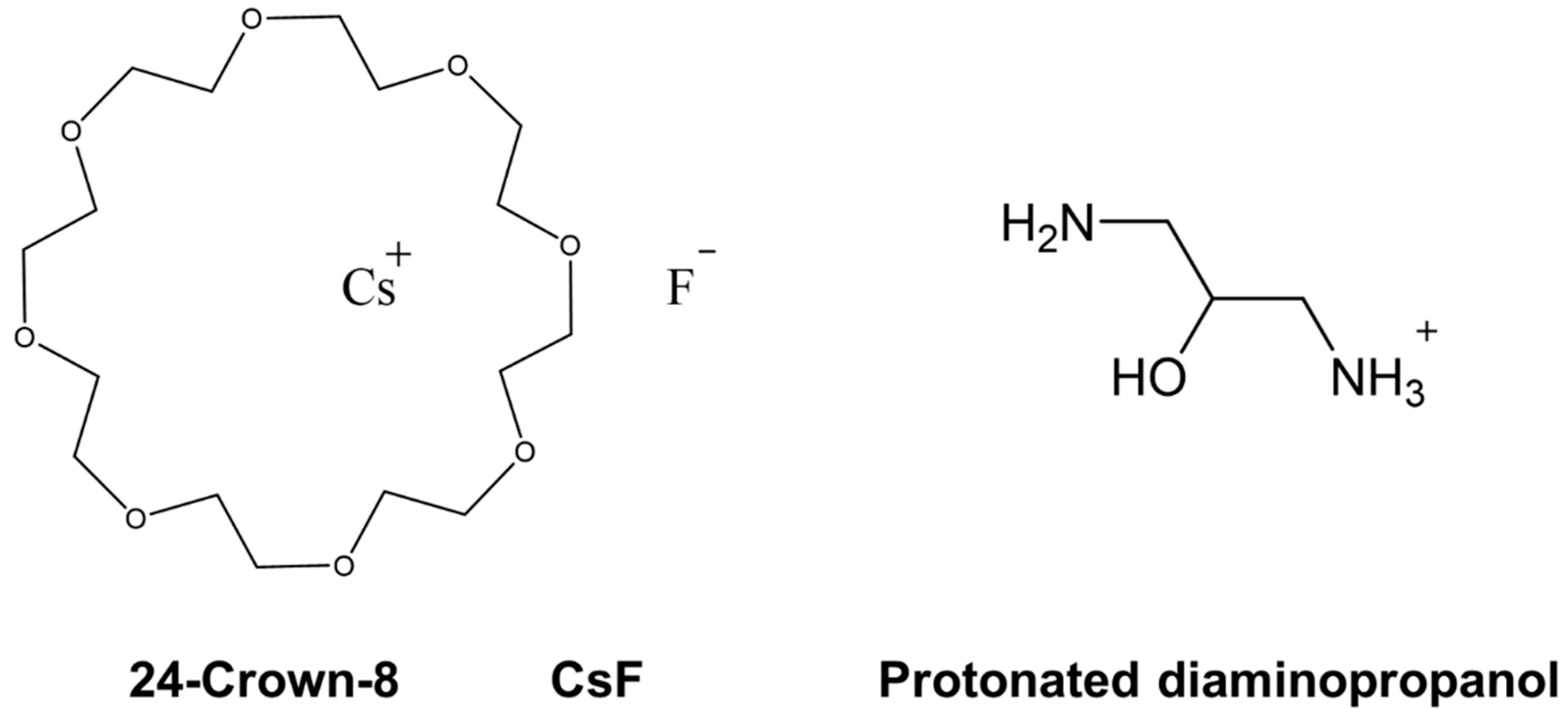
Delineating Host–Guest–Solvent Interactions in Solution from Gas-Phase Host–Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase
Abstract
1. Introduction
2. Results
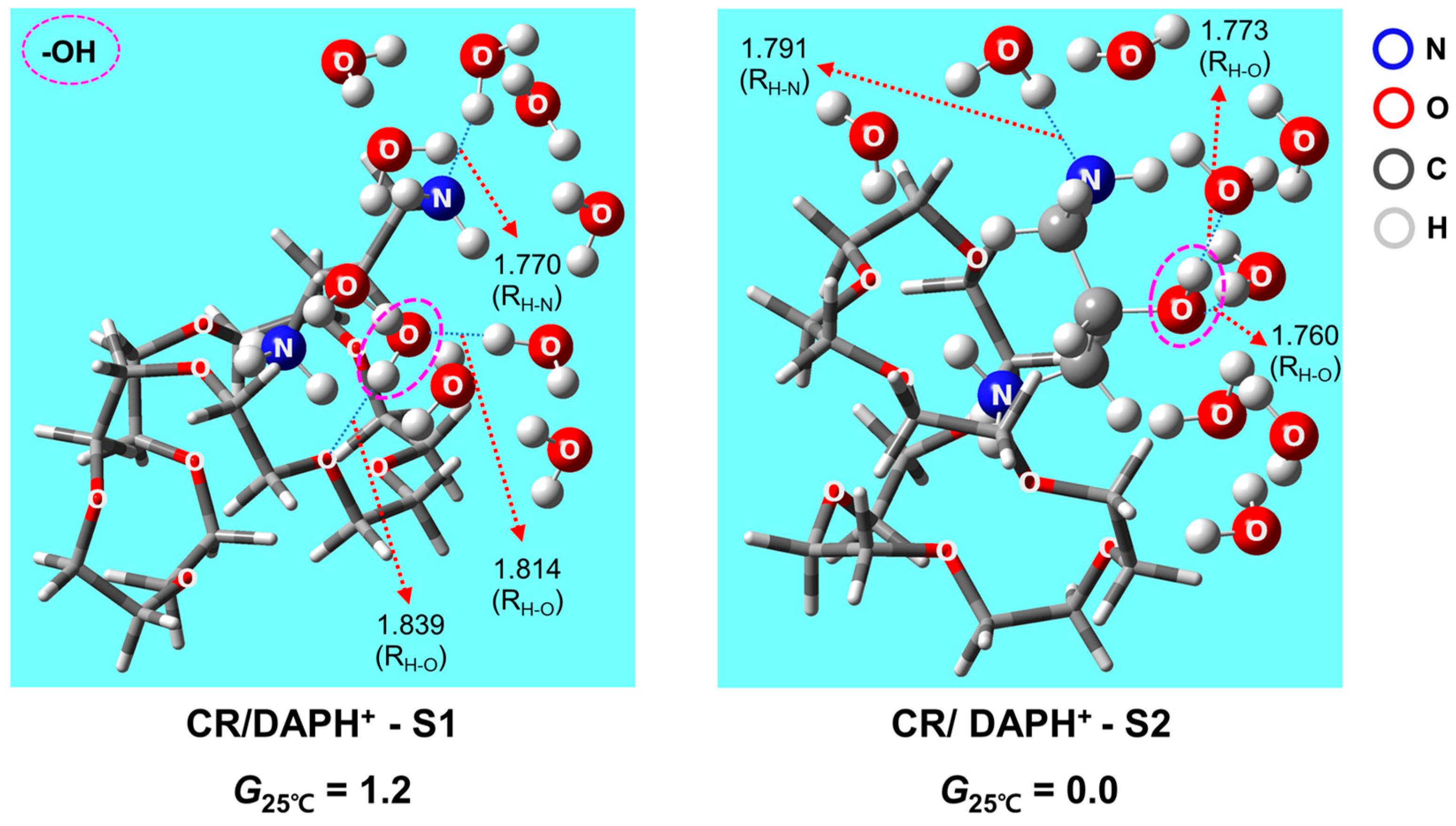
2.1. Structures and Thermodynamic Stability of CR/DAPH+ Non-Covalent Complex in Aqueous Solution
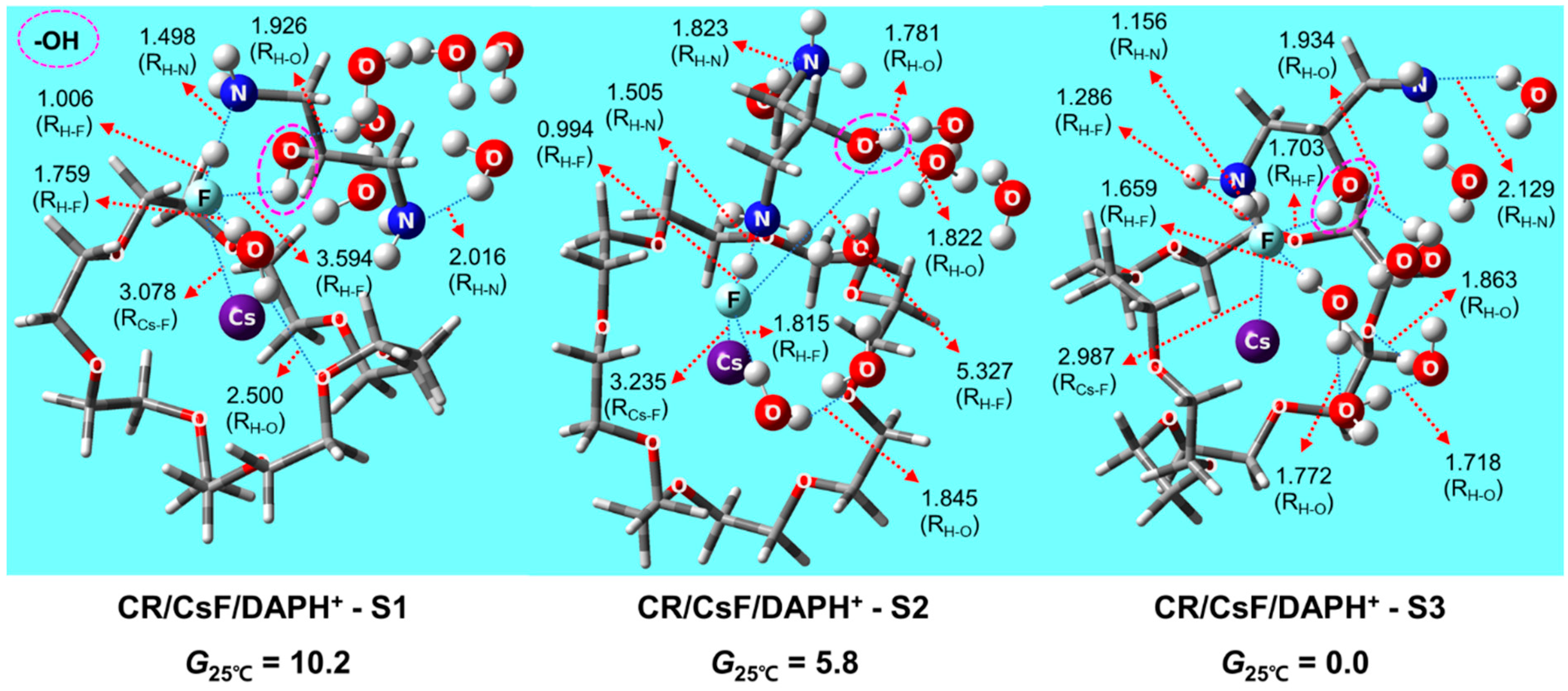
2.2. Structures and Thermodynamic Stability of CR/CsF/DAPHCl in Aqueous Solution
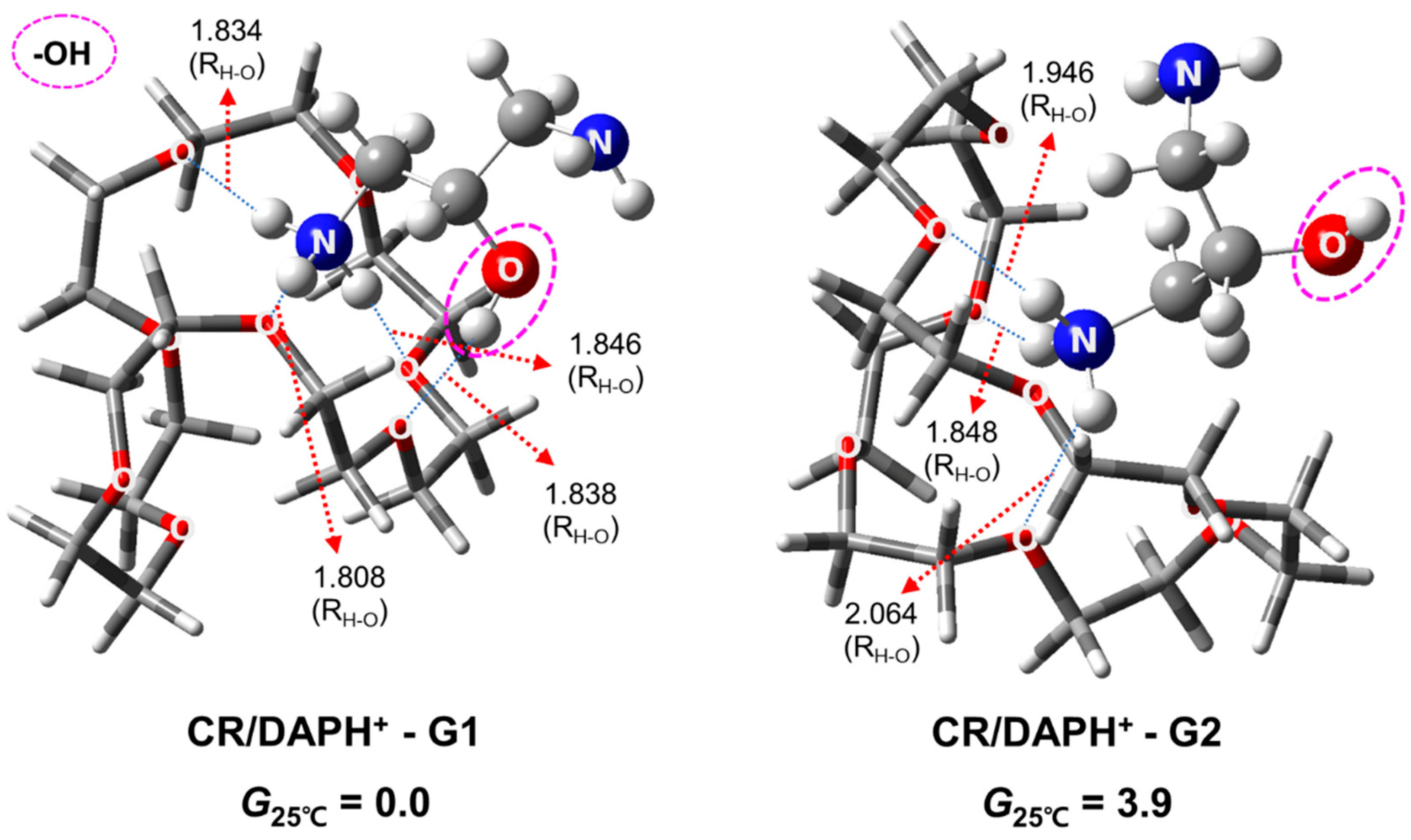
2.3. Structures and Relative Gibbs Free Energy of CR/DAPH+ in the Gas Phase
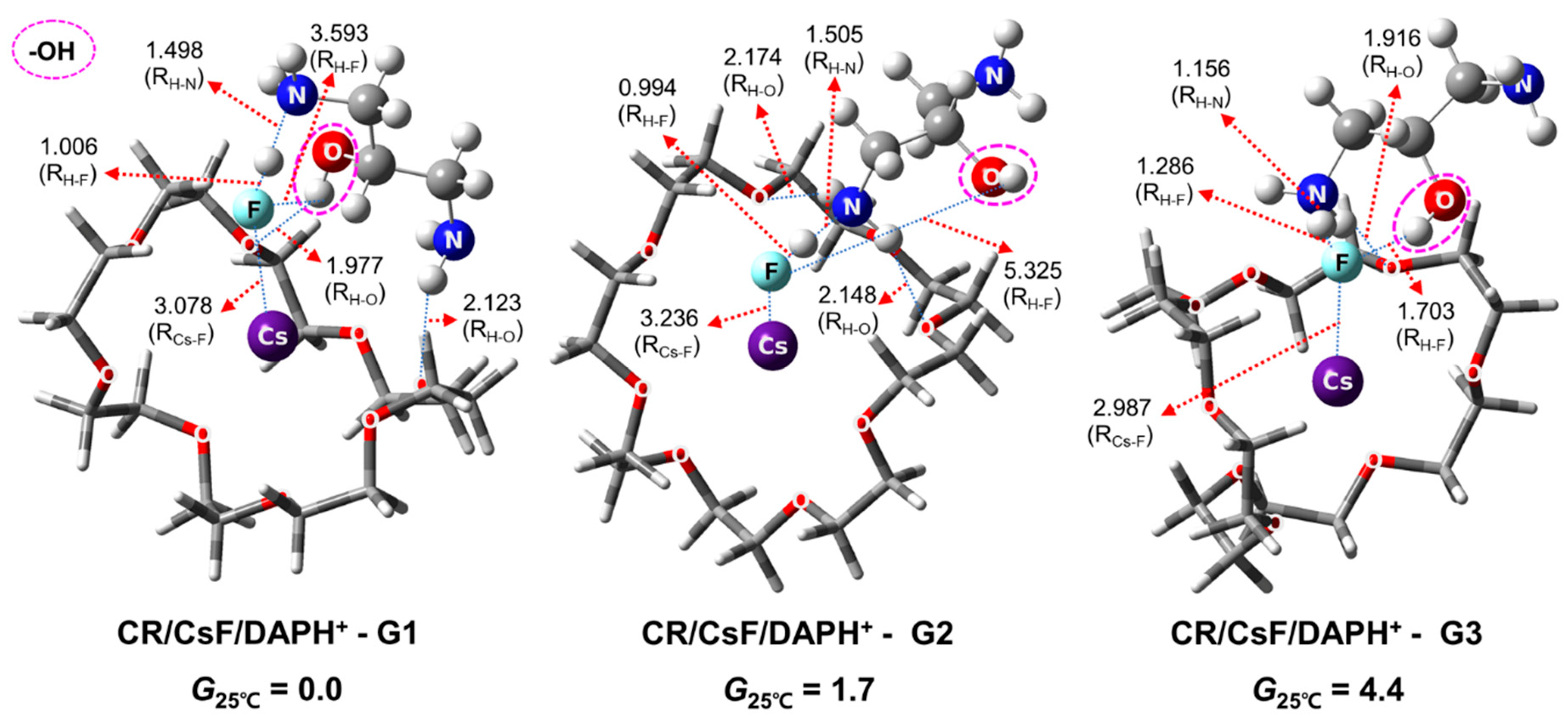
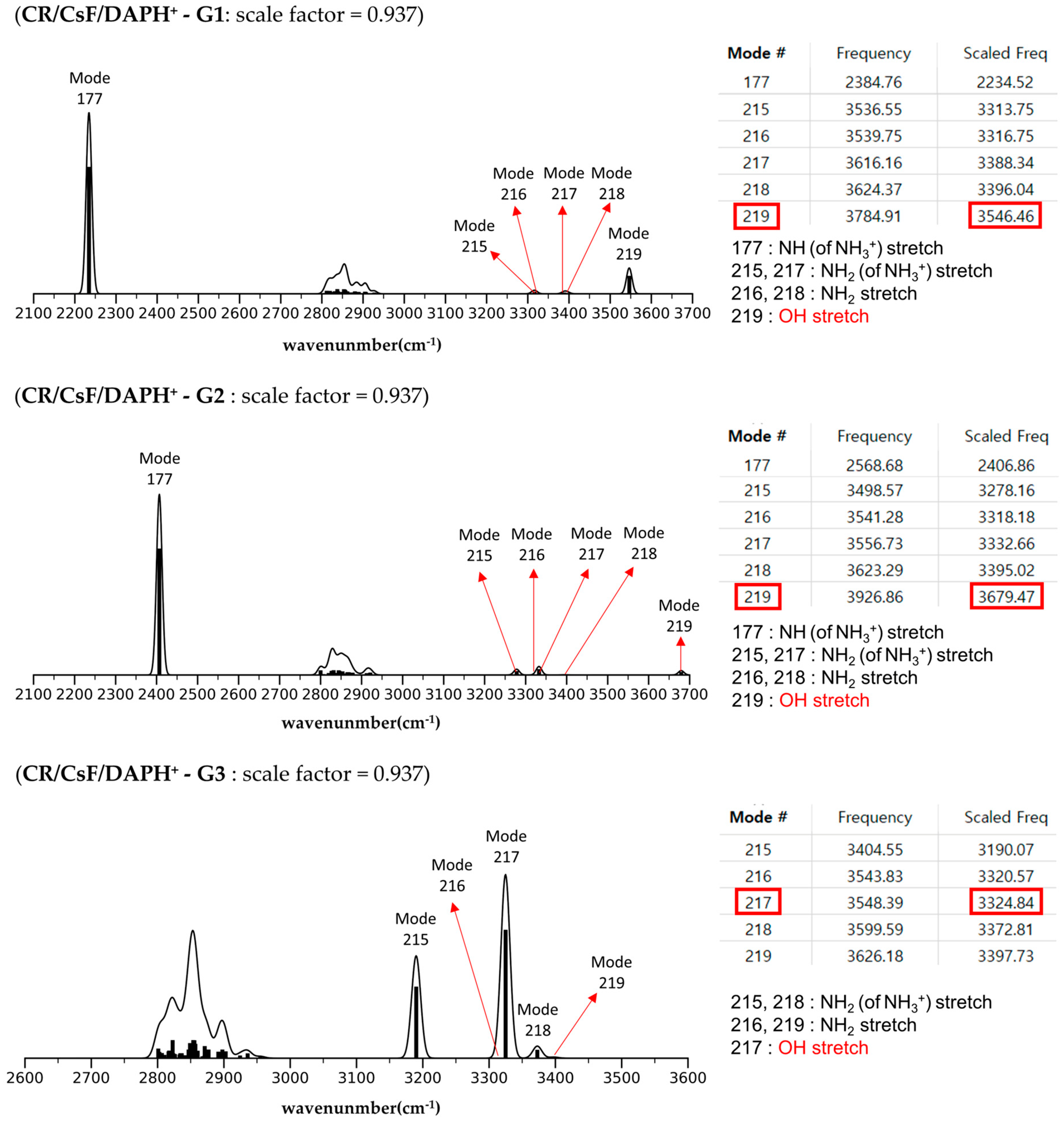
2.4. Structures and Relative Gibbs Free Energy of CR/CsF/DAPH+ in the Gas Phase
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry: Complexes between organic compounds simulate the substrate selectivity of enzymes. Science 1974, 183, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular chemistry at interfaces: Host–guest interactions for fabricating multifunctional biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Schalley, C.A. Supramolecular chemistry goes gas phase: The mass spectrometric examination of noncovalent interactions in host–guest chemistry and molecular recognition. Int. J. Mass Spectrom. 