Challenges and Opportunities in Hydrometallurgical Recovery of Germanium from Coal By-Products
Abstract
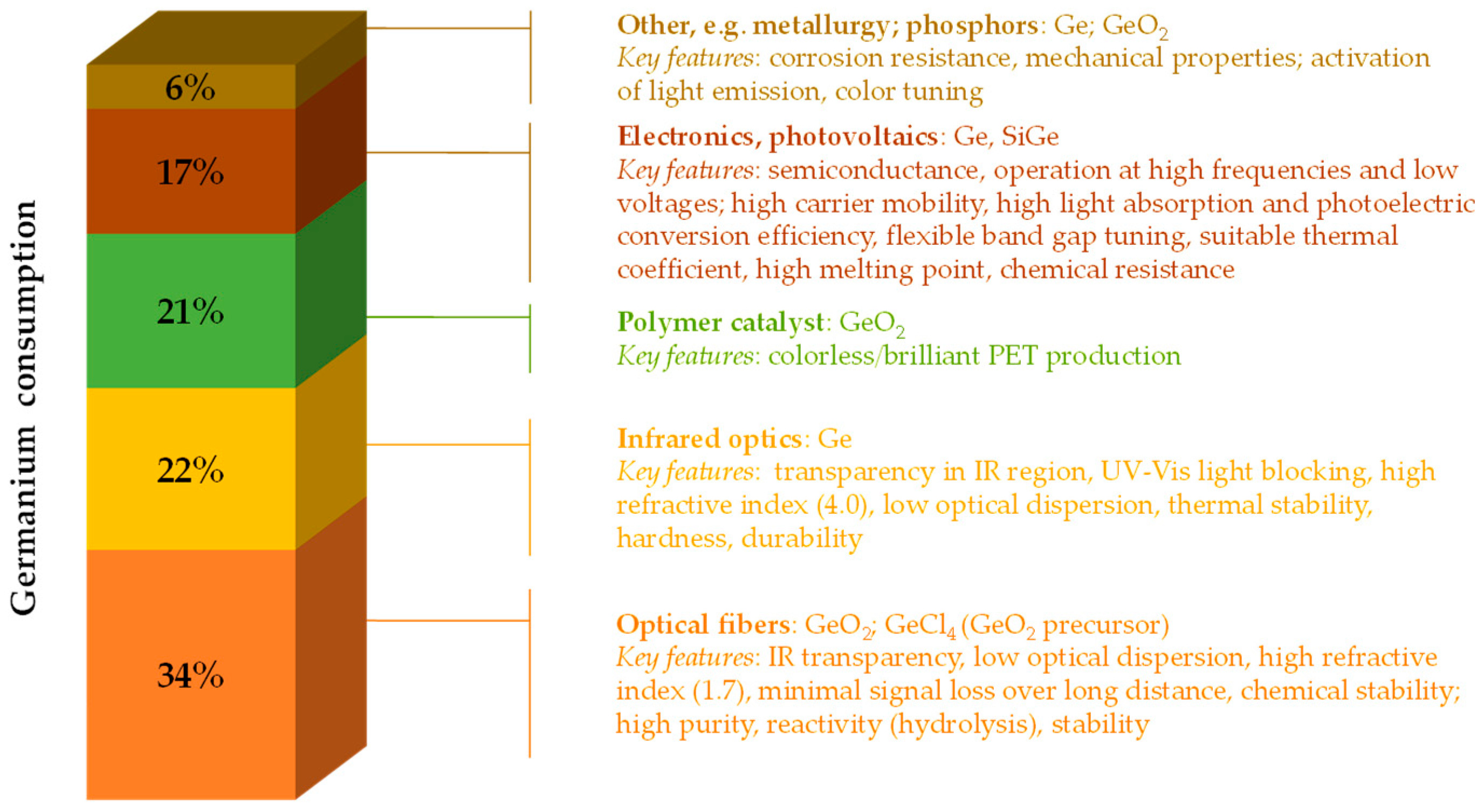
1. Introduction
2. Germanium in Coals
2.1. Global Reserves
2.2. Cut-Off Grade
2.3. Concentration
2.4. Occurrence Modes
2.5. Germanium Recovery
3. Germanium in Coal Gangue
4. Germanium in Coal Combustion Ashes
4.1. Concentration
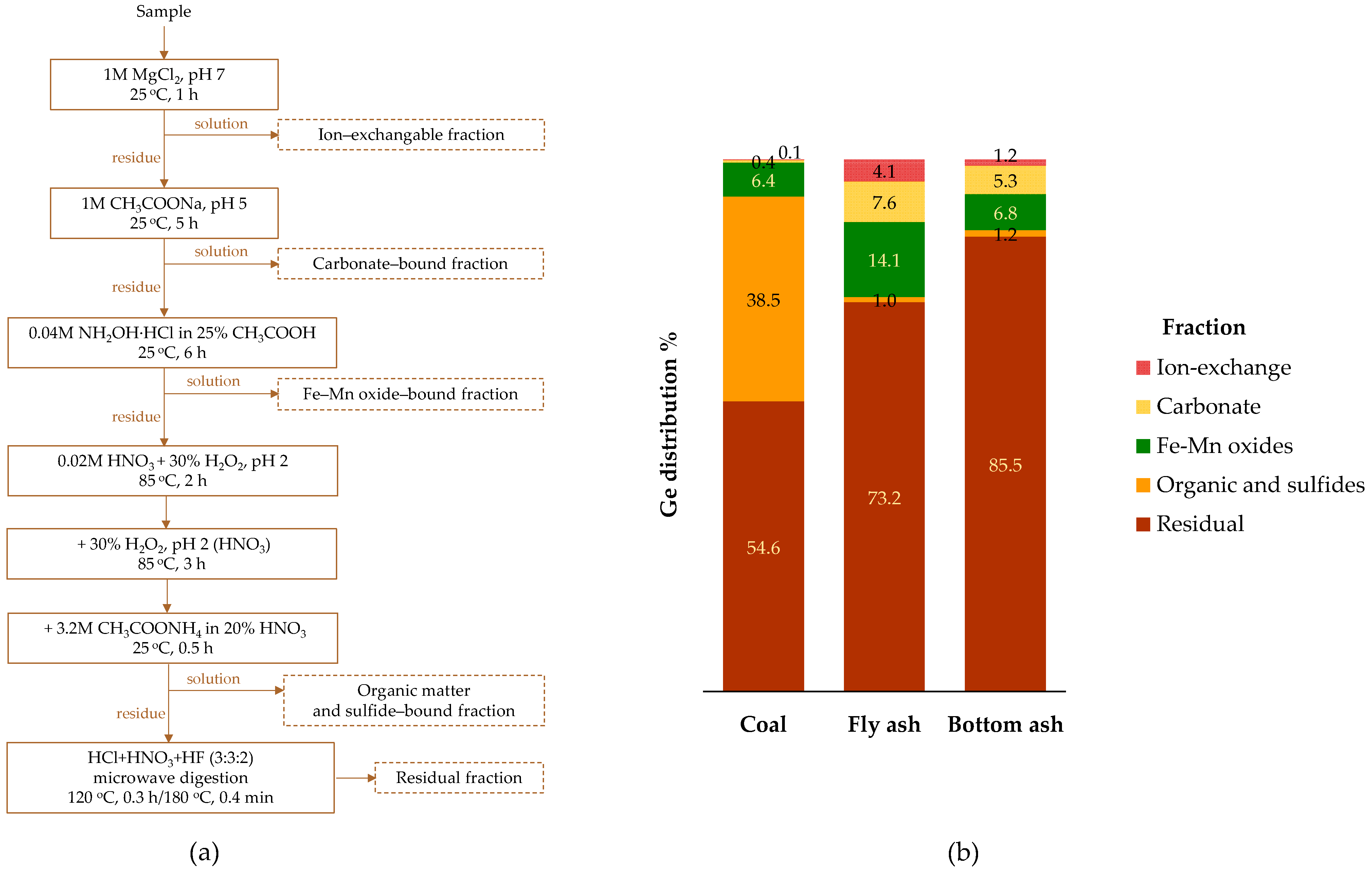
4.2. Occurrence Modes
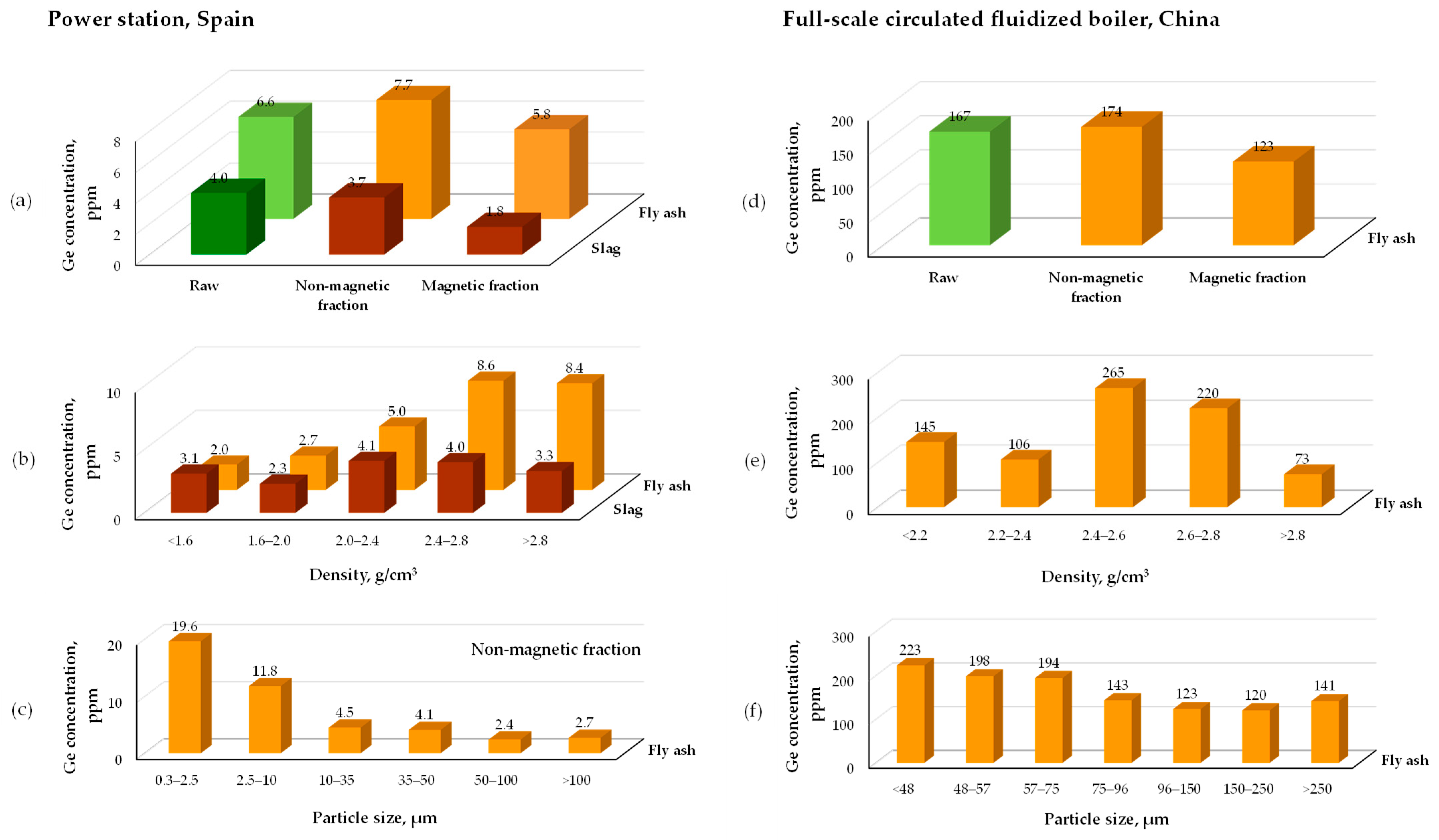
4.3. Physical Preconcentration
4.4. Industrial Recovery
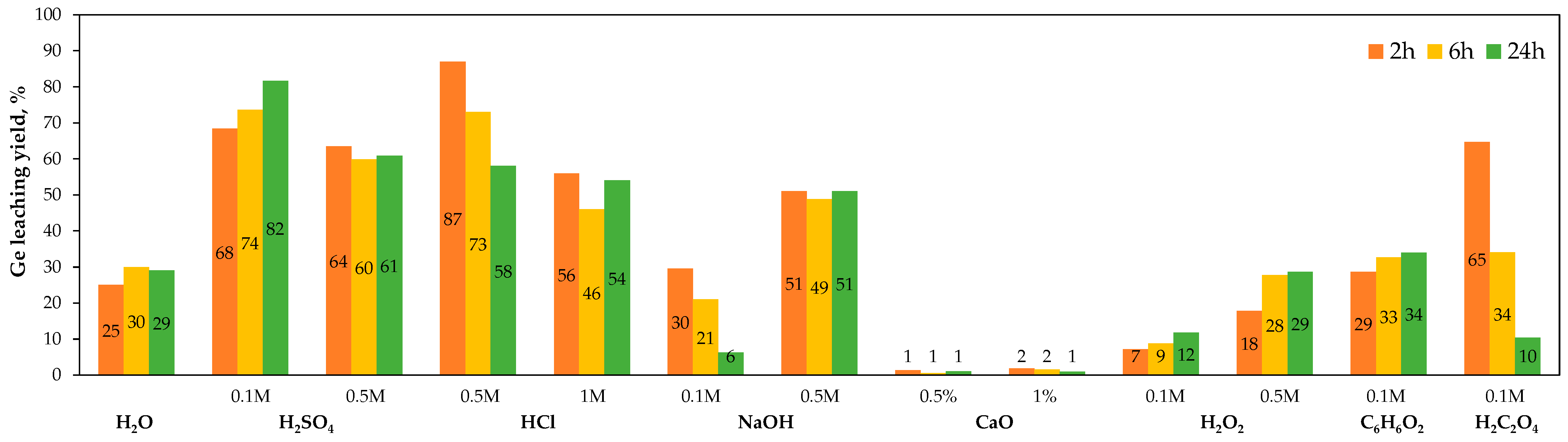
4.5. Laboratory Developments
5. Germanium in Coal Gasification Ashes
5.1. Concentration
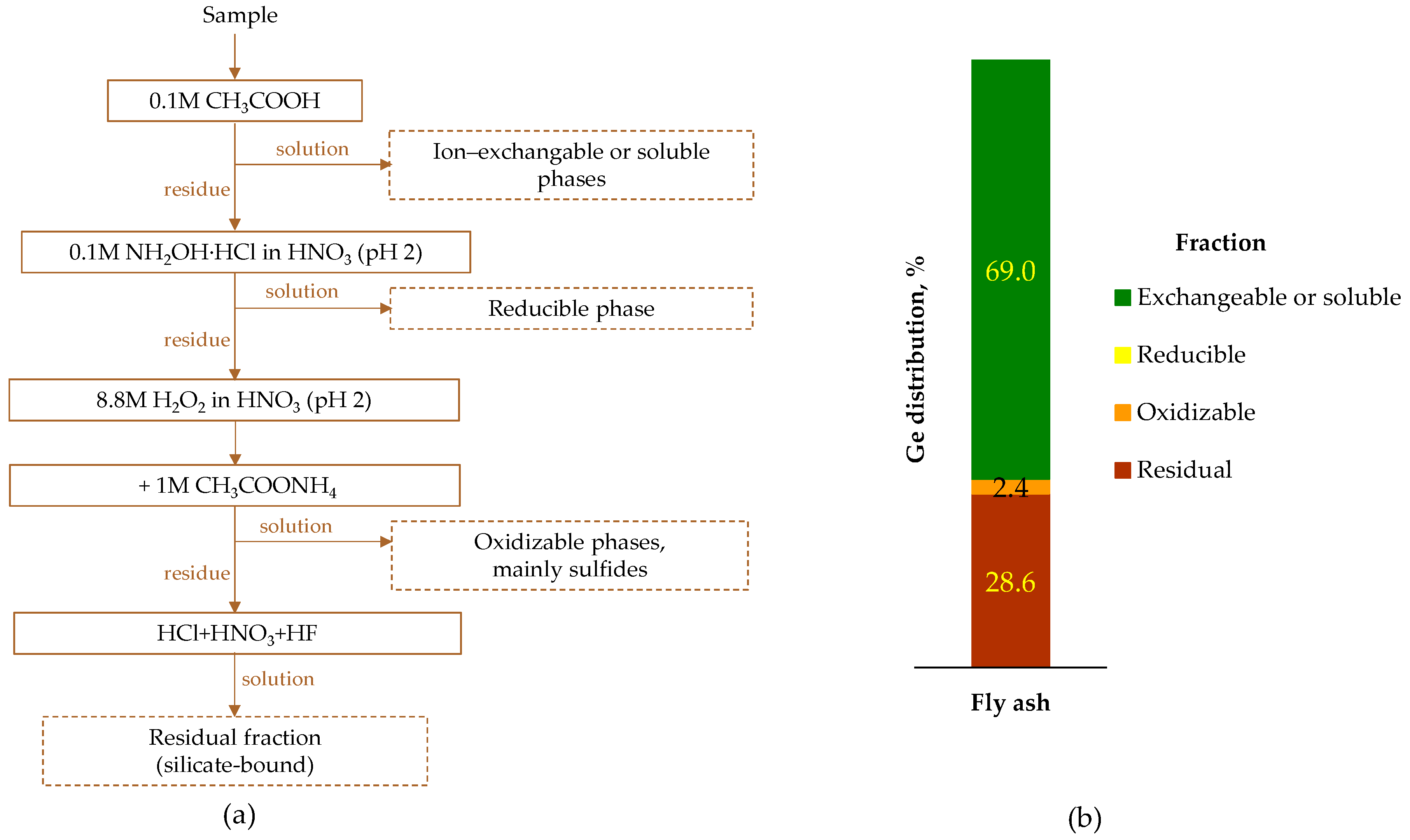
5.2. Occurrence Modes
5.3. Recovery
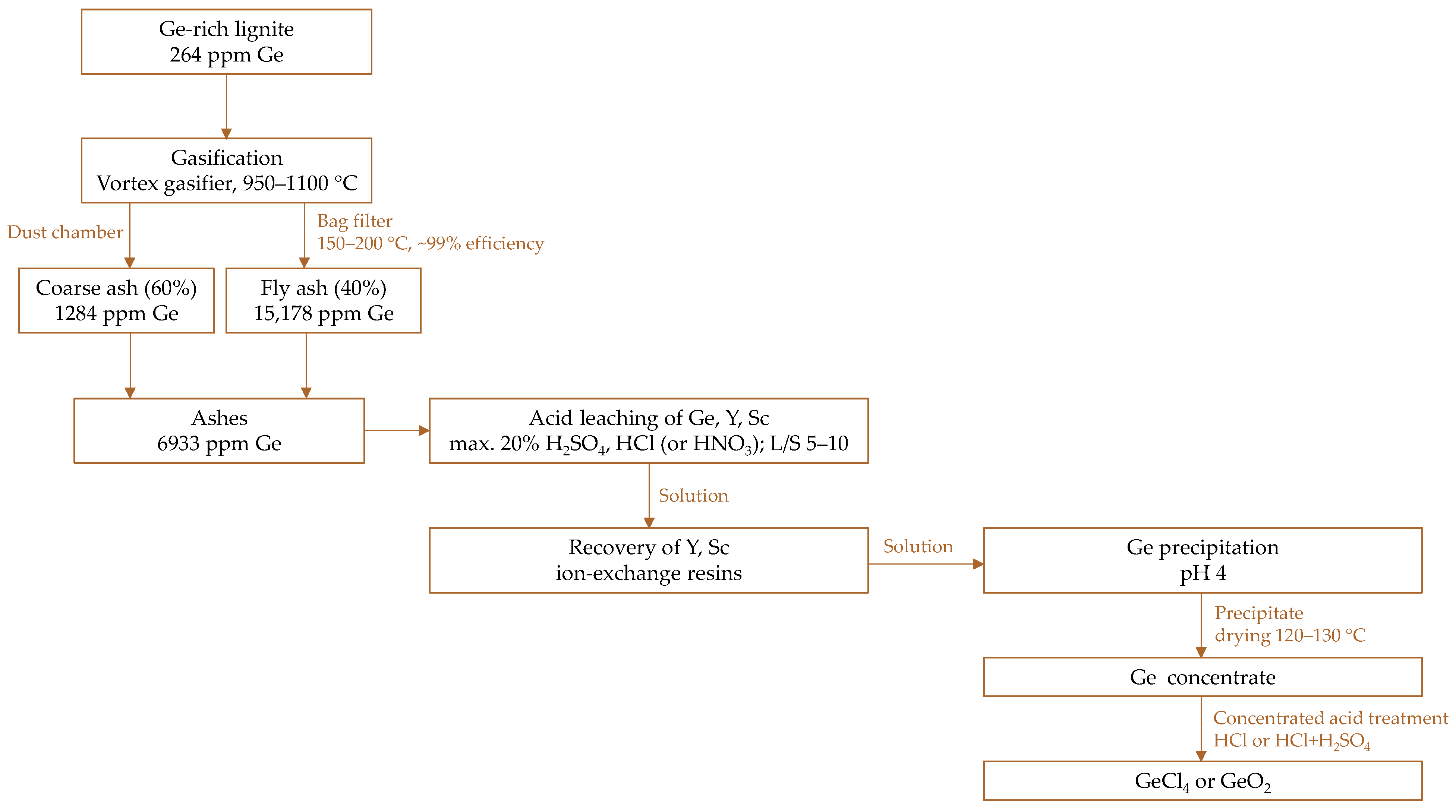
5.3.1. Case Study
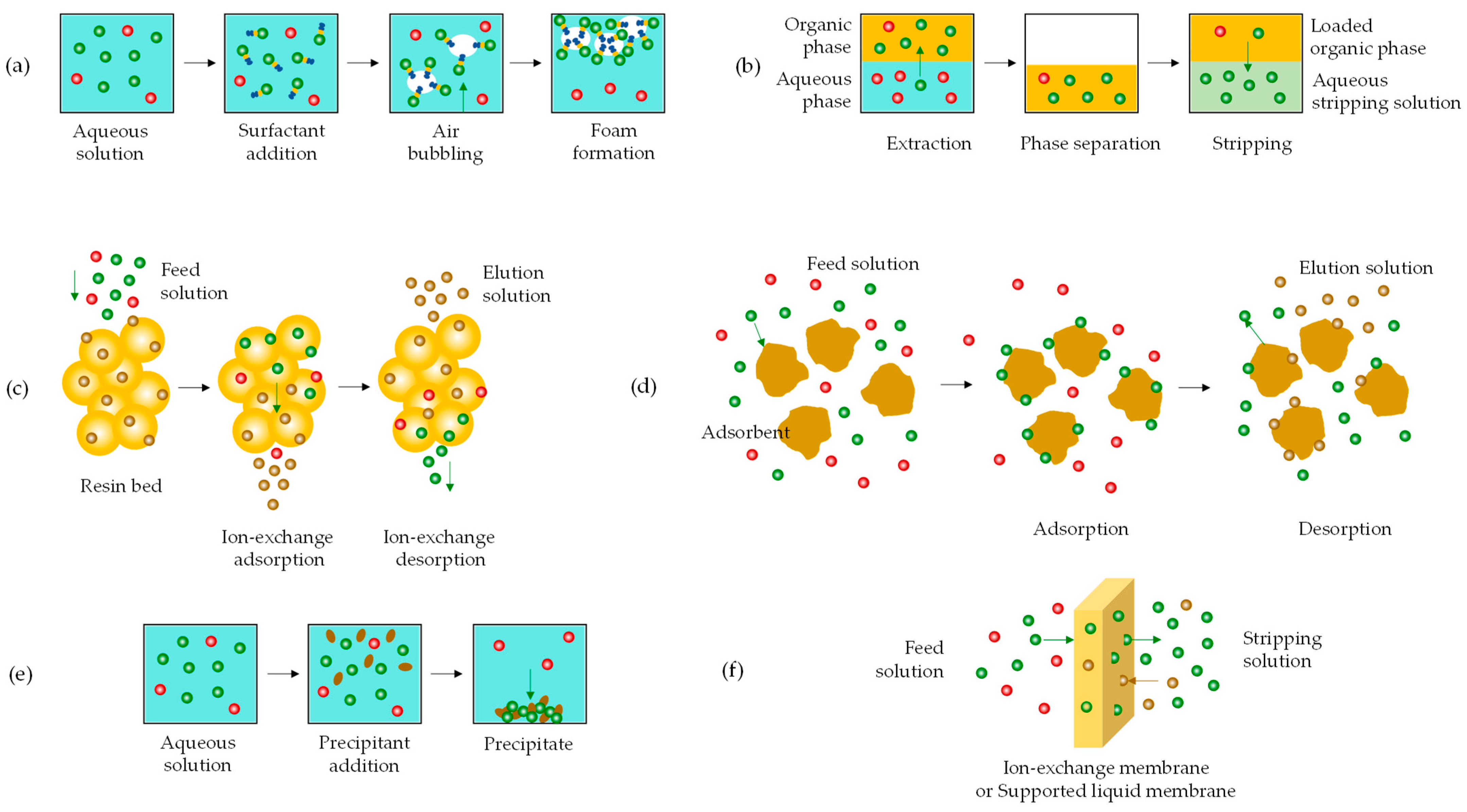
Leaching Step
From Laboratory to Pilot-Scale Implementation
- (i)
- germanium leaching from the fly ash
- (ii)
- complexation of germanium species with catechol
- (iii)
- formation of froth product with dodecylamine
- (i)
- hydrogen sulfide, H2S, a conventional and inexpensive compound already generated as a by-product in an IGCC plant:
- (ii)
- cetyltrimethylammonium bromide CTAB, a surfactant that forms crystalline molecular complexes with various aromatic compounds, such as catechol:
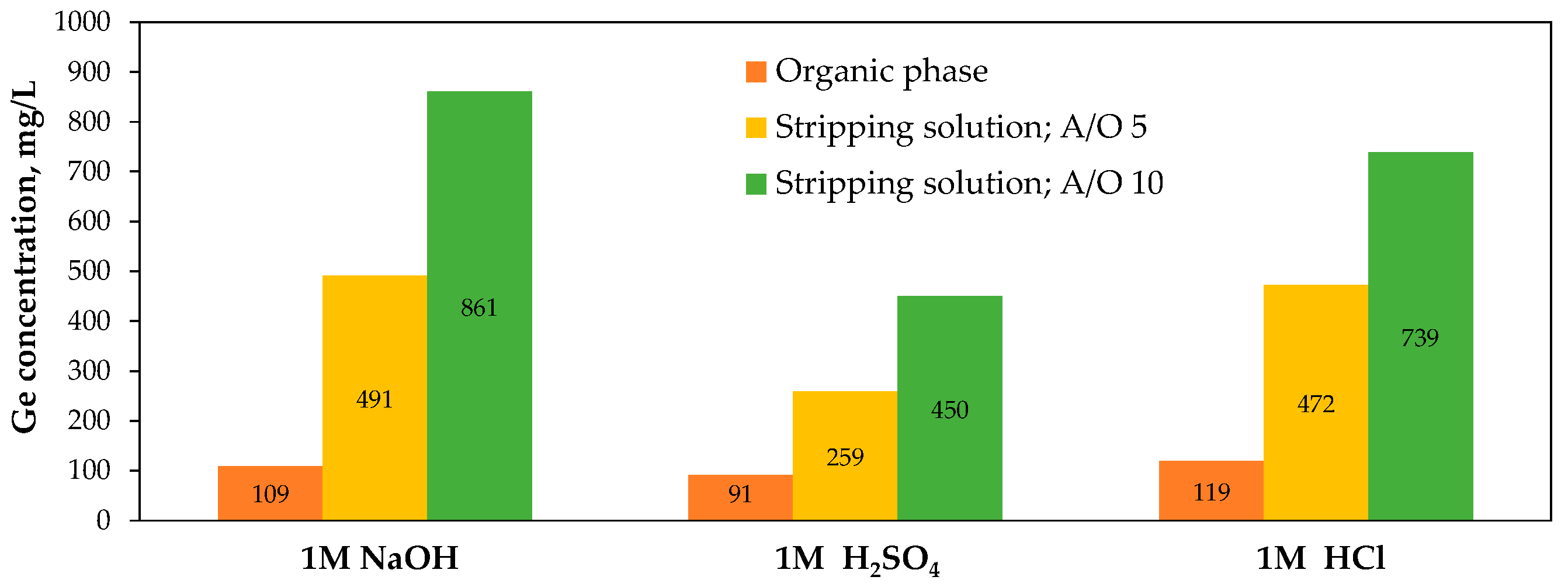
5.3.2. Alternative Approach
5.3.3. Innovations
6. Materials and Methods
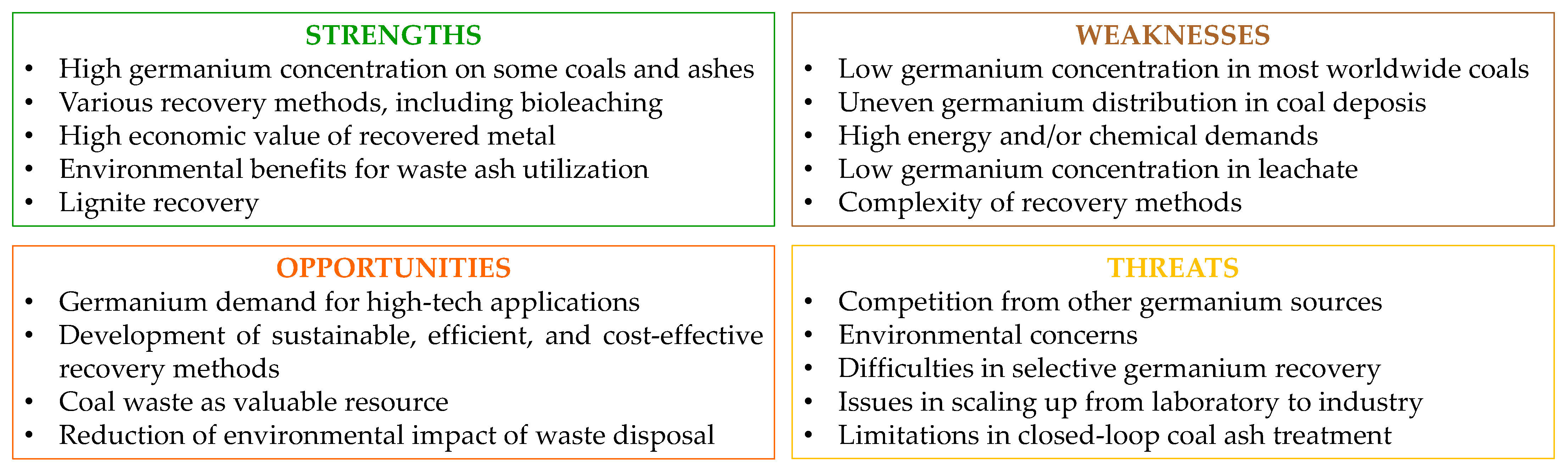
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haller, E.E. Germanium: From its discovery to SiGe device. Mater. Sci. Semicond. Process. 2006, 9, 408–422. [Google Scholar] [CrossRef]
