Regulation of Blood–Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer’s Disease and Brain Tumors
Abstract
1. Introduction
2. JNK
3. BBB
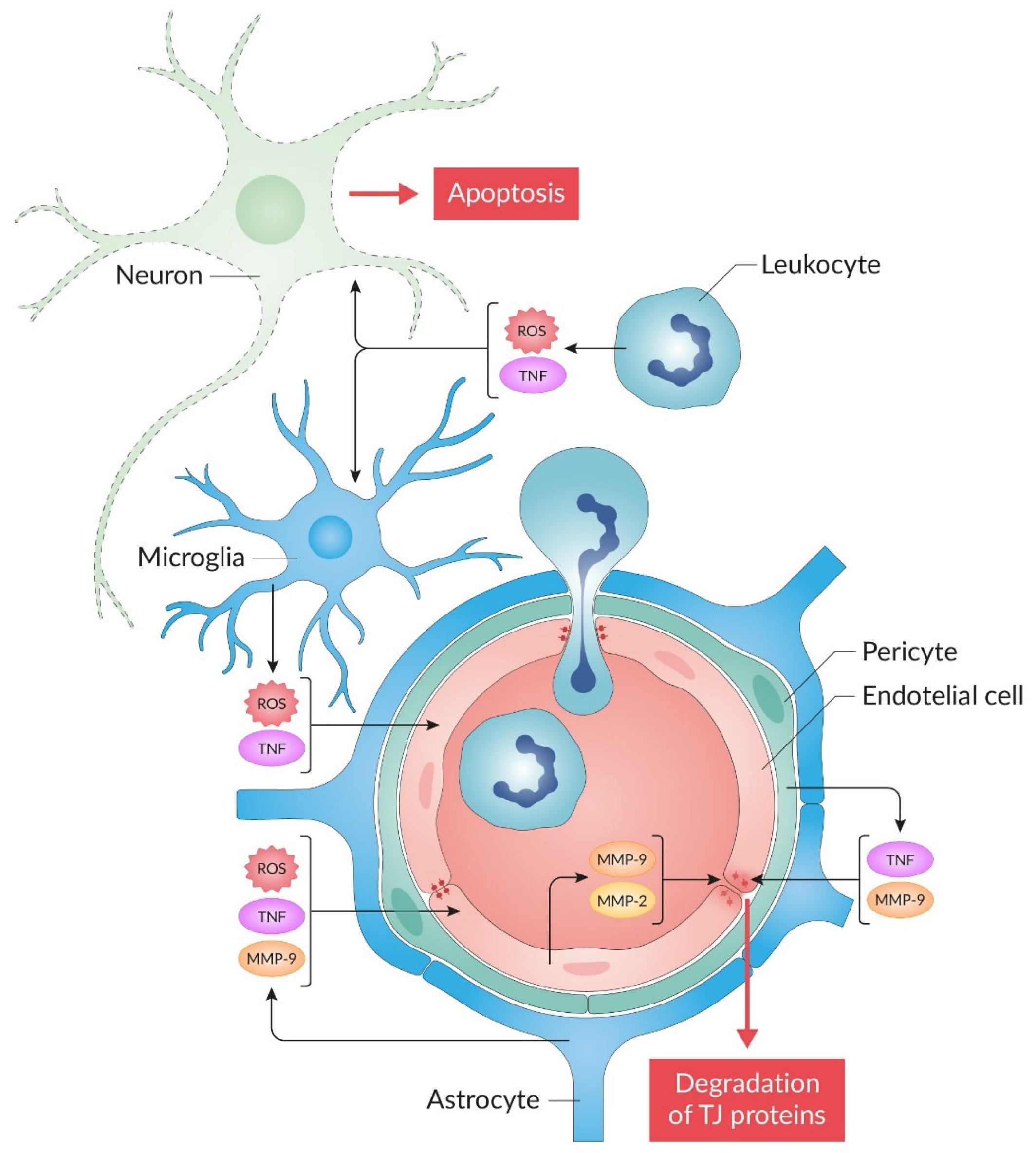
4. BBB Permeability in Ischemic Stroke and Neuroinflammation
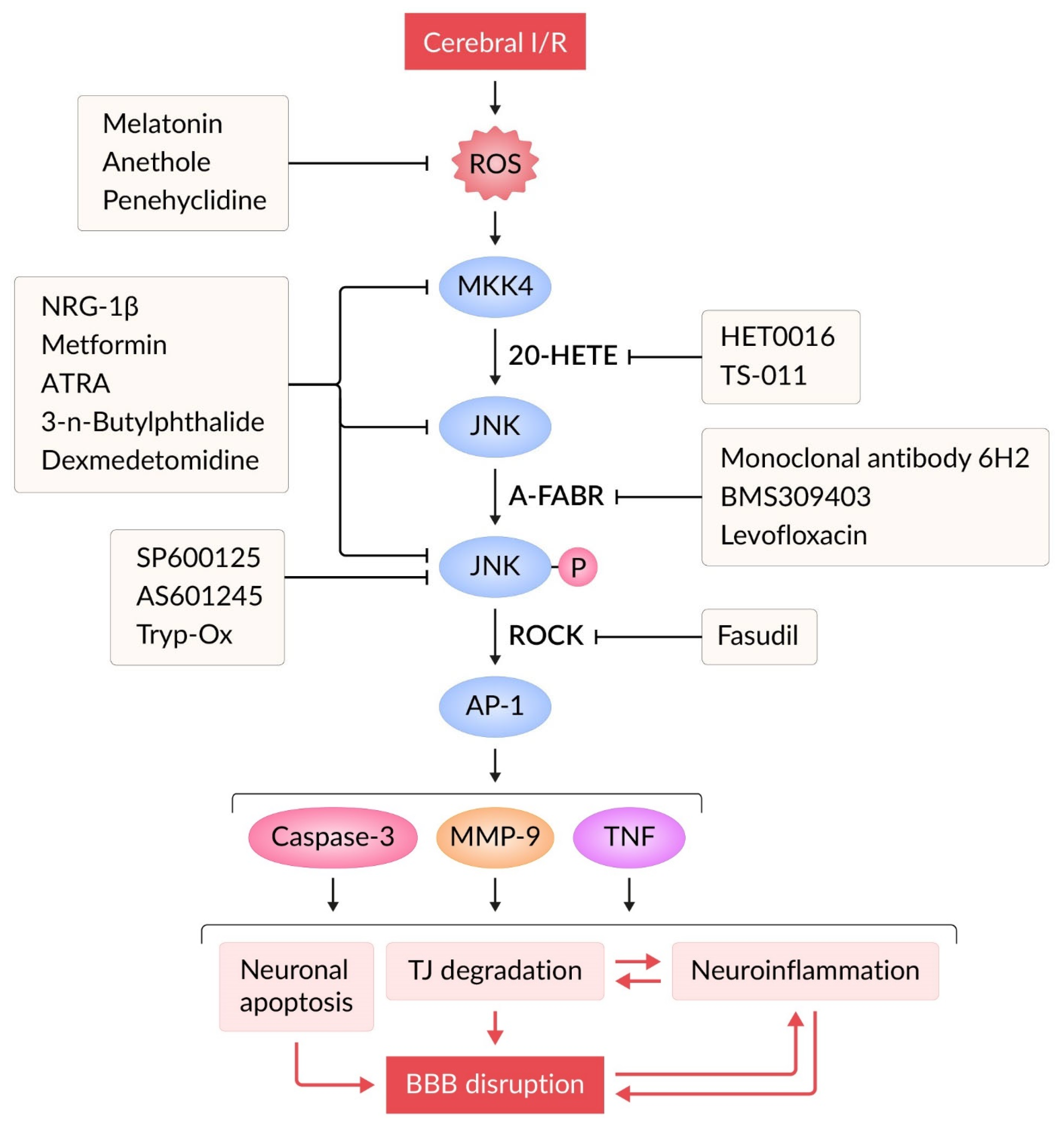
5. JNK Signaling and BBB Damage in Ischemic Stroke
6. Inhibition of JNK Signaling Pathway in Models of Ischemic Stroke
6.1. JNK Inhibitors
6.2. Indirect Modulators of JNK Signaling Pathway
7. JNK Signaling Pathway in BBB Permeability Impairment in AD
8. JNK Signaling Pathway in Blood–Tumor Barrier (BTB) Permeability
9. Limitations and Perspectives
- It appears that effective correctors of BBB dysfunction could be direct/indirect JNK inhibitors and other compounds that preserve and/or restore TJ structure. This issue requires further in-depth investigation.