2000, 194, 11–39. [Google Scholar] [CrossRef]
- Ortega-Caballero, F.; Rousseau, C.; Christensen, B.; Petersen, T.E.; Bols, M. Remarkable supramolecular catalysis of glycoside hydrolysis by a cyclodextrin cyanohydrin. J. Am. Chem. Soc. 2005, 127, 3238–3239. [Google Scholar] [CrossRef]
- Jiao, D.; Geng, J.; Loh, X.J.; Das, D.; Lee, T.; Scherman, O.A. Supramolecular peptide amphiphile vesicles through host–guest complexation. Angew. Chem. Int. Ed. 2012, 51, 9633–9637. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.-H.; Li, L.; Luo, Y.-L.; Xu, F.; Chen, Y. Dual Stimuli-Responsive Supramolecular Self-Assemblies Based on the Host–Guest Interaction Between β-Cyclodextrin and Azobenzene for Cellular Drug Release. Mol. Pharm. 2020, 17, 1100–1113. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive host–guest functional systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Kebarle, P.; Verkerk, U.H. Electrospray: From ions in solution to ions in the gas phase, what we know now. Mass Spectrom. Rev. 2009, 28, 898–917. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Barbu-Debus, K.L.; Scuderi, D.; Zehnecker-Rentien, A. Mass Spectrometry Study and Infrared Spectroscopy of the Complex Between Camphor and the Two Enantiomers of Protonated Alanine: The Role of Higher-Energy Conformers in the Enantioselectivity of the Dissociation Rate Constants. Chirality 2013, 25, 436–443. [Google Scholar] [CrossRef]
- Heo, K.S.; Ryoo, J.J. Synthesis and Application of New Calix[4]Arenes-containing (R)-phenylglycinol Chiral Stationary Phases for Enantioseparation. Bull. Korean Chem. Soc. 2020, 41, 753–758. [Google Scholar] [CrossRef]
- Shahgaldian, P.; Pieles, U. Cyclodextrin derivatives as chiral supramolecular receptors for enantioselective sensing. Sensors 2006, 6, 593–615. [Google Scholar] [CrossRef]
- Avilés-Moreno, J.R.; Quesada-Moreno, M.M.; López-González, J.J.; Martínez-Haya, B. Chiral Recognition of Amino Acid Enantiomers by a Crown Ether: Chiroptical IR-VCD Response and Computational Study. J. Phys. Chem. B 2013, 117, 9362–9370. [Google Scholar] [CrossRef]
