Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies
Abstract
1. Introduction
- Targeted composition—the glass must incorporate specific macro- and micronutrients to address existing soil deficiencies effectively;
- Amorphous structure—the material should exist in a fully vitreous state to enhance its solubility and reactivity compared to its crystalline counterparts;
- Selective solubility—rather than dissolving readily in water, the glass should respond to acidic exudates from plant roots and soil microorganisms, ensuring nutrients are released only when needed;
- Slow release—nutrient leaching should occur gradually throughout the entire plant development cycle, providing a sustained supply of essential elements.
2. Results and Discussion
2.1. Preliminary Glass Investigation
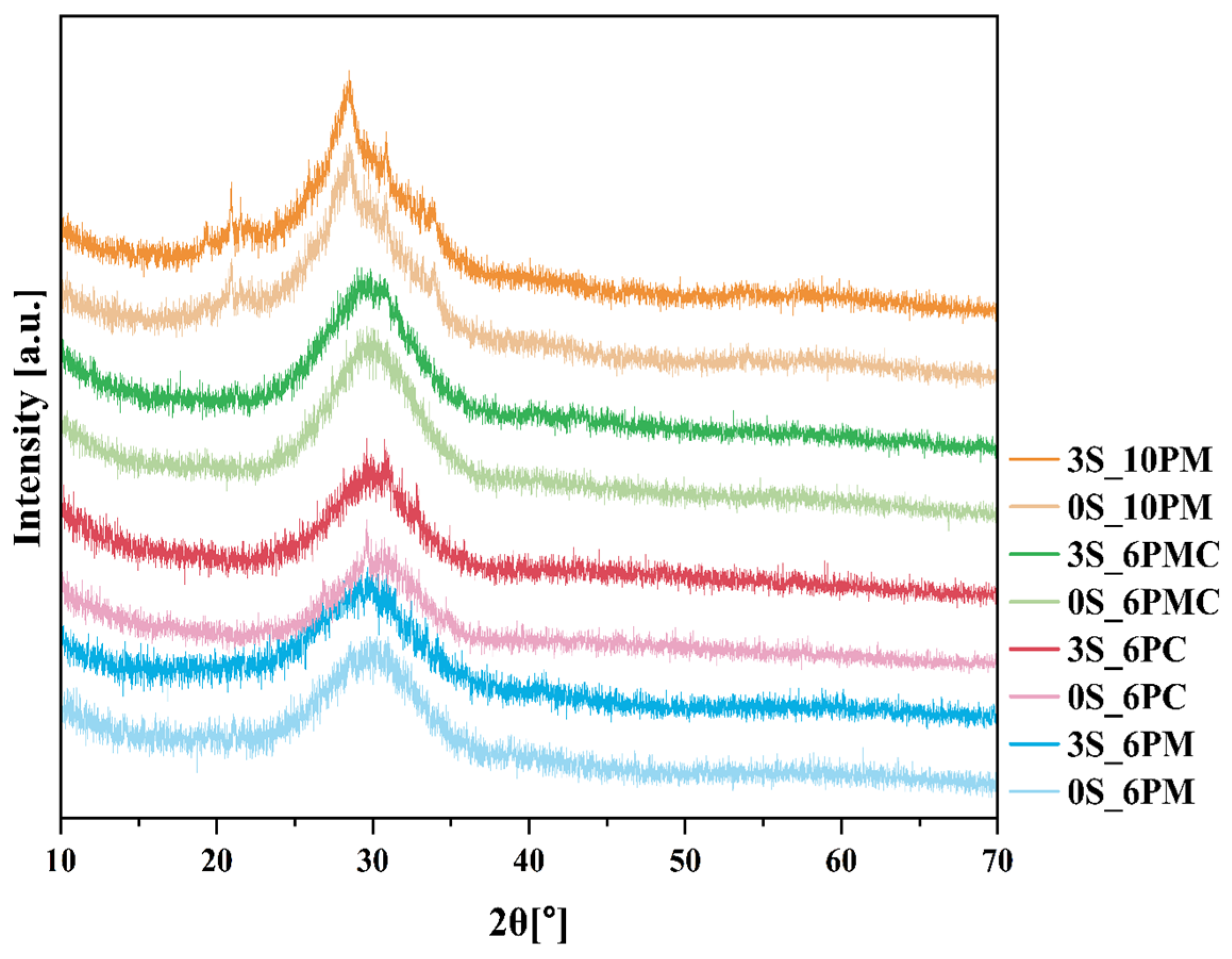
2.1.1. Assessing the Amorphous Nature of Glasses (XRD)
2.1.2. Characterization of Thermal Properties
2.1.3. Structural Analysis of Glasses via 29Si and 31P MAS-NMR Spectroscopy
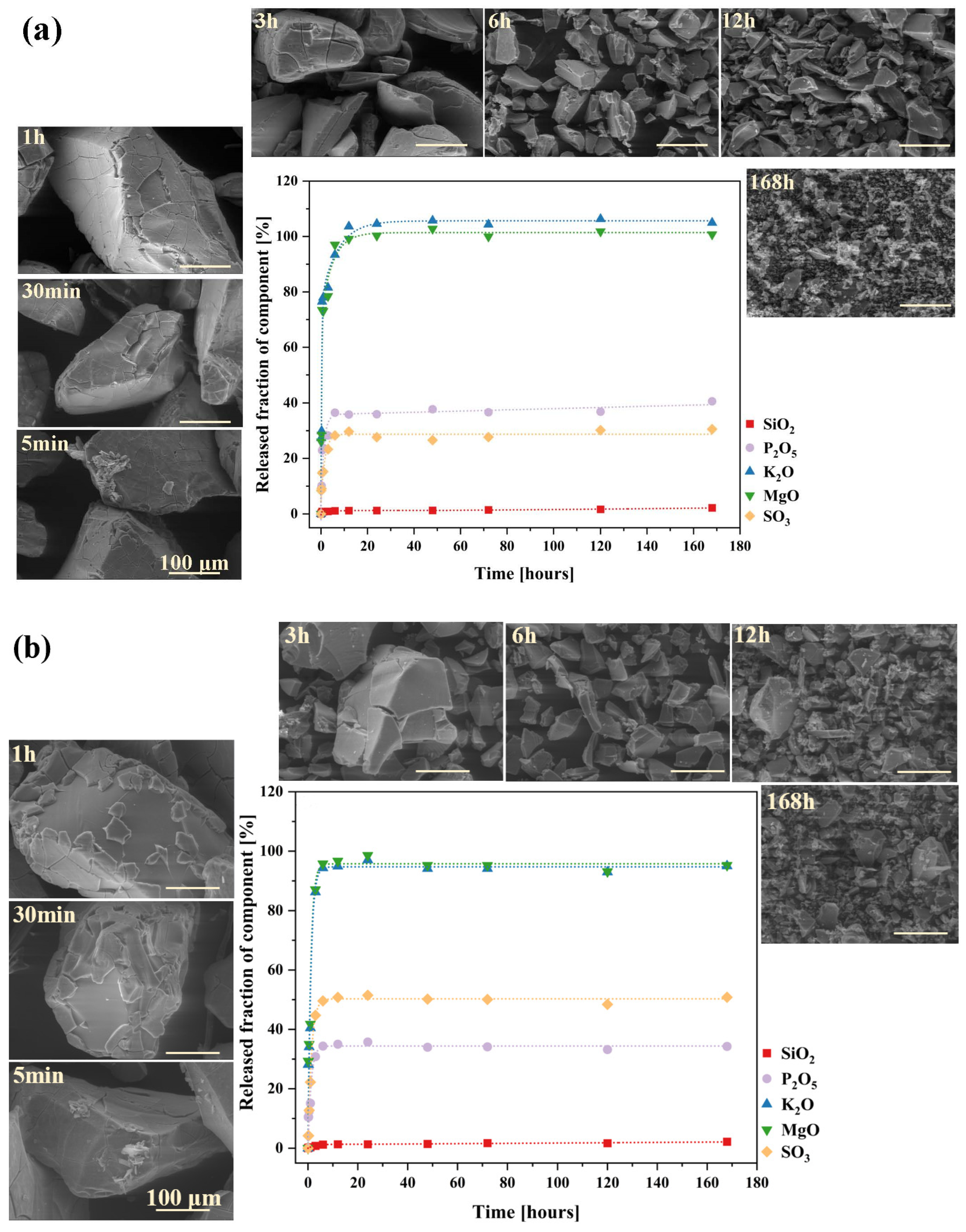
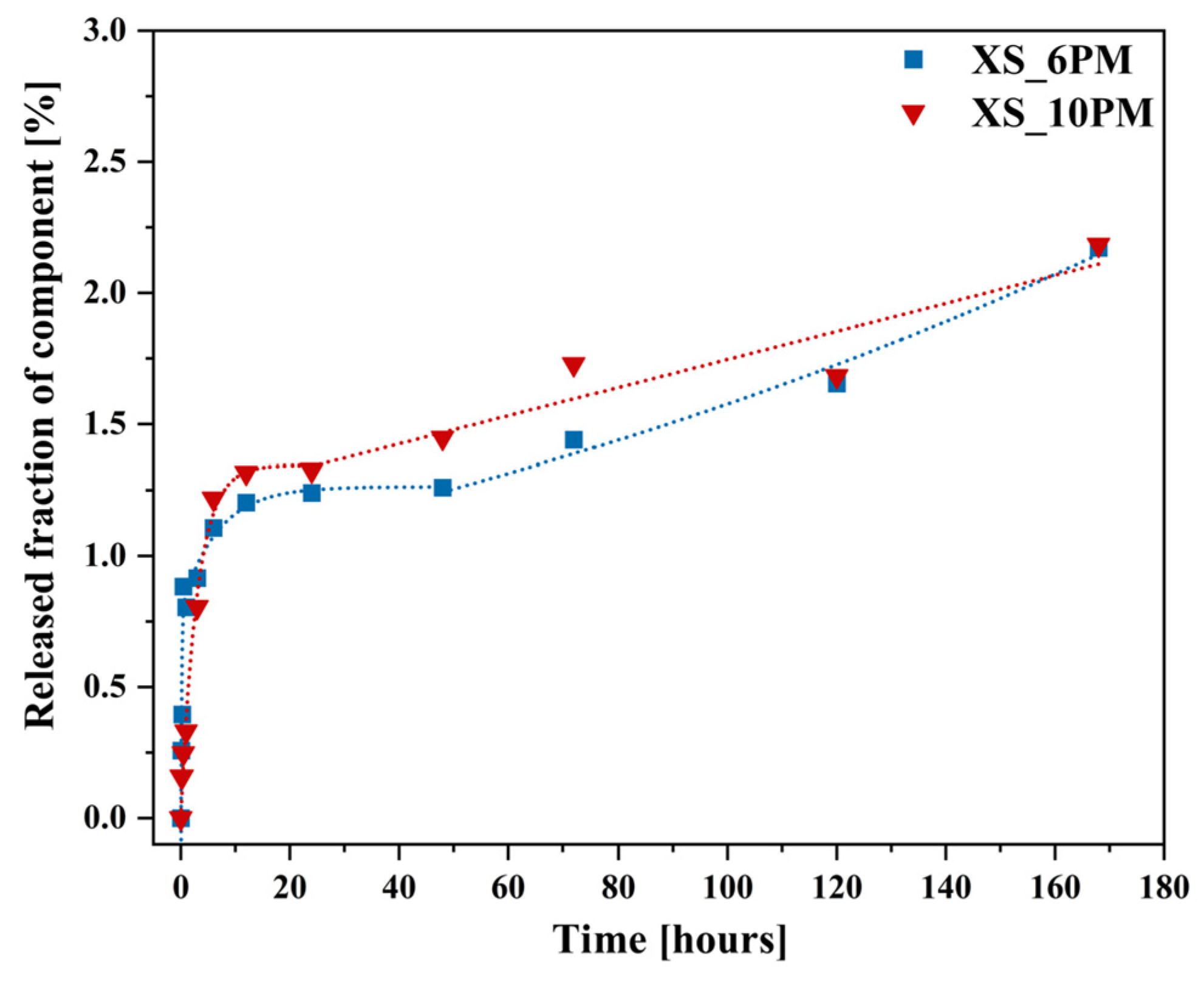
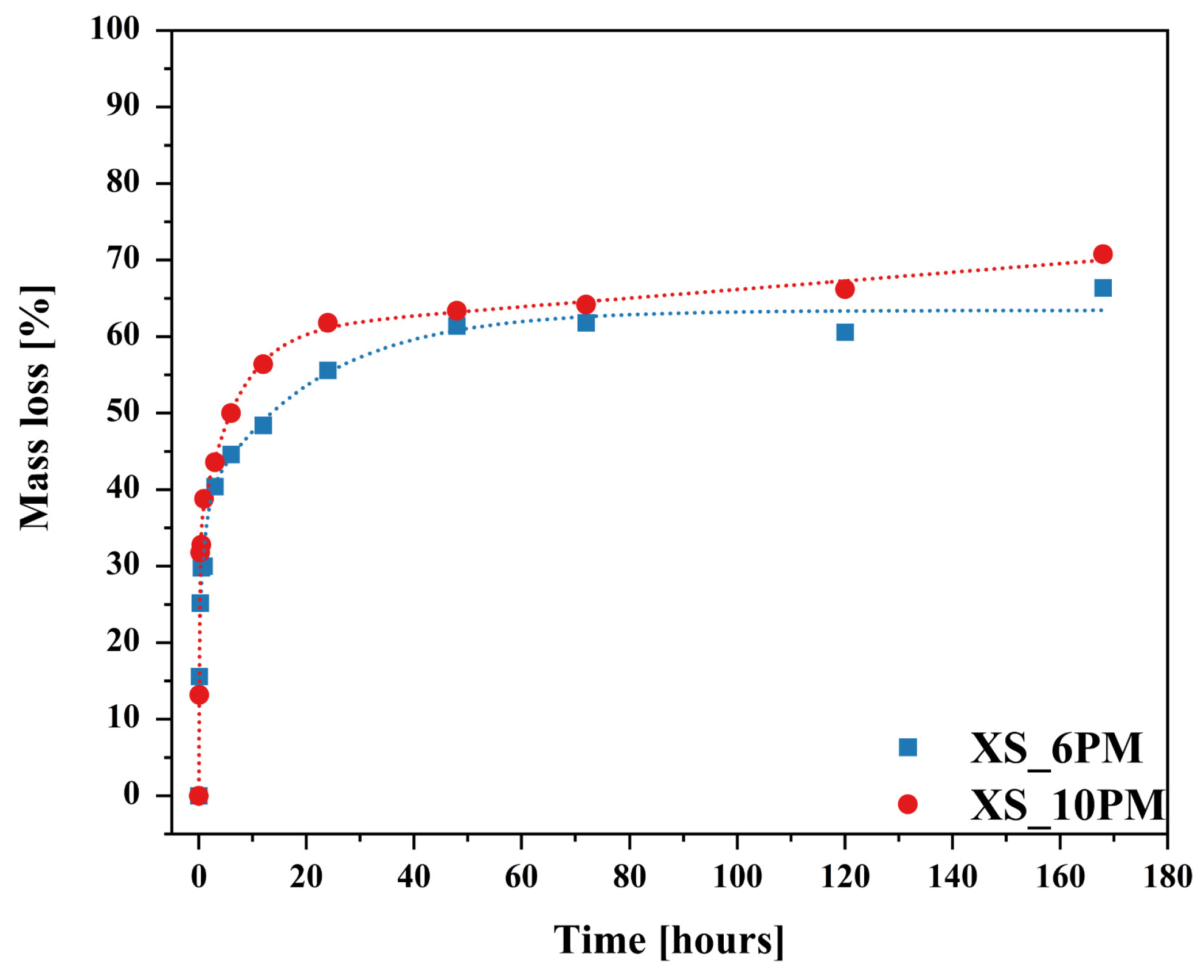
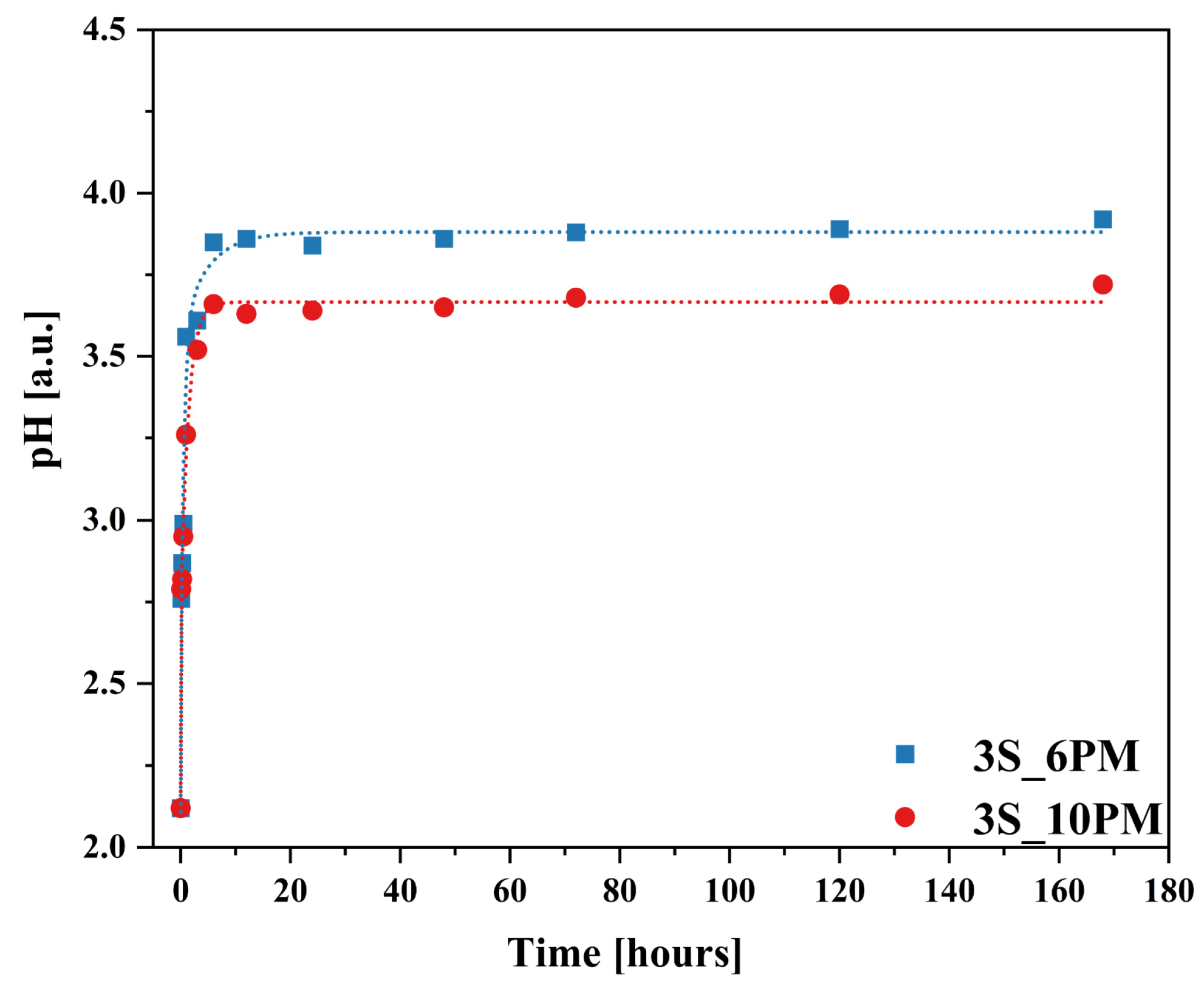
2.2. Glass Dissolution Studies (‘In Vitro’ Experiment)
2.3. Simulation of Long-Term Glass Dissolution (Dissolution Kinetic Studies)
- The glasses should exhibit the highest possible sulfate capacity without exceeding the critical [SO42−] ion concentration, beyond which the precipitation of a highly soluble sulfate-bearing crystalline phase occurs (as observed in the XS_6PM system at a nominal 5 mol.% sulfate loading [80,81]), leading to its immediate release into the environment when the material is used in its intended applications;
- The glasses should contain comparable concentrations of sulfate ions;
3. Conclusions
4. Materials and Methods
4.1. Glass Design, Synthesis, and Preliminary Characterization (XRF, XRD, DSC, MAS-NMR)
- XS_6PM system: 41SiO2∙6P2O5∙20K2O∙33MgO∙XSO3
- XS_6PC system: 41SiO2∙6P2O5∙20K2O∙33CaO∙XSO3
- XS_6PMC system: 41SiO2∙6P2O5∙20K2O∙16.5MgO∙16.5CaO∙XSO3
- XS_10PM system: 41SiO2∙10P2O5∙20K2O∙29MgO∙XSO3
| No | SiO2 | P2O5 | K2O | MgO | CaO | SO3 |
|---|---|---|---|---|---|---|
| 0S_6PM | 41.42 (±0.40) | 6.75 (±0.09) | 20.42 (±0.00) | 30.17 (±0.45) | − | − |
| 1S_6PM | 37.39 (±0.41) | 5.96 (±0.09) | 19.53 (±0.00) | 31.72 (±46) | − | 0.84 (±0.12) |
| 3S_6PM | 40.00 (±0.41) | 6.92 (±0.09) | 20.63 (±0.00) | 32.67 (±0.46) | − | 1.84 (±0.14) |
| 5S_6PM | 41.60 (±0.40) | 6.49 (±0.40) | 17.69 (±0.00) | 33.44 (±0.40) | − | 1.59 (±0.40) |