- Rosenberg, E. Germanium: Environmental occurrence, importance and speciation. Rev. Environ. Sci. Biotechnol. 2009, 8, 29–57. [Google Scholar] [CrossRef]
- Benedikovic, D.; Virot, L.; Aubin, G.; Hartman, J.M.; Amar, F.; Le Roux, X.; Alonso-Ramos, C.; Cassan, E.; Marris-Morini, D.; Fedelli, J.M.; et al. Silicon-germanium receivers for short-wave-infrared optoelectronics and communications. Nanophotonics 2021, 10, 1059–1079. [Google Scholar] [CrossRef]
- Cook, B. Silicon-germanium: The legacy lives on. Energies 2022, 15, 2957. [Google Scholar] [CrossRef]
- Chen, H.; Wei, M.; He, Y.; Abed, J.; Teale, S.; Sargent, E.H.; Yang, Z. Germanium silicon oxide achieves multicoloures ultra-long phosphorescence and delayed fluorescence at high temperature. Nat. Commun. 2022, 13, 4438. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Zhang, Y.; Hao, J.; Meng, J.; Shi, N. High-performance Ge photodetectors on silicon photonics platform for optical interconnect. Sens. Actuators A Phys. 2024, 376, 115535. [Google Scholar] [CrossRef]
- Distribution of Germanium Consumption Worldwide in 2022, by End-Use. Statista. Available online: https://www.statista.com/statistics/1446296/distribution-of-germanium-consumption-worldwide-by-end-use/ (accessed on 17 February 2025).
- Metals and Minerals. Refined Germanium Production, 1956 to 2021. USGS—Mineral Commodity Summaries (2024), USGS—Historical Statistics for Mineral and Material Commodities (2023)—With Major Processing by Our World in Data. Available online: https://ourworldindata.org/metals-minerals (accessed on 17 February 2025).
- Distribution of Germanium Production Worldwide in 2022, by Country. Statista. Available online: https://www.statista.com/statistics/1445497/germanium-share-of-production-worldwide-by-country/ (accessed on 17 February 2025).
