Co-Deposited Proteins in Alzheimer’s Disease as a Potential Treasure Trove for Drug Repurposing
Abstract
1. Introduction
2. Results
2.1. FDA Approved Drugs and Their Protein Targets
2.2. Proteins Found on Amyloid Plaques
2.3. Potential for Drug Repurposing
3. Discussion
4. Materials and Methods
4.1. Co-Deposited Proteins
4.2. Drugs and Their Protein Targets
4.3. Protein-Protein Interactions
4.4. Network Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| APOE | Apolipoprotein E |
References
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the Global Burden of Alzheimer’s Disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Iqbal, K.; Grundke-Iqbal, I. Alzheimer Disease, a Multifactorial Disorder Seeking Multi-Therapies. Alzheimers Dement. 2010, 6, 420–424. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Folding Proteins in Fatal Ways. Nature 2003, 426, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid Nomenclature 2020: Update and Recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2020, 27, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nastou, K.C.; Nasi, G.I.; Tsiolaki, P.L.; Litou, Z.I.; Iconomidou, V.A. AmyCo: The Amyloidoses Collection. Amyloid 2019, 26, 112–117. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Spatharas, P.M.; Nasi, G.I.; Tsiolaki, P.L.; Theodoropoulou, M.K.; Papandreou, N.C.; Hoenger, A.; Trougakos, I.P.; Iconomidou, V.A. Clusterin in Alzheimer’s Disease: An Amyloidogenic Inhibitor of Amyloid Formation? Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166384. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s Disease: Accidental Encounters or Partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef]
- Goh, K.-I.; Cusick, M.E.; Valle, D.; Childs, B.; Vidal, M.; Barabási, A.-L. The Human Disease Network. Proc. Natl. Acad. Sci. USA 2007, 104, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Biza, K.V.; Nastou, K.C.; Tsiolaki, P.L.; Mastrokalou, C.V.; Hamodrakas, S.J.; Iconomidou, V.A. The Amyloid Interactome: Exploring Protein Aggregation. PLoS ONE 2017, 12, e0173163. [Google Scholar] [CrossRef]
- Hughes, J.; Rees, S.; Kalindjian, S.; Philpott, K. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Zakeri, N.S.; Pashazadeh, S.; MotieGhader, H. Drug Repurposing for Alzheimer’s Disease Based on Protein-Protein Interaction Network. Biomed. Res. Int. 2021, 2021, 1280237. [Google Scholar] [CrossRef] [PubMed]