| 0S_6PC | 39.11 (±0.44) | 6.29 (±0.09) | 18.88 (±0.00) | − | 33.63 (±0.71) | − |
| 1S_6PC | 38.28 (±0.45) | 6.14 (±0.10) | 17.21 (±0.00) | − | 35.79 (±0.73) | 1.00 (±0.16) |
| 3S_6PC | 37.90 (±0.45) | 6.46 (±0.09) | 18.40 (±0.00) | − | 35.12 (±0.72) | 2.05 (±0.13) |
| 5S_6PC | 43.25 (±0.45) | 6.10 (±0.01) | 15.03 (±0.00) | − | 37.42 (±0.72) | 1.39 (±0.15) |
| 0S_6PMC | 39.91 (±0.41) | 6.33 (±0.09) | 18.40 (±0.00) | 15.46 (±0.34) | 18.16 (±0.44) | − |
| 1S_6PMC | 38.20 (±0.42) | 6.33 (±0.09) | 19.59 (±0.00) | 15.53 (±0.33) | 18.09 (±0.56) | 0.95 (±0.12) |
| 3S_6PMC | 38.08 (±0.09) | 6.64 (±0.09) | 18.92 (±0.00) | 16.57 (±0.34) | 20.15 (±0.45) | 1.76 (±0.15) |
| 5S_6PMC | 41.70 (±0.42) | 6.46 (±0.09) | 16.41 (±0.00) | 16.32 (±0.35) | 20.89 (±0.56) | 1.40 (±0.14) |
| 0S_10PM | 38.11 (±0.40) | 11.01 (±0.08) | 21.56 (±0.00) | 29.12 (±0.44) | − | − |
| 1S_10PM | 39.00 (±0.42) | 10.89 (±0.09) | 19.95 (±0.00) | 28.98 (±0.50) | − | 0.25 (±0.05) |
| 3S_10PM | 39.37 (±0.42) | 11.02 (±0.09) | 21.14 (±0.00) | 29.35 (±0.47) | − | 0.83 (±0.10) |
| 5S_10PM | 42.29 (±0.41) | 11.48 (±0.08) | 22.29 (±0.00) | 24.71 (±0.46) | − | 0.48 (±0.07) |
4.2. Glass Dissolution Studies (‘In Vitro’ Conditions)
4.3. Experimental Approach to Glass Dissolution Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiantis, D.; Ginting, F.I.; Gusnidar; Nelson, M.; Minasny, B. Volcanic Ash, insecurity for the people but securing fertile soil for the future. Sustainability 2019, 11, 3072. [Google Scholar] [CrossRef]
- Neall, V.E. Land use and land cover. In Encyclopaedia of Life Support Systems; UNESCO: Paris, France, 2006; Volume 1, pp. 1–24. [Google Scholar]
- Stoch, L.; Wacławska, I.; Szumera, M. Phospho-silicate glasses, self-degradable in environment conditions. Adv. Mater. Res. 2008, 39–40, 335–340. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Vega, N.W.O. A review on beneficial effects of Rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. Nac. Agron.-Medellín 2007, 60, 3621–3643. [Google Scholar]
- Wang, H.-Y.; Shen, Q.-H.; Zhou, J.-M.; Wang, J.; Du, C.-W.; Chen, X.-Q. Plants use alternative strategies to utilize nonexchangeable potassium in minerals. Plant Soil 2011, 343, 209–220. [Google Scholar] [CrossRef]
