presentation/inspection tools, and under 20 ppb in lithographic scanners [7,8,9,10]. Therefore, NH3 adsorption at low concentrations is critical for semiconductor production. Despite issues such as equipment corrosion and high operating costs, acid and water scrubbing remain the most common methods for ammonia capture [11]. Porous framework materials (PFMs), especially metal–organic frameworks (MOFs), have gained significant attention over the past two decades due to their high specific surface area, adjustable pore size, and active sites, which can provide metal ions and organic ligands, offering significant potential in the field of gas adsorption [12].
In this review, we summarize industrial ammonia abatement techniques and compare the adsorption performances of mature ammonia adsorption materials and PFMs (Figure 1). The prospects for the development of high-performance ammonia adsorbents are also presented.
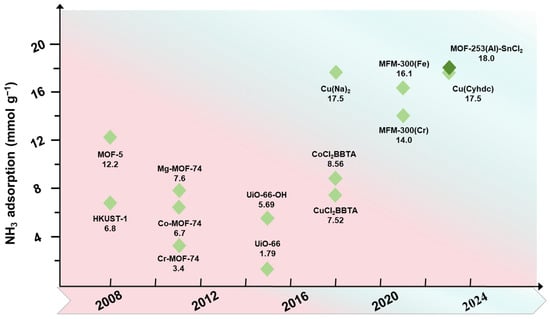
Figure 1.
Summary of recent developments in metal–organic frameworks for ammonia adsorption.
2. Ammonia Filtration Methods
Given the harmful effects of NH3 on the environment, capturing ambient ammonia gas is crucial from both an environmental and commercial perspective. Due to ammonia’s extremely high solubility in water (57.1 g L−1, 298 K, 1 atm), water scrubbing is the simplest and most common method for adsorbing it. This process involves absorbing NH3 using soft water, then distilling the solution to obtain concentrated ammonia water, and then recovering the NH3. However, despite its maturity and widespread application, water scrubbing is characterized by high water and energy consumption and a low recovery rate [13]. In contrast, optimized processes such as catalytic oxidation, membrane separation, biological treatment, washing, and adsorption methods offer more efficient and practical alternatives for ammonia capture [14,15,16,17,18,19].
2.1. Catalytic Oxidation Process
Catalytic oxidation uses highly selective catalysts to convert NH3 into N2, NOx, and H2O [14]. This reaction is considered one of the most significant heterogeneous catalytic processes in industrial and commercial applications. At higher reaction temperatures, NOx is mainly produced, while a mixture of N2, N2O, and NO forms when the oxidation reaction temperature is below 500 °C. Although catalytic oxidation is a simple, efficient, and stable method, it has a high energy demand and generates NOx, which is an exhaust gas that requires additional treatment [15].
The electrochemical oxidation of ammonia is a technique that converts ammonia into hydrazine (N2H4), nitrite (NO2−), and nitrate (NO3−) through the application of Pt-, Ir-, or Ni-based catalysts under an electric field [20]. This process can be conducted under an ambient temperature and pressure, thereby reducing the high energy demand of the traditional catalytic oxidation process. However, under prolonged high-potential operating conditions, the catalyst surface becomes susceptible to oxidative corrosion and irreversible phase transformations, which ultimately leads to the structural collapse of active sites and deterioration of the reaction kinetics. These degradation mechanisms pose critical challenges to the long-term operational stability and catalytic performance of the system [21].
2.2. Membrane Separation
Membranes have the advantages of lower maintenance requirements and being easier to install and operate than other traditional technologies. However, despite their significant advantages, membranes still have issues, such as low selectivity for target pollutants under high-throughput conditions, and further development is needed before membranes can be commercially applied [16]. In addition, the working stability of membranes under extreme conditions such as high temperatures, high pressure, and high pollution concentrations requires further study [22].
2.3. Biotechnology
Biotechnology mainly includes three NH3 treatment methods: biological purifiers, biological filters, and biological drip filters. Biological methods have the advantages of low pressure drops and operating costs; however, the accumulation of biomass and secondary waste remains a significant issue. Furthermore, bacterial discharge poses an environmental risk, further limiting the application of these filters [17].
2.4. Washing
Washing mainly relies on scrubbers, which are reactors that are filled with inert or inorganic fillers that have a large surface area and are highly porous. The gas that is to be separated is typically introduced counter-currently, allowing for the gas flow to be in close contact with the water and facilitating the transfer of NH3 from the gas phase to the liquid phase. In this process, the mass transfer rate is directly proportional to the NH3 concentration gradient between the gas and liquid phases [23]. Therefore, diluted sulfuric acid or phosphoric acid solutions are typically added to the water to maintain a pH below 4, thereby enhancing the mass transfer rate [24]. This method can withstand a wide temperature range, with the help of a robust scrubber. However, similarly to biological treatment methods, the scrubbing method faces high operational costs due to the need for extensive maintenance as a result of corrosion and structural degradation [18].
2.5. Adsorption
Among the various methods for NH3 purification, adsorption has gained attention and is of significant practical interest. Adsorption can be categorized into physisorption and chemisorption, each with distinct removal mechanisms. Physisorption is mainly driven by van der Waals forces between molecules, while chemisorption involves adsorption behavior that is driven by chemical bonding on the substance surface. Activated carbon (AC), zeolites, and metal oxides have been widely used as adsorption materials in recent years [19]. MOFs have received extensive attention over the past two decades due to their beneficial properties, such as a high specific surface area, large pore volume, and adjustable function, making them potential tools for NH3 adsorption and separation under different conditions [25].
In summary, although these technologies have proven to be viable ammonia abatement methods, there are technical and economic drawbacks in practical applications (Table 1). This facilitates the development of enhanced ammonia adsorption approaches.

Table 1.
Advantages and disadvantages of various industrial ammonia abatement techniques.
3. Commonly Used Ammonia Adsorbents
The adsorption method has emerged as a predominant technology for ammonia pollution control due to its operational flexibility, low energy consumption, and recyclability. Currently, widely used ammonia adsorbents include AC, as well as zeolite molecular sieves and similar materials, which can achieve the selective capture of ammonia molecules through mechanisms such as physical adsorption, chemical coordination, and acid–base neutralization (Figure 2). In this section, we systematically review extensively used ammonia adsorption materials for industrial applications. We subsequently critically analyze their core advantages and limitations, with the aim of providing scientific insights for material optimization and scenario-specific application strategies.