- The use of thrombolytic therapy and thromboextraction during the “therapeutic window” in patients with ischemic stroke can cause reperfusion in the ischemic zone [12]. Unfortunately, post-ischemic reperfusion promotes increased oxidative stress, inflammation, BBB disruption, brain tissue edema, and hemorrhagic transformation of the ischemic focus [14,15]. The mechanistic profile of JNK pathway inhibitors demonstrates their capacity to attenuate key pathological processes in I/R injury. Thus, recanalization leads to the appearance of neutrophils in the lesion zone [205]. JNK inhibitors may limit the proinflammatory potential of neutrophils [206]. Thrombolytic therapy is accompanied by additional activation of the MMP cascade [12]. Decreased activity of the JNK pathway during reperfusion weakens the expression and activation of MMPs [19,66,131]. During the reperfusion period, cerebral edema may increase [207]. Therapeutic administration of direct and indirect JNK inhibitors (Table 1, Table 2 and Table 3) mitigates cerebral edema formation during I/R injury. However, the impact of JNK inhibitors on hemorrhagic transformation following tPA-based thrombolytic therapy remains poorly characterized. This critical gap could be addressed through experimental studies using the MCAO model in conjunction with tPA administration [202,208].
- The contribution of BBB dysfunction in AD is underestimated. Increased BBB permeability in AD can have potentially catastrophic consequences for the homeostasis of the neural environment [209]. JNK is involved in numerous pathological processes that occur in AD. Although increased BBB permeability has been repeatedly shown in various in vivo AD models using dyes, in particular Evans blue [210,211,212], but there are no studies that have used this standardized method to confirm the involvement of the JNK signaling system in BBB regulation. Investigating JNK inhibitors as correctors of BBB dysfunction in this disease remains relevant.
- Undoubtedly, a promising direction is the study of selective JNK3 inhibitors as agents for protecting and restoring the BBB in models of I/R, AD, and brain tumors. Research into the effects of selective JNK inhibitors on BBB permeability in AD has just begun and, given the above, may become one of the promising directions for developing an innovative drug for treating AD [201].
- Loss of BBB integrity plays a critical role in the progression of brain tumors. In these tumors, the JNK signaling pathway is activated. However, studies on the effects of JNK inhibitors on BBB permeability remain in early stages. Furthermore, the feasibility of using JNK inhibitors to preserve BBB integrity has yet to be confirmed. It remains unclear whether mitigating BBB damage would improve outcomes in brain tumor therapy, highlighting the urgent need for interdisciplinary collaboration to resolve this critical gap in research.
- Most JNK inhibitors lack isoform-specific targeting, as structural similarities between isoforms hinder the development of precise therapeutics [213]. Future strategies must prioritize isoform-selective designs (e.g., targeting JNK2/3 over JNK1 or JNK3 over JNK1/2) to mitigate these risks while retaining therapeutic efficacy across CNS diseases [200,214].
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.; Jung, U.J.; Kim, S.R. The crucial role of the blood-brain barrier in neurodegenerative diseases: Mechanisms of disruption and therapeutic implications. J. Clin. Med. 2025, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. Influence of glioma cells on a new co-culture in vitro blood-brain barrier model for characterization and validation of permeability. Int. J. Pharm. 2015, 490, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Dourvetakis, K.D.; Cohen, J.; Valladares, D.S.; Joshi, R.S.; Kothuru, S.P.; Anderson, T.; Chinnappan, B.; Cheema, A.K.; Klimas, N.G.; et al. Neurovascular unit, neuroinflammation and neurodegeneration markers in brain disorders. Front. Cell. Neurosci. 2024, 18, 1491952. [Google Scholar] [CrossRef]
- Marchi, N.; Granata, T.; Ghosh, C.; Janigro, D. Blood-brain barrier dysfunction and epilepsy: Pathophysiologic role and therapeutic approaches. Epilepsia 2012, 53, 1877–1886. [Google Scholar] [CrossRef]
- Qu, X.Y.; Yang, R.C.; Tan, C.; Chen, H.C.; Wang, X.R. Astrocytes-secreted WNT5B disrupts the blood-brain barrier via ROR1/JNK/c-Jun cascade during meningitic Escherichia coli infection. Mol. Neurobiol. 2025, 62, 661–673. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, K.; He, Q.; Lei, Q.; Lu, W. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J. Neuroimmune Pharmacol. 2019, 14, 157–172. [Google Scholar] [CrossRef]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global stroke fact sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Barrett, K.M.; Lal, B.K.; Meschia, J.F. Stroke: Advances in medical therapy and acute stroke intervention. Curr. Cardiol. Rep. 2015, 17, 79. [Google Scholar] [CrossRef]
- Mathias, K.; Machado, R.S.; Stork, S.; dos Santos, D.; Joaquim, L.; Generoso, J.; Danielski, L.G.; Barichello, T.; Prophiro, J.S.; Petronilho, F. Blood-brain barrier permeability in the ischemic stroke: An update. Microvasc. Res. 2024, 151, 104621. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Y.; Wakhloo, A.K.; Fisher, M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.; Hoda, M.N.; Guo, X.Y.; Johnson, M.H.; Fagan, S.C.; Ergul, A. Comparative analysis of different methods of ischemia/reperfusion in hyperglycemic stroke outcomes: Interaction with tPA. Transl. Stroke Res. 2015, 6, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Jung, J.E.; Lo, E.H.; van Leyen, K. Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models. Brain Res. 2018, 1678, 123–128. [Google Scholar] [CrossRef]
- Wang, G.R.; Chen, Z.Y.; Song, Y.Y.; Wu, H.; Chen, M.; Lai, S.S.; Wu, X.J. Xueshuantong injection alleviates cerebral microcirculation disorder in middle cerebral artery occlusion/reperfusion rats by suppressing inflammation via JNK mediated JAK2/STAT3 and NF-κB signaling pathways. J. Ethnopharmacol. 2022, 298, 115592. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A. Global burden of stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhong, W.; Jiang, Z.; Tang, X.Q. New progress in the approaches for blood-brain barrier protection in acute ischemic stroke. Brain Res. Bull. 2019, 144, 46–57. [Google Scholar] [CrossRef]
- Liao, B.Y.; Geng, L.L.; Zhang, F.; Shu, L.L.; Wei, L.; Yeung, P.K.K.; Lam, K.S.L.; Chung, S.K.; Chang, J.L.; Vanhoutte, P.M.; et al. Adipocyte fatty acid-binding protein exacerbates cerebral ischaemia injury by disrupting the blood-brain barrier. Eur. Heart J. 2020, 41, 3169–3180. [Google Scholar] [CrossRef]
- Guan, X.; Wei, D.S.; Liang, Z.Z.; Xie, L.Y.; Wang, Y.F.; Huang, Z.J.; Wu, J.; Pang, T. FDCA attenuates neuroinflammation and brain Injury after cerebral ischemic stroke. ACS Chem. Neurosci. 2023, 14, 3839–3854. [Google Scholar] [CrossRef]