- Stoch, L.; Wacławska, I.; Ciecińska, M. Thermochemistry of biologically active glasses. J. Therm. Anal. Calorim. 2001, 65, 341–350. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Innovative formulations of phosphate glasses as controlled-release fertilizers to improve tomato crop growth, yield and fruit quality. Molecules 2021, 26, 3928. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Harech, M.A.; Anasser, I.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Advancing controlled-release phosphate glasses as sustainable fertilizers: Microelement-enhanced formulations and dissolution behavior analysis. J. Non-Cryst. Solids 2024, 625, 122765. [Google Scholar] [CrossRef]
- Ouis, M.A.; Abd-Eladl, M.; Abou-Baker, N.H. Evaluation of Agriglass as an Environment Friendly Slow Release Fertilizer. Silicon 2018, 10, 293–299. [Google Scholar] [CrossRef]
- Mandal, B.; Hazra, G.; Ghosh, G.K.; Das, T. Leaching pattern of phosphate glass fertilizers with different compositions under Soxhlet distillation conditions. Cerâmica 2020, 66, 250–255. [Google Scholar] [CrossRef]
- Sava, B.A.; Boroica, L.; Sava, M.; Elisa, M.; Vasiliu, C.I.; Nastase, F.; Nastase, C.; Medianu, R. Potassium phosphate glasses used as agro-fertilizers with controlled solubility. J. Optoelectron. Adv. Mater. 2011, 13, 1534–1541. [Google Scholar]
- Cacini, S.; Rinaldi, S.; Massa, D.; Nesi, B.; Epifani, R.; Trinchera, A. The effect of a glass matrix fertilizer and compost amendment on plant growth and mineral nutrition of two container-grown Rose spp. cultivars. Sci. Hortic. 2020, 274, 109660. [Google Scholar] [CrossRef]
- Waclawska, I.; Stoch, L. Thermal behaviour of silicate-phosphate glasses in relation to their biochemical activity. J. Therm. Anal. Calorim. 2001, 65, 141–146. [Google Scholar] [CrossRef]
- Wacławska, I.; Szumera, M. Thermal analysis of glasses for proecological applications. J. Therm. Anal. Calorim. 2003, 72, 1065–1072. [Google Scholar] [CrossRef]
- Szumera, M.; Wacławska, I. Spectroscopic and Thermal Studies of Silicate-Phosphate Glass. Proc. J. Therm. Anal. Calorim. 2007, 88, 151–156. [Google Scholar] [CrossRef]
- Szumera, M.; Wacławska, I. Effect of molybdenum addition on the thermal properties of silicate–phosphate glasses. J. Therm. Anal. Calorim. 2012, 109, 649–655. [Google Scholar] [CrossRef]
- Szumera, M.; Wacławska, I.; Sułowska, J. Thermal properties of MnO2 and SiO2 containing phosphate glasses. J. Therm. Anal. Calorim. 2016, 123, 1083–1089. [Google Scholar] [CrossRef][Green Version]