- Thomas, M.C.; Mitchell, T.W.; Harman, D.G.; Deeley, J.M.; Murphy, R.C.; Blanksby, S.J. Elucidation of double bond position in unsaturated lipids by ozone electrospray ionization mass spectrometry. Anal. Chem. 2007, 79, 5013–5022. [Google Scholar] [CrossRef]
- Yeni, O.; Ollivier, S.; Moge, B.; Ropartz, D.; Rogniaux, H.; Legentil, L.; Ferrières, V.; Compagnon, I. Ring-Size Memory of Galactose-Containing MS/MS Fragments: Application to the Detection of Galactofuranose in Oligosaccharides and Their Sequencing. J. Am. Chem. Soc. 2023, 145, 15180–15187. [Google Scholar] [CrossRef]
- Oomens, J.; Sartakov, B.G.; Meijer, G.; von Helden, G. Gas-phase infrared multiple photon dissociation spectroscopy of mass-selected molecular ions. Int. J. Mass Spectrom. 2006, 254, 1–19. [Google Scholar] [CrossRef]
- Bush, M.F.; O’Brien, J.T.; Prell, J.S.; Saykally, R.J.; Williams, E.R. Infrared spectroscopy of cationized arginine in the gas phase: Direct evidence for the transition from nonzwitterionic to zwitterionic structure. J. Am. Chem. Soc. 2007, 129, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Azargun, M.; Fridgen, T.D. Guanine tetrads: An IRMPD spectroscopy, energy resolved SORI-CID, and computational study of M (9-ethylguanine)4+(M= Li, Na, K, Rb, Cs) in the gas phase. Phys. Chem. Chem. Phys. 2015, 17, 25778–25785. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Oh, Y.-H.; Lee, S.; Kong, X. Distinguishing Gas Phase Lactose and Lactulose Complexed with Sodiated L-Arginine by IRMPD Spectroscopy and DFT Calculations. Phys. Chem. Chem. Phys. 2023, 25, 25116–25121. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Steill, J.D.; Oomens, J.; Jockusch, R.A. Infrared multiple photon dissociation action spectroscopy and computational studies of mass-selected gas-phase fluorescein and 2′,7′-dichlorofluorescein ions. J. Phys. Chem. A 2011, 115, 9739–9747. [Google Scholar] [CrossRef]
- Cheng, R.; Loire, E.; Fridgen, T.D. Hydrogen bonding in alkali metal cation-bound i-motif-like dimers of 1-methyl cytosine: An IRMPD spectroscopic and computational study. Phys. Chem. Chem. Phys. 2019, 21, 11103–11110.24. [Google Scholar] [CrossRef]
- Seo, J.; Hoffmann, W.; Warnke, S.; Bowers, M.T.; Pagel, K.; von Helden, G. Retention of native protein structures in the absence of solvent: A coupled ion mobility and spectroscopic study. Angew. Chem. Int. Ed. 2016, 55, 14173–14176. [Google Scholar] [CrossRef]
- Bush, M.F.; Forbes, M.W.; Jockusch, R.A.; Oomens, J.; Polfer, N.C.; Saykally, R.J.; Williams, E.R. Infrared spectroscopy of cationized lysine and ε-N-methyllysine in the gas phase: Effects of alkali-metal ion size and proton affinity on zwitterion stability. J. Phys. Chem. A 2007, 111, 7753–7760. [Google Scholar] [CrossRef]
- Burt, M.; Wilson, K.; Marta, R.; Hasan, M.; Hopkins, W.S.; McMahon, T. Assessing the impact of anion–π effects on phenylalanine ion structures using IRMPD spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 24223–24234. [Google Scholar] [CrossRef]
- Lee, S.-S.; Park, S.; Hong, Y.; Lee, J.U.; Kim, J.H.; Yoon, D.; Kong, X.; Lee, S.; Oh, H.B. Chiral differentiation of d- and l-alanine by permethylated β-cyclodextrin: IRMPD spectroscopy and DFT methods. Phys. Chem. Chem. Phys. 2017, 19, 14729–14737. [Google Scholar] [CrossRef]
- Lee, S.