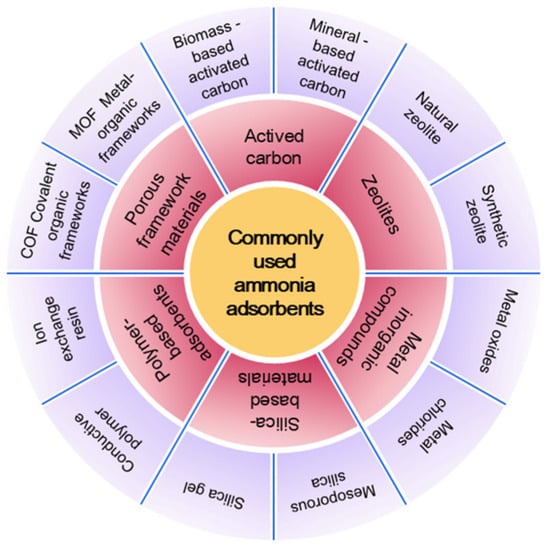
Figure 2.
Common types of ammonia adsorbents.
3.1. Activated Carbon
AC is mainly composed of C, H, O, and N, along with other elements. Due to its large specific surface area and rich pore structure, AC exhibits excellent adsorption performance for many pollutants, especially aromatic compounds such as benzene and toluene. AC can also be modified through methods such as washing, pickling, and alkali washing, according to the need to adsorb different types of impurities [26].
Huang et al. [27] determined the influence of the surface acidity of AC on the adsorption effect of ammonia gas through different modifications. The ammonia adsorption capacities of modified AC could be improved, with nitric acid-modified AC achieving the highest rate of ammonia penetration with 2.493 mmol g−1, which was significantly higher than that achieved by unmodified AC (0.134 mmol g−1). Qajar et al. [28] obtained a series of microporous carbons by oxidizing alcohols with CO2. Using the same nitric acid treatment method, the static adsorption capacity of NH3 increased from an initial 10 mmol g−1 to 17 mmol g−1. The strong acid–base interaction between ammonia and the surface functional groups of AC was found to be the main reason for the increased ammonia adsorption capacity.
Until now, a significant amount of AC, especially modified AC, has been used for gas adsorption and other needs, despite it exhibiting poor adsorption activity for ammonia and carrying the risk of braising under partial use conditions. These are issues that must be addressed in future research on AC materials.
3.2. Graphene Oxide
Unlike AC, graphene oxide (GO) consists of a honeycomb flat film, formed by means of the hybridization of carbon atoms with sp2. As a product of graphene stripping, GO has a large specific surface area and excellent two-dimensional structure. Zhang et al. [29] demonstrated through density functional theory (DFT) calculations that water molecules on a graphene surface in a humid environment promoted the conversion of NH3 to NH4+, resulting in a significant increase in adsorption energy from 31 to 45 meV (for pure NH3 on graphene under dry conditions) to 528–644 meV. Seredych et al. [30] prepared graphene by placing graphite powder in concentrated sulfuric acid with potassium permanganate, followed by calcination. The adsorption capacity of uncalcined graphene reached 3.59 mmol g−1. Moreover, the NH3 adsorption capacity of the GO decreased with the decrease in surface acidity after washing, indicating that the water vapor in the NH3 gas promoted the dissociation of carboxyl groups and interaction with NH3. However, the performance of graphene for ammonia adsorption under different humidity levels varies significantly, leading to a limited application scenario.
3.3. Zeolite
Zeolite is a crystalline material with a tetrahedral framework composed of silicon and aluminum with a highly porous structure. These structural characteristics give zeolites excellent ion exchange properties, adsorption selectivity, and thermal stability [31]. Bernal et al. [32] measured the adsorption effect of four different natural zeolites for NH3 and reported a maximum dynamic adsorption capacity of 0.831 mol g−1. In addition to natural zeolites, molecular sieves made from artificially modified zeolite can improve the properties of zeolites by adjusting the pore structure. Helminen et al. [33] found that the adsorption capacity of directionally synthesized 13X-zeolite reached 9.326 mmol g−1 at 93.8 kPa, which is higher than that of natural zeolites (5.904 mmol g−1). This was attributed to the increased surface activity of the zeolite materials. Witter et al. [34] found that zeolites could adsorb NH3 more effectively at lower pressures. However, unlike AC, the adsorption performance of zeolites for NH3 significantly decreased in the presence of water vapor. This was mainly because the presence of water molecules clogged the internal pores of the zeolites, leading to a decrease in their adsorption capacity and adsorption rate.
3.4. Metal Inorganic Compounds
Most metal inorganic compounds can react with ammonia through complexation reactions, forming coordination compounds, with notable examples including activated alumina and CaCl2 [35]. Helminen et al. [33] measured the ammonia adsorption capacities of three types of aluminum oxide adsorbents, Alumina VPO2, Alumina 1593, and Alumina 1597, and found that their adsorption capacities at 298.15 K and 100 kPa were 2.606, 2.159, and 3.008 mmol g−1, respectively. Kim et al. [36] synthesized mesoporous aluminum oxide BSMA using Al(NO3)3·9H2O as the alumina precursor, achieving an ammonia adsorption capacity of 3.77 mmol g−1. During the adsorption process, the ammonia first adsorbed on the outer surface of the BSMA and then adsorbed into the inner pores of the material. In addition, with a decreasing particle size, the adsorption performance increased, with an increasing number of effective sites.
Although metal inorganic compounds have good adsorption capacity for NH3, their slow adsorption kinetics, significant volume expansion, and difficult desorption limit their application [37,38]. Therefore, instead of directly using metal inorganic compounds as ammonia adsorbents, researchers have preferred to incorporate them into other materials as loading materials to enhance their ammonia adsorption performance.
In summary, these materials exhibit distinct advantages and limitations: activated carbon demonstrates a rapid adsorption capability owing to its high specific surface area and rich porous structure; however, it shows a low adsorption capacity for ammonia, meaning that it requires surface modification. Graphene oxide achieves strong chemisorption with ammonia through oxygen-containing functional groups, yet its practical performance is hindered by interlayer stacking. Zeolites achieve high adsorption selectivity due to their ordered microporous structure and ion exchange properties, but water will compete with ammonia, resulting in a reduced adsorption capacity in high-humidity environments. Although metal inorganic compounds have a high adsorption capacity and thermal stability, the difficulty of desorption and the volume expansion during the adsorption process significantly limit its large-scale utilization. Hence, researchers have developed novel ammonia adsorption materials to enhance ammonia adsorption capabilities.
