Separation and Recovery of Trace Silver from Sintering Filtrated Dust of Ferrous Metallurgy via Complexation Leaching
Abstract
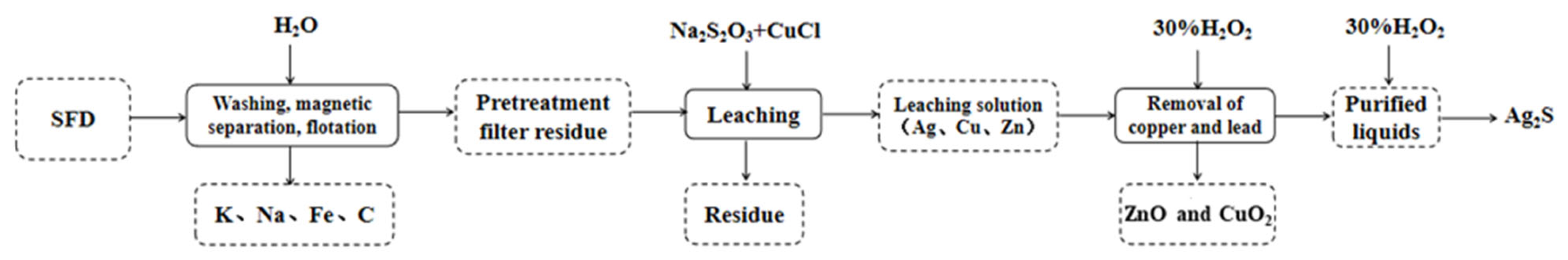
1. Introduction
2. Results and Discussion
2.1. Composition of SFD
2.2. Particle Size Analysis of SFD
2.3. Removal of Potassium from SFD
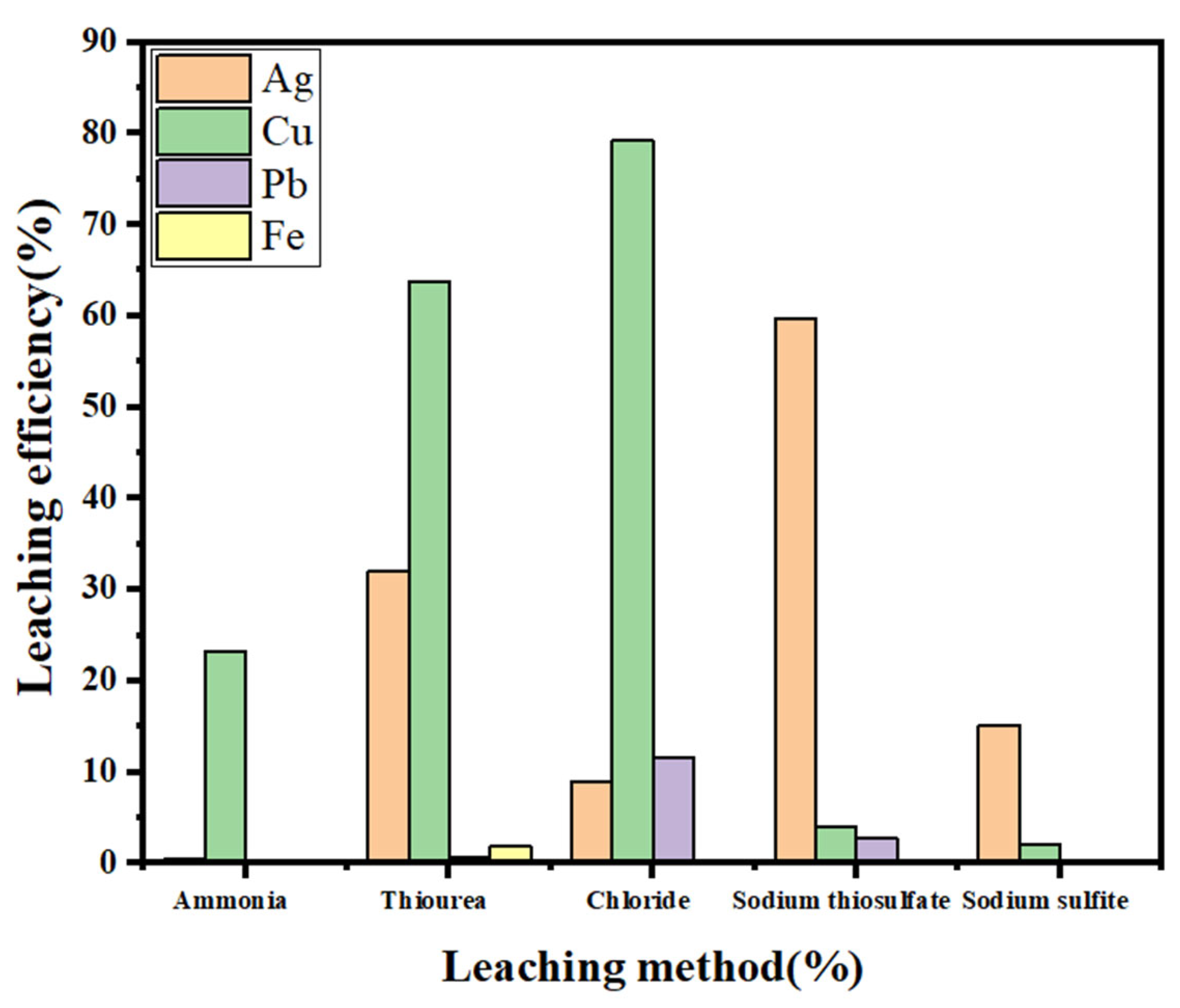
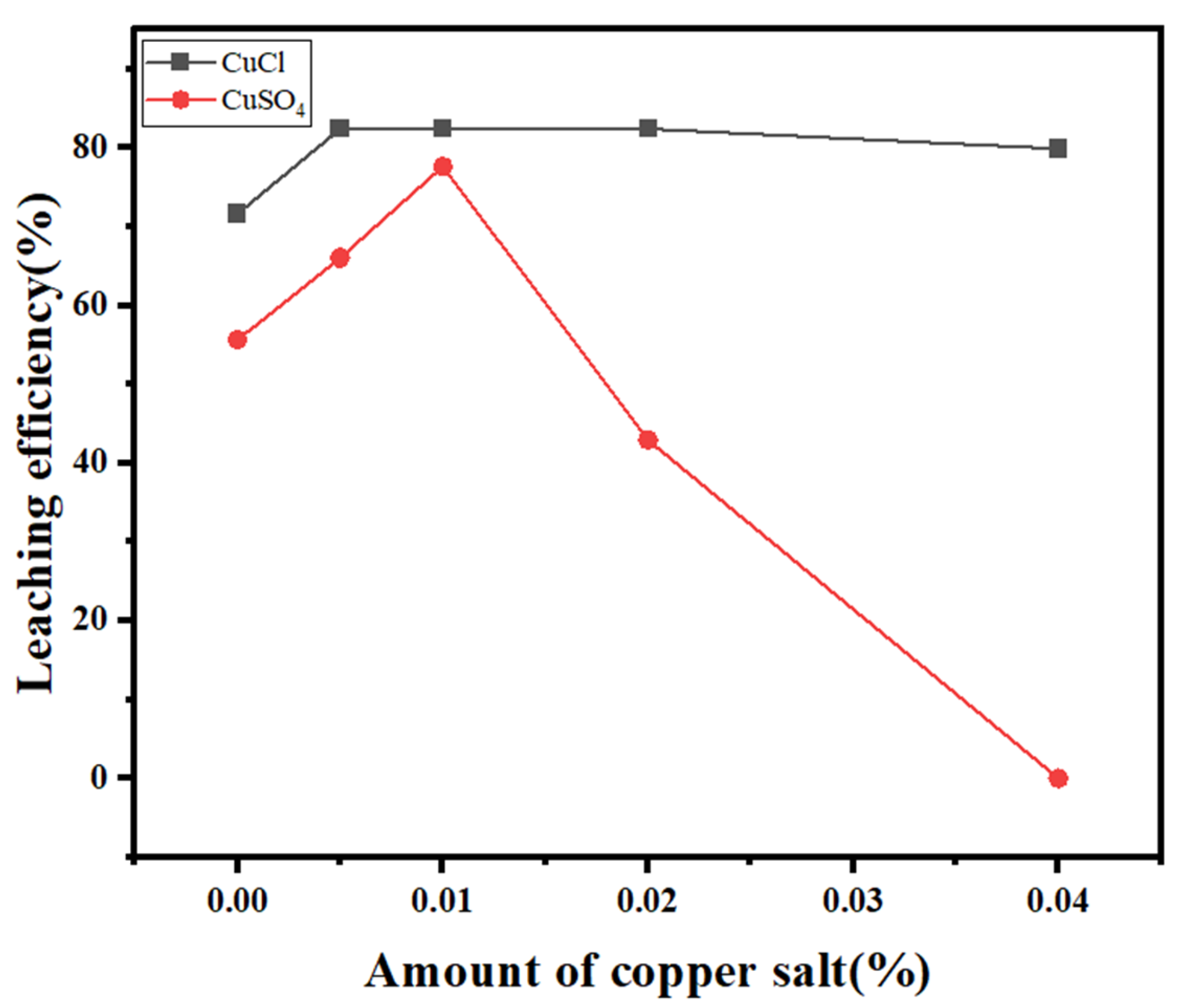
2.4. Na2S2O3-CuCl Leaching of SFD
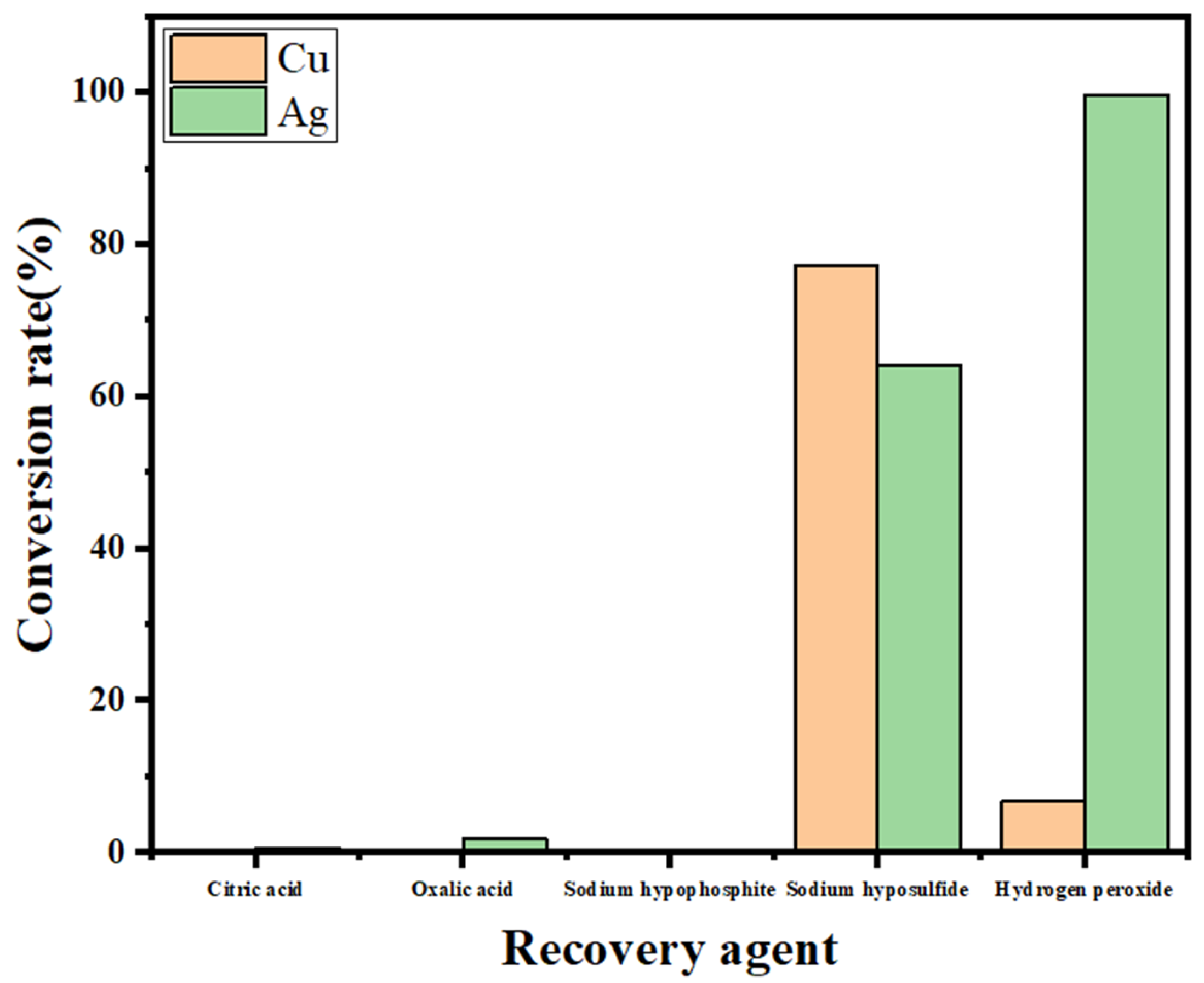
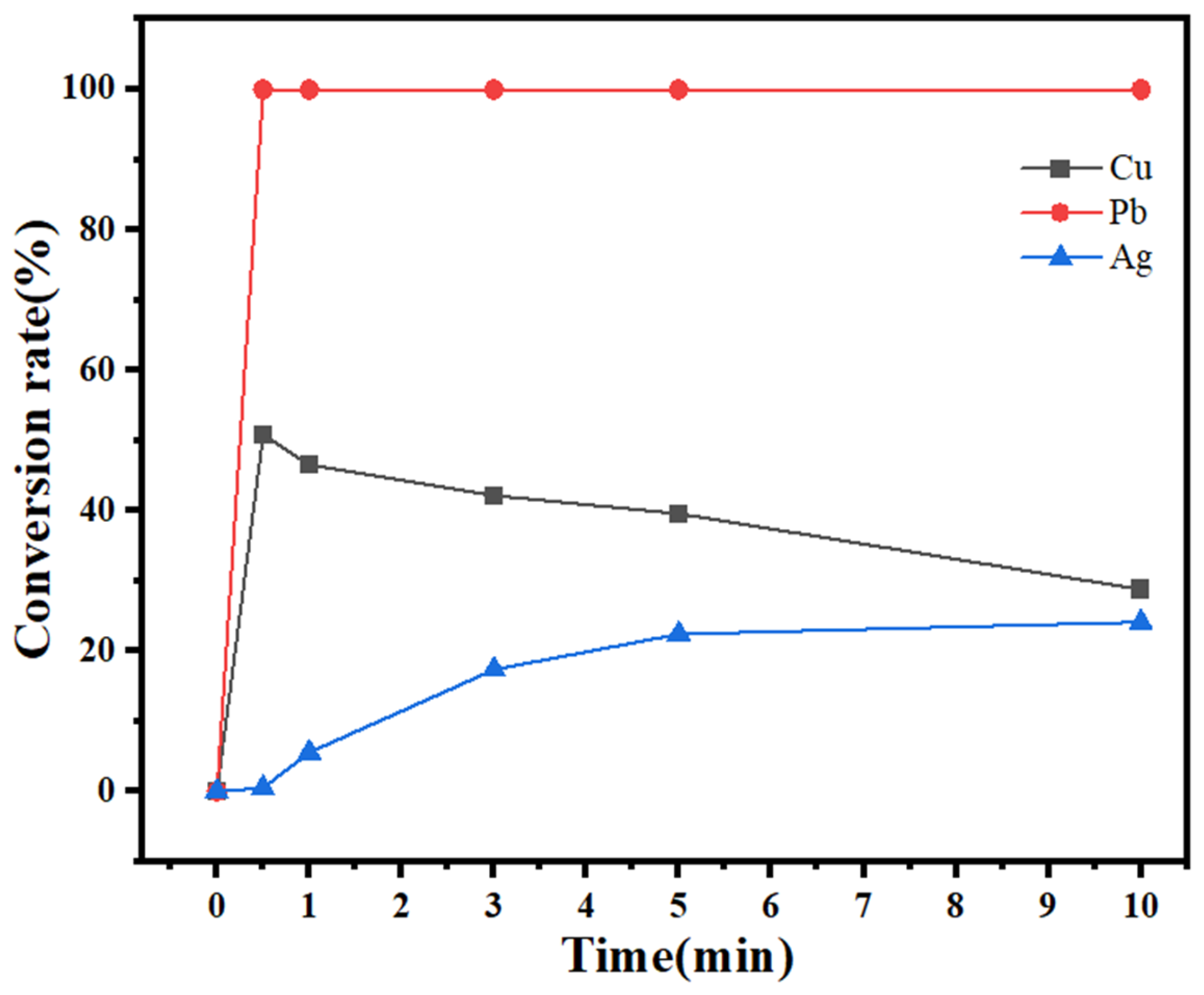
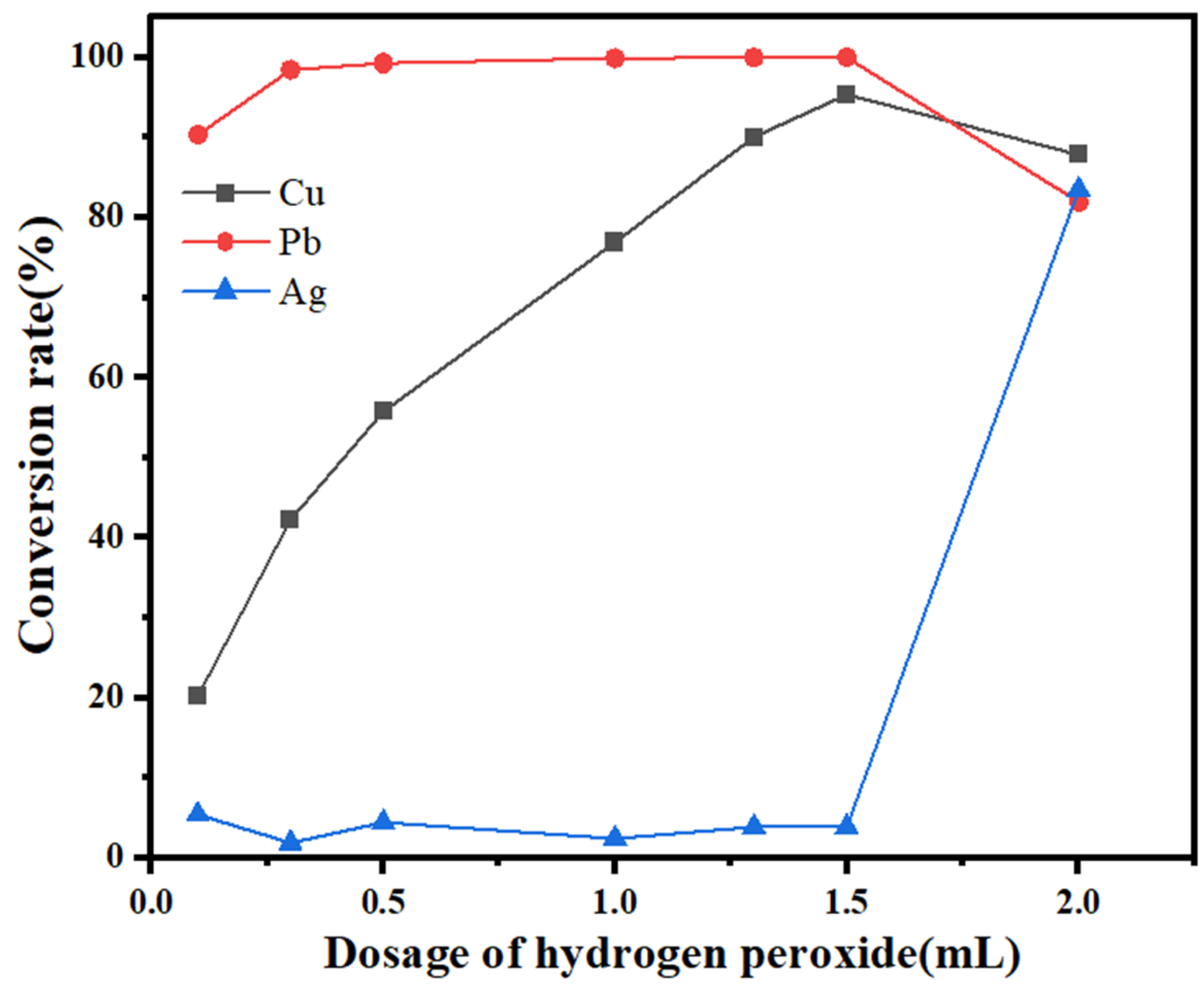
2.5. Removal of Copper and Lead from the Leaching Solution
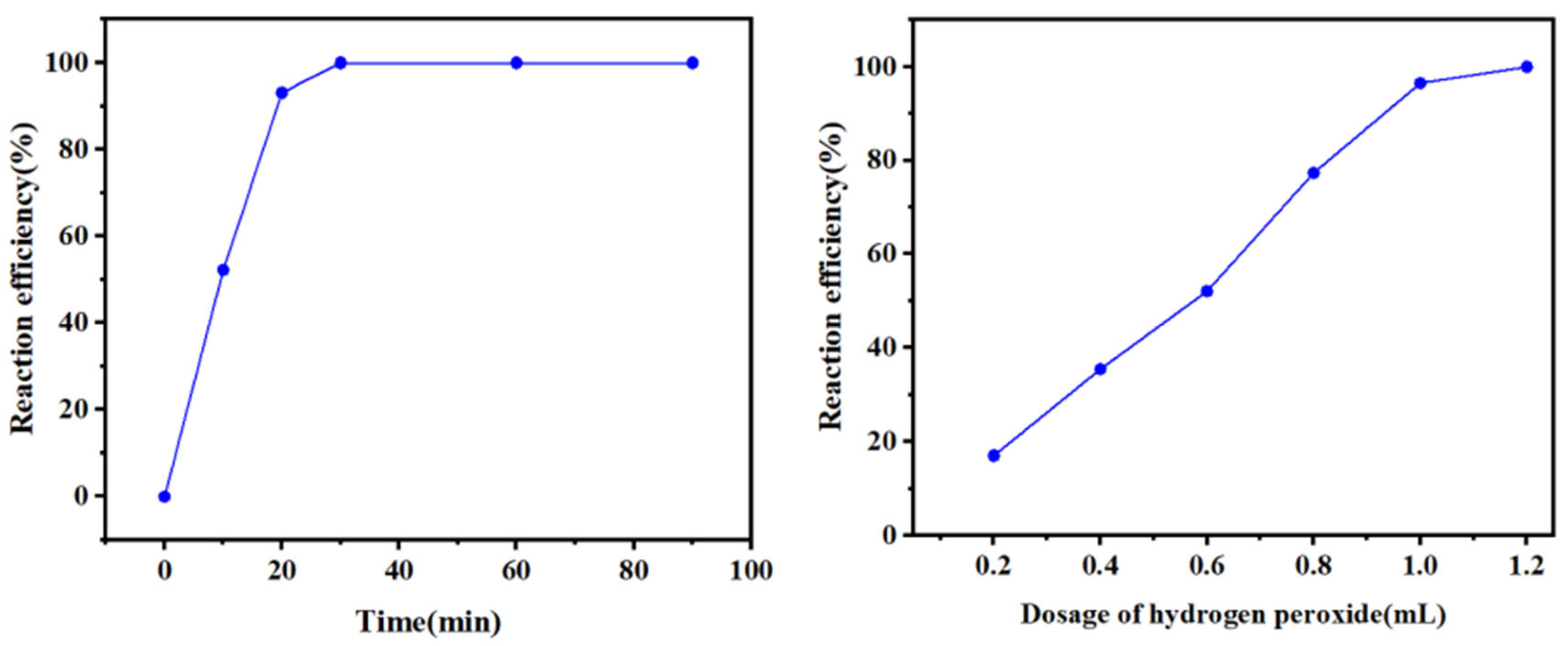
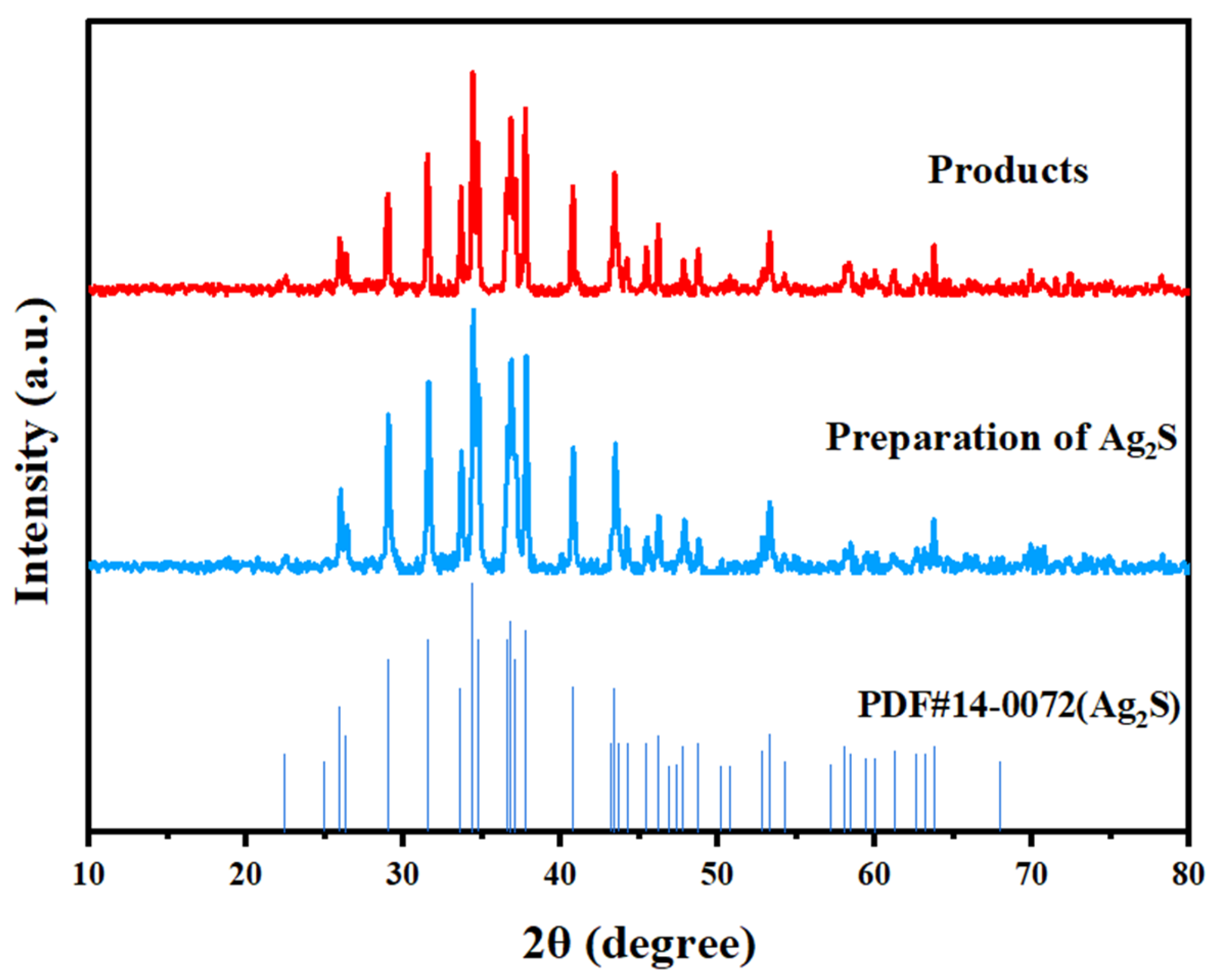
2.6. Separation and Recovery of Silver from Purified Liquids
3. Analysis of the Reaction Mechanism in the Recovery Process
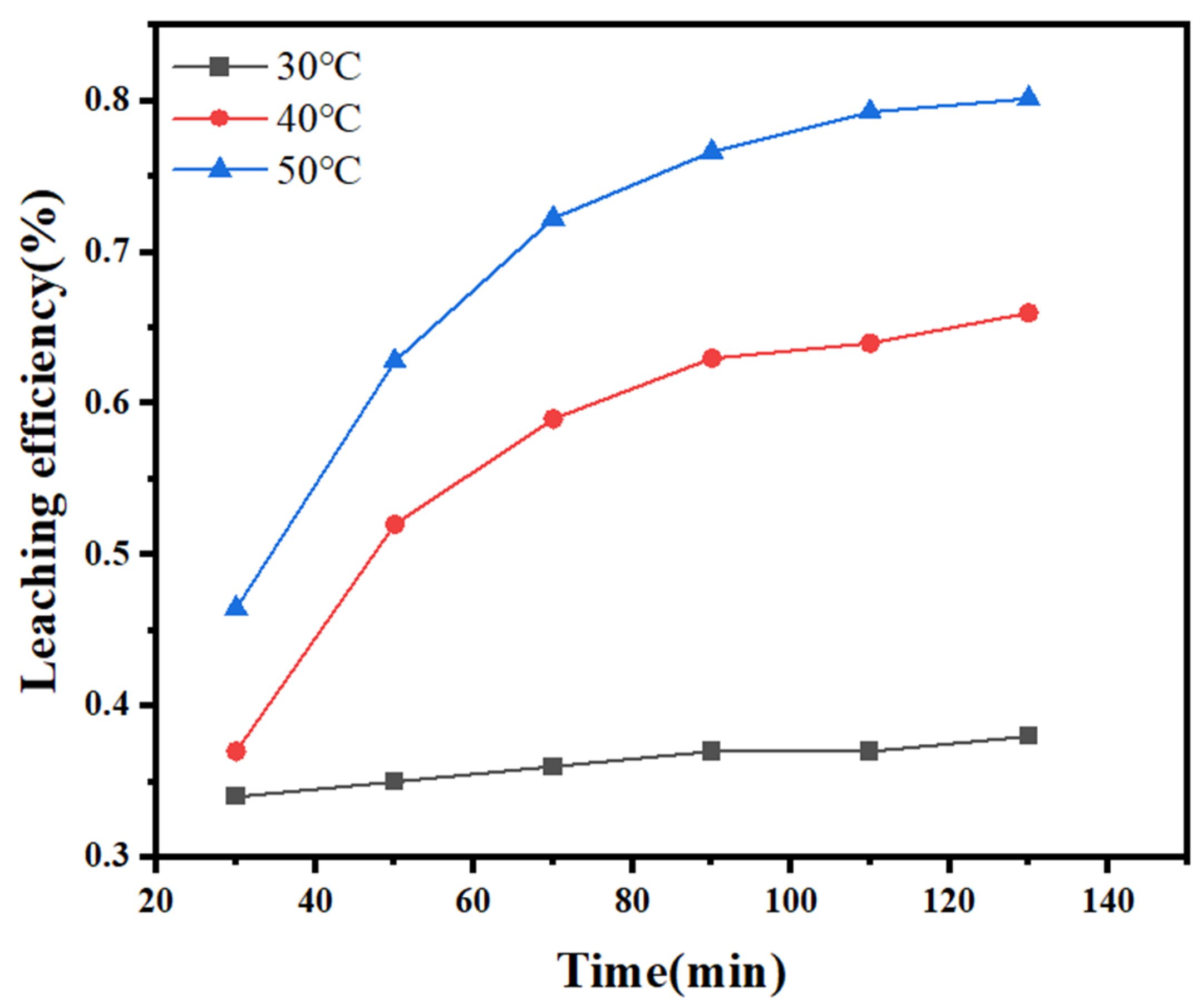
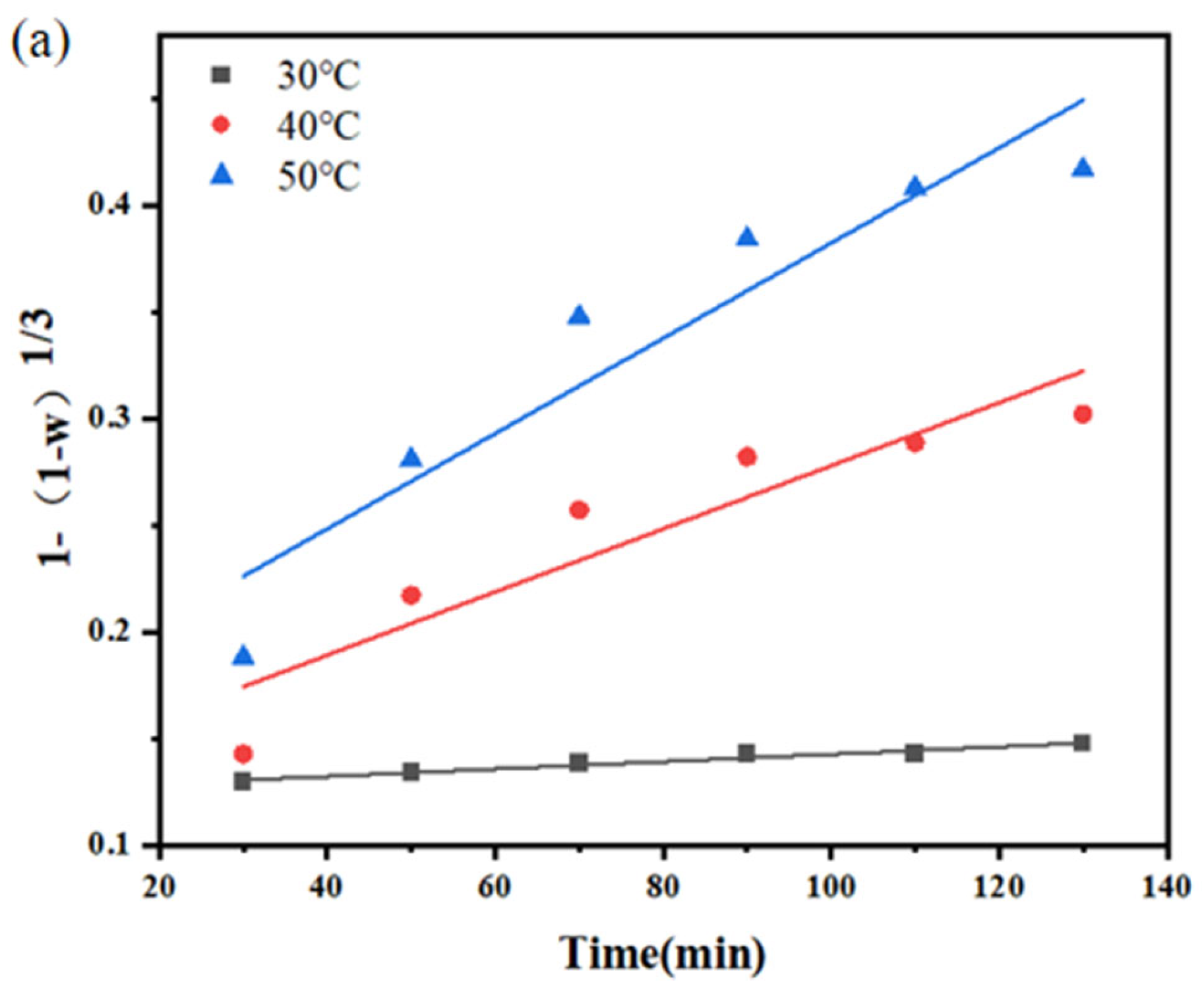
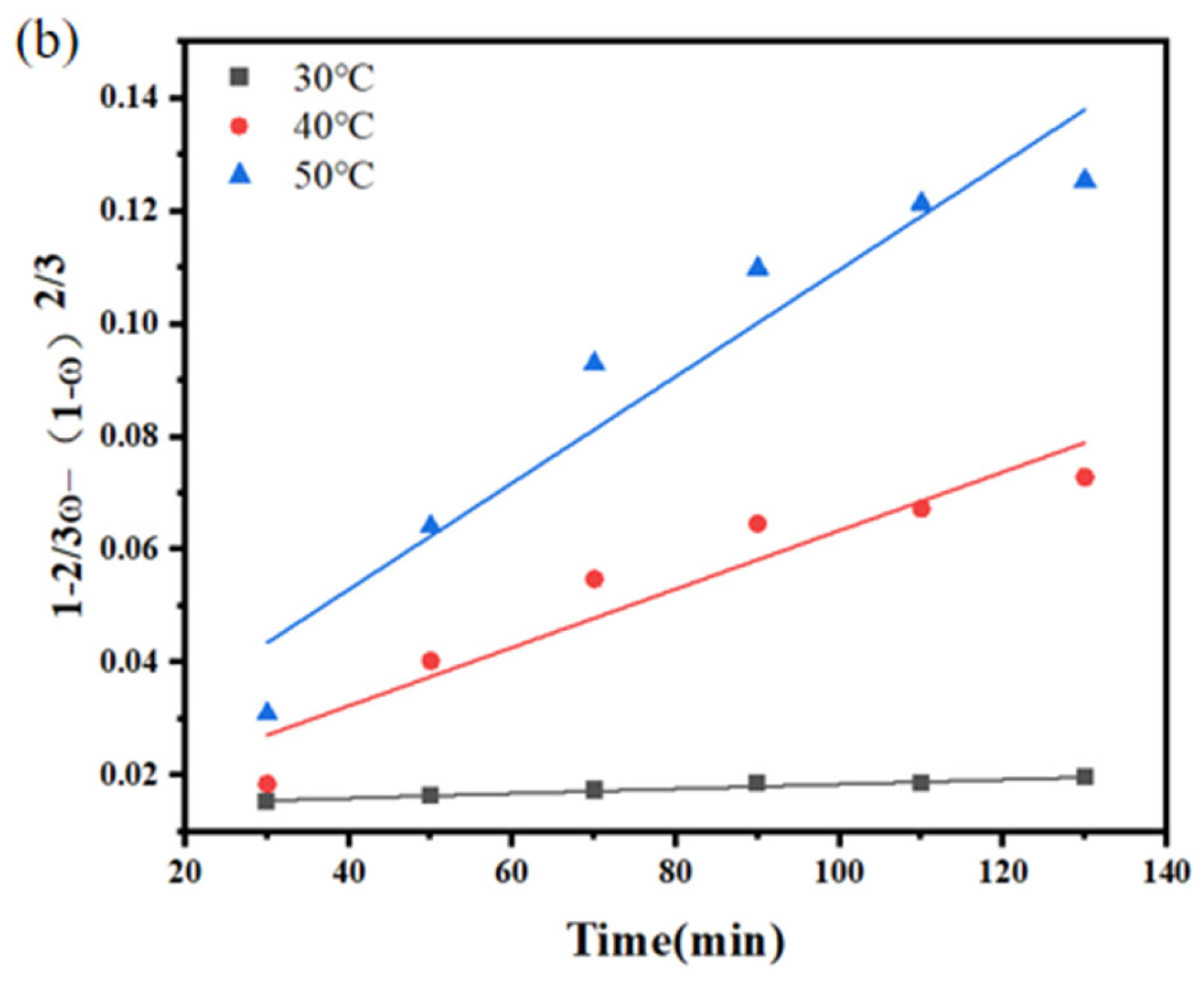
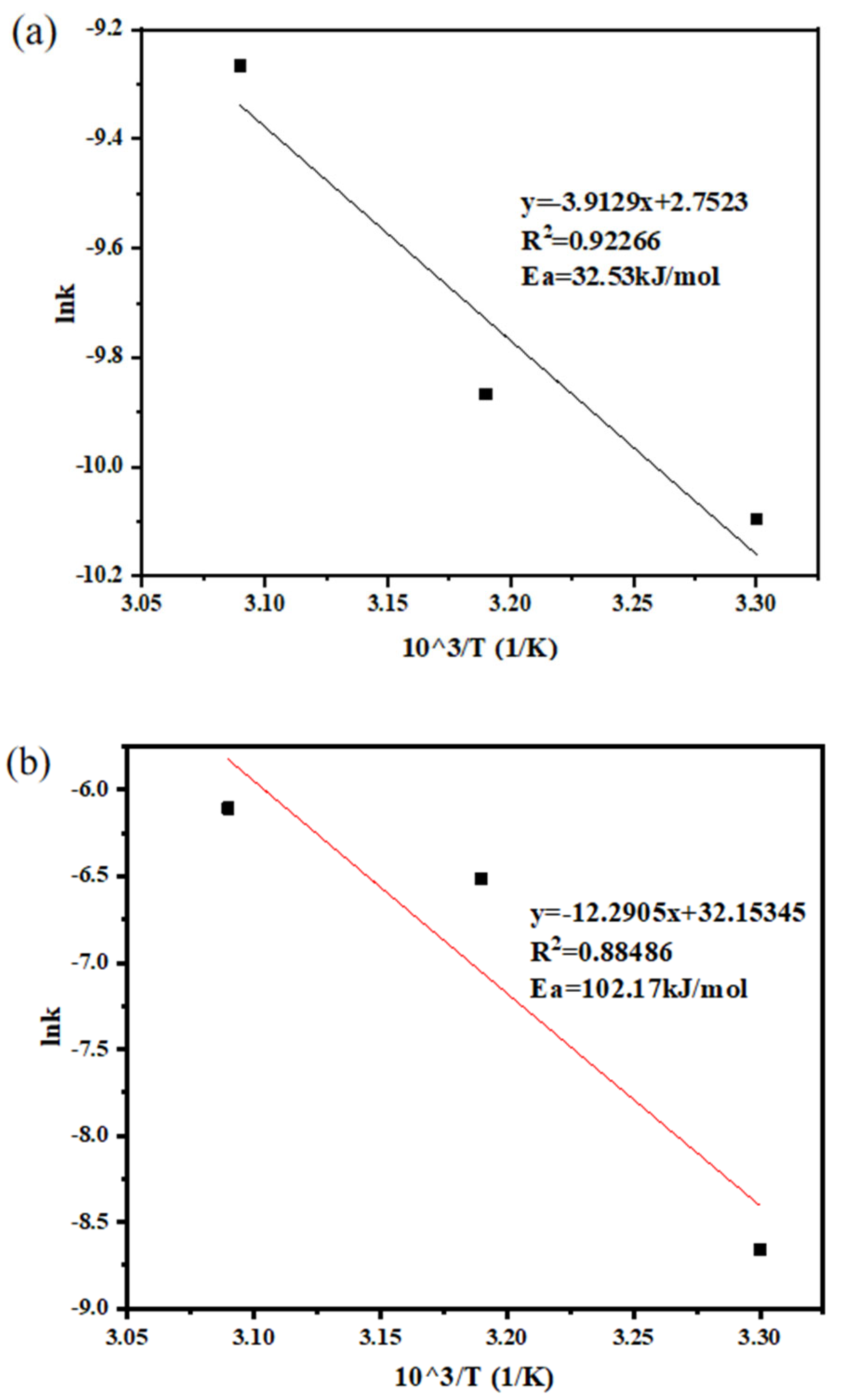
