Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism
Abstract
1. Introduction
2. Results and Discussion
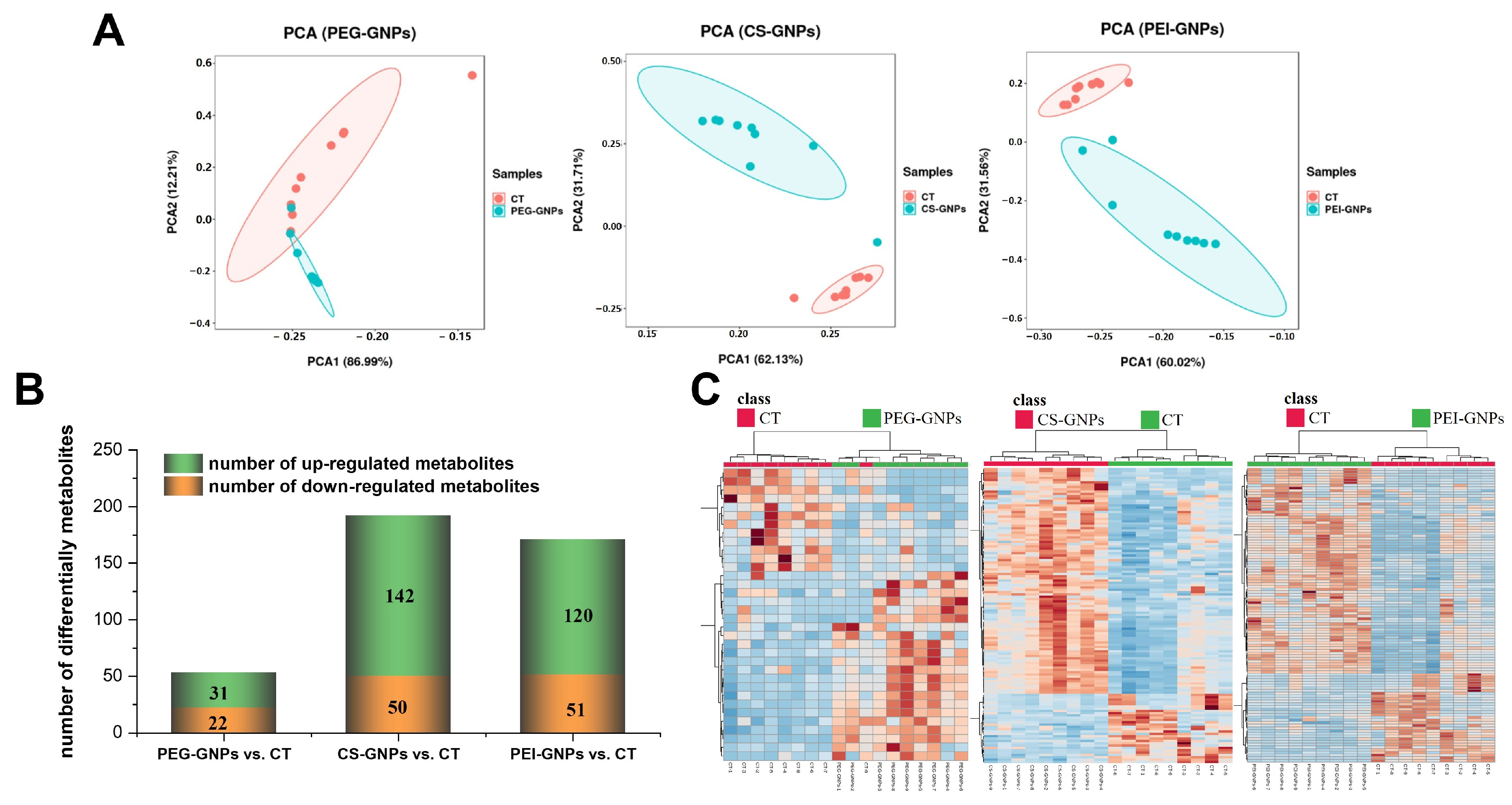
2.1. Hepatic Metabolites Changes After i.v. Injection of Functional GNPs
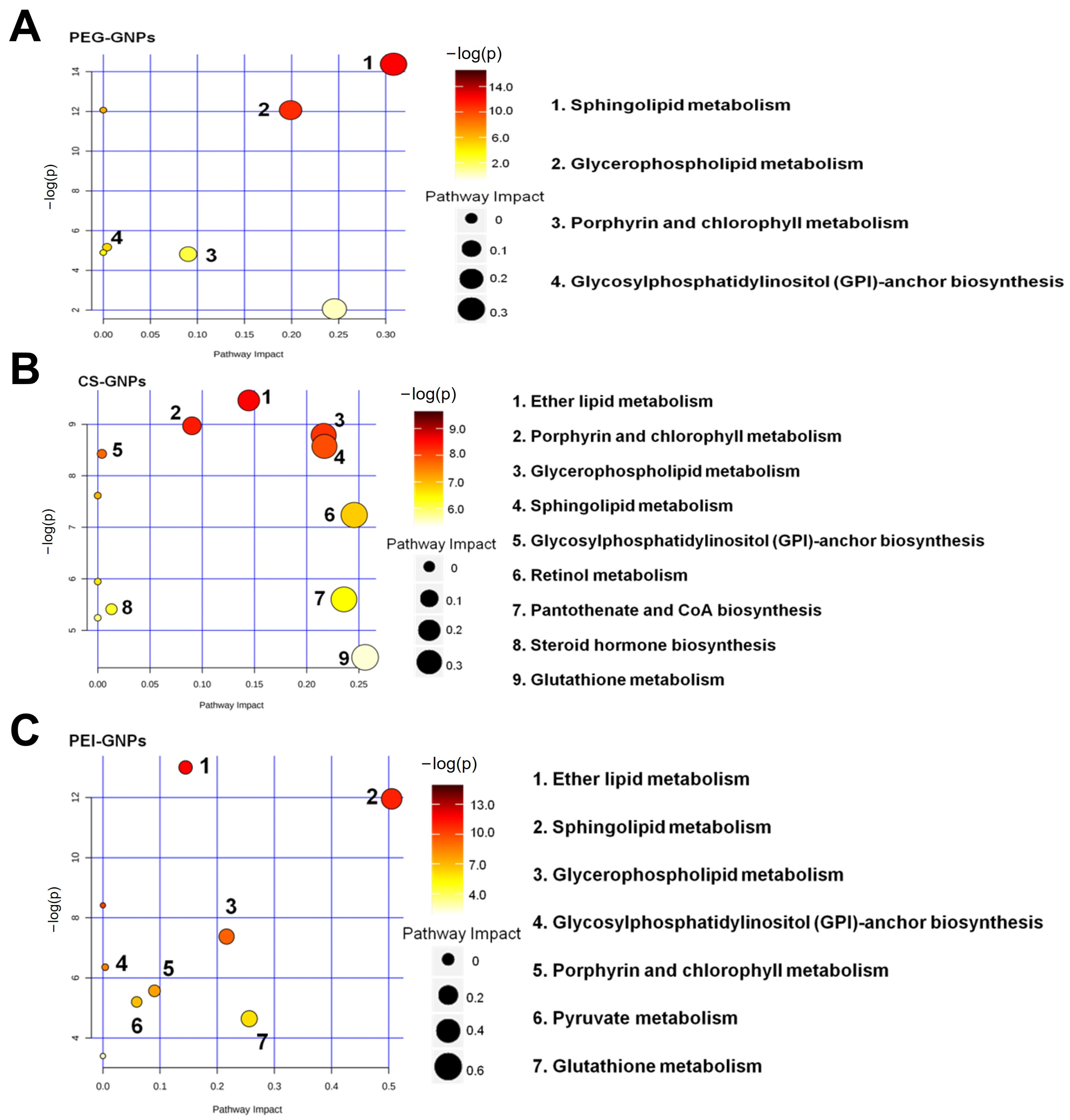
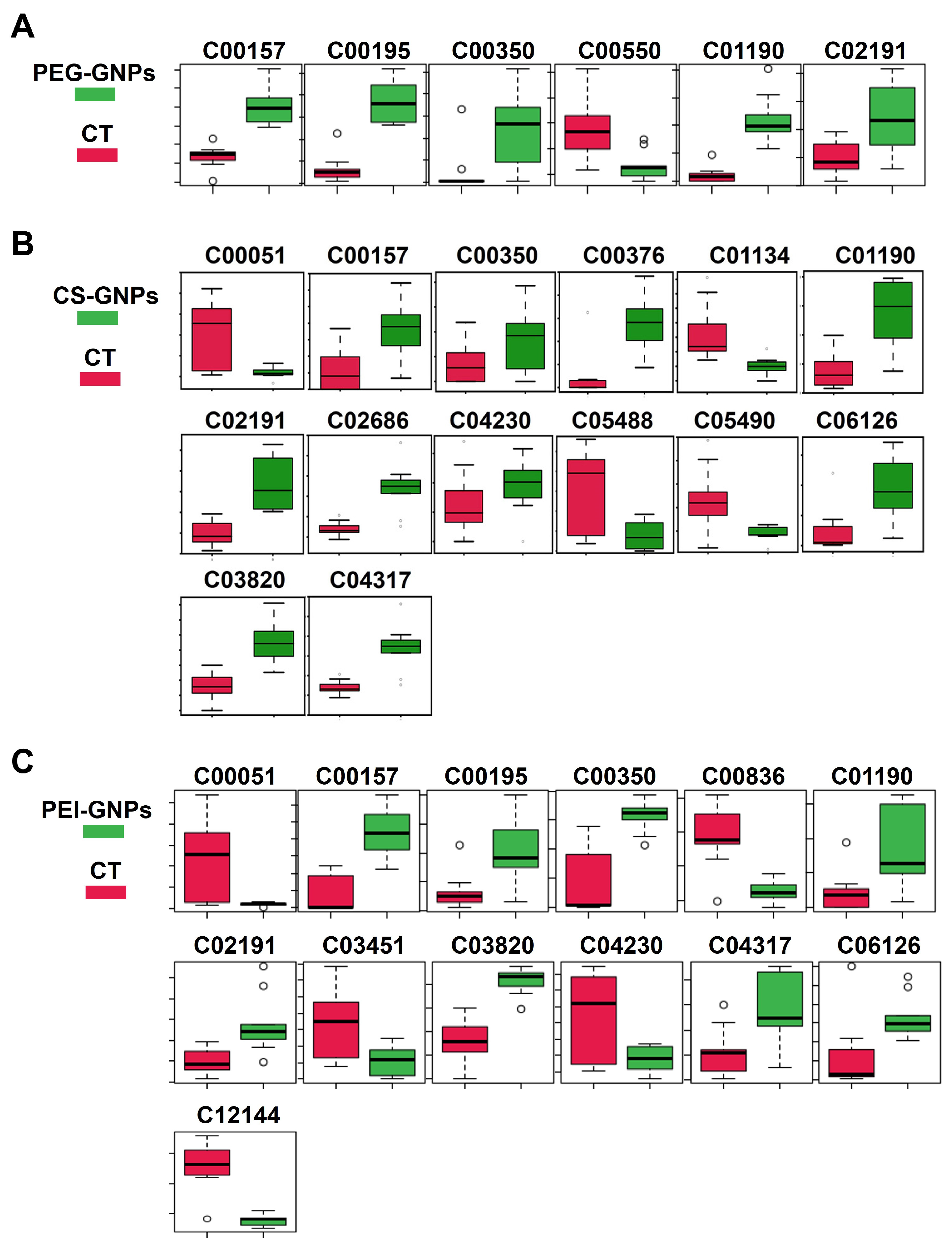
2.2. Signaling Pathway Analysis of Differentially Accumulated Metabolites
2.3. Transcriptomic and Metabolomics Integration Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Animal Experiment
3.3. Hepatic Metabolomic Analysis
3.4. Hepatic Transcriptomic Analysis
3.5. Integrated Analysis of Transcriptomic and Metabolomic: Pathway-Based Integration
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, I. Liver: Metabolic functions. Anaesth. Intensive Care Med. 2006, 7, 51–54. [Google Scholar] [CrossRef]
- Abulikemu, A.; Zhao, X.; Xu, H.; Li, Y.; Ma, R.; Yao, Q.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023, 59, 102569. [Google Scholar] [CrossRef] [PubMed]
- Ngo, W.; Ahmed, S.; Blackadar, C.; Bussin, B.; Ji, Q.; Mladjenovic, S.M.; Sepahi, Z.; Chan, W.C.W. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv. Drug Deliv. Rev. 2022, 185, 114238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnol. 2020, 18, 45. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhu, M.; Yu, H.; Chu, R.; Chen, W.; Wang, B.; Wang, M.; Zheng, L.; Chai, Z.; et al. Hepatic impacts of gold nanoparticles with different surface coatings as revealed by assessing the hepatic drug-metabolizing enzyme and lipid homeostasis in mice. NanoImpact 2020, 20, 100259. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Hariharan, K.; Patel, P.; Mehta, T. Surface modifications of gold nanoparticles: Stabilization and recent applications in cancer therapy. Pharm. Dev. Technol. 2022, 27, 665–683. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Zhu, M.; Wang, B.; Chen, W.; Zheng, L.; Wang, M.; Feng, W. Gold Nanoparticles Modified with Polyethyleneimine Disturbed the Activity of Drug-Metabolic Enzymes and Induced Inflammation-Mediated Liver Injury in Mice. Front. Pharmacol. 2021, 12, 706791. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Chen, W.; Zhu, M.; Yu, H.; Zheng, L.; Wang, B.; Wang, M.; Feng, W. PEG-GNPs aggravate MCD-induced steatohepatitic injury and liver fibrosis in mice through excessive lipid accumulation-mediated hepatic inflammatory damage. NanoImpact 2023, 31, 100469. [Google Scholar] [CrossRef]
- Wu, J.; Bai, X.; Yan, L.; Baimanov, D.; Cong, Y.; Quan, P.; Cai, R.; Guan, Y.; Bu, W.; Lin, B.; et al. Selective regulation of macrophage lipid metabolism via nanomaterials’ surface chemistry. Nat. Commun. 2024, 15, 8349. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Chen, C. The Nano–Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Acc. Chem. Res. 2019, 52, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Elci, S.G.; Jiang, Y.; Yan, B.; Kim, S.T.; Saha, K.; Moyano, D.F.; Tonga, G.Y.; Jackson, L.C.; Rotello, V.M.; Vachet, R.W. Surface Charge Controls the Suborgan Biodistributions of Gold Nanoparticles. ACS Nano 2016, 10, 5536–5542. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Y.; Al-Doaiss, A.; Alfaifi, M.; Shati, A.; Al-Kahtani, M.; Jarrar, B. The Influence of Five Metallic Nanoparticles on the Expression of Major Drug-Metabolizing Enzyme Genes with Correlation of Inflammation in Mouse Livers. Environ. Toxicol. Pharmacol. 2020, 80, 103449. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, J.; Li, J.; Xu, C.; Deng, H.; Wang, Y. An integrative imputation method based on multi-omics datasets. BMC Bioinform. 2016, 17, 247. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, M.; Bruschweiler-Li, L.; Brüschweiler, R. Nanoparticle-Assisted Removal of Protein in Human Serum for Metabolomics Studies. Anal. Chem. 2015, 88, 1003–1007. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Quintás, G.; Martínez-Sena, T.; Conde, I.; Pareja Ibars, E.; Kleinjans, J.; Castell, J.V. Metabolomic analysis to discriminate drug-induced liver injury (DILI) phenotypes. Arch. Toxicol. 2021, 95, 3049–3062. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Nunes de Paiva, M.J.; Menezes, H.C.; de Lourdes Cardeal, Z. Sampling and analysis of metabolomes in biological fluids. Anal. 2014, 139, 3683–3694. [Google Scholar] [CrossRef]
- Shaham-Niv, S.; Rencus-Lazar, S.; Gazit, E. Metabolite medicine offers a path beyond lists of metabolites. Commun. Chem. 2021, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Rao, S.; Weir, T.L.; O’Malia, J.; Bazan, M.; Brown, R.J.; Ryan, E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016, 4, 11. [Google Scholar] [CrossRef]