4. PFMs in Ammonia Adsorption
In recent years, PFMs, including covalent organic frameworks (COFs), hydrogen-bonded organic frameworks (HOFs), and metal–organic frameworks (MOFs), have emerged as promising materials for ammonia adsorption due to their high surface area, tunable pore structure, and excellent adsorption properties. COFs are an emerging class of crystalline porous materials that are composed of light elements (e.g., H, B, C, N, and O) which are connected via covalent bonds. Using state-of-the-art molecular design, COFs can exhibit a large surface area, unique porosity, high crystallinity, and tunable pore chemistry, with promising applications in the fields of catalysis, sensing, and gas adsorption [39,40,41]. HOFs are formed entirely from the self-assembly of weaker hydrogen bonds among organic molecules. The flexibility and reversibility of hydrogen bonds make it easy for HOFs to return to their initial state when the framework collapses after a stimulus response [42,43,44]. MOFs are formed through the coordination of transition metal ions with organic ligands. As a class of promising solid adsorbents, MOFs have received significant attention in gas separation due to their regular nanopores, adjustable porosity, high specific surface area, and easy functionalization [45,46,47,48]. Through chemical modification, pore engineering, and functional design, these materials can achieve high selectivity and the efficient storage of ammonia. For example, COF materials, with a highly ordered pore structure and chemical stability, have demonstrated excellent ammonia adsorption performance [49]. Furthermore, by introducing open metal sites or acidic functional groups, certain MOF materials can significantly enhance the interaction with ammonia, thereby improving their adsorption capacity [50]. In this section, we review the latest research progress in the field of ammonia adsorption using porous framework materials, focusing on their adsorption mechanisms, performance optimization strategies, and potential applications.
4.1. COFs and HOFs
Many COFs contain high-density Lewis acid sites that can interact with ammonia, resulting in excellent ammonia adsorption and storage. Doonan et al. [51] evaluated the ammonia adsorption effects of COF-10 and demonstrated that the static ammonia adsorption capacity of COF-10 at 298 K and 101 kPa was 15 mmol g−1, which was higher than those of other porous materials. Meanwhile, desorption was completed at 473.15 K under a vacuum environment. Because the material mainly adsorbed NH3 through Lewis acid–base interactions, it maintained a stable skeleton structure after multiple adsorption–desorption cycles (Figure 3).
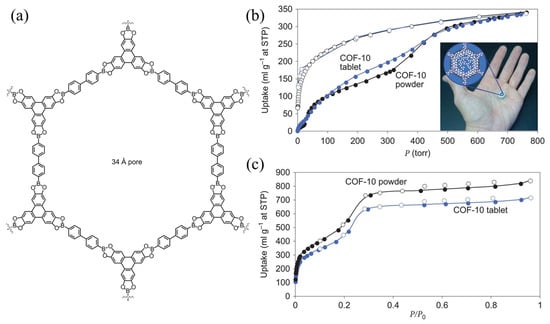
Figure 3.
(a) Schematic representation of COF-10; (b) ammonia uptake at 298 K for COF-10 and COF-10 tablets and pressed tablets of COF-10 loaded with ammonia; and (c) nitrogen gas adsorption isotherms (77 K), tested on the same COF-10 powder and COF-10 tablets (Solid symbols represent adsorption, hollow symbols represent desorption) [51].
Li et al. [52] synthesized a highly stable sulfonic acid COF using triformylphloroglucinol and diaminobenzene disulfuric acid. This COF retained its structural integrity after immersion in water, acid, and organic solvents for 48 h due to the covalent bonds that formed from the condensation of aldehyde and amine groups. The strong interaction between the sulfonic acid sites and ammonia molecules resulted in an ammonia adsorption capacity of 11.5 mmol g−1 at 298 K (Figure 4).
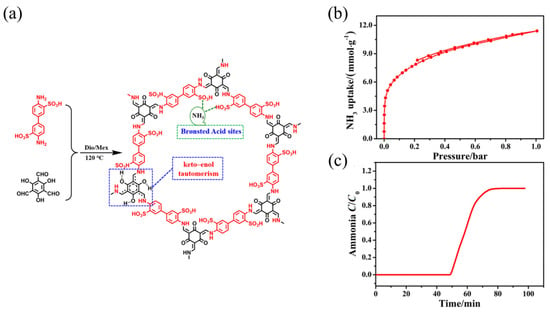
Figure 4.
(a) Synthesis and structure of TpBD-(SO3H)2; (b) NH3 adsorption isotherm of TpBD-(SO3H)2; and (c) NH3 breakthrough curve of TpBD-(SO3H)2 at 298 K [52].
Ma et al. [53] measured the ammonia adsorption capacity of HOF-102 to be 250 cm3 g−1 at 298 K and 1 atm (Figure 5). In addition, Song et al. [37] synthesized two carboxylic acids, HOF-101 and FDU-HOF-3, with an adaptive NH3 adsorption capacity and phase transition (Figure 5). The adsorption capacity of FDU-HOF-3 reached 8.13 mmol g−1 at 25 mbar, while FDU-HOF-3 exhibited significant potential for low-concentration ammonia capture and high-concentration ammonia storage.
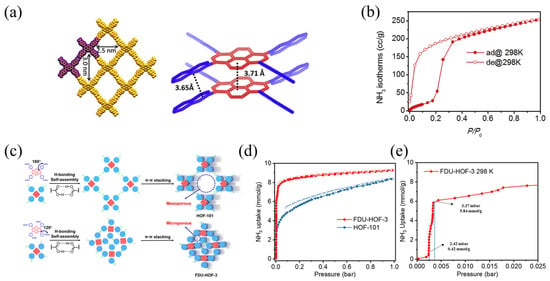
Figure 5.
(a) Crystal structures of HOF-102; (b) ammonia adsorption isotherm of HOF-102 at 298 K; (c) schematic of H-bonding self-assembly and π−π stacking in HOF-101 and FDU-HOF-3; (d) NH3 adsorption and desorption isotherms of HOF-101 and FDU-HOF-3 at 298 K; and (e) adsorption capacity of FDU-HOF-3 under low ammonia concentrations at 298 K (Solid symbols represent adsorption, hollow symbols represent desorption) [37,53].
Although COFs and HOFs differ in their structural construction, their widespread practical application remains limited by high synthesis costs, and addressing this challenge is crucial for commercialization (Table 2). In addition, their thermal and chemical stability must be improved. Choosing both simple and easily obtained monomers and enhancing the thermochemical stability are the main future research directions.

Table 2.