- Ji, F.T.; Liang, J.J.; Miao, L.P.; Wu, Q.; Cao, M.H. Propofol post-conditioning protects the blood brain barrier by decreasing matrix metalloproteinase-9 and aquaporin-4 expression and improves the neurobehavioral outcome in a rat model of focal cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2015, 12, 2049–2055. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kao, S.T.; Cheng, C.Y. Acorus tatarinowii Schott extract reduces cerebral edema caused by ischemia-reperfusion injury in rats: Involvement in regulation of astrocytic NKCC1/AQP4 and JNK/iNOS-mediated signaling. BMC Complement. Med. Ther. 2020, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Qi, J.H.; Su, Y.X.; Zhou, Y.; Wang, Q.; Huang, T.D.; Xue, D.D.; Zeng, Y.X.; Verkhratsky, A.; Zhou, B.J.; et al. Endothelial DR6 in blood-brain barrier malfunction in Alzheimer’s disease. Cell Death Dis. 2024, 15, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Wang, X.J.; Xie, J.W.; Liang, B.; Wu, J.H. Suppression of angiotensin-(1–7) on the disruption of blood-brain barrier in rat of brain glioma. Pathol. Oncol. Res. 2019, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.; Singh, S.; Singh, T.G. Pharmacological modulation of cytokines correlating neuroinflammatory cascades in epileptogenesis. Mol. Biol. Rep. 2022, 49, 1437–1452. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Hu, J.; Du, J.T.; Dordoe, C.; Zhou, Q.L.; Huang, W.T.; Guo, R.L.; Han, F.Y.; Guo, K.M.; et al. FGF20 protected against BBB disruption after traumatic brain injury by upregulating junction protein expression and inhibiting the inflammatory response. Front. Pharmacol. 2021, 11, 590669. [Google Scholar] [CrossRef]
- Meng, S.; Cao, H.; Huang, Y.C.; Shi, Z.Y.; Li, J.Y.; Wang, Y.N.; Zhang, Y.; Chen, S.N.; Shi, H.; Gao, Y.Q. ASK1-K716R reduces neuroinflammation and white matter injury via preserving blood-brain barrier integrity after traumatic brain injury. J. Neuroinflammation 2023, 20, 244. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chen, Y.H. Dexamethasone downregulates the expressions of MMP-9 and oxidative stress in mice with eosinophilic meningitis caused by Angiostrongylus cantonensis infection. Parasitology 2021, 148, 187–197. [Google Scholar] [CrossRef]
- Luo, C.L.; Li, Q.Q.; Chen, X.P.; Zhang, X.M.; Li, L.L.; Li, B.X.; Zhao, Z.Q.; Tao, L.Y. Lipoxin A attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013, 1502, 1–10. [Google Scholar] [CrossRef]
- Hung, T.C.; Huang, L.W.; Su, S.J.; Hsieh, B.S.; Cheng, H.L.; Hu, Y.C.; Chen, Y.H.; Hwang, C.C.; Chang, K.L. Hemeoxygenase-1 expression in response to arecoline-induced oxidative stress in human umbilical vein endothelial cells. Int. J. Cardiol. 2011, 151, 187–194. [Google Scholar] [CrossRef]
- Osaki, L.H.; Gama, P. MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int. J. Mol. Sci. 2013, 14, 10143–10161. [Google Scholar] [CrossRef]
- Knight, R.J.; Buxton, D.B. Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischemia and reperfusion in the perfused rat heart. Biochem. Biophys. Res. Commun. 1996, 218, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr. Opin. Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.C. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 427–436. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A.; Kobe, B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 1061–1095. [Google Scholar] [CrossRef]
- Duplain, H. Salvage of ischemic myocardium: A focus on JNK. In Hypoxia and Exercise; Advances in Experimental Medicine and Biology; Roach, R.C., Wagner, P.D., Hackett, P.H., Eds.; Springer: Boston, MA, USA, 2006; Volume 588, pp. 157–164. [Google Scholar]
- Bode, A.M.; Dong, Z.G. The functional contrariety of JNK. Mol. Carcinog. 2007, 46, 591–598. [Google Scholar] [CrossRef]
- Gupta, S.; Barrett, T.; Whitmarsh, A.J.; Cavanagh, J.; Sluss, H.K.; Derijard, B.; Davis, R.J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996, 15, 2760–2770. [Google Scholar] [CrossRef]
- Waetzig, V.; Herdegen, T. Context-specific inhibition of JNKs: Overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 2005, 26, 455–461. [Google Scholar] [CrossRef]
- Hibi, M.; Lin, A.N.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ichijo, H.; Korsmeyer, S.J. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol. Cell. Biol. 1999, 19, 8469–8478. [Google Scholar] [CrossRef]
- Chang, L.F.; Jones, Y.; Ellisman, M.H.; Goldstein, L.S.B.; Karin, M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 2003, 4, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kawasaki, H.; Arakawa, S.; Shimizu, K.; Shimizu, S.; Reiner, O.; Okano, H.; Nishina, S.; Azuma, N.; Penninger, J.M.; et al. Stress-activated protein kinase MKK7 regulates axon elongation in the developing cerebral cortex. J. Neurosci. 2011, 31, 16872–16883. [Google Scholar] [CrossRef] [PubMed]
- Kenney, A.M.; Kocsis, J.D. Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J. Neurosci. 1998, 18, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Nakamura, K. The c-Jun kinase/stress-activated pathway: Regulation, function and role in human disease. Biochim. Biophys. Acta 2007, 1773, 1341–1348. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-Terminal Kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef]
- Corrales, T.D.; Losada-Pérez, M.; Casas-Tintó, S. JNK pathway in CNS pathologies. Int. J. Mol. Sci. 2021, 22, 3883. [Google Scholar] [CrossRef]
- Ferrer, I.; Friguls, B.; Dalfó, E.; Justicia, C.; Planas, A.M. Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathol. Appl. Neurobiol. 2003, 29, 472–481. [Google Scholar] [CrossRef]
- Manning, A.M.; Davis, R.J. Targeting JNK for therapeutic benefit: From junk to gold? Nat. Rev. Drug Discov. 2003, 2, 554–565. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Smolyakova, V.I.; Aliev, O.I.; Trofimova, E.S.; Sherstoboev, E.Y.; Osipenko, A.N.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Neuroprotective effects of a novel inhibitor of c-Jun N-terminal kinase in the rat model of transient focal cerebral ischemia. Cells 2020, 9, 1860. [Google Scholar] [CrossRef]
- Repici, M.; Centeno, C.; Tomasi, S.; Forloni, G.; Bonny, C.; Vercelli, A.; Borsello, T. Time-course of c-Jun N-terminal kinase activation after cerebral ischemia and effect of D-JNKI1 on c-Jun and caspase-3 activation. Neuroscience 2007, 150, 40–49. [Google Scholar] [CrossRef]
- Herdegen, T.; Claret, F.X.; Kallunki, T.; Martin-Villalba, A.; Winter, C.; Hunter, T.; Karin, M. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J. Neurosci. 1998, 18, 5124–5135. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.R.; Liu, C.L.; Park, D.J. Alteration of MAP kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000, 20, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Tu, Y.F.; Huang, C.C.; Ho, C.J. JNK signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflammation 2012, 9, 175. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Signore, A.P.; Yin, W.; Cao, G.D.; Yin, X.M.; Sun, F.Y.; Luo, Y.M.; Graham, S.H.; Chen, J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J. Cereb. Blood Flow Metab. 2005, 25, 694–712. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Aliev, O.I.; Smol’iakova, V.I.; Fomina, T.I.; Osipenko, A.N.; Rydchenko, V.S.; Anfinogenova, Y.J.; Khlebnikov, A.I.; Schepetkin, I.A.; et al. Protective effects of a new c-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules 2019, 24, 1722. [Google Scholar] [CrossRef]
- Borsello, T.; Clarke, P.G.H.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.J.; Tao, H.; Wang, X.; Li, M.C. Targeting brain microvascular endothelial cells: A therapeutic approach to neuroprotection against stroke. Neural Regen. Res. 2015, 10, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Zhu, C.M.; Wang, D.L.; Chang, C.; Liu, A.F.; Zhou, J.; Yang, T.; Jiang, Y.F.; Li, X.; Jiang, W.J. Dexmedetomidine alleviates blood-brain barrier disruption in rats after cerebral ischemia-reperfusion by suppressing JNK and p38 MAPK signaling. Korean J. Physiol. Pharmacol. 2024, 28, 239–252. [Google Scholar] [CrossRef]
- Gareev, I.; Beylerli, O.; Yang, G.; Sun, J.X.; Pavlov, V.; Izmailov, A.; Shi, H.Z.; Zhao, S.G. The current state of MiRNAs as biomarkers and therapeutic tools. Clin. Exp. Med. 2020, 20, 349–359. [Google Scholar] [CrossRef]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Williams, S.N.; Haim, E.D.; Tajiri, N.; Hernandez-Ontiveros, D.G.; Frisina-Deyo, A.; Boffeli, S.M.; Sanberg, P.R.; Borlongan, C.V. Compromised blood-brain barrier competence in remote brain areas in ischemic stroke rats at the chronic stage. J. Comp. Neurol. 2014, 522, 3120–3137. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Cruz, M.E.; López-Valdés, H. Physiopathology of ischemic stroke and its modulation using memantine: Evidence from preclinical stroke. Neural Regen. Res. 2021, 16, 433–439. [Google Scholar] [CrossRef]