3.1. Kinetics of Sodium Thiosulfate Extraction
3.2. Mechanism of the Removal of Copper and Lead Impurities from the Leaching Solution
4. Materials and Methods
4.1. Main Instruments and Reagents
4.2. Experimental Methods
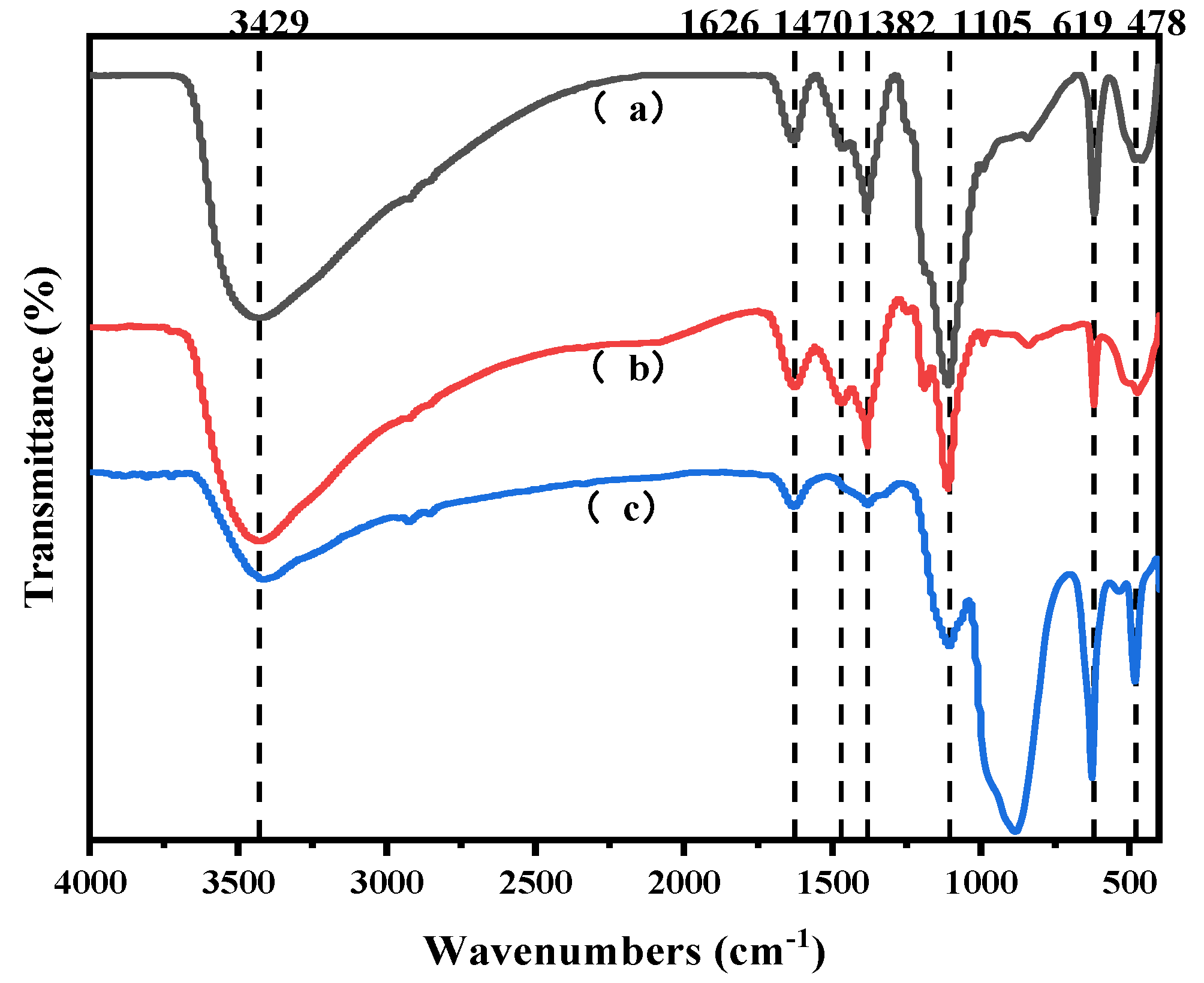
4.2.1. Chemical Composition and Physical Analysis of SFD
- (1)
- Physical phase analysis. The physical phases of the experimental raw materials, i.e., iron and steel metallurgical sintering ash, sintering ash after washing, filter residue after leaching, intermediate products, and final products, were detected and analyzed via a D/max 2550 X-ray diffractometer produced by the Nippon Mechanics Company. The test conditions were as follows: rotating anode, 18 kW; phototube voltage, 40 kV; phototube current, 300 mA; scanning angle range, 5–90°; step size, 0.02°; and scanning speed, 14°/min. The X-ray diffraction (XRD) patterns were analyzed via Jade5.0 software.
- (2)