Static adsorption capacities and specific surface areas of different types of COF and HOF materials.
4.2. MOF Materials
As a strong Lewis acid and Brønsted base, ammonia can react with most metal ions through coordination. As a result, many metal salts have a high theoretical ammonia adsorption capacity. However, the direct use of metal salts poses challenges, such as slow adsorption kinetics, volume expansions during adsorption, and difficult desorption [37,38]. Each metal unit in MOFs serves as a potential adsorption site for NH3; therefore, compared with metal salts, MOFs offer a superior choice for ammonia adsorption [45].
In 2008, Yaghi et al. [56] evaluated the ammonia adsorption performance of five MOFs, including MOF-5 and MOF-199. The study, which compared these MOFs to Calgon BPL activated carbon, revealed that the porosity of MOFs played a decisive role in their adsorption capacity, with MOF-199 exhibiting the highest dynamic ammonia adsorption capacity of 87 mg g−1, marking the first complete report of ammonia adsorption by MOFs.
Glover et al. [57] synthesized MOF-74 with four different metal centers, namely cobalt, magnesium, nickel, and zinc (Figure 6), and measured their ammonia adsorption properties. The study found that different metal centers significantly affected the adsorption results. Under the same drying conditions, Mg-MOF-74 reached the highest capacity of 7.6 mmol g−1, while Ni-MOF-74 had the lowest of only 2.3 mmol g−1. The experimental results revealed that the superior performances of Co-MOF-74 and Mg-MOF-74 were due to their coordinatively unsaturated sites. Desorption experiments demonstrated that Co-MOF-74 and Mg-MOF-74 retained 70% and 83% of the adsorbed ammonia, respectively, whereas Ni-MOF-74 and Zn-MOF-74 retained 51.3% and 58.6% after desorption under dry conditions.
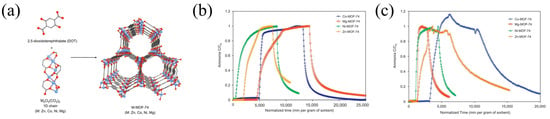
Figure 6.
(a) Structure of MOF-74; (b) ammonia breakthrough curves of MOF-74 under dry (RH = 0%) conditions; and (c) ammonia breakthrough curves under humid (RH = 80%) conditions [57].
Walton et al. [58] investigated the ammonia removal abilities of UiO-66 and its isomorphism and successfully synthesized six isomers of UiO-66 by changing the introduced ligands. Their results showed that the ammonia adsorption capacities of UiO-66-SO3H and UiO-66-(COOH)2 were lower than those of UiO-66-OH and UiO-66-NH2. UiO-66-OH had the highest NH3 adsorption capacity of 5.69 mmol g−1 under dry conditions (Figure 7) because the incorporation of larger functional groups such as carboxyl groups caused a decrease in material porosity. However, under humid conditions, the ammonia adsorption capacity of functionalized UiO-66 significantly decreased due to the competitive adsorption of water and ammonia molecules, with the highest adsorption capacity being only 5.69 mmol g−1.
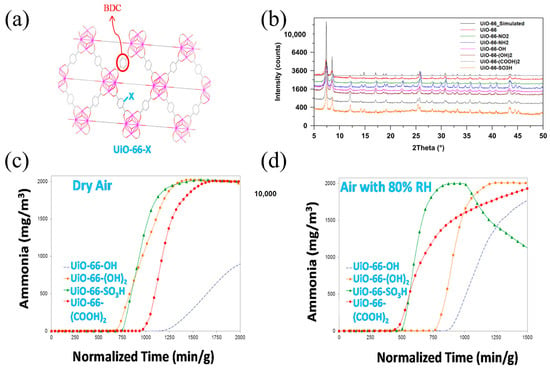
Figure 7.
(a) An illustration of the UiO-66-X framework structure; (b) PXRD patterns of as-synthesized UiO-66 and UiO-66-X variants; (c) NH3 breakthrough curves under dry air conditions; and (d) NH3 breakthrough curves under 80% RH air conditions (Solid symbols represent adsorption, hollow symbols represent desorption) [58].
Chen et al. [59] synthesized M-2(INA) (M = Cu, Co, Ni, and Cd) materials through dehydration with M(INA)2(H2O)4 and demonstrated that M-2(INA) could be reversibly converted to M(INA)2(H2O)2(NH3)2 under wet conditions. The dry NH3 adsorption capacity reached 12–13 mmol g−1, while the adsorption capacity under wet conditions and the same temperature conditions was 5–6 mmol g−1.
Rieth et al. [60] synthesized several microporous triazolate metal–organic frameworks containing open metal sites. The static ammonia adsorption capacities of CoCl2BBTA, NiCl2BBTA, and CuCl2BBTA (Figure 8) were 17.95, 14.68, and 19.79 mmol g−1, respectively. Although the adsorption capacity of BTDD reached 35 mmol g−1 at 263 K, due to the presence of more metal sites, BBTA with small pores exhibits a higher adsorption capacity than BTDD with large pores.
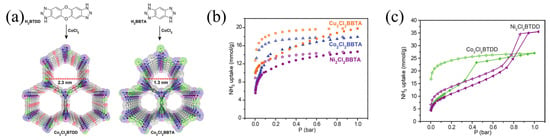
Figure 8.
(a) Synthesis and structure of Co2Cl2BTDD and Co2Cl2BBTA; (b) NH3 adsorption and desorption of Co2Cl2BBTA, Ni2Cl2BBTA, and Cu2Cl2BBTA; and (c) NH3 adsorption and desorption of Co2Cl2BTDD and Ni2Cl2BTDD at 298 K (Solid symbols represent adsorption, hollow symbols represent desorption) [60].
Chen et al. [61] evaluated the NH3 adsorption capacities of HKUST-1, MIL-100(Fe), and UiO-66 under low-pressure conditions, with the results showing that their static ammonia adsorption capacities were 17.73, 8.54, and 8.51 mmol g−1, respectively, at 0–0.13 P/P0. These findings were in agreement with the calculated results of HKUST-1 (−147.89 kJ mol−1) > MIL-100(Fe) (−80.28 kJ mol−1) > UiO-66 (−47.08 kJ mol−1).