- Wacławska, I.; Szumera, M.; Stoch, P.; Sitarz, M. Structural role of Fe in the soil active glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 728–732. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of silicon in mediating salt tolerance in plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Sharpley, A.N.; Withers, P.J.A.; Scott, J.T.; Haggard, B.E.; Neal, C. Phosphorus Mitigation to Control River Eutrophication: Murky Waters, Inconvenient Truths, and “Postnormal” Science. J. Environ. Qual. 2013, 42, 295–304. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Zellener, W.; Tubaña, B.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Plant Stress Reduction and Why This Element Is Not Used Routinely for Managing Plant Health. Plant Dis. 2021, 105, 2033–2049. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Yan, B.; Hou, Y. Effect of Soil Magnesium on Plants: A Review. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 022168. [Google Scholar] [CrossRef]
- Dahl, C.; Hell, R.; Knaff, D.; Leustek, T. (Eds.) Sulfur Metabolism in Phototrophic Organisms; Springer: Berlin, Germany, 2008; Volume 27. [Google Scholar]
- Jamal, A.; Moon, Y.S.; Abdin, M.Z. Sulphur—A general overview and interaction with nitrogen. Aust. J. Crop Sci. 2010, 4, 523–529. [Google Scholar]
- Stoch, L. Thermal analysis and thermochemistry of vitreous into crystalline state transition. J. Therm. Anal. Calorim. 2004, 77, 7–16. [Google Scholar] [CrossRef]
- Weinberg, M.C. Glass-forming ability and glass stability in simple systems. J. Non-Cryst. Solids 1994, 167, 81–88. [Google Scholar] [CrossRef]
- Turnbull, D. Under What Conditions Can a Glass Be Formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Zanotto, E.D.; Cassar, D.R. The race within supercooled liquids—Relaxation versus crystallization. J. Chem. Phys. 2018, 149, 024503. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, D.R. A Kinetic Treatment of Glass Transition. J. Non-Cryst. Solids 1972, 7, 337–348. [Google Scholar] [CrossRef]
- Richet, P.; Toplis, M.J. Thermodynamic aspects of the glass transition of silicates. Comptes Rendus l'Académie Sci.-Ser. IV-Phys. Astrophys. 2001, 2, 191–202. [Google Scholar] [CrossRef]
- Vogel, W. Introduction to Glass Science; Springer: New York, NY, USA, 1972. [Google Scholar] [CrossRef]
- Kawasaki, T.; Tanaka, H. Structural evolution in the aging process of supercooled colloidal liquids. Phys. Rev. E 2014, 89, 062315. [Google Scholar] [CrossRef]
- Johari, G.; Shanker, R.M. On determining the relaxation time of glass and amorphous pharmaceuticals’ stability from thermodynamic data. Thermochim. Acta 2010, 511, 89–95. [Google Scholar] [CrossRef]