- Savva, K.; Zachariou, M.; Bourdakou, M.M.; Dietis, N.; Spyrou, G.M. Network-Based Stage-Specific Drug Repurposing for Alzheimer’s Disease. Comput. Struct. Biotechnol. J. 2022, 20, 1427–1438. [Google Scholar] [CrossRef]
- Han, Z.; Xue, W.; Tao, L.; Zhu, F. Identification of Novel Immune-relevant Drug Target Genes for Alzheimer’s Disease by Combining Ontology Inference with Network Analysis. CNS Neurosci. Ther. 2018, 24, 1253–1263. [Google Scholar] [CrossRef]
- Siavelis, J.C.; Bourdakou, M.M.; Athanasiadis, E.I.; Spyrou, G.M.; Nikita, K.S. Bioinformatics Methods in Drug Repurposing for Alzheimer’s Disease. Brief. Bioinform. 2016, 17, 322–335. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, M.; Xin, J.; Liu, X.; Wang, J. Screening Novel Drug Candidates for Alzheimer’s Disease by an Integrated Network and Transcriptome Analysis. Bioinformatics 2020, 36, 4626–4632. [Google Scholar] [CrossRef]
- Lee, S.Y.; Song, M.-Y.; Kim, D.; Park, C.; Park, D.K.; Kim, D.G.; Yoo, J.S.; Kim, Y.H. A Proteotranscriptomic-Based Computational Drug-Repositioning Method for Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1653. [Google Scholar] [CrossRef]
- Myers, A.J.; Goate, A.M. The Genetics of Late-Onset Alzheimer’s Disease. Curr. Opin. Neurol. 2001, 14, 433–440. [Google Scholar] [CrossRef]
- Zhang, S.; Janciauskiene, S. Multi-Functional Capability of Proteins: Alpha1-Antichymotrypsin and the Correlation with Alzheimer’s Disease. J. Alzheimers Dis. 2002, 4, 115–122. [Google Scholar] [CrossRef]
- Mueller-Steiner, S.; Zhou, Y.; Arai, H.; Roberson, E.D.; Sun, B.; Chen, J.; Wang, X.; Yu, G.; Esposito, L.; Mucke, L.; et al. Antiamyloidogenic and Neuroprotective Functions of Cathepsin B: Implications for Alzheimer’s Disease. Neuron 2006, 51, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Tramutola, A.; Perluigi, M. Cathepsin D as a Therapeutic Target in Alzheimer’s Disease. Expert. Opin. Ther. Targets 2016, 20, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Lyra E Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.d.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Kolstoe, S.E.; Ridha, B.H.; Bellotti, V.; Wang, N.; Robinson, C.V.; Crutch, S.J.; Keir, G.; Kukkastenvehmas, R.; Gallimore, J.R.; Hutchinson, W.L.; et al. Molecular Dissection of Alzheimer’s Disease Neuropathology by Depletion of Serum Amyloid P Component. Proc. Natl. Acad. Sci. USA 2009, 106, 7619–7623. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.M.; Ngo, C.; Cummings, J.; Wight, T.N.; Snow, A.D. Perlecan Binds to the Beta-Amyloid Proteins (A Beta) of Alzheimer’s Disease, Accelerates A Beta Fibril Formation, and Maintains A Beta Fibril Stability. J. Neurochem. 1997, 69, 2452–2465. [Google Scholar] [CrossRef]