Synder et al. [62] reported an air-stable and crystalline framework, Cu(cyhdc) (cyhdc2− = trans1,4-cyclohexanedicarboxylate), with a saturated coordination Cu(II) site. Due to the reversible temperature- and pressure-dependent co-phase transition of NH3 on the material, Cu(cyhdc) could adsorb NH3 more selectively than N2 or H2. At 298 K, about 16 mmol g−1 was gradually absorbed at 80 mbar, reaching a plateau above 100 mbar. At 1 bar, the maximum adsorption capacity was 17.5 mmol g−1, corresponding to the adsorption of four equivalents of NH3 per Cu site (Figure 9). During desorption, a significant lag was observed in the Cu(NH3)4(cyhdc) isotherm, and only half of the NH3 was desorbed at 1 mbar, indicating that intermediates were present, with two NH3 per Cu during desorption, and blue Cu(NH3)4(cyhdc) transformed into purple during desorption. A single-crystal X-ray diffraction analysis confirmed the formation of a one-dimensional Cu(NH3)2(cyhdc) solid, where two trans-NH3 and two trans-carboxylic acid ligands coordinated through a single oxygen atom in the Cu(II) central coordination of each square plane. Meanwhile, at lower pressures, the desorption plateaued at one ammonia per Cu, which could be attributed to the formation of Cu(NH3)(cyhdc).
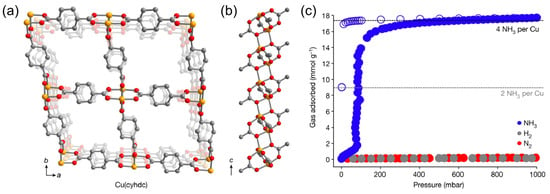
Figure 9.
(a) Illustration of the Cu(cyhdc) framework structure; (b) side view of the one-dimensional copper paddlewheel chain motif defining the pore vertices; and (c) NH3 adsorption and desorption isotherms at 298 K for Cu(cyhdc) [62].
Han et al. [63] synthesized a robust MOF structure, MFM-300(M) (M = Fe, V, Cr, and In), for NH3 adsorption, which had a high absorption rate of 17.3 mmol g−1 and could maintain an adsorption capacity of 16.1 mmol g−1 after 20 cycles at 273 K and 1 bar. In addition, MFM-300(CrVIII) demonstrated better NH3 adsorption stability in wet environments than MFM-300(FeVIII), indicating that even the saturation coordination of the metal center significantly affected the stability of the MOF in the presence of guest molecules.
The ammonia adsorption abilities of numerous MOFs have been evaluated (Table 3). Although the feasibility of MOFs for ammonia adsorption has been demonstrated experimentally, ensuring their commercialization or industrialization remains challenging, and the stability, reversibility, durability, and production cost of these materials cannot be ignored. Moreover, MOFs with a high NH3 adsorption capacity are generally composed of Cu2+ and Zn2+ nodes, which are prone to structural collapse during adsorption due to the strong coordination between metal nodes and NH3 [37,38]. Many high-capacity MOFs also exhibit reduced adsorption performance under varying environmental conditions, limiting their practical applicability. Therefore, rather than relying solely on MOFs, the development of functional MOF composite materials presents a promising alternative for improving their stability and performance under real-world conditions.

Table 3.
Static adsorption capacities and specific surfaces area of different types of MOF materials.
4.3. PFM Composite Materials
The combination of porous structural materials with chemical adsorbents has been shown to enhance both mass transfer and ammonia adsorption properties [66]. Inspired by these strategies, novel PFM composites can be designed to enhance the adsorption capacity of traditional PFMs. Approaches for this include functionalization with acidic groups that impart moderate hydrophobicity, anchoring halides or metal aggregates, and adjusting the pore size relative to the critical diameter.
Yang et al. [55] synthesized [CaOOC]17-COF, [MnOOC]17-COF, and [SrOOC]17-COF by immersing the COF in CaCl2, MnCl2, and SrCl2 solutions. They then anchored metals into the COF through coordination interactions to obtain composite materials. After modification, the [SrOOC]17-COF with the highest adsorption capacity achieved 14.3 mmol g−1 at 298 K, significantly surpassing that of the unmodified [HOOC]17-COF (6.85 mmol g−1). This novel approach enables the development of tailor-made porous materials with tunable pore-engineered surfaces for ammonia uptake.
Petit et al. [67] synthesized composites of MOF-5 and GO at different proportions and tested the adsorption capacity of ammonia under dry conditions. The dynamic adsorption capacity of the composite was increased to 82 mg g−1 at 1000 ppm and 450 mL min−1 due to its high porosity; however, excessive ammonia adsorption eventually led to structural collapse. More et al. [68] adopted a similar strategy by introducing partially reduced graphene oxide (rGO) into Zn-BDC and optimizing it for NH3 sensing at low concentrations, with a reaction time of 60/120 s at 20 ppm.
Han et al. [69] synthesized a composite material, [CAM][Cl]@MIL-101(Cr), based on MIL-101(Cr) and ionic liquids (ILs). According to their scanning electron microscopy (SEM) results, the ILs deposited irregular translucent layers on the MIL-101(Cr) surface, while no translucent layer was observed using transmission electron microscopy (TEM). The uniform distribution of Cl and N in the material, revealed by means of EDS, indicated that the ILs were uniformly distributed on the micron scale (Figure 10), and the saturated adsorption capacity of [CAM][Cl]@MIL-101(Cr)-30% reached 11.61 mmol g−1, which was much higher than the 8.03 mmol g−1 of the pristine MIL-101(Cr). The separation selectivity for NH3/CO2 (SNH3/CO2) of the composite was 3266, which was 3.13 times higher than that of MIL-101 (Cr).
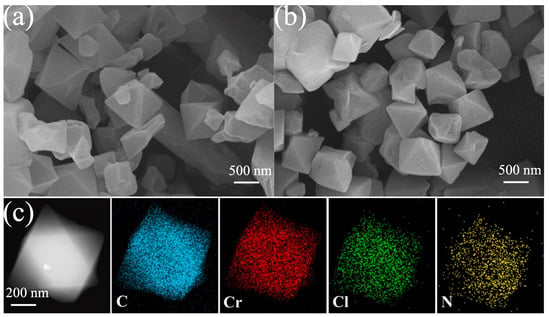
Figure 10.
(a) SEM images of MIL-101(Cr); (b) SEM images of [CAM][Cl]@MIL-101(Cr)-30%; and (c) TEM images and elemental mapping of [CAM][Cl]@MIL-101(Cr)-30% [69].