- Riechers, B.; Roed, L.A.; Mehri, S.; Ingebrigtsen, T.S.; Hecksher, T.; Dyre, J.C.; Niss, K. Predicting nonlinear physical aging of glasses from equilibrium relaxation via the material time. Sci. Adv. 2022, 8, eabl9809. [Google Scholar] [CrossRef]
- Krishnan, N.M.A.; Wang, B.; Le Pape, Y.; Sant, G.; Bauchy, M. Irradiation-driven amorphous-to-glassy transition in quartz: The crucial role of the medium-range order in crystallization. Phys. Rev. Mater. 2017, 1, 053405. [Google Scholar] [CrossRef]
- Stoch, L. Flexibility of structure and glass-forming ability: A chemical approach. Glass Phys. Chem. 2001, 27, 167–174. [Google Scholar] [CrossRef]
- Stoch, L. Flexibility of structure as a criterion of glass formation and stability. Opt. Appl. 2000, 30, 647–655. [Google Scholar]
- Manara, D.; Grandjean, A.; Pinet, O.; Dussossoy, J.; Neuville, D. Sulfur behavior in silicate glasses and melts: Implications for sulfate incorporation in nuclear waste glasses as a function of alkali cation and V2O5 content. J. Non-Cryst. Solids 2007, 353, 12–23. [Google Scholar] [CrossRef]
- Diallo, B.; Topka, K.C.; Puyo, M.; Lebesgue, C.; Genevois, C.; Laloo, R.; Samelor, D.; Lecoq, H.; Allix, M.; Vergnes, H.; et al. Network hydration, ordering and composition interplay of chemical vapor deposited amorphous silica films from tetraethyl orthosilicate. J. Mater. Res. Technol. 2021, 13, 534–547. [Google Scholar] [CrossRef]
- Awazu, K.; Kawazoe, H. Strained Si–O–Si bonds in amorphous SiO2 materials: A family member of active centers in radio, photo, and chemical responses. J. Appl. Phys. 2003, 94, 6243–6262. [Google Scholar] [CrossRef]
- Fabert, M.; Ojha, N.; Erasmus, E.; Hannula, M.; Hokka, M.; Hyttinen, J.; Rocherullé, J.; Sigalas, I.; Massera, J. Crystallization and sintering of borosilicate bioactive glasses for application in tissue engineering. J. Mater. Chem. B 2017, 5, 4514–4525. [Google Scholar] [CrossRef] [PubMed]
- Filho, O.P.; Latorre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Sitarz, M.; Bulat, K.; Szumera, M. Influence of modifiers and glass-forming ions on the crystallization of glasses of the NaCaPO4–SiO2 system. J. Therm. Anal. Calorim. 2012, 109, 577–584. [Google Scholar] [CrossRef]
- Montazerian, M.; Shearer, A.; Mauro, J.C. Perspectives on the impact of crystallization in bioactive glasses and glass-ceramics. Int. J. Ceram. Eng. Sci. 2024, 6, e10194. [Google Scholar] [CrossRef]
- Weaver, J.L.; DePriest, P.T.; Plymale, A.E.; Pearce, C.I.; Arey, B.; Koestler, R.J. Microbial interactions with silicate glasses. npj Mater. Degrad. 2021, 5, 11. [Google Scholar] [CrossRef]
- Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Astolfi, S.; Lehto, N.; Robinson, B.; Brunetto, G.; Terzano, R.; Cesco, S. Nutrient availability in the rhizosphere: A review. Acta Hortic. 2018, 1217, 13–28. [Google Scholar] [CrossRef]
- Vancea, C.; Mosoarca, G.; Popa, S. A sustainable solution to obtain p-k-mn glass fertilizers from cheap and readily available wastes. Int. J. Environ. Res. Public Health 2021, 18, 6585. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Gent, M.P.N.; Parrish, Z.D.; White, J.C. Nutrient Uptake among Subspecies of Cucurbita Pepo L. Is Related to Exudation of Citric Acid. JASHS 2005, 130, 782–788. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Gin, S.; Delaye, J.-M.; Angeli, F.; Schuller, S. Aqueous alteration of silicate glass: State of knowledge and perspectives. NPJ Mater. Degrad. 2021, 5, 42. [Google Scholar] [CrossRef]
- Zanini, R.; Franceschin, G.; Cattaruzza, E.; Traviglia, A. A review of glass corrosion: The unique contribution of studying ancient glass to validate glass alteration models. npj Mater. Degrad. 2023, 7, 38. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive glasses—Structure and properties. Angew. Chem. Int. Ed. Engl. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Tilocca, A.; Cormack, A.N.; de Leeuw, N.H. The structure of bioactive silicate glasses: New insight from molecular dynamics simulations. Chem. Mater. 2007, 19, 95–103. [Google Scholar] [CrossRef]
- Wacławska, I.; Środa, M.; Stoch, L. Thermal Methods Applied to the Glass Transition of Mixed Network AlPO4-BPO4-SiO2 Glasses. Proc. J. Therm. Anal. Calorim. 2001, 65, 661–667. [Google Scholar] [CrossRef]
- Waclawska, I.; Szumera, M. Reactivity of silicate–phosphate glasses in soil environment. J. Alloys Compd. 2009, 468, 246–253. [Google Scholar] [CrossRef]
- Edén, M. The split network analysis for exploring composition–structure correlations in multi-component glasses: I. Rationalizing bioactivity-composition trends of bioglasses. J. Non-Cryst. Solids 2011, 357, 1595–1602. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Henderson, G.S. Glasses: Alkali and Alkaline-Earth Silicates. Encycl. Mater. Tech. Ceram. Glas. 2021, 2, 462–482. [Google Scholar] [CrossRef]
- Ting, H.; Page, S.J.; Poologasundarampillai, G.; Chen, S.; Yu, B.; Hanna, J.V.; Jones, J.R. Phosphate content affects structure and bioactivity of sol-gel silicate bioactive glasses. Int. J. Appl. Glass Sci. 2017, 8, 372–382. [Google Scholar] [CrossRef]