- Elemental analysis. A total of 0.1000 g of SFD was accurately weighed, completely covered with 2 g of sodium hydroxide, and calcined in a nickel crucible at 700 °C for 2 h. Then, the solid in the crucible was dissolved with 4 mol/L hydrochloric acid and filtered, and the solution was fixed to 100 mL. The solution was diluted several times and analyzed by an atomic absorption spectrometer to determine the content of each major valent metal in the SFD.
- (3)
- Particle size analysis. Using anhydrous ethanol as the dispersing medium and nitrogen as the carrier, the SFD after washing, drying, and sieving was subjected to particle size analysis via a Mastersizer 2000 laser particle size analyzer.
- (4)
- Structural analysis. A Nicolet 6700 Fourier transform infrared spectrometer was used to detect and analyze the structure of the experimental raw materials, i.e., iron and steel metallurgical sintering ash, sintering ash after washing, filtrate residue after leaching, intermediate products, and final products. The instrument test conditions were as follows: resolution, 4 cm−1; sample prepared by a KBr press sheet; scanning, four times; and scanning range, 4000–400 cm−1.
4.2.2. Complexing Agent Selection
4.2.3. Experimental Method of Complexation Leaching via the Sodium Thiosulfate Method
5. Conclusions
- (1)
- The leaching effects of different complexing agents and complexing auxiliaries on each metal element in sintered ash were compared through experiments, the possible reasons for the large difference in the silver leaching rates caused by different methods were analyzed and explored, and the Na2S2O3-CuCl leaching system was finally determined.
- (2)
- Through one-factor experimental exploration and optimization of the Na2S2O3-CuCl co-complexation method, the recommended leaching conditions were obtained as follows: a reaction time of 120 min, a reaction temperature of 60 °C, an L/S of 6:1, a Na2S2O3 concentration of 45 g/L, and a CuCl dosage of 5.0 mol/L. The silver leaching rate under these conditions reached 84.3%, and the main impurity in the sintered ash was copper metal. The leaching rate of lead from the sintered ash was less than 5%, and iron was not leached, resulting in selective leaching of silver.
- (3)
- The optimum conditions for the removal of impurity ions were determined to be as follows: a reaction time of 1 min and a 30% H2O2 dosage of 1.5 mL/100 mL of solution. Under these conditions, the conversion rate of the impurity ion lead reached 100%, the copper conversion rate reached 95%, and the silver loss rate was within 4%. The optimum recovery conditions were as follows: a reaction time of 20 min and a 30% H2O2 dosage of 0.6 mL/50 mL of solution. Under these conditions, silver at a low concentration could be completely converted, and the conversion rate of the remaining copper in the solution was less than 5%. The product obtained by filtration was silver sulfide, the purity of which was determined by titration to be approximately 97%.