Wang et al. [70] designed and synthesized a composite material by anchoring three metal chlorides (NiCl2, CoCl2, and SnCl2) to the bipyridine group in MOF-253(Al). The highest NH3 absorption rate was that of MOF-253(Al)-NiCl2-2 at 18 mmol g−1, which was 3.33 times that of the original MOF-253(Al) (5.5 mmol g−1), and the adsorption kinetics were significantly faster than those of the original MOF-253(Al) (Figure 11). In addition, the material demonstrated excellent NH3 selectivity, with SNH3/N2 and SNH3/H2 coefficients of 5708 and 2320, respectively. According to the infrared spectroscopy, X-ray photoelectron spectroscopy (XPS), and DFT studies, this excellent absorption and selectivity was attributed to the coordination of the nitrogen atom of NH3 with Ni2+ and the synergistic interaction of the hydrogen bonds of NH3 with guest Cl− and carboxyl O atoms on the MOF.
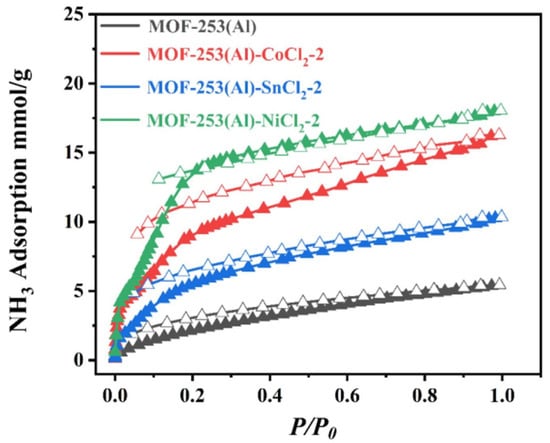
Figure 11.
NH3 adsorption isotherm of MOF-253(Al) and three functionalized MOFs (Solid symbols represent adsorption, hollow symbols represent desorption) [70].
Li et al. [48] impregnated ionic liquids in Al-fum, and the ammonia adsorption capacity results revealed that the capacities of [C2N][Zn3Cl7]@Al-fum-60% at 80 °C and 100 °C were 9.9 and 8.8 mmol g−1, respectively. Therefore, using the coordination interaction between ammonia and the ionic liquid Zn center is a promising method for promoting ammonia adsorption.
PFM composites, engineered by integrating PFMs with functional materials, address the inherent limitations of pristine PFMs and exhibit synergistically enhanced adsorption capacity, catalytic activity, and stability. However, current challenges lie in the poor interfacial compatibility and inhomogeneity of composites, which may obscure active sites or increase the mass transfer resistance. Additionally, complex fabrication processes often elevate costs and hinder scalable production, while the long-term cyclability of certain composite systems remains inadequately validated. Future research should prioritize interface engineering and sustainable synthesis strategies to reconcile performance enhancement with practical applicability.
5. Conclusions and Future Perspectives
In this review, we introduced several key NH3 capture techniques, focusing on physisorption and chemisorption for ammonia removal. We also compared the research progress, adsorption capacity, and advantages and disadvantages of materials such as AC, zeolite molecular sieves, COFs, and MOFs. With tunable porosity, a high specific surface area, and the ability to incorporate various functional groups, PFMs offer multiple mechanisms for ammonia adsorption, including physical and chemical adsorption and hydrogen bonding, making them highly promising materials for efficient NH3 capture. Moreover, their well-defined channels allow for precise structural modifications to enhance their adsorption capacity. These methods include increasing the number of acidic functional groups to expose more acidic sites, loading efficient adsorbents, and substitutions with more suitable metal groups and organic ligands.
Despite these advantages, PFMs face significant challenges in ammonia adsorption/desorption, including high production costs, lengthy preparation cycles, and poor stability and durability. Although these materials exhibit excellent ammonia adsorption performance under ambient temperature and pressure conditions, performance degradation in high-humidity environments remains a critical issue. In addition, the ammonia adsorption mechanisms of some PFMs remain poorly understood, and explorations of their microscopic interaction mechanisms, as well as an explanation of their structural collapse, remain lacking, limiting the further development of PFMs for ammonia adsorption. Future research should explore the structural optimization and functional enhancement of PFMs further to improve their ammonia adsorption performance in complex environments and promote their practical applications in industrial waste gas treatment, ammonia storage, and separation. We anticipate the development of highly efficient, stable, and easily synthesized PFMs that can adapt to real-world conditions, along with innovative design strategies to maximize their impact in environmental remediation and related fields.
Author Contributions
Literature search, writing, and creation of figures and tables: W.Y., W.W., Y.L., B.Z. and J.X. and review, editing, and supervision: S.X. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, Grant No. 22102008.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PFMs | Porous framework materials |
| MOFs | Metal–organic frameworks |
| COFs | Covalent organic frameworks |
| HOFs | Hydrogen-bonded organic frameworks |
| AC | Activated carbon |
| GO | Graphene oxide |
References
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Demirhan, C.D.; Tso, W.W.; Powell, J.B.; Pistikopoulos, E.N. Sustainable ammonia production through process synthesis and global optimization. Aiche J. 2019, 65, 16498. [Google Scholar] [CrossRef]
- Hoepfner, M.; Volkamer, R.; Grabowski, U.; Grutter, M.; Orphal, J.; Stiller, G.; von Clarmann, T.; Wetzel, G. First detection of ammonia NH3 in the Asian summer monsoon upper troposphere. Atmos. Chem. Phys. 2016, 16, 14357–14369. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Chen, J.; Zhou, X. Adsorption of SO2 and NH3 onto copper/graphene nanosheets composites: Statistical physics interpretations, thermodynamic investigations, and site energy distribution analyses. Chem. Eng. J. 2022, 446, 137224. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Zhong, F.-Y.; Liu, Y.; Huang, K. Effective and reversible capture of NH3 by ethylamine hydrochloride plus glycerol deep eutectic solvents. ACS Sustain. Chem. Eng. 2019, 7, 10552–10560. [Google Scholar] [CrossRef]
- Travlou, N.A.; Bandosz, T.J. N-doped polymeric resin-derived porous carbons as efficient ammonia removal and detection media. Carbon 2017, 117, 228–239. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, B.W.; Cheong, J.P.; Lee, S.K. Relationships between indoor and outdoor air quality during the summer seasons in Korea. Atmos. Environ. 1997, 31, 1689–1693. [Google Scholar] [CrossRef]