- Patel, M.; Karamalidis, A.K. Germanium: A review of its US demand, uses, resources, chemistry, and separation technologies. Sep. Purif. Technol. 2021, 275, 118981. [Google Scholar] [CrossRef]
- Guzik, K.; Burkiewicz, A.; Szlugaj, J. The EU’s demand for selected critical raw materials used in the photovoltaic industry. Miner. Resour. Manag. 2022, 38, 31–59. [Google Scholar]
- Sverdrup, H.U.; Haraldsson, H.V. Assessing the long-term sustainability of germanium supply and price using the WORLD7 integrated assessment model. Biophys. Econ. Sustain. 2024, 9, 5. [Google Scholar] [CrossRef]
- Wong, M.; Li, J.; Zeng, X. Uncovering the germanium sustainability up to 2050 in China. Front. Environ. Sci. Eng. 2025, 19, 25. [Google Scholar] [CrossRef]
- National Minerals Information Center. Germanium. In Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; pp. 80–81. [Google Scholar] [CrossRef]
- Nassar, N.T.; Shojaeddini, E.; Alonso, E.; Jaskula, B.; Tolcin, A. Quantifying Potential Effects of China’s Gallium and Germanium Export Restrictions on the U.E. Economy; U.S. Geological Survey: Reston, VA, USA, 2024.
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L_202401252 (accessed on 20 February 2025).
- Umicore and STL Sign Partnership Related to Germanium Recycling from Big Hill’s Mining Waste Materials in DRC. Umicore. Available online: https://www.umicore.com/en/media/newsroom/umicore-and-stl-sign-partnership-related-to-germanium-recycling/ (accessed on 20 February 2025).
- Tossou, E. DR Congo: Gécamines Starts Exporting Germanium from New Lubumbashi Plant. Ecofin Agency. Available online: https://www.ecofinagency.com/mining/1610-46023-dr-congo-gecamines-starts-exporting-germanium-from-new-lubumbashi-plant (accessed on 20 February 2025).