- Wang, Y.B.; Yuan, T.L.; Lyu, T.J.; Zhang, L.; Wang, M.; He, Z.Y.; Wang, Y.J.; Li, Z.X. Mechanism of inflammatory response and therapeutic effects of stem cells in ischemic stroke: Current evidence and future perspectives. Neural Regen. Res. 2025, 20, 67–81. [Google Scholar] [CrossRef]
- Gao, H.M.; Chen, H.; Cui, G.Y.; Hu, J.X. Damage mechanism and therapy progress of the blood-brain barrier after ischemic stroke. Cell Biosci. 2023, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Slota, J.A.; Booth, S.A. MicroRNAs in neuroinflammation: Implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and stroke: An overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- del Zoppo, G.J. Focus on research—Stroke and neurovascular protection. N. Engl. J. Med. 2006, 354, 553–555. [Google Scholar] [CrossRef]
- Li, Y.; Zou, C.Y.; Chen, C.; Li, S.X.; Zhu, Z.Y.; Fan, Q.Y.; Pang, R.; Li, F.S.; Chen, Z.A.; Wang, Z.H.; et al. Myeloid-derived MIF drives RIPK1-mediated cerebromicrovascular endothelial cell death to exacerbate ischemic brain injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2219091120. [Google Scholar] [CrossRef]
- Zhou, D.X.; Huang, Z.; Zhu, X.X.; Hong, T.; Zhao, Y.L. Circular RNA 0025984 ameliorates ischemic stroke injury and protects astrocytes through miR-143-3p/TET1/ORP150 pathway. Mol. Neurobiol. 2021, 58, 5937–5953. [Google Scholar] [CrossRef]
- Wang, J.P.; Fu, X.J.; Zhang, D.; Yu, L.; Lu, Z.F.; Gao, Y.F.; Liu, X.L.; Man, J.; Li, S.J.; Li, N.; et al. Effects of crenolanib, a nonselective inhibitor of PDGFR, in a mouse model of transient middle cerebral artery occlusion. Neuroscience 2017, 364, 202–211. [Google Scholar] [CrossRef]
- Dammann, O.; Durum, S.; Leviton, A. Do white cells matter in white matter damage? Trends Neurosci. 2001, 24, 320–324. [Google Scholar] [CrossRef]
- Volpe, J.J. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann. Neurol. 2011, 70, 525–529. [Google Scholar] [CrossRef]
- Kacimi, R.; Giffard, R.G.; Yenari, M.A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J. Inflamm. 2011, 8, 7. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, H.; Zhang, Y.F.; Dong, Y.X.; Yang, F.; Wu, X.Y.; Liu, H.T. Dexmedetomidine protects hippocampal neurons against hypoxia/reoxygenation-induced apoptosis through activation HIF-1α/p53 signaling. Life Sci. 2019, 232, 116611. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.T.; Kasner, S.E.; Saver, J.; Remmel, K.S.; Jauch, E.C.; BRAIN Study Group. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: The Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 2009, 40, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Han, S.W.; Lee, S.K. Free radicals as triggers of brain edema formation after stroke. Free Radic. Biol. Med. 2005, 39, 51–70. [Google Scholar] [CrossRef]
- Pun, P.B.L.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef]
- Sun, L.Z.; Zhuang, W.D.; Xu, X.Q.; Yang, J.; Teng, J.; Zhang, F.X. The effect of injection of EGb 761 into the lateral ventricle on hippocampal cell apoptosis and stem cell stimulation in situ of the ischemic/reperfusion rat model. Neurosci. Lett. 2013, 555, 123–128. [Google Scholar] [CrossRef]
- Cao, J.; Semenova, M.M.; Solovyan, V.T.; Han, J.H.; Coffey, E.T.; Courtney, M.J. Distinct requirements for p38α and c-jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J. Biol. Chem. 2004, 279, 35903–35913. [Google Scholar] [CrossRef]
- Harris, C.A.; Deshmukh, M.; Tsui-Pierchala, B.; Maroney, A.C.; Johnson, E.M. Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 (KT7515) preserves metabolism and growth of trophic factor-deprived neurons. J. Neurosci. 2002, 22, 103–113. [Google Scholar] [CrossRef]
- Minden, A.; Lin, A.; McMahon, M.; Langecarter, C.; Derijard, B.; Davis, R.J.; Johnson, G.L.; Karin, M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 1994, 266, 1719–1723. [Google Scholar] [CrossRef]
- Nijboer, C.H.; van der Kooij, M.A.; van Bel, F.; Ohl, F.; Heijnen, C.J.; Kavelaars, A. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2010, 24, 812–821. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Agostini, B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol. Ther. 2014, 144, 202–225. [Google Scholar] [CrossRef]
- Varfolomeev, E.E.; Ashkenazi, A. Tumor necrosis factor: An apoptosis JuNKie? Cell 2004, 116, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.Y.; Whitmarsh, A.J.; Yang, D.D.; Liao, G.H.; Schloemer, A.J.; Dong, C.; Bao, J.; Banasiak, K.J.; Haddad, G.G.; Flavell, R.A.; et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.H.; Pei, D.S.; Liu, X.M.; Wang, X.T.; Xu, T.L.; Zhang, G.Y. Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res. 2006, 1092, 36–46. [Google Scholar] [CrossRef]
- Guan, Q.H.; Pei, D.S.; Zong, Y.Y.; Xu, T.L.; Zhang, G.Y. Neuroprotection against ischemic brain injury by a small peptide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and non-nuclear pathways. Neuroscience 2006, 139, 609–627. [Google Scholar] [CrossRef]
- Karahashi, H.; Michelsen, K.S.; Arditi, M. Lipopolysaccharide-induced apoptosis in transformed bovine brain endothelial cells and human dermal microvessel endothelial cells: The role of JNK. J. Immunol. 2009, 182, 7280–7286. [Google Scholar] [CrossRef]
- Pirianov, G.; Jesurasa, A.; Mehmet, H. Developmentally regulated changes in c-Jun N-terminal kinase signalling determine the apoptotic response of oligodendrocyte lineage cells. Cell Death Differ. 2006, 13, 531–533. [Google Scholar] [CrossRef][Green Version]
- Wallace, B.K.; Jelks, K.A.; O’Donnell, M.E. Ischemia-induced stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransport involves p38 and JNK MAP kinases. Am. J. Physiol. Cell Physiol. 2012, 302, C505–C517. [Google Scholar] [CrossRef]
- Uesugi, M.; Nakajima, K.; Tohyama, Y.; Kohsaka, S.; Kurihara, T. Nonparticipation of nuclear factor kappa B (NFκB) in the signaling cascade of c-Jun N-terminal kinase (JNK)- and p38 mitogen-activated protein kinase (p38MAPK)-dependent tumor necrosis factor alpha (TNFα) induction in lipopolysaccharide (LIPS)-stimulated microglia. Brain Res. 2006, 1073–1074, 48–59. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Lu, J.; Sivakumar, V.; Ling, E.A.; Kaur, C. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Pathol. 2008, 18, 387–400. [Google Scholar] [CrossRef]
- Pan, W.H.; Kastin, A.J. Tumor necrosis factor and stroke: Role of the blood-brain barrier. Prog. Neurobiol. 2007, 83, 363–374. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhu, Y.M.; Chen, L.; Qu, Y.Y.; Zhu, Y.L. The protective effect of nordihydroguaiaretic acid on cerebral ischemia/reperfusion injury is mediated by the JNK pathway. Brain Res. 2012, 1445, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Manhas, N.; Rahubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.C.W.; Huang, H.M.; Tzen, J.T.C.; Jeng, K.C.G. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J. Neurosci. Res. 2003, 74, 123–133. [Google Scholar] [CrossRef]
- Hou, R.C.W.; Wu, C.C.; Yang, C.H.; Jeng, K.C.G. Protective effects of sesamin and sesamolin on murine BV-2 microglia cell line under hypoxia. Neurosci. Lett. 2004, 367, 10–13. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wu, X.M.; Yu, S.S.; Fauzee, N.J.S.; Wu, J.X.; Li, L.; Zhao, J.; Zhao, Y. Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol. Pharm. Bull. 2012, 35, 164–170. [Google Scholar] [CrossRef]
- Urrutia, A.; Rubio-Araiz, A.; Gutierrez-Lopez, M.D.; ElAli, A.; Hermann, D.M.; O’Shea, E.; Colado, M.I. A study on the effect of JNK inhibitor, SP600125, on the disruption of blood-brain barrier induced by methamphetamine. Neurobiol. Dis. 2013, 50, 49–58. [Google Scholar] [CrossRef]