- (4)
- The leaching kinetics of the Na2S2O3-CuCl system and the mechanism of the removal of copper and lead impurities from the leaching solution were investigated. The removal process was divided into three steps: first, the generation of hydroxyl radicals from H2O2 catalyzed by cuprous thiosulfate; second, the reaction of hydroxyl radicals with hydrogen peroxide to generate superoxide radicals; and third, the combination of superoxide radicals with cuprous thiosulfate to generate insoluble intermediates of ternary metal ion–peroxo structure–ligand complexes.
- (5)
- The process is easy to perform, is environmentally friendly, and significantly improves the silver recovery efficiency, providing a new technological pathway for the recovery of silver from industrial wastes, which is highly important for realizing the sustainable use of resources.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz, L.A.; Lister, T.E.; Parkman, J.A.; Clark, G.G. Comprehensive process for the recovery of value and critical materials from electronic waste. J. Clean. Prod. 2016, 125, 236–244. [Google Scholar] [CrossRef]
- Bakas, I.; Fischer, C.; Haselsteiner, S.; McKinnon, D.; Milios, L.; Harding, A.; Kosmol, J.; Plepys, A.; Tojo, N.; Wilts, H.; et al. Present and Potential Future Recycling of Critical Metals in WEEE; Copenhagen Resource Institute: Copenhagen, Denmark, 2014. [Google Scholar]
- Zientek, M.L.; Loferski, P.J. Platinum-Group Elements: So Many Excellent Properties; USGS Mineral Resources Program: Reston, VA, USA, 2014. [Google Scholar]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Z.; Xu, J.; Huang, B. Effect of Al2O3 phase on the catalytic performance for HCHO oxidation over Ag/Al2O3 catalysts. Appl. Catal. Gen. 2020, 602, 117705. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Yu, Y.; He, H. Role of silver species in H2-NH3-SCR of NOx over Ag/Al2O3 catalysts: Operando spectroscopy and DFT calculations. J. Catal. 2021, 395, 1–9. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Lin, J.; Wang, Y.; Shi, X.; Yu, Y.; He, H. Unraveling the mechanism of ammonia selective catalytic oxidation on Ag/Al2O3 catalysts by operando spectroscopy. ACS Catal. 2021, 11, 5506–5516. [Google Scholar] [CrossRef]
- Miranzadeh, M.; Afshari, F.; Khataei, B.; Kassaee, M.Z. Adsorption and photocatalytic removal of arsenic from water by a porous and magnetic nanocomposite: Ag/TiO2/Fe3O4. Adv. J. Chem.-Sect. A 2020, 3, 408–421. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Hong, L.K.; Park, K.T.; Kim, Y.E.; Tan, D.; Jeon, Y.E.; Park, J.E.; Youn, M.H.; Jeong, S.K.; Park, J.; Ko, Y.N.; et al. Ag/C composite catalysts derived from spray pyrolysis for efficient electrochemical CO2 reduction. Chem. Eng. J. 2022, 431, 133384. [Google Scholar] [CrossRef]
- Chmielewski, T.; Gibas, K.; Borowski, K.; Adamski, Z.; Wozniak, B.; Muszer, A. Chloride leaching of silver and lead from a solid residue after atmospheric leaching of flotation copper concentrates. Physicochem. Probl. Miner. Process. 2017, 53, 893–907. [Google Scholar]
- Lei, C.; Yan, B.; Chen, T.; Wang, X.L.; Xiao, X.M. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J. Clean. Prod. 2018, 181, 408–415. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Extraction of metals from waste printed circuit boards (WPCBs) in H2SO4-CuSO4-NaCl solutions. Hydrometallurgy 2013, 139, 30–38. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Cupric chloride leaching (HCl-CuCl2-NaCl) of metals from waste printed circuit boards (WPCBs). Int. J. Miner. Process. 2015, 134, 89–96. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegli’o, F. Overview on hydrometallurgical procedures for silver recovery from various wastes. J. Environ. Chem. Eng. 2018, 6, 2932–2938. [Google Scholar] [CrossRef]
- Jadhav, V.U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Rao, M.D.; Meshram, A.; Singh, K.K. Recovery of silver from waste printed circuit boards (WPCBs) through hydrometallurgical route: A review. Environ. Chall. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Sethurajan, M.M.; Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Tunsu, C.; Retegan, T. Hydrometallurgical processes for the recovery of metals from WEEE. In WEEE Recycling: Research, Development, and Policies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 139–175. [Google Scholar]