- Cui, L.; Jiang, Y. Quantitative analysis of the U.S. chip embargo and China’s export controls on GaGe and graphite. Comp. Ind. Eng. 2025, 200, 110860. [Google Scholar] [CrossRef]
- Mei, Y.; Geng, Y.; Chen, Z.; Xiao, S.; Gao, Z. Ensuring the sustainable supply of semiconductor material: A case of germanium in China. Int. J. Prod. Econ. 2024, 271, 109231. [Google Scholar] [CrossRef]
- Germanium Prices. Strategic Metal Invest. Available online: https://strategicmetalsinvest.com/germanium-prices/ (accessed on 11 March 2025).
- Höll, R.; Kling, M.; Schroll, E. Metalogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.P.; Gutzmer, J. On the geological availability of germanium. Miner. Depos. 2014, 49, 471–486. [Google Scholar] [CrossRef]
- Geng, X.; Liu, Y.; Zhang, W.; Wang, L.; Wen, J.; Sun, J. Recent advances in the recovery of germanium during the zinc refining process. Chem. Eng. J. 2022, 446, 137445. [Google Scholar] [CrossRef]
- Meshram, P.; Abhilash. Strategies for recycling of primary and secondary resources for germanium extraction. Min. Metall. Explor. 2022, 39, 389–707. [Google Scholar] [CrossRef]
- Haghighi, H.K.; Irannajad, M. Roadmap for recycling of germanium from various resources: Reviews in recent developments and feasibility views. Environ. Sci. Pollut. Res. 2022, 29, 48126–48151. [Google Scholar] [CrossRef] [PubMed]
- Germanium. SCRREEN3. Available online: https://scrreen.eu/crms-2023/ (accessed on 20 February 2025).
- Schrön, W. Formation of nickel-iron meteorites by chemical fluid transport. Sci. Res. 2019, 1–22. [Google Scholar] [CrossRef]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry. The Crust; Rudnick, R.L., Ed.; Elsevier: Oxford, UK, 2005; Volume 3, pp. 1–64. [Google Scholar]
- Filella, M.; Rodriguez-Murillo, J.C. Germanium in the environment: Current knowledge and gap identification. Soil Environ. Health 2025, 3, 100132. [Google Scholar] [CrossRef]
- Jabłońska-Czapla, M.; Grygoryć, K.; Rachwał, M.; Fornalcyk, A.; Willner, J. Germanium speciations study in soil from an electronic waste processing plant area. J. Soils Sediments 2023, 23, 3362–3375. [Google Scholar] [CrossRef]
- Wiche, O.; Zertani, V.; Hentschel, W.; Achtziger, R.; Midula, P. Germanium and rare earth elements in topsoil and soil-grown plants on different land use types in the mining area of Freiberg (Germany). J. Geochem. Explor. 2017, 175, 120–129. [Google Scholar] [CrossRef]
- Sarosiek, J.; Kosiba, P. The effects of water and hydrosol chemistry on the accumulation of beryllium and germanium in selected species of macrohydrophytes. Water Air Soil Pollut. 1993, 69, 405–411. [Google Scholar] [CrossRef]
- Dobrzyński, D.; Karasiński, J.; Tetfejer, K.; Tupys, A.; Słaby, E.; Stępień, M. Germanium and its isotopes as indicators of hydrogeochemical conditions in a terrestial geothermal system (Karkonosze granitoid, Sudets, Poland). Appl. Geochem. 2024, 174, 106138. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L.; Chen, T.T. Host minerals for gallium-germanium ores of the Apex mine, Utah. Econ. Geol. 1986, 81, 945–950. [Google Scholar] [CrossRef]
- Wiche, O.; Szekely, B.; Moschner, C.; Heilmeier, H. Germanium in the soil-plant system—A review. Environ. Sci. Pollut. Res. 2018, 25, 31938–31956. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Robla, J.I. Some recent advances in germanium recovery from various resources. Metals 2024, 14, 559. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on germanium resources and its extraction by hydrometallurgical method. Miner. Process. Extr. Metall. Rev. 2021, 42, 406–426. [Google Scholar] [CrossRef]
- Robertz, B.; Verhelle, J.; Schurmans, M. The primary and secondary production of germanium: A life-cycle assessment of different process alternatives. JOM 2015, 67, 412–424. [Google Scholar] [CrossRef]
- Australia Government. Department of Industry, Science and Resources. Australia’s Critical Minerals List and Strategic Material List. Available online: https://www.industry.gov.au/publications/australias-critical-minerals-list-and-strategic-materials-list (accessed on 20 February 2025).
- Ontario’s Critical Minerals Strategy: 2022–2027. Available online: https://www.ontario.ca/page/ontarios-critical-minerals-strategy-2022-2027-unlocking-potential-drive-economic-recovery-prosperity (accessed on 20 February 2025).
- Critical Minerals Policy Tracker. Available online: https://www.iea.org/data-and-statistics/data-tools/critical-minerals-policy-tracker (accessed on 21 February 2025).
- Critical Minerals for India. Report of the Committee on Identification of Critical Minerals. Ministry of Mines, India. 2023. Available online: https://mines.gov.in/admin/download/649d4212cceb01688027666.pdf (accessed on 21 February 2025).
- U.S. Geological Survey, Department of the Interior. 2022 Final list of critical minerals. Fed. Regist. 2022, 37, 10381. [Google Scholar]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R.B. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Morgan, G.; Davies, G.R. Germanium and gallium in coal ash and flue dust. J. Soc. Chem. Ind. 1937, 56, 717–721. [Google Scholar] [CrossRef]
- Lin, R.; Soong, Y.; Granite, E.J. Evaluation of trace elements in U.S. coals using USGS COALQUAL database version 3.0. Part II: Non-REY critical elements. Int. J. Coal Geol. 2018, 192, 39–50. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Li, Y.; Pan, S.; Ning, S.; Shao, L.; Jing, Z.; Wang, Z. Coal measure metallogeny: Metallogenic system and implication for resource and environment. Sci. China Earth Sci. 2022, 65, 1211–1228. [Google Scholar] [CrossRef]
- Shpirt, M.Y.; Nukenov, D.N.; Punanova, S.A.; Visaliev, M.Y. Principles of the production of valuable metal compounds from fossil fuels. Solid Fuel Chem. 2013, 47, 71–82. [Google Scholar] [CrossRef]
- Salikhov, V.A.; Strakhov, V.M.; Yedilbaev, A.I. Assessing the content of nonferrous and rare metals in nonclinkering Kazakh coal. Coke Chem. 2021, 64, 279–283. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Tewalt, S.J.; Belkin, H.E.; SanFilipo, J.R.; Merrill, M.D.; Palmer, C.A.; Warwick, P.D.; Karlsen, A.W.; Finkelman, R.B.; Park, A.J. Chemical Analyses in the World Coal Quality Inventory, Version 1: U.S. Geological Survey Open—File Report 2010–1196. U.S. Geological Survey: Reston, VA, USA, 2010. Available online: https://pubs.usgs.gov/of/2010/1196/ (accessed on 25 February 2025).
- Kunstmann, F.H.; Hammersma, J.C. The occurrence of germanium in South African coal and derived products. J. Chem. Metall. Min. Soc. S. Afr. 1955, 56, 11–22. [Google Scholar]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X.; Jiang, Y.; Wang, X.; Gronostaeva, T.; Li, X.; et al. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. Geol. Rev. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Hu, R.Z.; Qi, H.W.; Zhou, M.F.; Su, W.C.; Bi, X.W.; Peng, J.T.; Zhong, H. Geological and geochemical constrains on the origin of the giant Lincang coal seam-hosted germanium deposit, Yunnan, SW China: A review. Ore Geol. Rev. 2009, 36, 221–234. [Google Scholar] [CrossRef]
- Huang, W.; Wan, H.; Du, G.; Sun, L.; Ma, Y.; Tang, X.; Wi, W.; Qin, S. Research on element geochemical characteristics of coal-Ge deposit in Shengli Coalfield, Inner Mongolia, China. Earth Sci. Front. 2008, 15, 55–64. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Izquierdo, M.; Wang, Z. New data on mineralogy and geochemistry of high-Ge coals in the Yimin coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2014, 125, 10–21. [Google Scholar] [CrossRef]
- Auguścik, J.; Wasilewska-Błaszczyk, M.; Wójtowicz, J.; Paszek, M. Variability in the content of critical elements (Be, Co, Ga and Ge) in bituminous coal of the Lublin Coal Basin. Biul. Państw. Inst. Geol. 2016, 466, 7–16. [Google Scholar] [CrossRef]