- Cheng, H.B.; Men, Y.J.; An, Y.Q.; Yu, J.G.; Zhang, G.S.; Li, J.M.; Wang, X.L.; Sun, G.Z.; Wu, Y. Overexpression of endothelial S1pr2 promotes blood-brain barrier disruption via JNK/c-Jun/MMP-9 pathway after traumatic brain injury in both in vivo and in vitro models. Front. Pharmacol. 2024, 15, 1448570. [Google Scholar] [CrossRef]
- Nagyoszi, P.; Wilhelm, I.; Farkas, A.E.; Fazakas, C.; Dung, N.T.K.; Haskó, J.; Krizbai, I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010, 57, 556–564. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Dai, R.R.; Zhou, Y.Q.; Fu, H.; Meng, Q. TLR2 ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-κB signaling pathways in primary brain microvascular endothelial cells. Neurochem. Res. 2018, 43, 1897–1904. [Google Scholar] [CrossRef]
- Li, M.H.; Tian, X.C.; An, R.D.; Yang, M.; Zhang, Q.; Xiang, F.; Liu, H.L.; Wang, Y.C.; Xu, L.; Dong, Z. All-trans retinoic acid ameliorates the early experimental cerebral ischemia-reperfusion injury in rats by inhibiting the loss of the blood-brain barrier via the JNK/p38MAPK signaling pathway. Neurochem. Res. 2018, 43, 1283–1296. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Teng, L.; Zhang, R.; Sun, J.P.; Guo, Y.L. NRG-1β exerts neuroprotective effects against ischemia reperfusion-induced injury in rats through the JNK signaling pathway. Neuroscience 2017, 362, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Atochin, D.N.; Zanoza, S.O.; Gaidarzhy, N.M.; Lyakhov, S.A.; et al. Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Smol’yakova, V.I.; Aliev, O.I.; Anishchenko, A.M.; Ulyakhina, O.A.; Trofimova, E.S.; Ligacheva, A.A.; Anfinogenova, N.D.; Osipenko, A.N.; et al. Neuroprotective effects of tryptanthrin-6-oxime in a rat model of transient focal cerebral ischemia. Pharmaceuticals 2023, 16, 1057. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, G.A.; Smolyakova, V.I.; Plotnikov, M.B.; Aliev, O.I.; Ulyakhina, O.A.; Osipenko, A.N.; Kovrizhina, A.R.; Khlebnikov, A.I. Effect of tryptanthrin and its oxime on blood-brain barrier permeability in cerebral infarction in rats. Bull. Exp. Biol. Med. 2025, 179, 174–179. [Google Scholar] [CrossRef]
- Ritter, A.M.V.; Domiciano, T.P.; Verri, W.A.; Zarpelon, A.C.; da Silva, L.G.; Barbosa, C.P.; Natali, M.R.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef]
- Kang, P.; Kim, K.Y.; Lee, H.S.; Min, S.S.; Seol, G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013, 93, 955–961. [Google Scholar] [CrossRef]
- Lim, J.Y.; Ker, C.J.; Lai, N.M.; Romantsik, O.; Fiander, M.; Tan, K. Dexmedetomidine for analgesia and sedation in newborn infants receiving mechanical ventilation. Cochrane Database Syst. Rev. 2024, 5, CD012361. [Google Scholar] [CrossRef]
- Ye, Q.Y.; Li, X.; Gao, W.; Gao, J.Y.; Zheng, L.P.; Zhang, M.M.; Yang, F.G.; Li, H.L. Role of Rho-associated kinases and their inhibitor fasudil in neurodegenerative diseases. Front. Neurosci. 2024, 18, 1481983. [Google Scholar] [CrossRef]
- Yang, S.L.; Xu, D.K.; Zhang, D.H.; Huang, X.W.; Li, S.M.; Wang, Y.; Lu, J.; Wang, D.M.; Guo, Z.N.; Yang, Y.; et al. Levofloxacin alleviates blood-brain barrier disruption following cerebral ischemia and reperfusion via directly inhibiting A-FABP. Eur. J. Pharmacol. 2024, 963, 176275. [Google Scholar] [CrossRef]
- Wang, Y.; Law, W.K.; Hu, J.S.; Lin, H.Q.; Ip, T.M.; Wan, D.C.C. Discovery of FDA-approved drugs as inhibitors of fatty acid binding protein 4 using molecular docking screening. J. Chem. Inf. Model. 2014, 54, 3046–3050. [Google Scholar] [CrossRef]
- Meester-Smoor, M.A.; Janssen, M.; ter Haar, W.M.; van Wely, K.H.M.; Aarnoudse, A.; van Oord, G.; van Tilburg, G.B.A.; Zwarthoff, E.C. The ETS family member TEL binds to nuclear receptors RAR and RXR and represses gene activation. PLoS ONE 2011, 6, e23620. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, F.; Huang, L.J.; Zhang, Y.; Peng, Y.C.; Xing, C.H.; Feng, Y.P.; Wang, X.L.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A promising therapeutic agent for ischemic stroke. CNS Neurol. Disord. Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.C.; Zhao, W.H.; Cao, D.X.; Shi, Q.Q.; Wang, X.L. Novel neuroprotectant chiral 3-n-butylphthalide inhibits tandem-pore-domain potassium channel TREK-1. Acta Pharmacol. Sin. 2011, 32, 182–187. [Google Scholar] [CrossRef]
- Cao, H.J.; Yu, D.M.; Zhang, T.Z.; Zhou, J.; Chen, K.Y.; Ge, J.; Pei, L. Protective effect of penehyclidine hydrochloride on lipopolysaccharide-induced acute kidney injury in rat. Genet. Mol. Res. 2015, 14, 9334–9342. [Google Scholar] [CrossRef]
- Yu, C.C.; Wang, J.K. Neuroprotective effect of penehyclidine hydrochloride on focal cerebral ischemia-reperfusion injury. Neural Regen. Res. 2013, 8, 622–632. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.; Absalom, A.R. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Sato, M.; Ishii, T.; Kobayashi-Matsunaga, Y.; Amada, H.; Taniguchi, K.; Miyata, N.; Kameo, K. Discovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorganic Med. Chem. Lett. 2001, 11, 2993–2995. [Google Scholar] [CrossRef]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlowwalden, L.R. Melatonin—A highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Liao, B.Y.; Yang, S.L.; Geng, L.L.; Zong, J.Y.; Zhang, Z.X.; Jiang, M.X.; Jiang, X.; Li, S.M.; Xu, A.M.; Chang, J.L.; et al. Development of a therapeutic monoclonal antibody against circulating adipocyte fatty acid binding protein to treat ischaemic stroke. Br. J. Pharmacol. 2024, 181, 1238–1255. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. Anethole pretreatment modulates cerebral ischemia/reperfusion: The role of JNK, p38, MMP-2 and MMP-9 pathways. Pharmaceuticals 2023, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, F.F.; Yang, B.B. MicroRNA152-3p protects against ischemia/reperfusion-induced BBB destruction possibly targeting the MAP3K2/JNK/c-Jun pathway. Neurochem. Res. 2023, 48, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, D.; Lu, Z.F.; Man, J.; Zhang, Z.; Fu, X.J.; Cui, K.F.; Wang, J.P. Metformin protects against pericyte apoptosis and promotes neurogenesis through suppressing JNK p38 MAPK signalling activation in ischemia/reperfusion injury. Neurosci. Lett. 2022, 783, 136708. [Google Scholar] [CrossRef]
- Yan, R.Y.; Wang, S.J.; Yao, G.T.; Liu, Z.G.; Xiao, N. The protective effect and its mechanism of 3-n-butylphthalide pretreatment on cerebral ischemia reperfusion injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5275–5282. [Google Scholar]
- Shu, Y.; Yang, Y.; Zhang, P.B. Neuroprotective effects of penehyclidine hydrochloride against cerebral ischemia/reperfusion injury in mice. Brain Res. Bull. 2016, 121, 115–123. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, H.; Qu, Y.Y.; Xiao, X.J.; Zhu, Y.L. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014, 1544, 45–53. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Lee, W.T.; Park, K.A.; Lee, K.M.; Lee, J.E. The beneficial effect of melatonin in brain endothelial cells against oxygen-glucose deprivation followed by reperfusion-induced injury. Oxidative Med. Cell. Longev. 2014, 2014, 639531. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Lange, A.R.; Lowry, T.F.; Taheri, M.R.; Birks, E.K.; Hudetz, A.G.; Narayanan, J.; Falck, J.R.; Okamoto, H.; Roman, R.J.; et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ. Res. 2000, 87, 60–65. [Google Scholar] [CrossRef]
- Omura, T.; Tanaka, Y.; Miyata, N.; Koizumi, C.; Sakurai, T.; Fukasawa, M.; Hachiuma, K.; Minagawa, T.; Susumu, T.; Yoshida, S.; et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 2006, 37, 1307–1313. [Google Scholar] [CrossRef]
- Toth, P.; Csiszar, A.; Sosnowska, D.; Tucsek, Z.; Cseplo, P.; Springo, Z.; Tarantini, S.; Sonntag, W.E.; Ungvari, Z.; Koller, A. Treatment with the cytochrome P450 ω-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br. J. Pharmacol. 2013, 168, 1878–1888. [Google Scholar] [CrossRef]