-S.; Lee, J.U.; Oh, J.H.; Park, S.; Hong, Y.; Min, B.K.; Lee, H.H.L.; Kim, H.I.; Kong, X.; Lee, S.; et al. Chiral differentiation of D- and L-isoleucine using permethylated β-cyclodextrin: Infrared multiple photon dissociation spectroscopy, ion-mobility mass spectrometry, and DFT calculations. Phys. Chem. Chem. Phys. 2018, 20, 30428–30436. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Lee, S.Y.; Kong, X.; Oh, H.B.; Lee, S. Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown-8/Protonated Serine Non-Covalent Complexes in the Gas Phase vs. in Solution: Quantum Chemical Analysis. ACS Omega 2024, 9, 23793–23801. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Oh, Y.-H.; Park, S.; Lee, S.-S.; Oh, H.B.; Lee, S. Unveiling Host-Guest-Solvent Interactions in Solution by Identifying Highly Unstable Host-Guest Configurations in Thermal Non-Equilibrium Gas Phase. Sci. Rep. 2022, 27, 5702. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.G.; Oh, Y.-H.; Park, T.H.; Lee, S.-S.; Kim, D.W.; Lee, S. Contact ion-pair SN2 reactions activated by Lewis Base Phase transfer catalysts. Nat. Commun. 2025, 16, 1236. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Stedwell, C.N.; Galindo, J.F.; Gulyuz, K.; Roitberg, A.E.; Polfer, N.C. Crown Complexation of Protonated Amino Acids: Influence on IRMPD Spectra. J. Phys. Chem. A 2013, 117, 1181–1188. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.-H.; Lee, S.Y.; Oh, H.B.; Lee, S. Delineating Host–Guest–Solvent Interactions in Solution from Gas-Phase Host–Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase. Molecules 2025, 30, 1723. https://doi.org/10.3390/molecules30081723
Oh Y-H, Lee SY, Oh HB, Lee S. Delineating Host–Guest–Solvent Interactions in Solution from Gas-Phase Host–Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase. Molecules. 2025; 30(8):1723. https://doi.org/10.3390/molecules30081723
Chicago/Turabian StyleOh, Young-Ho, So Yeon Lee, Han Bin Oh, and Sungyul Lee. 2025. "Delineating Host–Guest–Solvent Interactions in Solution from Gas-Phase Host–Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase" Molecules 30, no. 8: 1723. https://doi.org/10.3390/molecules30081723
APA StyleOh, Y.-H., Lee, S. Y., Oh, H. B., & Lee, S. (2025). Delineating Host–Guest–Solvent Interactions in Solution from Gas-Phase Host–Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase. Molecules, 30(8), 1723. https://doi.org/10.3390/molecules30081723