- Makowska, D.; Wierońska, F.; Strugała, A.; Kosowska, K. Germanium content in Polish hard coals. E3S Web Conf. 2016, 10, 00121. [Google Scholar] [CrossRef]
- Etschmann, B.; Liu, W.; Li, K.; Dai, S.; Reith, F.; Falconer, D.; Kerr, G.; Paterson, D.; Howard, D.; Kappen, P.; et al. Enrichment of germanium and associated arsenic and tungsten in coal and roll-front uranium deposits. Chem. Geol. 2017, 463, 29–49. [Google Scholar] [CrossRef]
- Vyalov, V.I.; Oleinikova, G.A.; Nastavkin, A.V. Distribution of germanium in coals of the Pavlovsk deposit. Solid Fuel Chem. 2020, 54, 163–169. [Google Scholar] [CrossRef]
- Jiu, B.; Huang, W.; Sun, Q. Distribution characteristics and enrichment model of germanium in coal: An example from the Yimin Coalfield, Hailar Basin, China. Nat. Resour. Res. 2021, 30, 725–740. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Wei, Q.; Rimmer, S.M. Acid solubility and affinities of trace elements in the high-Ge coals from Wulantuga (Inner Mongolia) and Lincang (Yunnan Province), China. Int. J. Coal Geol. 2017, 178, 39–55. [Google Scholar] [CrossRef]
- Admakin, L.A. Concentration of germanium in lignite deposits. Coke Chem. 2019, 62, 437–446. [Google Scholar] [CrossRef]
- Vyalov, V.I.; Nastavkin, A.V.; Shishov, E.P. Distribution of industrially valuable trace elements associated with germanium in the coals of the Pavlovsk deposit (Spetsugli section). Solid Fuel Chem. 2021, 55, 14–25. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A. Gallium and germanium in selected Indiana coals. Int. J. Coal Geol. 2012, 94, 302–313. [Google Scholar] [CrossRef]
- Das, A.; Kumar, R.; Patel, S.S.; Saha, C.; Das, A.; Mandal, H.; Patel, R.S. Geochemical variations of major, trace, rare earth elements in some Gondwana and Eocene coals of India with a comparison of their germanium, lithium, and mercury content. Geochemistry 2023, 83, 125960. [Google Scholar] [CrossRef]
- Spears, D.A.; Tewalt, S.J. The geochemistry of environmentally important trace elements in UK coals, with special reference to the Parkgate coal in the Yorkshire-Nottinghamshire Coalfield, UK. Int. J. Coal Geol. 2009, 80, 157–166. [Google Scholar] [CrossRef]
- Spears, D.A.; Zheng, Y. Geochemistry and origin of elements in some UK coals. Int. J. Coal Geol. 1999, 38, 161–179. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Chekryzhov, I.Y.; Spears, D.A.; Ilenok, S.S.; Soktoev, B.R.; Popov, N.Y. Geology, geochemistry, mineralogy and genesis of the Spetsugli high-germanium coal deposit in the Pavlovsk coalfield, Russian Far East. Ore Geol. Rev. 2021, 139, 104537. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Spears, D.A.; Ilenok, S.S.; Chekryzhov, I.Y.; Ivanov, V.P. Modes of occurrence of germanium and tungsten in the Spetsugli high-germanium germanium ore field, Pavlovka brown coal deposit, Russian Far East. Ore Geol. Rev. 2021, 132, 103986. [Google Scholar] [CrossRef]
- Zhang, X.; Querol, X.; Alastuey, A.; Juan, R.; Plana, F.; Lopez-Soler, A.; Du, G.; Martynov, V.V. Geochemistry and mineralogy of the Cretaceous Wulantuga high-germanium coal deposit in Shengli coal field, Inner Mongolia, Northeastern China. Int. J. Coal Geol. 2006, 66, 119–136. [Google Scholar] [CrossRef]
- Du, G.; Zhuang, X.; Querol, X.; Izequierdo, M.; Alastuey, A.; Moreno, T.; Font, O. Ge distribution in the Wulantuga high-germanium coal deposit in the Shengli coalfield, Inner Mongolia, northeastern China. Int. J. Coal Geol. 2009, 78, 16–26. [Google Scholar] [CrossRef]
- Hower, J.C.; Ruppert, L.F.; Williams, D.A. Controls on boron and germanium distribution in the low-sulfur Amos coal bed, Western Centucky coalfield, USA. Int. J. Coal Geol. 2002, 53, 27–42. [Google Scholar] [CrossRef]
- Hower, J.C.; Eble, C.F.; O’Keefe, J.M.K.; Dai, S.; Wang, P.; Xie, P.; Liu, J.; Ward, C.R.; French, D. Petrology, palynology, and geochemistry of Gray Hawk coal (early Pennsylvanian, Langsettian) in Eastern Kentucky, USA. Minerals 2015, 5, 592–622. [Google Scholar] [CrossRef]
- Jiu, B.; Huang, W.; Li, Y. The origin migration, and accumulation mechanism of germanium and the metallgenic model of coal-hosted Ge ore deposits in Wulantuga, Erlian Basin, China. J. Geochem. Explor. 2021, 226, 106779. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, S.; Zhang, S. Concentrations and modes of occurrence of some potentially valuable and toxic elements in the No. 5 coal from the Yanzishan Mine, Datong Coalfield, Shanxi Province, China. Energy Explor. Exploit. 2019, 37, 1694–1720. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Liu, G.; Zhan, R.; Hu, G. Characteristics and geological significance of germanium in Taiyuan coal formation of Huainan Coalfield, Anhui, China. Int. J. Coal Sci. Technol. 2020, 7, 662–675. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, C.; Zhang, J.; Arbuzov, S.I.; Spears, D.A. Discovery of Ge bearing mineral in a Carboniferous coal from the Yuzhou Coalfield, southern part of the North China Basin. Energy Explor. Exploit. 2023, 41, 2007–2015. [Google Scholar] [CrossRef]
- Guo, W.; Nechaev, V.P.; Yan, X.; Yang, C.; He, X.; Shan, K.; Nechaeva, E.V. New data on geology and germanium mineralization in the Hunchun Basin, northeastern China. Ore Geol. Rev. 2019, 107, 381–391. [Google Scholar] [CrossRef]
- Yudovich, Y.E. Notes of the marginal enrichment of germanium in coal beds. Int. J. Coal Geol. 2003, 56, 223–232. [Google Scholar] [CrossRef]
- Wei, Q.; Dai, S.; Costin, G. Electron probe microanalysis of major and trace elements in coals and their low-temperature ashes from Wulantuga and Lincang Ge ore deposits, China. Fuel 2018, 215, 1–12. [Google Scholar] [CrossRef]
- Wei, Q.; Cui, C.; Dai, S. Organic-association of Ge in the coal-hosted ore deposits: An experimental and theoretical approach. Ore Geol. Rev. 2020, 117, 103291. [Google Scholar] [CrossRef]
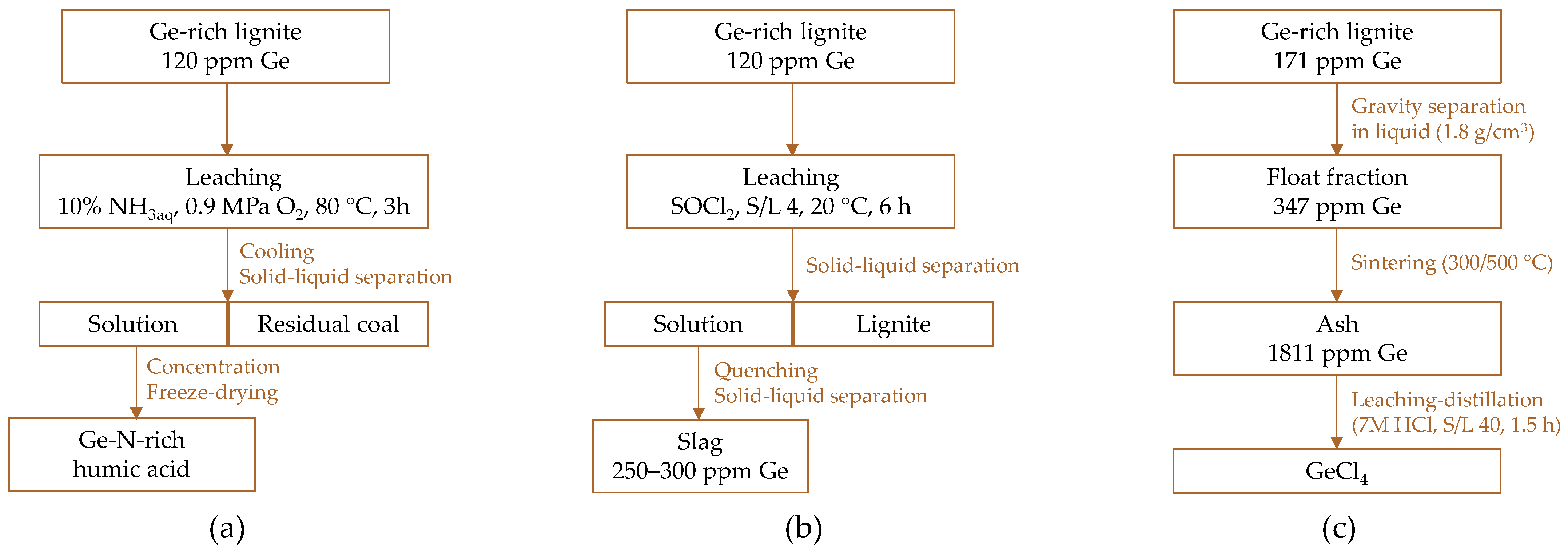
- Bo, W.; Wu, J.; Miao, Z.; Wan, K. Germanium extraction from lignite using gravity separation combined with low-temperature sintering and chlorinated destillation. Sep. Purif. Technol. 2024, 329, 125215. [Google Scholar] [CrossRef]
- Klika, Z.; Ambružová, L.; Sýkorová, I.; Seidlerová, J.; Kolomaznik, I. Critical evaluation of sequential extraction and sink-float method used for the determination of Ga and Ge affinity in lignite. Fuel 2009, 88, 1834–1841. [Google Scholar] [CrossRef]
- Mu, R.; Wang, S.; Wang, X.; Zhao, Y.; Dong, Z. Occurrence modes of critical metals (Li+ and Ge4+) in the organic molecular structures of coal: A density functional theory study. ACS Omega 2023, 8, 17264–17273. [Google Scholar] [CrossRef]
- Mu, R.; Wang, S.; Wang, X.; Su, H.; Shao, Y. Organic modes of occurrence and evolution mechanism of germanium and lithium in coal: Insights from density functional theory. Int. J. Coal Geol. 2025, 298, 104661. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Cao, D.; Xu, L.; Zhang, Y.; Wei, J.; Dong, B. Cooperative exploration model of coal-Ge deposit: A case study of the Wulantuga coal-ge deposit in Shengli coalfield, Inner Mongolia, China. Energy Explor. Exploit. 2024, 42, 1666–1683. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Khudyakov, D.S.; Beilina, N.Y.; Trukhina, M.A.; Kozlov, A.M.; Zhagfarov, F.G. Solid fossil fuels as a source of trace elements. Solid Fuel Chem. 2022, 56, 1–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Teng, D.; Fan, G.; Cao, Y.; Liu, J.; Li, P. Co-extraction of germanium and nitrogen-rich humic acid from germanium-rich lignite by ammonooxidation. Fuel 2024, 366, 131361. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, G.; Zhou, G.; Teng, D.; Nan, H.; Li, P.; Cao, Y.; Liu, J. Kill two birds with one stone: Efficient leaching of germanium and recovery of lignite from germanium-rich lignite by thionyl chloride. Sep. Pur. Technol. 2025, 354, 128703. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, B.; Dang, J.; Zhao, Y.; Liang, D.; Xie, Q. Migration, transformation, and enrichment of strategic metal elements including Li, Ga, Ge, In, and Re during the coal pyrolysis process. J. Anal. Appl. Pyrolysis 2025, 186, 106953. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Miao, Z.; Xu, E.; Han, Y.; Ding, L. Extraction and volatilization mechanism of germanium (Ge) during lignite pyrolysis. Sep. Purif. Technol. 2025, 354, 128915. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risk of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Rudnik, E. Coal and coal by-products as unconventional lithium sources: A review on occurrence modes and hydrometallurgical strategies for metal recovery. Minerals 2024, 14, 849. [Google Scholar] [CrossRef]