- Kaur, G.; Levy, E. Cystatin C in Alzheimer’s Disease. Front. Mol. Neurosci. 2012, 5, 79. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s Disease Drug Development Pipeline: 2022. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12295. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I. The Essential Elements of Alzheimer’s Disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Islam, F.; Nafady, M.H.; Islam, M.R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, M.H.; Akhtar, M.F.; Perveen, A.; Md Ashraf, G.; et al. Resveratrol and Neuroprotection: An Insight into Prospective Therapeutic Approaches against Alzheimer’s Disease from Bench to Bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A Randomized, Double-Blind, Placebo-Controlled Trial of Resveratrol for Alzheimer Disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Sabayan, B.; Farshchi, S.; Zamiri, N.; Sabayan, B. Can Tetrathiomolybdate Be a Potential Agent against Alzheimer Disease? A Hypothesis Based on Abnormal Copper Homeostasis in Brain. Alzheimer Dis. Assoc. Disord. 2010, 24, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Harris, C.J.; Cobb, K.E.; Domes, C.; Ralle, M.; Brewer, G.; Wadsworth, T.L. A Copper-Lowering Strategy Attenuates Amyloid Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 21, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.J.; Huber, B.R.; Silverman, M.A.; Cline, M.M.; Gill, T.B.; Cross, C.G.; Cook, D.G.; Minoshima, S. Intranasal Paclitaxel Alters Alzheimer’s Disease Phenotypic Features in 3xTg-AD Mice. J. Alzheimers Dis. 2021, 83, 379–394. [Google Scholar] [CrossRef]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, Microtubules and Chemoresistant Breast Cancer. Biochim. Biophys. Acta 2008, 1785, 96–132. [Google Scholar] [CrossRef]
- Rojo, L.E.; Alzate-Morales, J.; Saavedra, I.N.; Davies, P.; Maccioni, R.B. Selective Interaction of Lansoprazole and Astemizole with Tau Polymers: Potential New Clinical Use in Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 19, 573–589. [Google Scholar] [CrossRef]