- Zeng, H.; Jiang, Q.; Liu, Z.; Li, X.; Ren, J.; Chen, G.; Liu, F.; Peng, S. Unique sodium phosphosilicate glasses designed through extended topological constraint theory. J. Phys. Chem. B 2014, 118, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- das Dores, D.R.S.; da Silva, V.R.; Silva, G.H.; De Oliveira, V.H.; Ludwig, Z.M.d.C.; Bell, M.J.V.; Ludwig, V.; Pontuschka, W.M.; dos Anjos, V.C. On the influence of low silica content on phosphates glasses doped with Erbium Ions. Quarks Braz. Electron. J. Phys. Chem. Mater. Sci. 2020, 3, 41–49. [Google Scholar] [CrossRef]
- Wallace, K.E.; Hill, R.G.; Pembroke, J.T.; Brown, C.J.; Hatton, P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci. Mater. Med. 1999, 10, 697–701. [Google Scholar] [CrossRef]
- Stoch, L. The significance of structural factors in thermal dissociation of solids. Thermochim. Acta 1985, 92, 97–100. [Google Scholar] [CrossRef]
- Laura, S.; Mountjoy, G. Homogeneity of modifier ion distributions and the mixed alkaline earth effect in MgO–CaO–SiO2 silicate glasses using molecular dynamics. Eur. J. Glass Sci. Technol. Part B 2018, 58, 165–170. [Google Scholar]
- Yang, R.; Liu, H.; Ding, Z.; Zheng, J.; Mauro, J.C.; Kim, S.H.; Zheng, Q. Chemical durability of borosilicate pharmaceutical glasses: Mixed alkaline earth effect with varying [MgO]/[CaO] ratio. J. Am. Ceram. Soc. 2021, 104, 3973–3981. [Google Scholar] [CrossRef]
- Garza-Garcia, M.; Lopez-Cuevas, J.; Gutierrez-Chavarria, C.A.; Rendon-Angeles, J.C.; Valle-Fuentes, J.F. Study of a Mixed Alkaline–Earth Effect on Some Properties of Glasses of the CaO-MgO-Al2O3-SiO2 System. Bol. Soc. Esp. Ceram. Vidr. 2007, 46, 153–162. [Google Scholar]
- Manara, D.; Grandjean, A.; Pinet, O.; Dussossoy, J.; Neuville, D. Factors influencing the sulphate incorporation in radioactive waste glasses. MRS Proc. 2006, 932, 377–384. [Google Scholar] [CrossRef]
- Hill, R.G.; Brauer, D.S. Predicting the bioactivity of glasses using the network connectivity or split network models. J. Non-Cryst. Solids 2011, 357, 3884–3887. [Google Scholar] [CrossRef]
- Stoch, L. Structure and Crystallization of Multicomponent Glasses. High Temp. Mater. Processes 1992, 10, 245–264. [Google Scholar] [CrossRef]
- O’donnell, M.D.; Watts, S.; Law, R.; Hill, R. Effect of P2O5 content in two series of soda lime phosphosilicate glasses on structure and properties—Part I: NMR. J. Non-Cryst. Solids 2008, 354, 3554–3560. [Google Scholar] [CrossRef]
- Deng, B.; Shi, Y.; Zhou, Q.; Bauchy, M. Revealing the structural role of MgO in aluminosilicate glasses. Acta Mater. 2022, 222, 117417. [Google Scholar] [CrossRef]