- Rudnik, E. Review on gallium in coal and coal waste materials: Exploring strategies for hydrometallurgical metal recovery. Molecules 2024, 29, 5919. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Potentially useful elements (Al, Fe, Ga, Ge, U) in coal gangue: A case study in Weibei coal mining area, Shaanxi Province, northwestern China. Environ. Sci. Pollut. Res. 2018, 25, 11893–11904. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, B.; Zhou, C. Experimental investigation on gallium and germanium migration in coal gangue combustion. Minerals 2024, 14, 476. [Google Scholar] [CrossRef]
- Ramme, B.W.; Tharaniyil, M.P. Coal Combustion Products Utilization Handbook, 3rd ed.; We Energies: Milwaukee, WI, USA, 2013. [Google Scholar]
- Zhu, Q. Coal Sampling and Analysis Standards; IEA Clean Coal Centre: London, UK, 2014. [Google Scholar]
- Zhou, C.; Du, J.; Zhang, Y.; Sun, J.; Wu, W.; Liu, G. Redistribution and transformation mechanisms of gallium and germanium during coal combustion. Fuel 2021, 305, 121532. [Google Scholar] [CrossRef]
- Clarke, L.B.; Sloss, L.L. Trace Elements—Emissions from Coal Combustion and Gasification; IEA Coal Research: London, UK, 1992. [Google Scholar]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Depoi, F.S.; Pozebon, D.; Kalkreuth, W.D. Chemical characterization of feed coals and combustion-by-products from Brazilian power plants. Int. J. Coal Geol. 2008, 76, 227–236. [Google Scholar] [CrossRef]
- Yossifova, M.G. Mineral and inorganic chemical composition of the Pernik coal, Bulgaria. Int. J. Coal Geol. 2007, 72, 268–292. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.; Li, S.; Wang, J.; Qi, D.; Lu, Q.; Xing, J. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant. J. Clean. Prod. 2022, 324, 130910. [Google Scholar] [CrossRef]
- Lin, M.; Bai, G.; Duan, P.; Xu, J.; Duan, D.; Li, Z. Perspective of comprehensive exploitation of the valuable elements of Chinese coal. Energy Explor. Exploit. 2013, 31, 623–627. [Google Scholar] [CrossRef]
- Papadoyannis, I.N.; Matis, K.A.; Zoumboulis, A.I. Extraction and flameless AAS determination of germanium in lignite fly ash. Anal. Lett. 1985, 18, 2467–2475. [Google Scholar] [CrossRef]
- Georgakopoulos, A.; Filippidis, A.; Kassoli-Fournaraki, A.; Iordanidis, A.; Fernández-Turiel, J.L.; LLorens, J.F.; Gimeno, D. Environmentally important elements in fly ashes and their leachates of the power stations of Greece. Energy Sources 2002, 24, 83–91. [Google Scholar] [CrossRef]
- Karan, K.K.; Masto, R.E.; Kumar, S.; Agarwalla, H.; Bari, S. Prospect for recycling critical elements in combustion residues of coal, lignite, and biomass feedstocks. Miner. Eng. 2024, 219, 109063. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicova, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Medina, A.; Gamero, P.; Querol, X.; Moreno, N.; De Leon, B.; Almanza, M.; Vargas, G.; Izquierdo, M.; Font, O. Fly ash from a Mexican mineral coal I: Mineralogical and chemical characterization. J. Hazard. Mater. 2010, 181, 82–90. [Google Scholar] [CrossRef]
- Plewa, M. Pierwiastki śladowe w węglu Lubelskiego Zagłębia Węglowego. In Metodyka Rozpoznawania i Dokumentowania Złóż Kopalin Stałych; Nieć, M., Kokesz, Z., Eds.; Wydawnictwo AGH: Kraków, Poland, 1990. [Google Scholar]
- Arbuzov, S.I.; Spears, D.A.; Vergunov, A.V.; Ilenok, S.S.; Mezhibor, A.M.; Ivanov, V.P.; Zarubina, N.A. Geochemistry, mineralogy and genesis of rare metal (Nb-Ta-Zr-Hf-Y-REE-Ga) coals of the seam XI in the south of Kuznetsk Basin, Russia. Ore Geol. Rev. 2019, 113, 103073. [Google Scholar] [CrossRef]
- Vergunov, A.V.; Arbuzov, S.I.; Spears, D.A.; Kholodov, A.S.; Ilenok, S.S. Mineralogy and geochemistry of rare metal (Zr-Nb-Hf-Ta-REE-Ga) coals of the seam XXX of the Izykh Coalfield, Minusinsk Basin, Russia: Implications for more widespread rare metal mineralization in North Asia. Int. J. Coal Geol. 2024, 289, 104542. [Google Scholar] [CrossRef]
- Querol, X.; Umaña, J.C.; Alastuey, A.; Bertrana, C.; Lopez-Soler, A.; Plana, F. Physicochemical characterization of Spanish fly ashes. Energy Sources 1999, 21, 883–898. [Google Scholar]
- Moscoco-Pérez, C.; Moreda-Piñeiro, J.; López-Mahia, P.; Muniategui-Lorenzo, S.; Fernández- Fernández, E.; Prada-Rodriguez, D. Direct determination of Ge in hot spring waters and coal fly ash samples by hydride generation-ETAAS. Talanta 2004, 64, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Fernández-Turiel, J.L.; López-Soler, A. Trace elements in coal and their behaviour during combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Alastuey, A.; Jiménez, A.; Plana, F.; Querol, X.; Suárez-Ruiz, I. Geochemistry, mineralogy, and technological properties of the main Stephanian (Carboniferous) coal seams from the Puertollano Basin, Spain. Int. J. Coal. Geol. 2001, 45, 247–265. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Onacak, T.; Gayer, R.A.; Goldsmith, S. Mineralogy and geochemistry of feed coals and their combustion residues from the Cayirhan power plant, Ankara, Turkey. Appl. Geochem. 2001, 16, 911–919. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Oskay, R.G.; Gayer, R.A. Mineralogy and geochemistry of feed coals and combustion residues of the Kangal power plant (Sivas, Turkey). Turk. J. Earth Sci. 2019, 28, 438–456. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Yiğitler, Ö.; Işerli, S.; Querol, X.; Mastalerz, M.; Oskay, R.G.; Hower, J.C. Mineralogy and geochemistry of feed coals and combustion residues from Tunçbilek and Seyitömer coal-fired power plants in western Turkey. Coal Comb. Gasif. Prod. 2019, 11, 18–31. [Google Scholar]
- Stadnichenko, T.; Murata, K.J.; Zubovic, P.; Hufschmidt, E.L. Concentration of Germanium in the Ash of American Coals. A Progress Report; Geological Survey: Washington, DC, USA, 1953.
- Frandsen, F.; Dam–Johansen, K.; Rasmussen, P. Trace elements from combustion and gasification of coal—An equilibrium approach. Prog. Energy Combust. Sci. 1994, 20, 115–138. [Google Scholar] [CrossRef]
- Li, C.; Zhou, C.; Li, W.; Zhu, W.; Shi, J.; Liu, G. Enrichment of critical elements from coal fly ash by the combination of physical separations. Fuel 2023, 336, 127156. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Li, W.; Sun, J.; Li, Q.; Wu, W. Distribution and preconcentration of critical elements from coal fly ash by integrated physical separations. Int. J. Coal Geol. 2022, 261, 104095. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, L.; French, D.; Graham, I.; Wei, Q.; Dai, S.; Feng, L. Revisiting sustainable resources in the combustion products of alumina-rich coal: Critical metal (Li, Ga, Nb, and REY) potential of ash from the Togtoh Power Plant, Inner Mongolia, China. Sci. Total Environ. 2024, 950, 175056. [Google Scholar] [CrossRef]
- Torma, A.E.; Jiang, H. Extraction processes for gallium and germanium. Miner. Process. Extr. Metall. Rev. 1991, 7, 235–258. [Google Scholar] [CrossRef]