- Pei, J.; Yin, Y.; Liu, J. Long-term indoor gas pollutant monitor of new dormitories with natural ventilation. Energy Build. 2016, 129, 514–523. [Google Scholar] [CrossRef]
- Bai, Z.; Dong, Y.; Wang, Z.; Zhu, T. Emission of ammonia from indoor concrete wall and assessment of human exposure. Environ. Int. 2006, 32, 303–311. [Google Scholar] [CrossRef]
- Lopez-Aparicio, S.; Smolik, J.; Maskova, L.; Souckova, M.; Grontoft, T.; Ondrackova, L.; Stankiewicz, J. Relationship of indoor and outdoor air pollutants in a naturally ventilated historical building envelope. Build. Environ. 2011, 46, 1460–1468. [Google Scholar] [CrossRef]
- Cheng, N.-N.; Li, Z.-L.; Lan, H.-C.; Xu, W.-L.; Jiang, W.-J.; Huang, K.; Peng, H.-L. Deep eutectic solvents with multiple weak acid sites for highly efficient, reversible and selective absorption of ammonia. Sep. Purif. Technol. 2021, 269, 118791–118799. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem.-Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, S.; Chen, N.; Shang, D.; Zhang, X.; Li, J. Research progress of ammonia adsorption materials. Chin. J. Process Eng. 2019, 19, 14–24. [Google Scholar]
- Sun, M.; Wang, S.; Li, Y.; Xu, H.; Chen, Y. Promotion of catalytic performance by adding W into Pt/ZrO2 catalyst for selective catalytic oxidation of ammonia. Appl. Surf. Sci. 2017, 402, 323–329. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In situDRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Dumont, E.; Hamon, L.; Lagadec, S.; Landrain, P.; Landrain, B.; Andres, Y. NH3 biofiltration of piggery air. J. Environ. Manag. 2014, 140, 26–32. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Melse, R.W.; Ogink, N.W.M. Air scrubbing techniques for ammonia and odor reduction at livestock operations: Review of on-farm research in the Netherlands. Trans. ASAE 2005, 48, 2303–2313. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, Y.; Ding, J.; Zhou, H.; Zhang, Y.; Feng, Q.; Zhang, X.; Chen, K.; Wang, J.; Chen, Q.; et al. Tunable ammonia adsorption within metal-organic frameworks with different unsaturated metal sites. Molecules 2022, 27, 7847. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Xiong, H.; Cai, Y.; Zhang, B. Molecular catalysts for electrocatalytic ammonia oxidation. Sci. China-Chem. 2024, 67, 3976–3993. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.-J.; Dong, C.-L.; Wang, J.; Zhang, X.; Lyalin, A.; Taketsugu, T.; Chen, Z.; Guan, D.; Xu, X.; et al. Electrocatalytic ammonia oxidation to nitrite and nitrate with NiOOH-Ni. Adv. Energy Mater. 2024, 14, 2401675. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Li, T.; Dietiker, J.-F.; Rogers, W.; Panday, R.; Gopalan, B.; Breault, G. Investigation of CO2 capture using solid sorbents in a fluidized bed reactor: Cold flow hydrodynamics. Powder Technol. 2016, 301, 1130–1143. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, S.-J.; Kim, K.-H.; Lee, S.-H.; Park, H.-S.; Jeon, B.-H. Removal of hazardous hexavalent chromium from aqueous phase using zirconium oxide-immobilized alginate beads. Appl. Geochem. 2018, 88, 113–121. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Ammonia adsorption and its effects on framework stability of MOF-5 and MOF-177. J. Colloid Interface Sci. 2010, 348, 615–620. [Google Scholar] [CrossRef]
- Teng, H.S.; Yeh, T.S.; Hsu, L.Y. Preparation of activated carbon from bituminous coal with phosphoric acid activation. Carbon 1998, 36, 1387–1395. [Google Scholar] [CrossRef]
- Huang, C.-C.; Li, H.-S.; Chen, C.-H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef]
- Qajar, A.; Peer, M.; Andalibi, M.R.; Rajagopalan, R.; Foley, H.C. Enhanced ammonia adsorption on functionalized nanoporous carbons. Microporous Mesoporous Mater. 2015, 218, 15–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Luo, W.; Yang, H.; He, Y.; Liu, Y.; Zhang, X.; Peng, G. Study on adsorption and desorption of ammonia on graphene. Nanoscale Res. Lett. 2015, 10, 359. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Mechanism of ammonia retention on graphite oxides: Role of surface chemistry and structure. J. Phys. Chem. C 2007, 111, 15596–15604. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, J.; Rappeport, S.; Zheng, Z.; Wang, Z.; Han, Z.; Langer, J.; Economy, J. Activated carbon fiber composites for gas phase ammonia adsorption. Microporous Mesoporous Mater. 2016, 234, 146–154. [Google Scholar] [CrossRef]
- Bernal, M.P.; Lopezreal, J.M. Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 1993, 43, 27–33. [Google Scholar] [CrossRef]
- Helminen, J.; Helenius, J.; Paatero, E.; Turunen, I. Adsorption equilibria of ammonia gas on inorganic and organic sorbents at 298.15 K. J. Chem. Eng. Data 2001, 46, 391–399. [Google Scholar] [CrossRef]
- Witter, E.; Kirchmann, H. Peat, zeolite and basalt as adsorbents of ammoniacal nitrogen during manure decomposition. Plant Soil 1989, 115, 43–52. [Google Scholar] [CrossRef]
- Taghipour, H.; Shahmansoury, M.R.; Bina, B.; Movahdian, H. Operational parameters in biofiltration of ammonia-contaminated air streams using compost-pieces of hard plastics filter media. Chem. Eng. J. 2008, 137, 198–204. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Vo, H.T.; Lee, G.; Kim, N.; Jang, S.; Joo, J.B. Bead-shaped mesoporous alumina adsorbents for adsorption of ammonia. Materials 2020, 13, 1375. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Wang, C.; Gao, X.; Zhou, Y.; Chen, B.; Li, P. Self-healing hydrogen-bonded organic frameworks for low-concentration ammonia capture. J. Am. Chem. Soc. 2023, 146, 627–634. [Google Scholar] [CrossRef]