- Li, J.; Sun, R.; Xu, H.; Wang, G. Integrative Metabolomics, Proteomics and Transcriptomics Analysis Reveals Liver Toxicity of Mesoporous Silica Nanoparticles. Front. Pharmacol. 2022, 13, 835359. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Vinken, M. Hepatotoxicity induced by nanomaterials: Mechanisms and in vitro models. Arch. Toxicol. 2020, 95, 27–52. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef]
- Gedda, M.R.; Babele, P.K.; Zahra, K.; Madhukar, P. Epigenetic Aspects of Engineered Nanomaterials: Is the Collateral Damage Inevitable? Front. Bioeng. Biotechnol. 2019, 7, 228. [Google Scholar] [CrossRef]
- Kinaret, P.; Ilves, M.; Fortino, V.; Rydman, E.; Karisola, P.; Lähde, A.; Koivisto, J.; Jokiniemi, J.; Wolff, H.; Savolainen, K.; et al. Inhalation and Oropharyngeal Aspiration Exposure to Rod-Like Carbon Nanotubes Induce Similar Airway Inflammation and Biological Responses in Mouse Lungs. ACS Nano 2017, 11, 291–303. [Google Scholar] [CrossRef]
- Perazzo, A.; Gallier, S.; Liuzzi, R.; Guido, S.; Caserta, S. Quantitative methods to detect phospholipids at the oil-water interface. Adv. Colloid Interface Sci. 2021, 290, 102392. [Google Scholar] [CrossRef]
- Eros, G.; Ibrahim, S.; Siebert, N.; Boros, M.; Vollmar, B. Oral phosphatidylcholine pretreatment alleviates the signs of experimental rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 43. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E.; Vance, D.E. Phospholipid biosynthesis in mammalian cells. Biochem. Cell Biol. 2004, 82, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Wellner, N.; Tsuboi, K.; Madsen, A.N.; Holst, B.; Diep, T.A.; Nakao, M.; Tokumura, A.; Burns, M.P.; Deutsch, D.G.; Ueda, N.; et al. Studies on the anorectic effect of N-acylphosphatidylethanolamine and phosphatidylethanolamine in mice. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.S.d.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- Hofmanová, J.; Ciganek, M.; Slavík, J.; Kozubík, A.; Stixová, L.; Vaculová, A.; Dušek, L.; Machala, M. Lipid alterations in human colon epithelial cells induced to differentiation and/or apoptosis by butyrate and polyunsaturated fatty acids. J. Nutr. Biochem. 2012, 23, 539–548. [Google Scholar] [CrossRef]
- Lee, S.H.; Ok, S.-H.; Kim, J.-Y.; Subbarao, R.B.; Bae, S.I.; Hwang, Y.; Park, K.-E.; Kim, J.W.; Sohn, J.T. Linolenic Acid Attenuates the Vasodilation Induced by Acetylcholine in Isolated Rat Aortae. Dose-Response 2019, 17, 1559325819894148. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Edin, M.L.; Wang, Y.; Luo, Y.; Wan, D.; Yang, H.; Song, C.-Q.; Xue, W.; Sanidad, K.Z.; et al. Targeted Metabolomics Identifies the Cytochrome P450 Monooxygenase Eicosanoid Pathway as a Novel Therapeutic Target of Colon Tumorigenesis. Cancer Res. 2019, 79, 1822–1830. [Google Scholar] [CrossRef]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef]
- Zhou, R.; Horai, R.; Mattapallil, M.J.; Caspi, R.R. A New Look at Immune Privilege of the Eye: Dual Role for the Vision-Related Molecule Retinoic Acid. J. Immunol. 2011, 187, 4170–4177. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Moussa, S.A.A.; Qaid, H.A.; Al-Ayed, M.S. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int. J. Nanomed. 2018, 13, 7931–7938. [Google Scholar] [CrossRef]