- Poloyac, S.M.; Zhang, Y.Q.; Bies, R.R.; Kochanek, P.M.; Graham, S.H. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Renic, M.; Klaus, J.A.; Omura, T.; Kawashima, N.; Onishi, M.; Miyata, N.; Koehler, R.C.; Harder, D.R.; Roman, R.J. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2009, 29, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef]
- Tso, A.W.K.; Lam, T.K.Y.; Xu, A.; Yiu, K.H.; Tse, H.F.; Li, L.S.W.; Law, L.S.C.; Cheung, B.M.Y.; Cheung, R.T.F.; Lam, K.S.L. Serum adipocyte fatty acid-binding protein associated with ischemic stroke and early death. Neurology 2011, 76, 1968–1975. [Google Scholar] [CrossRef]
- Tu, W.J.; Zeng, X.W.; Deng, A.J.; Zhao, S.J.; Luo, D.Z.; Ma, G.Z.; Wang, H.; Liu, Q. Circulating FABP4 (Fatty Acid-Binding Protein 4) is a novel prognostic biomarker in patients with acute ischemic stroke. Stroke 2017, 48, 1531–1538. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Jiang, P.J.; Li, M.G.; Liu, Q.Y.; Cao, Y.; Wang, S. Serum fatty acid binding protein 4 is positively associated with early stroke recurrence in nondiabetic ischemic stroke. Aging 2019, 11, 1977–1989. [Google Scholar] [CrossRef]
- Hui, X.Y.; Li, H.Y.; Zhou, Z.G.; Lam, K.S.L.; Xiao, Y.; Wu, D.H.; Ding, K.; Wang, Y.; Vanhoutte, P.M.; Xu, A.M. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J. Biol. Chem. 2010, 285, 10273–10280. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Wang, X.J.; Wang, X.T.; Zhao, Y.; Wang, L.M.; Chen, Z.Y. Retinoic acid ameliorates blood-brain barrier disruption following ischemic stroke in rats. Pharmacol. Res. 2015, 99, 125–136. [Google Scholar] [CrossRef]
- Zhou, T.B.; Qin, Y.H. The potential mechanism for the different expressions of gelatinases induced by all-trans retinoic acid in different cells. J. Recept. Signal Transduct. Res. 2012, 32, 129–133. [Google Scholar] [CrossRef]
- Engelhard, K.; Werner, C.; Eberspächer, E.; Pape, M.; Stegemann, U.; Kellermann, K.; Hollweck, R.; Hutzler, P.; Kochs, E. Influence of propofol on neuronal damage and apoptotic factors after incomplete cerebral ischemia and reperfusion in rats: A long-term observation. Anesthesiology 2004, 101, 912–917. [Google Scholar] [CrossRef]
- Wang, H.Y.; Luo, M.Q.; Li, C.; Wang, G.L. Propofol post-conditioning induced long-term neuroprotection and reduced internalization of AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/reperfusion. J. Neurochem. 2011, 119, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wang, G.L.; Yu, Y.H.; Wang, Y. The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. 2009, 1297, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Bao, T.H.; Han, J.H.; Yin, M.; Zhang, J.; Yan, Y.; Zhu, Y.H. Neuroprotection induced by post-conditioning following ischemia/reperfusion in mice is associated with altered microRNA expression. Mol. Med. Rep. 2016, 14, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.X.; Hu, L.B.; Zhu, L.; Shang, L.; Wang, X.C.; Liu, N.; Wen, N.N.; Hong, Y.; Fang, D.H. Metformin attenuates neurological deficit after intracerebral hemorrhage by inhibiting apoptosis, oxidative stress and neuroinflammation in rats. Neurochem. Res. 2017, 42, 2912–2920. [Google Scholar] [CrossRef]
- Laredo, F.; Plebanski, J.; Tedeschi, A. Pericytes: Problems and promises for CNS repair. Front. Cell. Neurosci. 2019, 13, 546. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Xu, Z.F.; Croslan, D.R.; Harris, A.E.; Ford, G.D.; Ford, B.D. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J. Cereb. Blood Flow Metab. 2006, 26, 527–535. [Google Scholar] [CrossRef]
- Ege, D. Action mechanisms of curcumin in Alzheimer’s disease and its brain targeted delivery. Materials 2021, 14, 3332. [Google Scholar] [CrossRef]
- Lee, R.L.; Funk, K.E. Imaging blood-brain barrier disruption in neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1144036. [Google Scholar] [CrossRef]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef]
- Frolich, L.; Kornhuber, J.; Ihl, R.; Fritze, J.; Maurer, K.; Riederer, P. Integrity of the blood-CSF barrier in dementia of Alzheimer type: CSF/serum ratios of albumin and IgG. Eur. Arch. Psychiatry Clin. Neurosci. 1991, 240, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Su, G.C.; Arendash, G.W.; Kalaria, R.N.; Bjugstad, K.B.; Mullan, M. Intravascular infusions of soluble β-amyloid compromise the blood-brain barrier, activate CNS glial cells and induce peripheral hemorrhage. Brain Res. 1999, 818, 105–117. [Google Scholar] [CrossRef]
- Ujiie, M.; Dickstein, D.L.; Carlow, D.A.; Jefferies, W.A. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 2003, 10, 463–470. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Stewart, P.A.; Hayakawa, K.; Akers, M.A.; Vinters, H.V. A morphometric study of the blood-brain barrier in Alzheimer’s disease. Lab. Investig. 1992, 67, 734–742. [Google Scholar]
- Tai, L.M.; Holloway, K.A.; Male, D.K.; Loughlin, A.J.; Romero, I.A. Amyloid-β-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J. Cell. Mol. Med. 2010, 14, 1101–1112. [Google Scholar] [CrossRef]
- Paik, S.; Somvanshi, R.K.; Kumar, U. Somatostatin maintains permeability and integrity of blood-brain barrier in β-amyloid induced toxicity. Mol. Neurobiol. 2019, 56, 292–306. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Shin, E.J.; Kim, H.J.; Kim, C.W.; Kim, M.J.; Heo, H.J. Anti-amnesic effect of walnut via the regulation of BBB function and neuro-inflammation in Aβ1-42-induced mice. Antioxidants 2020, 9, 976. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, J.; Zhang, Z.N.; Su, Q.; Guo, J.H. The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2355–2363. [Google Scholar] [CrossRef]
- Marco, S.; Skaper, S.D. Amyloid β-peptide 1–42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci. Lett. 2006, 401, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.S.; Mamo, J.C.L. Chylomicron amyloid-beta in the aetiology of Alzheimer’s disease. Atheroscler. Suppl. 2008, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.Y.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Sousa, N.; Palha, J.A. Blood-brain-barriers in aging and in Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 38. [Google Scholar] [CrossRef]
- Hayes, G.; Pinto, J.; Sparks, S.N.; Wang, C.X.Y.; Suri, S.; Bulte, D.P. Vascular smooth muscle cell dysfunction in neurodegeneration. Front. Neurosci. 2022, 16, 1010164. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Zhdankina, A.A.; Khlebnikov, A.I.; Kolosova, N.G. JNK signaling pathway activity alterations in the rat hippocampus: Effect of age, Alzheimer’s disease-like pathology development, and the JNK Inhibitor IQ-1S. Biokhimiia 2025, 90, 265–275. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Zheng, J.B.; Town, T.; Flavell, R.A.; Selkoe, D.J. Physiological regulation of the β-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J. Neurosci. 2005, 25, 5533–5543. [Google Scholar] [CrossRef]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. β-Amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001, 21, 7551–7560. [Google Scholar] [CrossRef]
- Togo, T.; Akiyama, H.; Iseki, E.; Kondo, H.; Ikeda, K.; Kato, M.; Oda, T.; Tsuchiya, K.; Kosaka, K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002, 124, 83–92. [Google Scholar] [CrossRef]
- Liu, Y.J.; Guo, D.W.; Tian, L.; Shang, D.S.; Zhao, W.D.; Li, B.; Fang, W.G.; Zhu, L.; Chen, Y.H. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-α-dependent. Neurobiol. Aging 2010, 31, 175–188. [Google Scholar] [CrossRef]