- Badiola, N.; Alcalde, V.; Pujol, A.; Münter, L.-M.; Multhaup, G.; Lleó, A.; Coma, M.; Soler-López, M.; Aloy, P. The Proton-Pump Inhibitor Lansoprazole Enhances Amyloid Beta Production. PLoS ONE 2013, 8, e58837. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gahlawat, A.; Kumar, R.N.; Singh, Y.P.; Modi, G.; Garg, P. Drug Repurposing for Alzheimer’s Disease: In Silico and in Vitro Investigation of FDA-Approved Drugs as Acetylcholinesterase Inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 2878–2892. [Google Scholar] [CrossRef]
- Rizou, A.E.I.; Nasi, G.I.; Paikopoulos, Y.; Bezantakou, D.S.; Vraila, K.D.; Spatharas, P.M.; Dimaki, V.D.; Papandreou, N.C.; Lamari, F.N.; Chondrogianni, N.; et al. A Multilevel Study of Eupatorin and Scutellarein as Anti-Amyloid Agents in Alzheimer’s Disease. Biomedicines 2023, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Apostolakou, A.E.; Sula, X.K.; Nastou, K.C.; Nasi, G.I.; Iconomidou, V.A. Exploring the Conservation of Alzheimer-Related Pathways between H. Sapiens and C. Elegans: A Network Alignment Approach. Sci. Rep. 2021, 11, 4572. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; del-Toro, N.; et al. The MIntAct Project—IntAct as a Common Curation Platform for 11 Molecular Interaction Databases. Nucleic Acids Res. 2014, 42, D358–D363. [Google Scholar] [CrossRef]
- Porras, P.; Barrera, E.; Bridge, A.; del-Toro, N.; Cesareni, G.; Duesbury, M.; Hermjakob, H.; Iannuccelli, M.; Jurisica, I.; Kotlyar, M.; et al. Towards a Unified Open Access Dataset of Molecular Interactions. Nat. Commun. 2020, 11, 6144. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ragueneau, E.; Shrivastava, A.; Morris, J.H.; Del-Toro, N.; Hermjakob, H.; Porras, P. IntAct App: A Cytoscape Application for Molecular Interaction Network Visualisation and Analysis. Bioinformatics 2021, 37, 3684–3685. [Google Scholar] [CrossRef]
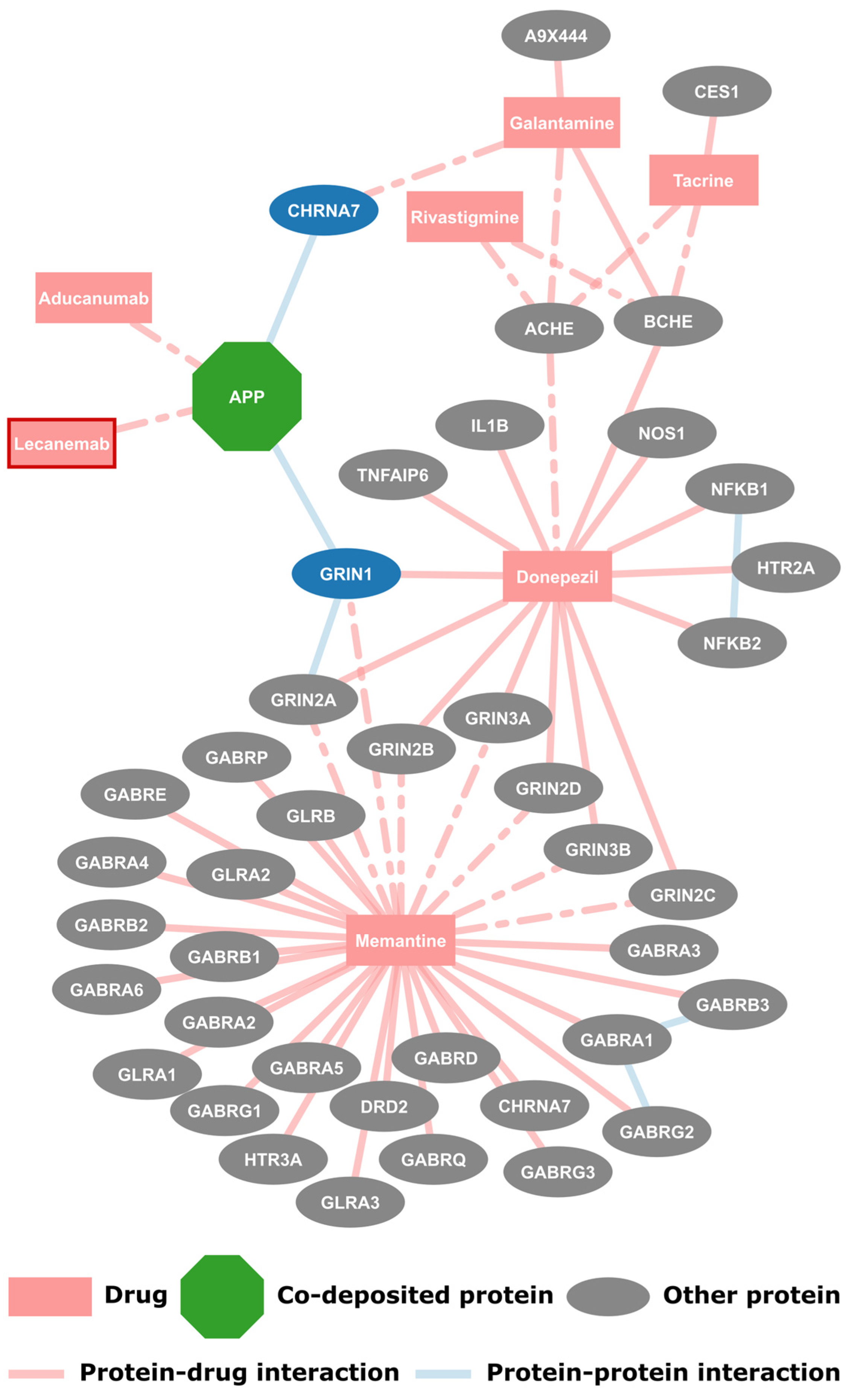
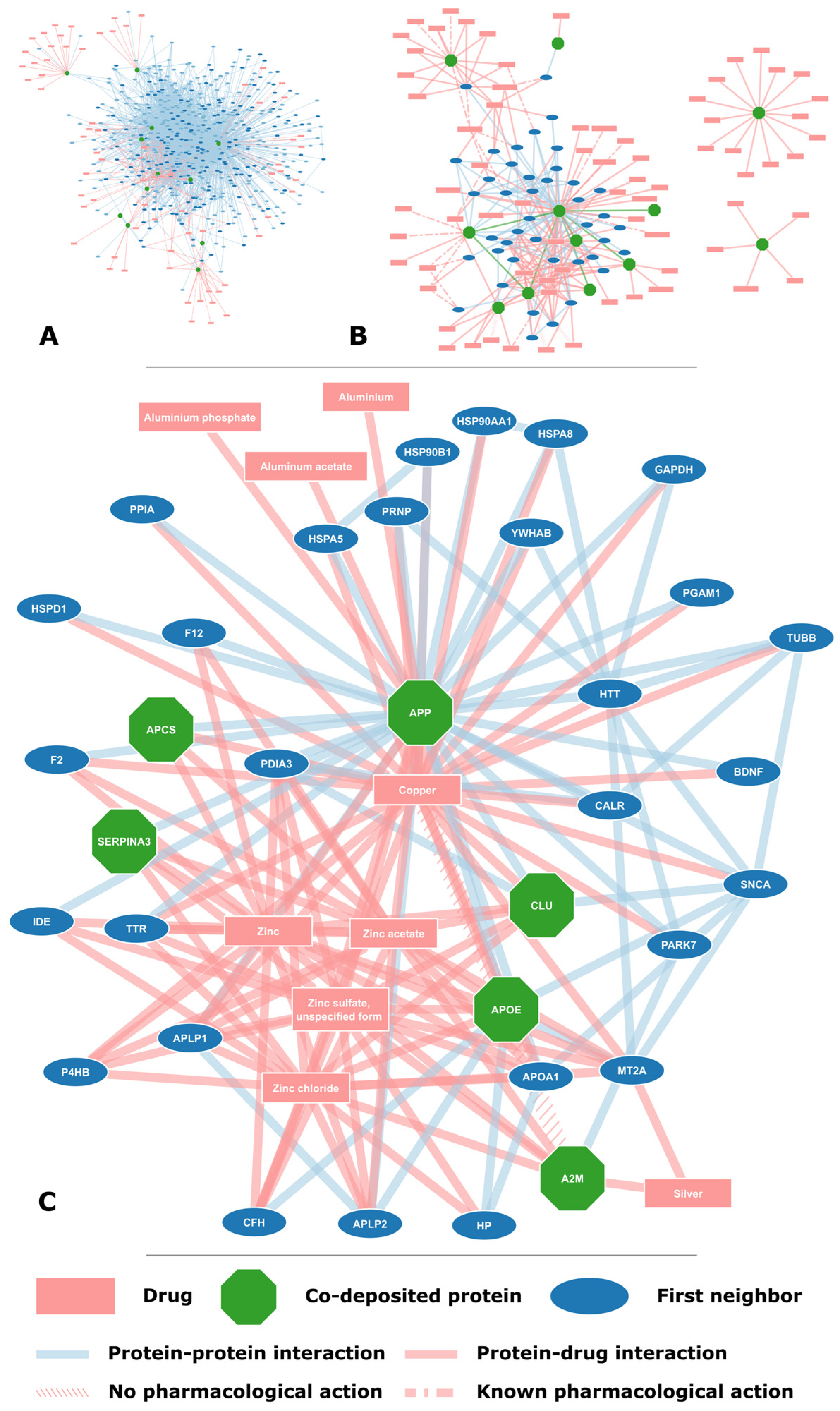

| Gene Name | Accession | Protein Name | Neighbors | Drugs | Interacts with APP |
|---|---|---|---|---|---|
| APP | P05067 | Amyloid-β precursor protein | 386 | 22 | - |
| A2M | P01023 | Alpha-2-macroglobulin | 55 | 11 | no |
| APOE | P02649 | Apolipoprotein E | 43 | 7 | yes |
| MAPT | P10636 | Microtubule-associated protein tau | 39 | 5 | yes |
| CTSD | P07339 | Cathepsin D | 22 | 5 * | no |
| CLU | P10909 | Clusterin | 10 | 5 | yes |
| SERPINA3 | P01011 | Alpha-1-antichymotrypsin | 8 | 4 | yes |
| CTSB | P07858 | Cathepsin B | 5 | 15 * | no |
| IL6 | P05231 | Interleukin-6 | 5 | 13 | no |
| APCS | P02743 | Serum amyloid P-component | 3 | 6 | yes |
| HSPG2 | P98160 | Basement membrane-specific heparan sulfate proteoglycan core protein | 3 | 1 | no |
| CST3 | P01034 | Cystatin-C | 2 | - | no |