- Waters, R.F.; Kenworthy, H. Extraction of Germanium and Gallium from Coal Fly Ash and Phosphorus Furnace Flue Dust; US Department of Interior, Bureau of Mines: Washington, DC, USA, 1967.
- Lisowyj, B.; Hitchcock, D.C.; Epstein, H. Process for the Recovery of Gallium and Germanium from Coal Fly Ash. U.S. Patent 4,678,647, 7 July 1987. [Google Scholar]
- Xu, D.; Chen, Y.; Guo, H.; Liu, H.; Xue, Y.; Zhang, W.; Sun, Y. Review of germanium recovery technologies from coal. Appl. Mech. Mater. 2013, 423–426, 565–573. [Google Scholar] [CrossRef]
- Tao, J.; Tao, Z.; Zhihong, L. Review on resources and recycling of germanium, with special focus on characteristics, mechanism and challenges of solvent extraction. J. Clean. Prod. 2021, 294, 126217. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Qureol, X.; Font, O.; Moreno, N. A review on the applications of coal combustion products in China. Int. Geol. Rev. 2018, 60, 671–716. [Google Scholar] [CrossRef]
- Chen, M.; Gong, Y.; Zhang, X.; Zhao, X.; Chen, T.; Batdelger, B.; Bold, T. Comprehensive analysis of thermochemical phase evolution and strategic recovery of valuable elements from high-alumina coal fly ash at different thermal conditions. Fuel 2024, 367, 131490. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Application of vacuum reduction and chlorinated distillation to enrich and prepare pure germanium from coal fly ash. J. Hazard. Mater. 2017, 321, 18–27. [Google Scholar] [CrossRef]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Shahidi, A.; Ghassa, S. A sustainable method for germanium, vanadium and lithium extraction from coal fly ash: Sodium salts roasting and organic acids leaching. Fuel 2022, 312, 122844. [Google Scholar] [CrossRef]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Ghassa, S.; Boroumand, Z.; Nosratabad, A.F. Spent-medium leaching of germanium, vanadium and lithium from coal fly ash with biogenic carboxylic acids and comparison with chemical leaching. Hydrometallurgy 2023, 217, 106038. [Google Scholar] [CrossRef]
- Li, C.; Zhou, C.; Li, W.; Zhu, W.; Shi, J.; Wu, L.; Liu, G. A two-stage process of alkali fusion and organic acid leaching for recovery of critical elements from coal fly ash. J. Ind. Eng. Chem. 2024, 138, 131–143. [Google Scholar] [CrossRef]
- Arroyo–Torralvo, F.; Fernandez–Pereira, C.; Font–Piqueras, O. By–products from the integrated gas combined cycle in IGCC systems. In Integrated Gasification Combined Cycle (IGCC) Technologies; Wang, T., Stiegel, G., Eds.; Elsevier: Oxford, UK, 2017; pp. 465–494. [Google Scholar]
- Fernández-Pereira, C.; Leiva, C.; Luna-Galiano, Y.; Vilches, L.F.; Arroyo, F. Improved recycling of a gasification fly ash: An integrated waste management approach within the framework of a Circular Economy. Waste Manag. 2024, 187, 31–38. [Google Scholar] [CrossRef]
- Shpirt, M.Y.; Stopani, O.I.; Lebedeva, L.N.; Kost, L.A.; Gorlov, E.G. Germanium production technology based on the conversion of germanium-bearing lignites. Solid Fuel Chem. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Saini, R.; Barma, S.D.; Rao, D.S.; Basu, S.; Mahajani, S.M. Applied mineralogical investigation on coal gasification ash. Waste Manag. 2023, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bielowicz, B. Selected critical raw materials in waste from coal gasification in Poland. Energies 2021, 14, 8071. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Huggins, F.E.; Chimenos, J.M.; Fernández, A.I.; Burgos, S.; Peña, F.G. Speciation of major and selected trace elements in IGCC fly ash. Fuel 2005, 84, 1364–1371. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C.; Bermejo, P. Demonstration plant equipment design and scale-up from pilot plant of a leaching and solvent extraction process. Minerals 2015, 5, 298–313. [Google Scholar] [CrossRef]
- Arroyo, F.; Font, O.; Chimenos, J.M.; Fernández-Pereira, C.; Querol, X.; Coca, P. IGCC fly ash valorization. Optimization of Ge and Ga recovery for an industrial application. Fuel Process. Technol. 2014, 124, 222–227. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; López-Soler, A.; Chimenos, J.M.; Fernández, A.; Burgos, S.; Peña, F.G. Ge extraction from gasification fly ash. Fuel 2005, 84, 1384–1392. [Google Scholar] [CrossRef]
- Hernández-Expósito, A.; Chimenos, J.M.; Fernández, A.; Font, O.; Querol, X.; Coca, P.; Peña, F.G. Ion flotation of germanium from fly ash aqueous leachates. Chem. Eng. J. 2006, 118, 69–75. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C. Hydrometallurgical recovery of germanium from coal gasification fly ash. Solvent extraction method. Ind. Eng. Chem. Res. 2008, 47, 3186–3191. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C. Hydrometallurgical recovery of germanium from coal gasification fly ash: Pilot plant scale evaluation. Ind. Eng. Chem. Res. 2009, 48, 3573–3579. [Google Scholar] [CrossRef]
- Arroyo, F.; Font, O.; Fernández-Pereira, C.; Querol, X.; Chimenos, J.M.; Zeegers, H. Germanium and gallium extraction from gasification fly ash: Optimization for up-scaling a recovery process. In Proceedings of the World of Coal Ash Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Arroyo, F.; Font, O.; Fernández–Pereira, C.; Querol, X.; Juan, R.J.; Ruiz, C.; Coca, P. Germanium recovery from gasification fly ash: Evaluation of end products obtained by precipitation methods. J. Hazard. Mater. 2009, 167, 582–588. [Google Scholar] [CrossRef]
- Arroyo, F.; Camacho, N.P.; Coca, P.; Fernández–Pereira, C. Recovery of germanium from coal fly ash leachate by precipitation. In Proceedings of the World of Coal Ash Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Arroyo–Torralvo, F.; Fernández–Pereira, C.; Campanario, M.C. Recovery of germanium from aqueous solutions by ion-exchange extraction of its catechol complex. Ind. Eng. Chem. Res. 2010, 49, 4817–4823. [Google Scholar] [CrossRef]
- Arroyo–Torralvo, F.; Fernández–Pereira, C. Recovery of germanium from real fly ash leachates by ion-exchange extraction. Miner. Eng. 2011, 24, 35–41. [Google Scholar] [CrossRef]
- Marco-Lozar, J.P.; Cazorls-Amorós, D.; Linares-Solano, A. A new strategy for germanium adsorption on activated carbon by complex formation. Carbon 2007, 45, 2519–2528. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.; de Valle-Zermeño, R.; Font, O.; Querol, X.; Coca, P. Arsenic and antimony removal by oxidative aqueous leaching of IGCC fly ash during germanium extraction. Fuel 2013, 112, 450–458. [Google Scholar] [CrossRef]
- Haghighi, H.K.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Recovery of germanium from leach solution of fly ash using solvent extraction with various extractants. Hydrometallurgy 2018, 175, 164–169. [Google Scholar] [CrossRef]
- Haghighi, H.K.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Non-dispersive selective extraction of germanium from fly ash leachates using membrane-based processes. Sep. Sci. Technol. 2019, 54, 2879–2894. [Google Scholar] [CrossRef]
- Patel, M.; Karamalidis, A.K. Microwave-assisted synthesis of catechol-based functionalized adsorbents for selective adsorption of critical element germanium. Chem. Eng. J. 2023, 475, 146367. [Google Scholar] [CrossRef]
- Wu, H.; Hu, L.; Zhang, Z.; Guo, W.; Chen, W.; Yu, J.; Feng, G.; Chi, R.; Pan, Z. Highly effective and selective recovery of germanium from coal acid leaching solution by trihydroxyl functionalized titanium dioxide. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135130. [Google Scholar] [CrossRef]

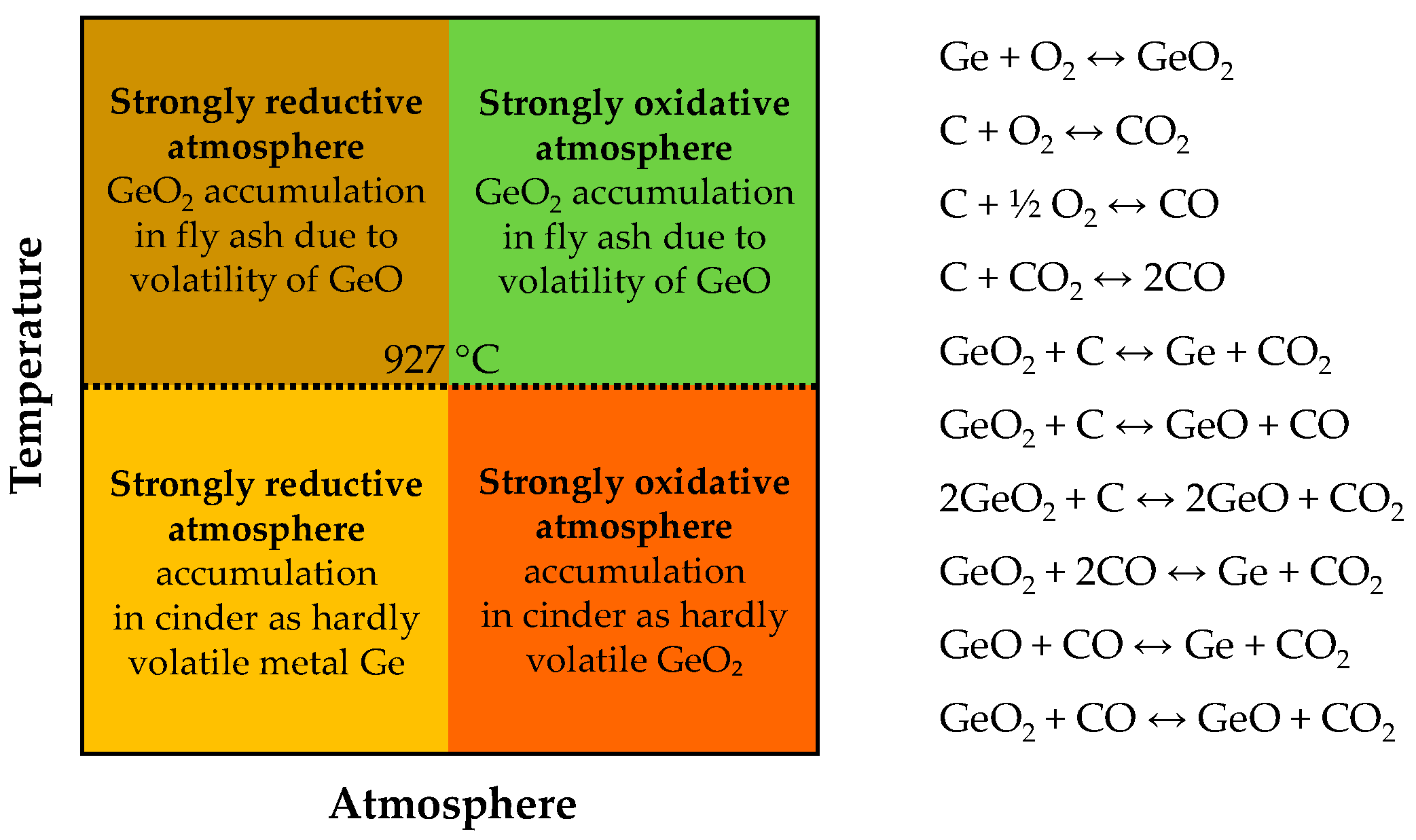
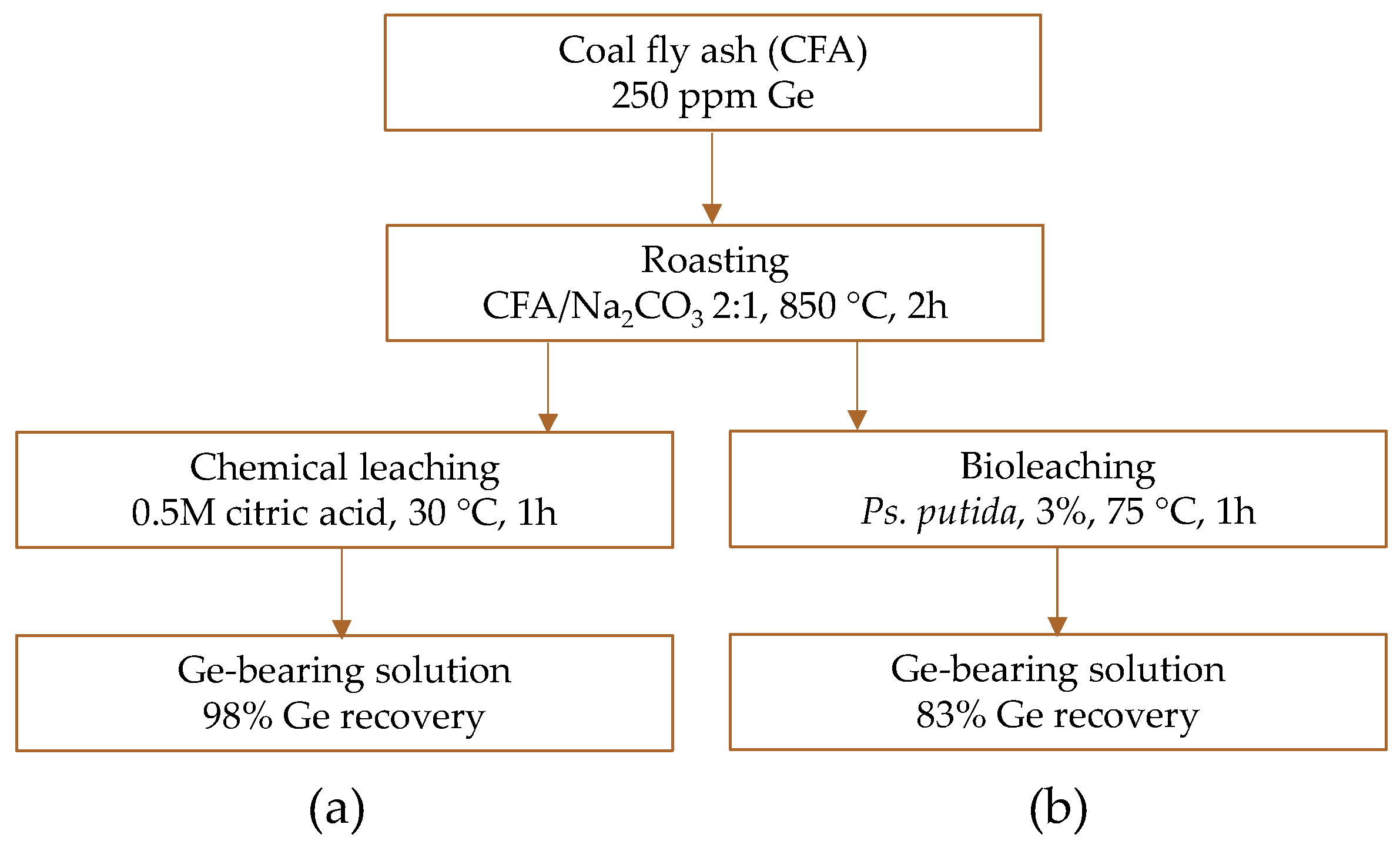
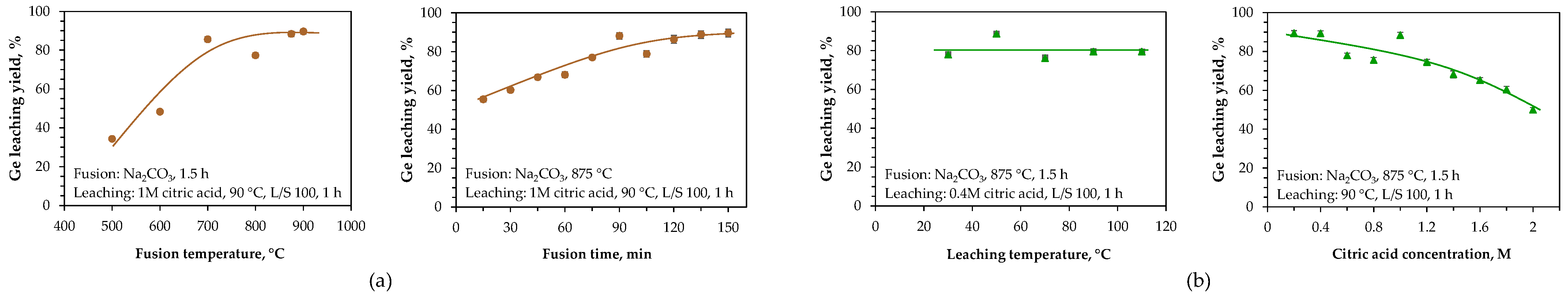


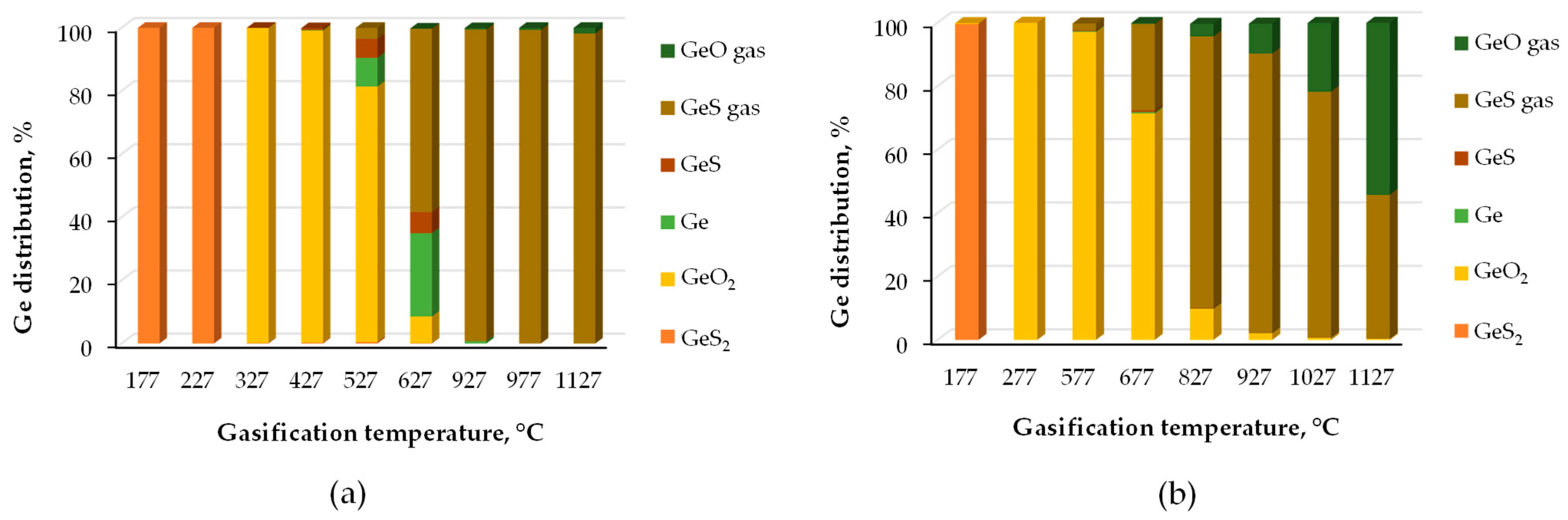

| Category | Source | Concentration Range (Median) | Unit | Ref. |
|---|---|---|---|---|
| Space | Meteorites | 0.01–2000 | ppm | [28,29] |