- Sun, B.; Zhang, X.L.; Kou, J.; Xing, Y. A review of gold extraction using noncyanide lixiviants: Fundamentals, advancements, and challenges toward alkaline sulfur-containing leaching agents. Int. J. Miner. Metall. Mater. 2020, 27, 417–431. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M.; Staunton, W.P. Effect of minerals on the stability of gold in copper ammoniacal thiosulfate solutions-The role of copper, silver and polythionates. Hydrometallurgy 2014, 143, 12–22. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.S.; Wang, W.Y.; Liu, W.Y.; Fu, Z.G.; Yang, Y.Q. Recovery of potassium chloride from blast furnace flue dust. RSC Adv. 2015, 5, 84901–84909. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, Z.G.; Wu, B.; Lv, N.; Zeng, L.L.; Yang, Y.Q. Recovery of potassium chloride from sintering EAF dust. Chin. J. Process. Eng. 2014, 14, 979–983. [Google Scholar]
- Qiao, Z.; Yang, Y.; Yang, T.; Yang, H.; Li, W.; Li, Z.; Wang, W.; Shen, Z.; Wu, K. Removal and recovery of potassium chloride from sintering filtrated dust of ferrous metallurgy by solvent crystallization and the phase equilibrium of quaternary solution system. J. Environ. Chem. Eng. 2023, 11, 111432. [Google Scholar] [CrossRef]
- Raschman, P.; Popovič, Ľ.; Fedoročková, A.; Kyslytsyna, M.; Sučik, G. Non-Porous shrinking particle model of leaching at low liquid-to-solid ratio. Hydrometallurgy 2019, 190, 105151. [Google Scholar] [CrossRef]
- Gbor, P.K.; Jia, C.Q. Critical evaluation of coupling particle size distribution with the shrinking core model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Puente-Siller, D.M.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. A kinetic thermodynamic study of silver leaching in thiosulfate-copper-ammonia-EDTA solutions. Hydrometallurgy 2013, 124–131. [Google Scholar] [CrossRef]
- Islas, H.; Flores, M.U.; Juárez, J.C.; Reyes, M.; Blanco, A.; Gutiérrez, E.J.; Aguilar, J.; Nolasco, M.C.; Rodríguez, I.; Reyes, I.A. Silver leaching from jarosite-type compounds using cyanide and non-cyanide lixiviants: A kinetic approach. Miner. Eng. 2021, 174, 107250. [Google Scholar] [CrossRef]
- Rivera, I.; Patiño, F.; Roca, A.; Cruells, M. Kinetics of metallic silver leaching in the O2-thiosulfate system. Hydrometallurgy 2015, 156, 63–70. [Google Scholar] [CrossRef]
- Li, X.B.; Wu, T.; Zhou, Q.-S.; Qi, T.-G.; Peng, Z.-H.; Liu, G.-H. Kinetics of oxidation roasting of molybdenite with different particle sizes. Trans. Nonferrous Met. Soc. China 2021, 31, 842–852. [Google Scholar] [CrossRef]
- Popovič, Ľ.; Raschman, P.; Spišák, J.; Fedoročková, A.; Sučik, G. Generalized shrinking particle model of leaching: Effect of the order of surface chemical reaction, liquid-to-solid ratio and non-ideal behaviour of the liquid phase. Hydrometallurgy 2020, 196, 105441. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Quan, X.J.; Feng, C.F.; Jian, C.Z. Study on liquid phase oxidation leaching of chromite by microwave enhanced roasting. J. Chongqing Univ. Technol. Nat. Sci. 2023, 37, 315–320. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Székely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid and Liquid-Solid Reactions; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Roldán-Contreras, E.; Salinas-Rodríguez, E.; Hernández-Ávila, J.; Cerecedo-Sáenz, E.; Rodríguez-Lugo, V.; Jeldres, R.I.; Toro, N. Leaching of Silver and Gold Contained in a Sedimentary Ore Using Sodium Thiosulfate: A Preliminary Kinetic Study. Metals 2020, 10, 159. [Google Scholar] [CrossRef]
- Alvarez, P.; Lagos, M.; Jeldres, R.I. Thiosulfate Leaching of Gold: A Review of Kinetic Models and Mechanistic Insights. Hydrometallurgy 2023, 223, 105978. [Google Scholar]
- Cui, Y.Q. Process and Mechanism Study of Silver Sulphide Ore Leaching by Ammonia-Free Thiosulphate Method. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2009. [Google Scholar]
- Cai, X. Mechanistic Study of Zinc Replacement of Silver in Ammonia-Free Thiosulfate Precious Liquid. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2022. [Google Scholar]
- Chen, X. Study on the Role and Influence of Common Minerals in the Process of Gold Leaching by Thiosulphate. Ph.D. Thesis, Northeastern University, Boston, MA, USA, 2020. [Google Scholar]
- Rajput, A.; Sharma, A.K.; Barman, S.K.; Saha, A.; Mukherjee, R. Valence tautomerism and delocalization in transition metal complexationes of o-amidophenolates and other redox-active ligands. Some recent results. Coord. Chem. Rev. 2020, 414, 213240. [Google Scholar] [CrossRef]