- Johnsen, R.E.; Jensen, P.B.; Norby, P.; Vegge, T. Temperature- and Pressure-Induced Changes in the Crystal Structure of Sr(NH3)8Cl2. J. Phys. Chem. C 2014, 118, 24349–24356. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, C.-W.; Aguila, B.; Perman, J.; Wang, S.; Huang, H.-Y.; Xiao, F.-S.; Ma, S. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 984–992. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Thallapally, P.K.; Ma, S. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 2018, 30, 1705479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Li, W.; Yang, Z.; Zhang, Y.; Zhao, J.; Chi, Z. Local dynamics in a hydrogen-bonded organic framework for adaptive guest accommodation with programmable luminescence. Chem 2023, 9, 1241–1254. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Exploring the coordination chemistry of MOF-graphite oxide composites and their applications as adsorbents. Dalton Trans. 2012, 41, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Binaeian, E.; El-Sayed, E.-S.M.; Matikolaei, M.K.; Yuan, D. Experimental strategies on enhancing toxic gases uptake of metal-organic frameworks. Coord. Chem. Rev. 2021, 430, 213738. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Wang, H.; Zhao, Y.; Xia, Q.; Qiu, J.; Wang, J. Regulating the pore microenvironment of microporous metal-organic frameworks for efficient adsorption of low-concentration ammonia. ACS Sustain. Chem. Eng. 2022, 10, 10945–10954. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Wang, Z.; Zhao, Y.; Xia, Q.; Qiu, J.; Wang, H.; Wang, J. Ionic liquid hybrid metal-organic frameworks for efficient adsorption and selective separation of ammonia at high temperature. Chem. Eng. J. 2023, 464, 142728. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, W.; Ma, H. Application and challenge of metal/covalent organic frameworks in ammonia sorption and separation. ChemPlusChem 2024, 89, e202400236. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Li, G.; Jiang, G.; Zhao, Y.; Li, L.; Li, K.; Liang, G.; Gao, S.; Xi, H.; et al. High-efficiency capture of ammonia using hierarchically porous Zr-MOF nanoarchitectures under ambient conditions: Thermodynamics, kinetics, and mechanisms. Chem. Eng. J. 2022, 440, 135764. [Google Scholar] [CrossRef]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, Y.; Shui, F.; Yi, M.; Zhang, Z.; Liu, X.; Zhang, L.; You, Z.; Yang, R.; Yang, S.; et al. Extremely stable sulfuric acid covalent organic framework for highly effective ammonia capture. Chin. J. Chem. 2022, 40, 2445–2450. [Google Scholar] [CrossRef]
- Ma, K.; Li, P.; Xin, J.H.; Chen, Y.; Chen, Z.; Goswami, S.; Liu, X.; Kato, S.; Chen, H.; Zhang, X.; et al. Ultrastable Mesoporous Hydrogen-Bonded Organic Framework-Based Fiber Composites toward Mustard Gas Detoxification. Cell Rep. Phys. Sci. 2020, 1, 100024. [Google Scholar] [CrossRef]
- Kang, D.W.; Kang, M.; Kim, H.; Choe, J.H.; Kim, D.W.; Park, J.R.; Lee, W.R.; Moon, D.; Hong, C.S. A hydrogen-bonded organic framework (HOF) with type IV NH3 adsorption behavior. Angew. Chem.-Int. Ed. 2019, 58, 16152–16155. [Google Scholar] [CrossRef]
- Yang, Y.; Faheem, M.; Wang, L.; Meng, Q.; Sha, H.; Yang, N.; Yuan, Y.; Zhu, G. Surface pore engineering of covalent organic frameworks for ammonia capture through synergistic multivariate and open metal site approaches. ACS Cent. Sci. 2018, 4, 748–754. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef]
- Glover, T.G.; Peterson, G.W.; Schindler, B.J.; Britt, D.; Yaghi, O. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Jasuja, H.; Peterson, G.W.; Decoste, J.B.; Browe, M.A.; Walton, K.S. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air. Chem. Eng. Sci. 2015, 124, 118–124. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Li, J.; Ouyang, K.; Yang, J. Ammonia capture and flexible transformation of M-2(INA) (M = Cu, Co, Ni, Cd) series materials. J. Hazard. Mater. 2016, 306, 340–347. [Google Scholar] [CrossRef]
- Rieth, A.J.; Dincă, M. Controlled gas uptake in metal–organic frameworks with record ammonia sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef]
- Chen, R.; Liu, J. Competitive coadsorption of ammonia with water and sulfur dioxide on metal-organic frameworks at low pressure. Build. Environ. 2022, 207, 108421. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Turkiewicz, A.B.; Furukawa, H.; Paley, M.V.; Velasquez, E.O.; Dods, M.N.; Long, J.R. A ligand insertion mechanism for cooperative NH3 capture in metal–organic frameworks. Nature 2023, 613, 287–291. [Google Scholar] [CrossRef]
- Han, X.; Lu, W.; Chen, Y.; da Silva, I.; Li, J.; Lin, L.; Li, W.; Sheveleva, A.M.; Godfrey, H.G.W.; Lu, Z.; et al. High ammonia adsorption in MFM-300 materials: Dynamics and charge transfer in host–guest binding. J. Am. Chem. Soc. 2021, 143, 3153–3161. [Google Scholar] [CrossRef]
- Cao, R.; Chen, Z.; Chen, Y.; Idrees, K.B.; Hanna, S.L.; Wang, X.; Goetjen, T.A.; Sun, Q.; Islamoglu, T.; Farha, O.K. Benign integration of a Zn-azolate metal-organic framework onto textile fiber for ammonia capture. ACS Appl. Mater. Interfaces 2020, 12, 47747–47753. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Ma, K.; Chen, Z.; Wang, X.; Knapp, J.; Alayoglu, S.; Wang, F.; Xia, Q.; Li, Z.; et al. Zirconium-based metal-organic framework with 9-connected nodes for ammonia capture. ACS Appl. Nano Mater. 2019, 2, 6098–6102. [Google Scholar] [CrossRef]
- Wu, S.-F.; Yuan, B.-Z.; Wang, L.-W. MOF–ammonia working pairs in thermal energy conversion and storage. Nat. Rev. Mater. 2023, 8, 636–638. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF-graphite oxide composites: Combining the uniqueness of graphene layers and metal-organic frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- More, M.S.; Bodkhe, G.A.; Singh, F.; Kim, M.; Shirsat, M.D. Metal–organic framework-reduced graphene oxide (Zn-BDC@rGO) composite for selective discrimination among ammonia, carbon monoxide, and sulfur dioxide. Appl. Phys. A 2023, 129, 828. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Guo, M.; Fan, H.; Guo, Q.; Yu, G. MIL-101(Cr) loaded simple ILs for efficient ammonia capture and selective separation. Chem. Eng. J. 2023, 471, 144545. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xiong, D.; Li, Z.; Wang, H.; Xuan, X.; Wang, J. Metal chloride functionalized MOF-253(Al) for high-efficiency selective separation of ammonia from H2 and N2. Chem. Eng. J. 2023, 474, 145307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).