- Hirofumi, S.; Naochika, D.; Toshiro, O. Current Status and Perspectives in Ceramide-Targeting Molecular Medicine. Curr. Pharm. Des. 2005, 11, 2479–2487. [Google Scholar]
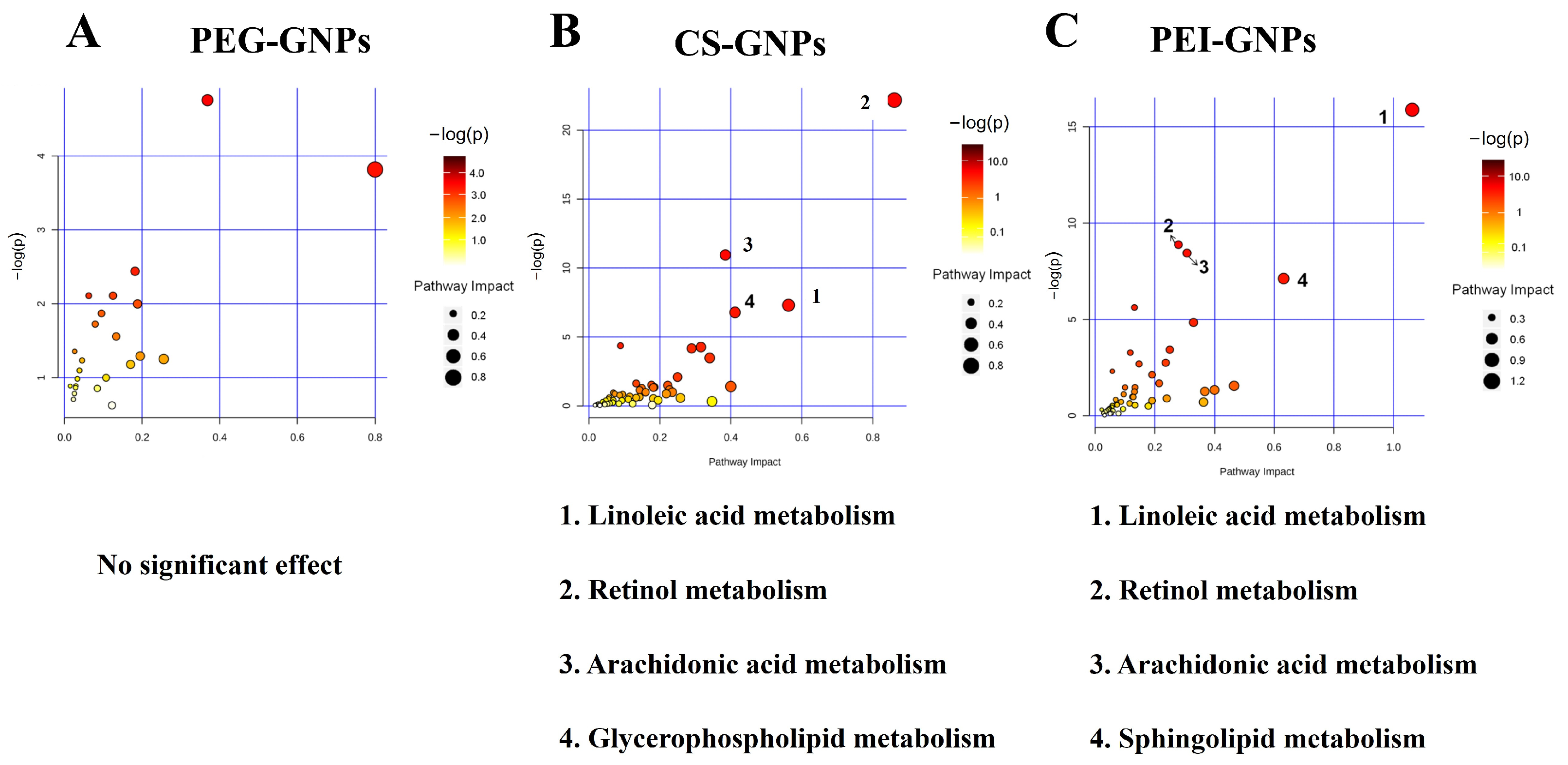

| Pathway Name | Hits | Total | p | FDR | Impact | Matched Features |
|---|---|---|---|---|---|---|
| PEG-GNPS | ||||||
| Sphingolipid metabolism | 3 | 58 | 0.008593 | 0.722 | 0.36842 | cpd:C00550; cpd:C00195; cpd:C01190 |
| Glycosphingolipid biosynthesis-globo and isoglobo series | 2 | 31 | 0.021983 | 0.923 | 0.8 | mmu:239559; mmu:26879 |
| CS-GNPs | ||||||
| Retinol metabolism | 12 | 44 | 2.34 × 10−10 | 1.97 × 10−8 | 0.86047 | cpd:C00376; mmu:11529; mmu:27400; mmu:17252; mmu:107141; mmu:13096; mmu:107605; mmu:13117; mmu:13118; mmu:13119; mmu:277753; mm:666168 |
| Arachidonic acid metabolism | 10 | 79 | 1.76 × 10−5 | 7.40 × 10−4 | 0.38462 | cpd:C00157; mmu:107141; mmu:13096; mmu:74519; mmu:13117; mmu:13118; mmu:13119; mmu:277753; mmu:666168; mmu:11684 |
| Linoleic acid metabolism | 4 | 17 | 0.000675 | 1.89 × 10−2 | 0.5625 | cpd:C00157; mmu:107141; mmu:13096; mmu:74519 |
| Glycerophospholipid metabolism | 8 | 86 | 0.001137 | 2.39 × 10−2 | 0.41176 | cpd:C00350; cpd:C00157; cpd:C04230; mmu:212862; mmu:64898; mmu:104759; mmu:18777; mmu:71760 |
| PEI-GNPS | ||||||
| Linoleic acid metabolism | 7 | 17 | 1.26 × 10−7 | 1.06 × 10−5 | 1.0625 | cpd:C00157; mmu:13097; mmu:13098; mmu:13099; mmu:74519; mmu:13106; mmu:337924 |