- Fromigué, O.; Hamidouche, Z.; Marie, P.J. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J. Biol. Chem. 2008, 283, 30549–30556. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Q.; Ma, Y.F.; Wang, J.L.; Brantley-Sieders, D.; Chen, J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014, 74, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, H.; Tnani, M.; Okamoto, I.; Kenyon, L.C.; Emlet, D.R.; Holgado-Madruga, M.; Lanham, I.S.; Joynes, C.J.; Vo, K.T.; Wong, A.J. Constitutively active forms of c-Jun NH2-terminal kinase are expressed in primary glial tumors. Cancer Res. 2003, 63, 250–255. [Google Scholar]
- Cui, J.; Han, S.Y.; Wang, C.L.; Su, W.W.; Harshyne, L.; Holgado-Madruga, M.; Wong, A.J. c-Jun NH2-terminal kinase 2α2 promotes the tumorigenicity of human glioblastoma cells. Cancer Res. 2006, 66, 10024–10031. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Kenyon, L.C.; Godwin, A.K.; James, D.C.; Emlet, D.R.; Okamoto, I.; Tnani, M.; Holgado-Madruga, M.; Moscatello, D.K.; Wong, A.J. Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene 2002, 21, 5038–5046. [Google Scholar] [CrossRef]
- Chen, X.R.; Hao, A.J.; Li, X.; Ye, K.Q.; Zhao, C.G.; Yang, H.R.; Ma, H.H.; Hu, L.; Zhao, Z.Y.; Hu, L.Z.; et al. Activation of JNK and p38 MAPK mediated by ZDHHC17 drives glioblastoma multiforme development and malignant progression. Theranostics 2020, 10, 998–1015. [Google Scholar] [CrossRef]
- Matsuda, K.; Sato, A.; Okada, M.; Shibuya, K.; Seino, S.; Suzuki, K.; Watanabe, E.; Narita, Y.; Shibui, S.; Kayama, T.; et al. Targeting JNK for therapeutic depletion of stem-like glioblastoma cells. Sci. Rep. 2012, 2, 516. [Google Scholar] [CrossRef]
- Portela, M.; Venkataramani, V.; Fahey-Lozano, N.; Seco, E.; Losada-Perez, M.; Winkler, F.; Casas-Tintó, S. Glioblastoma cells vampirize WNT from neurons and trigger a JNK/MMP signaling loop that enhances glioblastoma progression and neurodegeneration. PLoS Biol. 2019, 17, e3000545. [Google Scholar] [CrossRef]
- Kose, C.; Uslu, S.; Calik-kocaturk, D.; Ozdil, B.; Aktug, H. JNK inhibition modulates the cytoskeleton, hypoxia, and neurogenesis on the protein level in glioblastoma cells and astrocytes: An immunofluorescence study. Turk. Neurosurg. 2023, 33, 982–989. [Google Scholar] [CrossRef]
- Ye, L.Y.; Sun, L.X.; Zhong, X.H.; Chen, X.S.; Hu, S.; Xu, R.R.; Zeng, X.N.; Xie, W.P.; Kong, H. The structure of blood-tumor barrier and distribution of chemotherapeutic drugs in non-small cell lung cancer brain metastases. Cancer Cell Int. 2021, 21, 556. [Google Scholar] [CrossRef]
- Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zaczynski, A.; Zawadzki, M. Breaking barriers in neuro-oncology: A scoping literature review on invasive and non-invasive techniques for blood-brain barrier disruption. Cancers 2024, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.I.; Rathi, S.; Zhang, W.Q.; Zhang, W.J.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB heterogeneity: A new paradigm for drug delivery to brain tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.C.S.; Pellerino, A.; Soffietti, R.; Ruda, R. Blood-brain barrier in brain tumors: Biology and clinical relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Sprowls, S.A.; Arsiwala, T.A.; Bumgarner, J.R.; Shah, N.; Lateef, S.S.; Kielkowski, B.N.; Lockman, P.R. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer 2019, 5, 495–505. [Google Scholar] [CrossRef]
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 2022, 12, 4734–4752. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Kim, W.; Shi, M.N.; Kiliclioglu, M.; Bayram, C.; Bolar, I.; Tozlu, Ö.; Baba, C.; Yuksel, N.; et al. Multi-tissue network analysis reveals the effect of JNK inhibition on dietary sucrose-induced metabolic dysfunction in rats. eLife 2025, 13, RP98427. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A.; Arthur, P.G. Inhibitors of c-Jun N-terminal kinases—JuNK no more? BBA Proteins Proteom. 2008, 1784, 76–93. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Karpenko, O.S.; Kovrizhina, A.R.; Kirpotina, L.N.; Khlebnikov, A.I.; Chekal, S.I.; Radudik, A.V.; Shybinska, M.O.; Quinn, M.T. Novel tryptanthrin derivatives with selectivity as c-Jun N-terminal kinase (JNK) 3 inhibitors. Molecules 2023, 28, 4806. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Jun, J.; Moon, J.; Oh, J.; Bhujbal, S.P.; Hah, J.M. Targeting JNK3 for Alzheimer’s disease: Design and synthesis of novel inhibitors with aryl group diversity utilizing wide pocket. Eur. J. Med. Chem. 2025, 285, 117209. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.C.; Chen, Q.X.; Wang, J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: Mechanisms, models, and biomarkers. Mol. Neurobiol. 2015, 52, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, V.V.; Schepetkin, I.A.; Bazan, L.V.; Gainutdinov, K.L.; Kovrizhina, A.R.; Atochin, D.N.; Khlebnikov, A.I. Evaluation of nitric oxide-donating properties of 11H-indeno[1,2-b]quinoxalin-11-one oxime (IQ-1) by electron paramagnetic resonance spectroscopy. Molecules 2024, 29, 3820. [Google Scholar] [CrossRef] [PubMed]
- Utepbergenov, D.I.; Mertsch, K.; Sporbert, A.; Tenz, K.; Paul, M.; Haseloff, R.F.; Blasig, I.E. Nitric oxide protects blood-brain barrier in vitro from hypoxia/reoxygenation-mediated injury. FEBS Lett. 1998, 424, 197–201. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.L.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Li, L.F.; Yu, L.Y.; Quinn, D.A. Ventilation-induced neutrophil infiltration depends on c-Jun N-terminal kinase. Am. J. Respir. Crit. Care Med. 2004, 169, 518–524. [Google Scholar] [CrossRef]
- Qi, L.J.; Wang, F.; Sun, X.J.; Li, H.; Zhang, K.; Li, J.A. Recent advances in tissue repair of the blood-brain barrier after stroke. J. Tissue Eng. 2024, 15, 1–25. [Google Scholar] [CrossRef]
- dela Peña, I.C.; Yoo, A.; Tajiri, N.; Acosta, S.A.; Ji, X.M.; Kaneko, Y.; Borlongan, C.V. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J. Cereb. Blood Flow Metab. 2015, 35, 338–346. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Yanase, D.; Noguchi-Shinohara, M.; Ono, K.; Yoshita, M.; Yamada, M. Blood-brain barrier permeability correlates with medial temporal lobe atrophy but not with amyloid-β protein transport across the blood-brain barrier in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2007, 23, 241–245. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Mitra, C.; Bhardwaj, D.; Burge, K.Y.; Parthasarathy, S. Alzheimer’s disease markers in aged ApoE-PON1 deficient mice. J. Alzheimer’s Dis. 2019, 67, 1353–1365. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Yi, S.Q.; Li, T.P.; Pan, X.; Wang, G.G.; Liu, X.; Li, M.; Min, J.; Le, H.H.; Tang, Z.Y. Calpeptin improves the cognitive function in Alzheimer’s disease-like complications of diabetes mellitus rats by regulating TXNIP/NLRP3 inflammasome. J. Diabetes Investig. 2024, 15, 1365–1376. [Google Scholar] [CrossRef]
- Lynch, M.; Heinen, S.; Markham-Coultes, K.; O’Reilly, M.; Van Slyke, P.; Dumont, D.J.; Hynynen, K.; Aubert, I. Vasculotide restores the blood-brain barrier after focused ultrasound-induced permeability in a mouse model of Alzheimer’s disease. Int. J. Med. Sci. 2021, 18, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Probst, G.D.; Bowers, S.; Sealy, J.M.; Truong, A.P.; Hom, R.K.; Galemmo, R.A.; Konradi, A.W.; Sham, H.L.; Quincy, D.A.; Pan, H.; et al. Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorganic Med. Chem. Lett. 2011, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Iqbal, S.; Hernandez, P.; Park, H.; LoGrasso, P.V.; Feng, Y.B. Design and synthesis of highly potent and isoform selective JNK3 inhibitors: SAR studies on aminopyrazole derivatives. J. Med. Chem. 2014, 57, 10013–10030. [Google Scholar] [CrossRef]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.H.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef]
- Shimizu, F.; Nakamori, M. Blood-brain barrier disruption in neuroimmunological disease. Int. J. Mol. Sci. 2024, 25, 10625. [Google Scholar] [CrossRef]