| Associated with AD and Related Processes | No or Limited Known Association with AD | ||||
|---|---|---|---|---|---|
| DrugBank ID | Drug Name | Type | DrugBank ID | Drug Name | Type |
| DB00626 | Bacitracin | antibiotic | DB05470 | VX-702 | anti-cytokine |
| DB00637 | Astemizole | antihistamine | DB05744 | CRx-139 | anti-inflammatory |
| DB02709 | Resveratrol | antioxidant | DB12140 | Dilmapimod | anti-inflammatory |
| DB06782 | Dimercaprol | chelator | DB09221 | Polaprezinc | antioxidant |
| DB05088 | Tetrathiomolybdate | chelator | DB11886 | Infigratinib | cancer treatment |
| DB00515 | Cisplatin | chemotherapy | DB00746 | Deferoxamine | chelator |
| DB01229 | Paclitaxel | chemotherapy | DB05513 | Atiprimod | chemotherapy |
| DB01248 | Docetaxel | chemotherapy | DB11967 | Binimetinib | chemotherapy |
| DB05846 | Mito-4509 | estrogen | DB06796 | Mangafodipir | contrast agent |
| DB00877 | Sirolimus | immunosuppressant | DB13127 | Olokizumab | monoclonal antibody |
| DB00102 | Becaplermin | protein based therapy | DB09036 | Siltuximab | monoclonal antibody |
| DB08888 | Ocriplasmin | protein based therapy | DB14962 | Trastuzumab deruxtecan | monoclonal antibody |
| DB00039 | Palifermin | protein based therapy | DB05017 | YSIL6 | T-cell inhibitor |
| DB03754 | Tromethamine | proton acceptor | |||
| DB00448 | Lansoprazole | proton pump inhibitor | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolakou, A.E.; Douska, D.E.; Litou, Z.I.; Trougakos, I.P.; Iconomidou, V.A. Co-Deposited Proteins in Alzheimer’s Disease as a Potential Treasure Trove for Drug Repurposing. Molecules 2025, 30, 1736. https://doi.org/10.3390/molecules30081736
Apostolakou AE, Douska DE, Litou ZI, Trougakos IP, Iconomidou VA. Co-Deposited Proteins in Alzheimer’s Disease as a Potential Treasure Trove for Drug Repurposing. Molecules. 2025; 30(8):1736. https://doi.org/10.3390/molecules30081736
Chicago/Turabian StyleApostolakou, Avgi E., Dimitra E. Douska, Zoi I. Litou, Ioannis P. Trougakos, and Vassiliki A. Iconomidou. 2025. "Co-Deposited Proteins in Alzheimer’s Disease as a Potential Treasure Trove for Drug Repurposing" Molecules 30, no. 8: 1736. https://doi.org/10.3390/molecules30081736
APA StyleApostolakou, A. E., Douska, D. E., Litou, Z. I., Trougakos, I. P., & Iconomidou, V. A. (2025). Co-Deposited Proteins in Alzheimer’s Disease as a Potential Treasure Trove for Drug Repurposing. Molecules, 30(8), 1736. https://doi.org/10.3390/molecules30081736