- Jannesari, L.; Akhavan, O.; Hosseini, H.R.M.; Bakhshi, B. Graphene/CuO2 Nanoshuttles with controllable release of oxygen nanobubbles promoting interruption of bacterial respiration. ACS Appl. Mater. Interfaces 2020, 12, 35813–35825. [Google Scholar] [CrossRef] [PubMed]
- Orbán, M.; Kurin-Csörgei, K.; Rábai, G.; Epstein, I.R. Mechanistic studies of oscillatory copper(II) catalyzed oxidation reactions of sulfur compounds. Chem. Eng. Sci. 2000, 55, 267–273. [Google Scholar] [CrossRef]
- Navarro, C.; Diaz, M.; Villa-Garcia, M.A. Physico-Chemical characterization of steel slag. Study of its behavior under simulated environmental conditions. Environ. Sci. Technol. 2010, 44, 5383. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Lui, X. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. The reduction of copper(II) and the oxidation of thiosulfate and oxysulfur anions in gold leaching solutions. Hydrometallurgy 2003, 70, 163–173. [Google Scholar] [CrossRef]
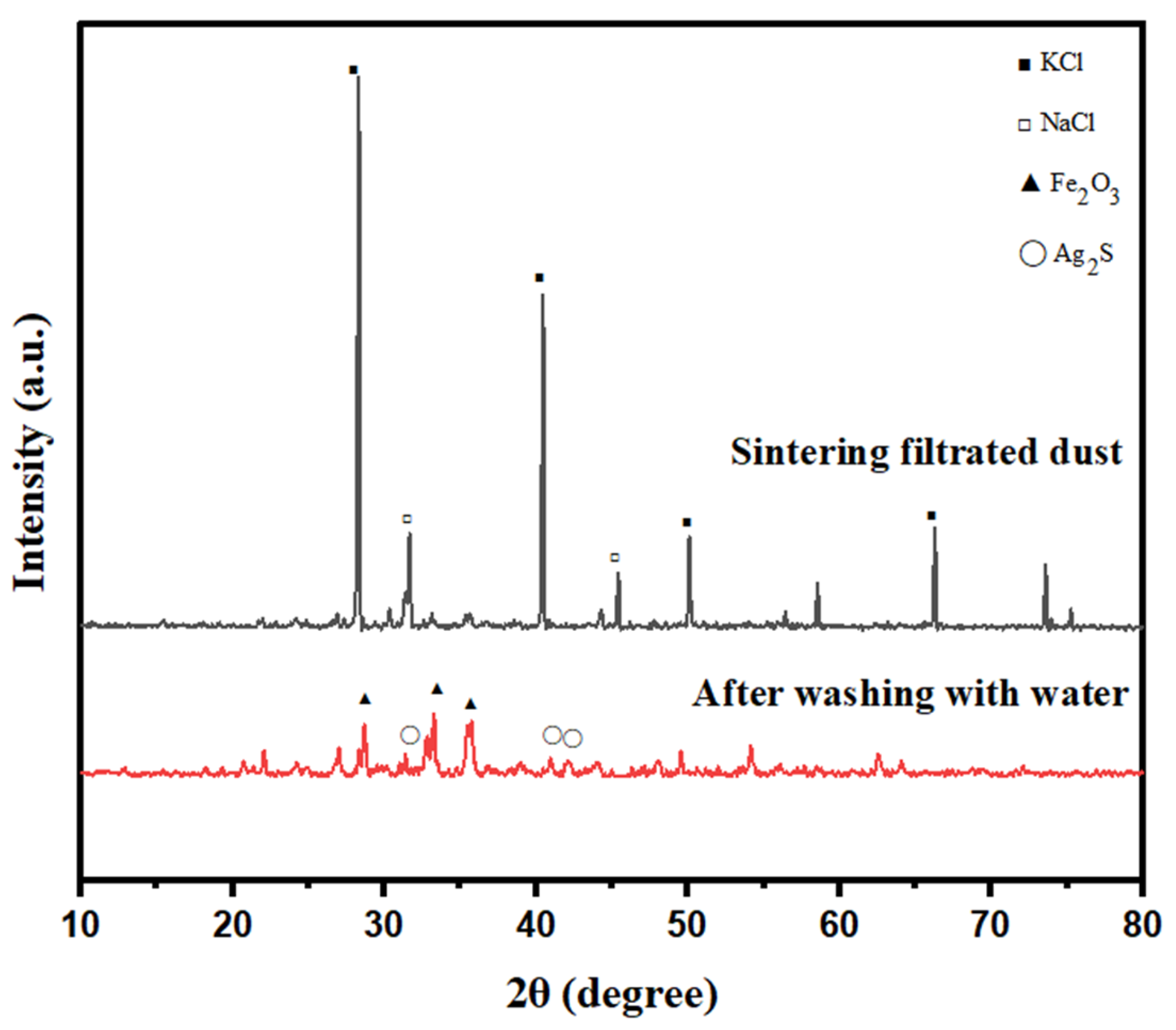

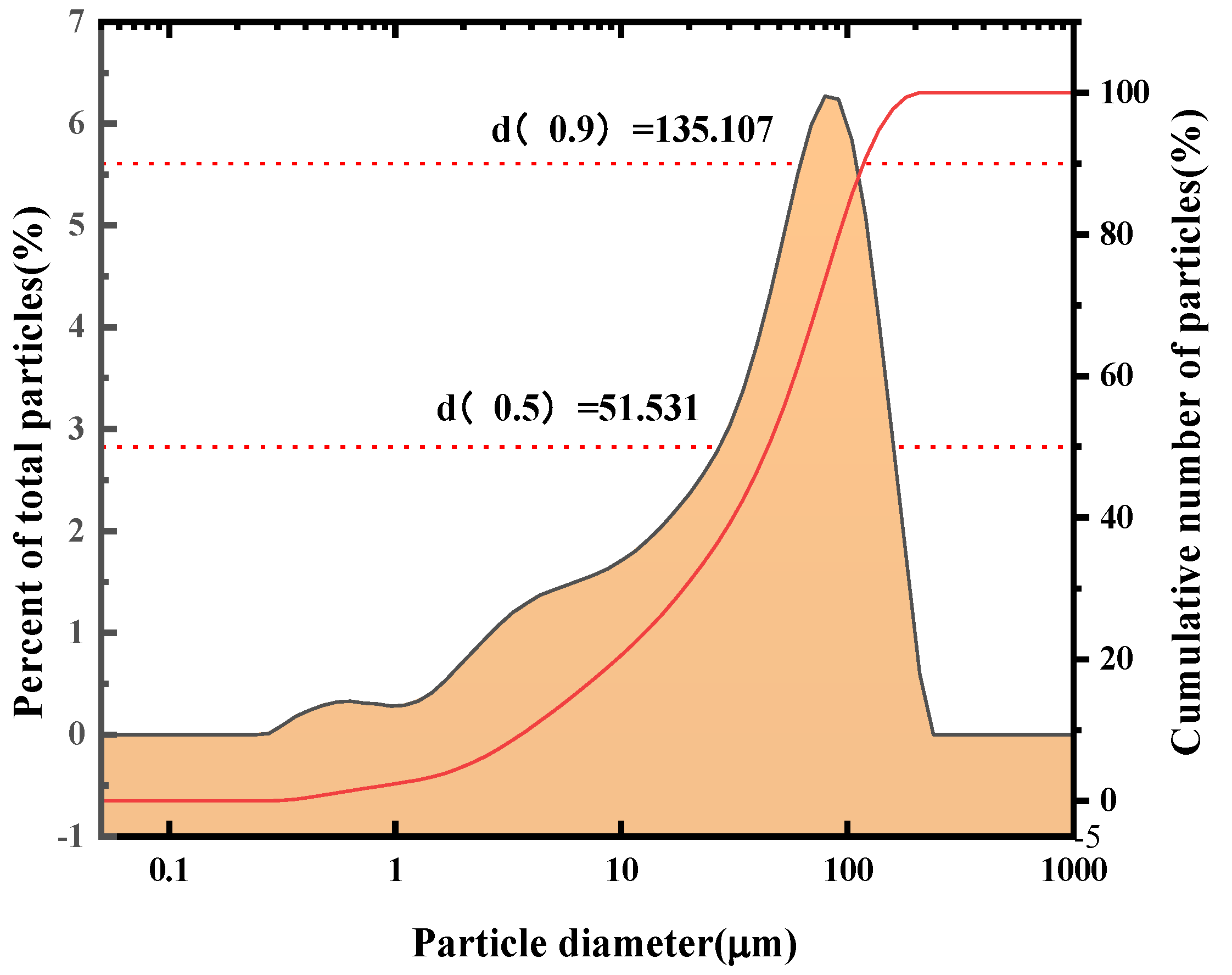
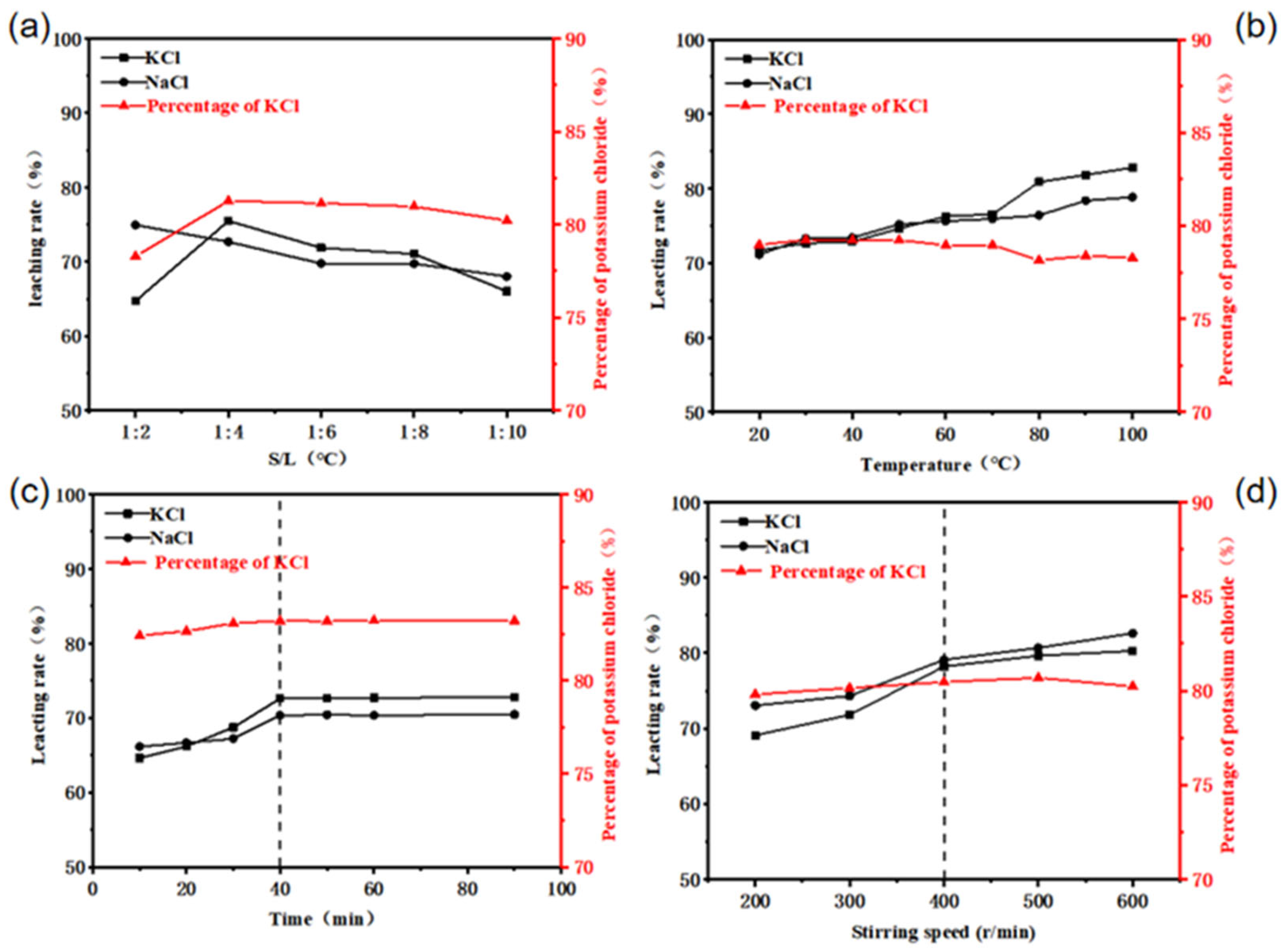
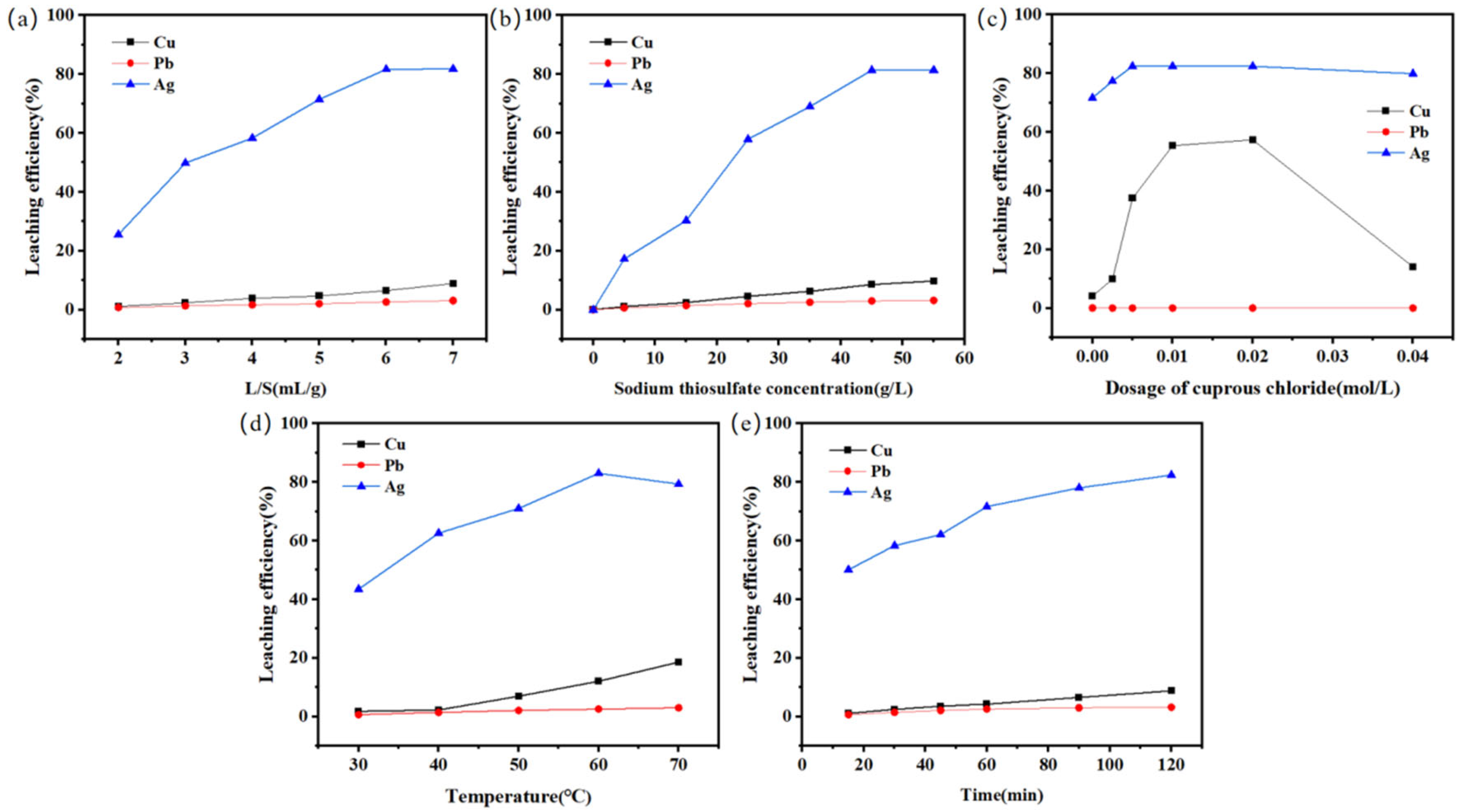
| Element | K | Sodium Carbonate (Chemistry) | Fe | Pb | Cu | Ag |
|---|---|---|---|---|---|---|
| Raw ash content (%) | 18.11 | 3.2 | 16.33 | 1.78 | 0.29 | 160 (g/t) |
| Washed ash content (%) | 1.3 | 0.6 | 65.3 | 7.1 | 1.2 | 500 (g/t) |
| Median Diameter (μm) d(0.5) | Volume Average Particle Size (μm) D[4,3] | Surface Area Average Particle Size (μm) D[3,2] | Specific Surface Area (m2/g) |
|---|---|---|---|
| 51.1 | 61.1 | 9.7 | 0.6 |
| Temperature (°C) | Reaction Time (min) | Efficiency Constant k1 | R2 |
|---|---|---|---|
| 30 | 30–130 | 1.73 × 10−5 | 0.9624 |
| 40 | 30–130 | 1.48 × 10−5 | 0.8593 |
| 50 | 30–130 | 2.23 × 10−5 | 0.8912 |
| Temperature (°C) | Reaction Time (min) | Efficiency Constant k2 | R2 |
|---|---|---|---|
| 30 | 30–130 | 4.13 × 10−5 | 0.9650 |
| 40 | 30–130 | 5.19 × 10−5 | 0.8993 |
| 50 | 30–130 | 9.45 × 10−5 | 0.9189 |
| Apparatus | Specification | Manufacturer |
|---|---|---|
| Atomic Absorption Spectrometer | ZA3000 | Hitachi High-tech Co., Ltd., Tokyo, Japan |
| Rotary Evaporator | RE-2000A | Shanghai Yarong Co., Ltd., Shanghai, China |
| Circulating water vacuum pump | SHB-Ⅲ | Zhengzhou Chengke Industry and Trade Co., Ltd., Zhengzhou, China |
| X-ray diffraction (XRD) | D/max 2550 | Rigaku Co., Ltd., Tokyo, Japan |
| Inductively Coupled Plasma Optical Emission Spectrometers (ICP-OES) | Optima 3000 | Perkin-Elmer Co., Ltd., Waltham, MA, USA |
| Laser Particle Sizer | Mastersizer 2000 | Malvern Instruments Co., Ltd., Malvern, UK. |
| Electric Blast Dryer | 101–2AB | Tianjin Teste Instruments Co., Ltd., Tianjin, China |
| Infrared spectrometer | Nicolet 6700 | Thermo Nicolet Co., Ltd., Madison, WI, USA |
| Reagent | Specification | Manufacturer |
|---|---|---|
| Sodium dodecylbenzene sulfonate | AR | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Sodium hyposulfide | AR | Hunan Huihong Reagent Co., Ltd., Changsha, China |
| Sodium sulfurous acid | AR | Hunan Huihong Reagent Co., Ltd., Changsha, China |
| Cuprous chloride | AR | Shanghai Maclean Reagent Co., Ltd., Shanghai, China |
| Ammonia | AR | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| 30% Hydrogen peroxide | AR | Hunan Huihong Reagent Co., Ltd., Changsha, China |
| Silver nitefficiency | AR | Guangzhou Jinzhujiang Chemical Co., Ltd., Guangzhou, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Yang, Y.; Zhang, T.; Chen, W. Separation and Recovery of Trace Silver from Sintering Filtrated Dust of Ferrous Metallurgy via Complexation Leaching. Molecules 2025, 30, 1339. https://doi.org/10.3390/molecules30061339
Qiao Z, Yang Y, Zhang T, Chen W. Separation and Recovery of Trace Silver from Sintering Filtrated Dust of Ferrous Metallurgy via Complexation Leaching. Molecules. 2025; 30(6):1339. https://doi.org/10.3390/molecules30061339
Chicago/Turabian StyleQiao, Zhiqiang, Yunquan Yang, Tian Zhang, and Weishun Chen. 2025. "Separation and Recovery of Trace Silver from Sintering Filtrated Dust of Ferrous Metallurgy via Complexation Leaching" Molecules 30, no. 6: 1339. https://doi.org/10.3390/molecules30061339
APA StyleQiao, Z., Yang, Y., Zhang, T., & Chen, W. (2025). Separation and Recovery of Trace Silver from Sintering Filtrated Dust of Ferrous Metallurgy via Complexation Leaching. Molecules, 30(6), 1339. https://doi.org/10.3390/molecules30061339