| Retinol metabolism | 7 | 44 | 0.000139 | 5.84 × 10−3 | 0.27907 | mmu:337924; mmu:13088; mmu:13089; mmu:13094; mmu:13097; mmu:13098; mmu:13099 |
| Arachidonic acid metabolism | 9 | 79 | 0.000215 | 6.02 × 10−3 | 0.30769 | cpd:C00157; mmu:13088; mmu:13089; mmu:13094; mmu:13097; mmu:13098; mmu:13099; mmu:74519; mmu:13106 |
| Sphingolipid metabolism | 7 | 58 | 0.000807 | 1.69 × 10−2 | 0.63158 | cpd:C00836; cpd:C00195; cpd:C06126; cpd:C01190; cpd:C12144; mmu:58994; mmu:20773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Liang, S.; Zhang, T.; Zhang, M.; Fang, H.; Tian, J.; Liu, J.; Peng, Y.; Zheng, L.; Wang, B.; et al. Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism. Molecules 2025, 30, 1727. https://doi.org/10.3390/molecules30081727
Ding X, Liang S, Zhang T, Zhang M, Fang H, Tian J, Liu J, Peng Y, Zheng L, Wang B, et al. Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism. Molecules. 2025; 30(8):1727. https://doi.org/10.3390/molecules30081727
Chicago/Turabian StyleDing, Xinyu, Shanshan Liang, Tingfeng Zhang, Minglu Zhang, Hao Fang, Jiale Tian, Jinke Liu, Yuyuan Peng, Lingna Zheng, Bing Wang, and et al. 2025. "Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism" Molecules 30, no. 8: 1727. https://doi.org/10.3390/molecules30081727
APA StyleDing, X., Liang, S., Zhang, T., Zhang, M., Fang, H., Tian, J., Liu, J., Peng, Y., Zheng, L., Wang, B., & Feng, W. (2025). Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism. Molecules, 30(8), 1727. https://doi.org/10.3390/molecules30081727