| Earth | Core | (37) | ppm | [22] |
| Bulk | (13.8) | ppm | [22,29] | |
| Continental crust | 1.0–1.7 (1.4) | ppm | [29,30,31] | |
| Environments | Soils * | 0.02–379 (2.5) | ppm | [31,32,33] |
| Rivers * | 0.7–1487 (9–11) | ppt | [31,34] | |
| Lakes | 0.06–6.8 | ppt | [31] | |
| Hydrothermal waters | 0.006–50 | ppm | [22,31,35] | |
| Oceans | 50–70 (60) | ppt | [29] | |
| Ge-bearing minerals | Silicates | 0.3–700 | ppm | [29] |
| Sulfides | 0.05–5000 | ppm | [22,29] | |
| Oxides, hydroxides | 0.3–10,000 | ppm | [29,36] | |
| Plants | Field soil | 0.001–2.8 | ppm | [37] |
| Greenhouse, soil amended with Ge | 6.8–2086 | ppm | [37] | |
| Aquatic environment * | 7–220 | ppm | [34] |
| Continent | Country | Coal Type | Concentration (Mean), ppm | Refs. |
|---|---|---|---|---|
| World | – | hard (bituminous, anthracite) | (2.4 ± 0.2) | [54] |
| brown (subbituminous, lignite) | (2.0 ± 0.1) | [54] | ||
| Africa | Bostwana | no data | 0.9–9 | [55] |
| Nigeria | bituminous, subbituminous | 0.2–5 | [55] | |
| Tanzania | subbituminous or no data | 1–10 | [55] | |
| South Africa | no data | 1–16 | [56] | |
| Antarctica | - | bituminous–subbituminous | 1–81 (11) | [55] |
| Asia | Afghanistan | bituminous, lignite | 0.2–11 | [55] |
| China | bituminous, subbituminous, lignite | 1–2500 (2.7) | [46] | |
| India | bituminous, lignite | 2–343 | [55,71] | |
| Mongolia | bituminous, subbituminous, lignite | 0.1–1.5 | [55] | |
| Vietnam | anthracite | 0.3–1 | [55] | |
| Australia & Oceania | Australia | bituminous, lignite | 0.5–58 | [55,63] |
| New Zealand | bituminous, subbituminous, lignite | 0.1–3 | [55] | |
| Europe | Czech Republic | bituminous, subbituminous, lignite | 0.1–631 (27) | [55] |
| Hungary | bituminous, subbituminous, lignite | 0.3–5 | [55] | |
| Poland | bituminous | 0.2–2 | [61,62] | |
| Turkey | bituminous, subbituminous, lignite | 0.2–23 (3.7) | [55] | |
| United Kingdom | bituminous | 1–48 (8) | [55,72,73] | |
| North America | Canada | no data | 0.1–2 | [55] |
| United States | bituminous, subbituminous, lignite | 0.03–235 (5.5/7.2) | [49,70] | |
| South America | Argentina | bituminous | 0.7–6 | [55] |
| Brazil | bituminous, subbituminous | 1–99 (13) | [55] | |
| Colombia | bituminous, subbituminous | 0.3–7 | [55] | |
| Peru | anthracite | 0.1–2 | [55] | |
| Venezuela | no data | 0.1–20 (3) | [55] |
| Mine | Sampling Location in Coal Seam | ||
|---|---|---|---|
| Roof | Parting | Floor | |
| Bindong | 10–35 | 4–28 | 8–38 |
| Guojiahe | 10–45 | 1–33 | 8–38 |
| Yuanzigou | 1–33 | 1–33 | 2–35 |
| Zhaoxian | 5–31 | 4–30 | 8–33 |
| Region | Coal | Coal Ash | Fly Ash | Bottom Ash (Slag) | Refs. |
|---|---|---|---|---|---|
| Brazil, power plants | 1.0–6.4 | – | 1.6–41.8 | 1.1–7.2 | [108] |
| Bulgaria, Pernik (feed coal) | <2.0 | <3.7 | – | – | [109] |
| Bulgaria, Pernik (high-grade coal) | <4.5 | <24 | – | – | [109] |
| China, Chongqing power plant | – | – | 4.5 | 1.3 (1.4) | [110] |
| China, Lincang | 1294/825 | 3902/7100 | 38,964 | (96.3) | [57,111] |
| China, Mangniuhai Coalfield | 107 ± 45 | – | 302 ± 58 | 184 ± 74 | [105] |
| China, Wulantuga | 273/727 | 2820/1643 | – | – | [57,111] |
| Greece, Kardia | – | 154–186 L | 1.4 P | – | [112,113] |
| Greece, Megalopolis | – | 134–185 L | 3.3 P | – | [112,113] |
| India, Jharkhand power plant | – | 7.2 L/18.6 | – | – | [114] |
| Italy, power plants | – | – | 7–18 | – | [115] |
| Mexico, Coahuila power plant | – | – | 12 | – | [116] |
| Netherlands, power plants | – | – | 4–35 | – | [115] |
| Poland, Lublin Coal Basin | – | 50–1720 | – | – | [117] |
| Russia, Kuznetsk Basin | 0.9 | 7.5 | – | – | [118] |
| Russia, Minusinsk Basin | 1.5/1.2 | 14.3/11.4 | – | – | [119] |
| Russia, Spetsugli | 1025 | 4906 | 1120/24,600 | (270) | [57] |
| Russia, Western Siberia | 10–67/39–198 L | 62–263/2389–6500 L | – | – | [68] |
| Spain, power plants | 1.5 | – | 1–132 | (4.0) | [120,121,122] |
| Spain, Puertollano Basin | 1–56 | 61 | – | – | [123] |
| South Africa | 56.5/122 | 1135/1330 | 7.5–111 P | – | [56] |
| Turkey, Cayirhan power plant | 3.3 ± 1.3 | 6.6 ± 2.6 | 6.2 ± 1.9 | 2.4 ± 0.3 | [124] |
| Turkey, Kangal power plant | 0.1–1.0 | – | 0.4–2.0 | 0.2–2.6 | [125] |
| Turkey, Seyitömer power plant | 0.01–1.4 | – | 1.2–3.6 | 0.01–1.2 | [126] |
| Turkey, Tunçbilek power plant | 0.7–2.4 | – | 1.3–4.9 | 0.9–1.8 | [126] |
| United Kingdom | 51–70 | 230–1500 | 40–14,000 P | – | [48] |
| USA, Colorado | – | 5–400 | – | – | [127] |
| USA, Kentucky (Gray Hawk) | 6.97 | 175 | – | – | [79] |
| USA, Montana | – | 5–47,000 | – | – | [127] |
| USA, North Dakota | – | 5–430 | – | – | [127] |
| USA, Ohio | – | 5–2600 | – | – | [127] |
| USA, Wyoming | – | 5–650 | – | – | [127] |
| Process Details | Plant Localization | ||
|---|---|---|---|
| Wulantuga, China | Lincang, China | Spetsugli, Russia | |
| Designed capacity | 100 t/y | 39–48 t/y | 21 t/y |
| Coal combustion | vortex furnace | chain conveyor furnace | flare-layered boiler |
| Combustion intensity | 156 kg/m2 | no data | no data |
| Boiler temperature | 1200 °C | no data | no data |
| Fly ash collection | baghouse filter | baghouse fabric filter, 110–120 °C, efficiency 99.8% | no data |
| Ge concentration in fly ash | 14,870 ppm | 38,964 ppm | 24,600 ppm |
| Ge concentration on an ash basis | 35,170 ppm | 46,580 ppm | 29,078 ppm |
| Products | different, including high-purity zone-refined germanium ingots (99.99999–99.999999% Ge) | no data | no data |
| Extraction Method | Leaching Rate | Recovery Method | Recovery Rate | Ref. |
|---|---|---|---|---|
| Acid leaching | no data | Solvent extraction (dihydroxamic acid in sulfonated kerosene) | 99% | [137] * |
| H2SO4 leaching | no data | Salt pyrolysis to produce GeO2 | no data | [137] * |
| H2SO4 + acid (or salt) leaching | 85–90% | no data | no data | [137] * |
| Two-step acid leaching: (1) dilute for Ge, (2) concentrated for Al | no data | High-temperature decomposition of double salt | no data | [137] * |
| Ash chlorination with NH4Cl, HCl leaching | no data | Solvent extraction (dihydroxamic acid) | min. 95% | [137] * |
| Ash suspended in 8–9.6 M HCl in a foam column (air bubbling), room temperature | no data | Absorption of evaporated GaCl4 in a foam column with alkali solution or nonpolar solvent (e.g., CCl4, gasoline). HCl addition followed by GeCl4 distillation. | no data | [93] |
| Ash reductive smelting (to 3–4% Ge alloy), leaching in FeCl3 + Cl2 | no data | Addition of H2SO4 (to 14 M) to chloride Ge-bearing solution, GeCl4 distillation | no data | [93] |
| Region/Source | Coal Type | Coal | Fly Ash | Slag (Bottom Ash) | Refs. |
|---|---|---|---|---|---|
| Poland/Laboratory | bituminous | 0.2–0.3 | 0.8–1.0 | 0.3–0.4 | [147] |
| Poland/Laboratory | lignite | 0.2 | 0.8 | 0.5 | [147] |
| Russia/Laboratory | lignite | 263 | 15,178/1284 * | 317 | [145] |
| Spain/IGCC plant | bituminous + petcoke | – | 194–420 | – | [148,149] |
| Spain/IGCC plant | bituminous (+petcoke) | 10.7–21 | 174–356 | – | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnik, E. Challenges and Opportunities in Hydrometallurgical Recovery of Germanium from Coal By-Products. Molecules 2025, 30, 1695. https://doi.org/10.3390/molecules30081695
Rudnik E. Challenges and Opportunities in Hydrometallurgical Recovery of Germanium from Coal By-Products. Molecules. 2025; 30(8):1695. https://doi.org/10.3390/molecules30081695
Chicago/Turabian StyleRudnik, Ewa. 2025. "Challenges and Opportunities in Hydrometallurgical Recovery of Germanium from Coal By-Products" Molecules 30, no. 8: 1695. https://doi.org/10.3390/molecules30081695
APA StyleRudnik, E. (2025). Challenges and Opportunities in Hydrometallurgical Recovery of Germanium from Coal By-Products. Molecules, 30(8), 1695. https://doi.org/10.3390/molecules30081695