- Toyama, K.; Spin, J.M.; Mogi, M.; Tsao, P.S. Therapeutic perspective on vascular cognitive impairment. Pharmacol. Res. 2019, 146, 104266. [Google Scholar] [CrossRef]
- Appunni, S.; Gupta, D.; Rubens, M.; Ramamoorthy, V.; Singh, H.N.; Swarup, V. Deregulated protein kinases: Friend and foe in ischemic stroke. Mol. Neurobiol. 2021, 58, 6471–6489. [Google Scholar] [CrossRef]
| Inhibitor | Dosage | Biological Effect | Ref. |
|---|---|---|---|
| SP600125/SB203580 | 0.3 mg/0.3 mg, i.c.v. | ↓ BBB permeability, brain water content, infarct volume, neurological deficits | [111] |
| SP600125 | 1 mg/kg, i.c.v. | ↓ BBB permeability, infarct volume, neuronal apoptosis, JNK activity, p-c-Jun | [112] |
| Tryp-Ox | 10 mg/kg, i.p. | ↓ BBB permeability | [115] |
| Common Name | Chemical Structure | Chemical Class/Main Pharmacological Mechanism | Ref. |
|---|---|---|---|
| Anethol | 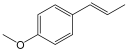 | Phenylpropanoid with antimicrobial and immunomodulate activity. | [116,117] |
| Dexmedetomidine | 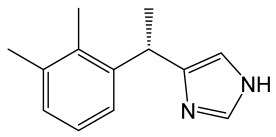 | Imidazole derivative with analgesic and sedative properties, agonist of α2-adrenergic receptors. | [118] |
| Fasudil | 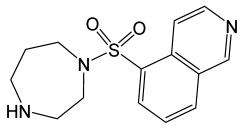 | Isoquinoline derivative, potent Rho-kinase inhibitor and vasodilator. | [119] |
| Levofloxacin | 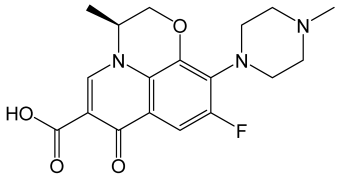 | Broad-spectrum, third-generation fluoroquinolone antibiotic used to treat bacterial infections, displayed A-FABP inhibitory activities. | [120,121] |
| All-trans retinoic acid | 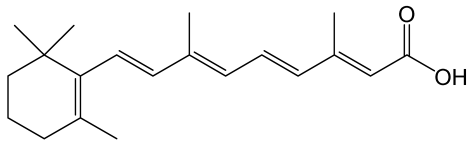 | The vitamin A derivative binds to two nuclear receptors in keratinocytes: retinoic acid receptor and retinoid X receptor. | [122] |
| 3-n-Butylphthalide | 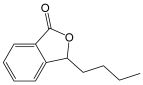 | Benzofuran derivative, an inhibitor of TWIK-related expressing K+ channel 1(TREK-1) with antioxidant, anti-inflammation, and anti-apoptosis activities. | [123,124] |
| Penehyclidine hydrochloride |  | Quinuclidine compound, an anticholinergic agent, a selective antagonist of muscarinic M1 and M3 acetylcholine receptors, with anti-inflammation and neuroprotection effects. | [125,126] |
| Propofol | 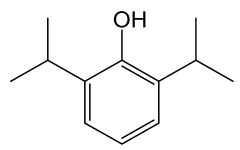 | Phenol derivate with anesthetic effect; potentiates the inhibitory effects of the neurotransmitter gamma-aminobutyric acid (GABA) by binding to and activating GABA receptors in the CNS. | [127] |
| HET0016 | 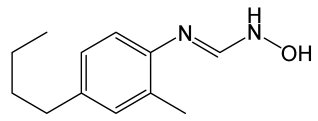 | N-hydroxy-N’-(4-butyl-2-methylphenyl)-formamidine, a potent and selective 20-HETE synthase inhibitor. | [128] |
| Melatonin | 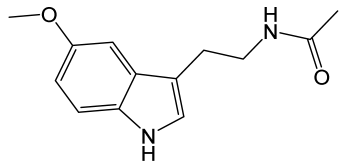 | Derivative of serotonin, a hormone, which regulates the body’s sleep–wake cycles by interacting with the suprachiasmatic nucleus of the hypothalamus and the retina. | [129] |
| Metformin | 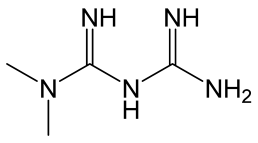 | Biguanide derivative. Reduces glucose production in the liver by inhibiting the enzyme complex I in the mitochondria with following AMPK (AMP-activated protein kinase) activation. | [130] |
| Substance | Animals (Model)/In Vitro (Cells) | Dosage/Concentration | Biological Effect | Ref. |
|---|---|---|---|---|
| Dexmedetomidine | Rats (MCAO/R) | 9 µg/kg, i.v. | ↓ BBB permeability, neuroinflammation, MMP-9 level, JNK and p38 signaling | [66] |
| Antibodies against A-FABP | Mice (MCAO/R) | 1.8 and 3.6 mg/kg | ↓ BBB permeability, cerebral edema, infarct volume, neurological deficits, mortality, MMP-9, p-JNK, p-c-Jun | [131] |
| In vitro (Mϕ) | 1 μg/mL | ↓ MMP-9 level and p-JNK | [131] | |
| A-FABP inhibitor | Mice (MCAO/R) | 15 mg/kg, p.o. | ↓ BBB permeability, cerebral edema, infarct volume, neuronal apoptosis, neurological deficits, mortality, JNK/c-Jun signaling; ↑ occludin and ZO-1 expression in brain tissue | [18] |
| Levofloxacin | Mice (MCAO/R) | 30 mg/kg, i.v. | ↓ BBB permeability, neuroinflammation, neurological deficits, mortality | [120] |
| In vitro (Mϕ) | 30 μM | ↓ A-FABP-induced JNK activity | [120] | |
| Anethole | Rats (MCAO/R) | 125 and 250 mg/kg, p.o. | ↓ BBB permeability, cerebral edema, neurological deficits, neuroinflammation, MMP-9, TNF, IL-6, IL-1β, and NF-κB levels, JNK and p38 signaling | [132] |
| miR-152-3p | Rats (MCAO/R) | 5 mL of 100 mM solution, i.v. | ↓ BBB permeability, infarct volume, neurological deficits; ↑ claudin-5 and occludin expression | [133] |
| In vitro (bEnd.3 cells) | N.S. | ↓ Apoptosis, JNK activity | [133] | |
| Fasudil | Rats (MCAO/R) | 40 mg/kg, i.v. | ↓ BBB permeability, ischemic volume, neurological deficits, neuroinflammation, MMP-9 level, JNK and p38 signaling; ↑ ZO-1 and occludin expression | [19] |
| Metformin | Mice (MCAO/R) | 200 mg/kg, i.p. | ↓ BBB permeability, cerebral edema, neurological deficits, apoptosis of pericytes, JNK and p38 activation; ↑ neoneurogenesis | [134] |
| All-trans retinoic acid | Rats (MCAO/R) | 10 and 30 mg/kg, i.p. | ↓ BBB permeability, ischemic volume, neurological deficits, degradation of TJ proteins, MMP-9 expression and activity, JNK and p38 activity | [111] |
| 3-n-Butylphthalide | Rats (MCAO/R) | 75 mg/kg, p.o. | ↓ BBB permeability, cerebral edema, infarct volume, neuronal apoptosis, ROS production, MDA, JNK and p38 activation; ↑ SOD activity | [135] |
| Penehyclidine hydrochloride | Mice (MCAO/R) | 0.1 and 1 mg/kg, i.p. | ↓ BBB permeability, brain edema, neurological deficits, infarct volume, neuronal apoptosis, ROS production, TNF, IL-1β, p-JNK, p-p38, p-c-Jun; ↑ SOD and GSH-Px activity | [136] |
| Propofol | Rats (MCAO/R) | 20–40 mg/kg/h, i.v. | ↓ BBB permeability, cerebral edema, aquaporin-4/MMP-9-positive cells, JNK activity | [20] |
| HET0016 | Rats (MCAO/R) | 1 mg/kg, i.v. | ↓ BBB permeability, MMP-9, JNK and c-Jun activation; ↑ claudin-5 and ZO-1 expression | [137] |
| Neuregulin-1β | Rats (MCAO/R) | 2 μg/kg, i.c. | ↓ BBB permeability, infarct volume, neurological deficits, neuronal apoptosis, p-MMK4, p-JNK, p-c-Jun | [112] |
| Melatonin | OGD/R (in vitro, bEnd.3 cells) | 10 and 100 nM | ↓ ROS production, p-JNK; ↑ claudin-5 expression | [138] |
| Substance | Model | Concentration/Dosage | Biological Effect | Ref. |
|---|---|---|---|---|
| Models of Alzheimer’s Disease | ||||
| SP600125 | hCMEC/D3 cells, Aβ1–40 | 50 µM | ↓ intercellular permeability in hCMEC/D3 cells↑ occludin expression | [168] |
| Somatostatin | hCMEC/D3 cells, Aβ1–42 | 0.4–10 µM | ↓ intercellular permeability in hCMEC/D3 cells; p-JNK and MMP-2 | [169] |
| J. regia L. extract | Mice, Aβ1–40 i.c.v. | 20 mg/kg | ↓ p-JNK↑ claudin-5 and ZO-1 expression | [170] |
| Models of Brain Tumor | ||||
| SP600125 | RBE4 with U87 cells (co-culture, in vitro model) | N.S. | ↑ claudin-5 and ZO-1 expression | [23] |
| Rats (glioblastoma, in vivo model) | N.S. | ↓ BBB permeability | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikov, M.B.; Anishchenko, A.M.; Khlebnikov, A.I.; Schepetkin, I.A. Regulation of Blood–Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer’s Disease and Brain Tumors. Molecules 2025, 30, 2353. https://doi.org/10.3390/molecules30112353
Plotnikov MB, Anishchenko AM, Khlebnikov AI, Schepetkin IA. Regulation of Blood–Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer’s Disease and Brain Tumors. Molecules. 2025; 30(11):2353. https://doi.org/10.3390/molecules30112353
Chicago/Turabian StylePlotnikov, Mark B., Anna M. Anishchenko, Andrei I. Khlebnikov, and Igor A. Schepetkin. 2025. "Regulation of Blood–Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer’s Disease and Brain Tumors" Molecules 30, no. 11: 2353. https://doi.org/10.3390/molecules30112353
APA StylePlotnikov, M. B., Anishchenko, A. M., Khlebnikov, A. I., & Schepetkin, I. A. (2025). Regulation of Blood–Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer’s Disease and Brain Tumors. Molecules, 30(11), 2353. https://doi.org/10.3390/molecules30112353