- Jha, P.; Singh, K. Effect of MgO on bioactivity, hardness, structural and optical properties of SiO2–K2O–CaO–MgO glasses. Ceram. Int. 2016, 42, 436–444. [Google Scholar] [CrossRef]
- Berezicka, A.; Sułowska, J.; Jeleń, P.; Lach, R.; Szumera, M. Thermal behaviour of sulphate-bearing multicomponent silicate–phosphate glasses. J. Therm. Anal. Calorim. 2023, 148, 1381–1405. [Google Scholar] [CrossRef]
- Berezicka, A.; Szumera, M.; Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Stępień, J.; Zając, M.; Pollastri, S.; Olivi, L. Unraveling the nature of sulfur-bearing silicate-phosphate glasses: Insights from multi-spectroscopic (Raman, MIR, 29Si, 31P MAS-NMR, XAS, XANES) investigation. Ceram. Int. 2022, 48, 4238–4254. [Google Scholar] [CrossRef]
- Berezicka, A.; Sułowska, J.; Jeleń, P.; Komarek, S.; Szumera, M. Effect of sulfur addition on glass-forming ability, structure and thermal stability of multicomponent silicate-phosphate glasses. J. Therm. Anal. Calorim. 2024, 149, 10405–10427. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Hu, J.-Y.; Neuber, N.; Bochtler, B.; Adam, B.; Riegler, S.S.; Frey, M.; Ruschel, L.; Lu, W.-F.; Feng, A.-H.; et al. Effect of sulfur on the glass-forming ability, phase transformation, and thermal stability of Cu-Zr-Al bulk metallic glass. Acta Mater. 2021, 212, 116923. [Google Scholar] [CrossRef]
- Reben, M.; Wacławska, I.; Paluszkiewicz, C.; Środa, M. Thermal and Structural Studies of Nanocrystallization of Oxyfluoride Glasses. Proc. J. Therm. Anal. Calorim. 2007, 88, 285–289. [Google Scholar] [CrossRef]
- Kjeldsen, J.; Smedskjaer, M.M.; Mauro, J.C.; Youngman, R.E.; Huang, L.; Yue, Y. Mixed alkaline earth effect in sodium aluminosilicate glasses. J. Non-Cryst. Solids 2013, 369, 61–68. [Google Scholar] [CrossRef]
- Rouxel, T.; Dély, N.; Sangleboeuf, J.C.; Dériano, S.; LeFloch, M.; Beuneu, B. Structure-property correlations in Y-Ca-Mg-sialon glasses: Physical and mechanical properties. J. Am. Ceram. Soc. 2005, 88, 889–896. [Google Scholar] [CrossRef]
- Jha, P.; Danewalia, S.S.; Singh, K. Influence of thermal stability on dielectric properties of SiO2–K2O–CaO–MgO glasses. J. Therm. Anal. Calorim. 2017, 128, 745–754. [Google Scholar] [CrossRef]
- Mysen, B.; Richet, P. Silicate Glasses and Melts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–720. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; Royal Society of Chemistry: London, UK, 2005; Volume 1. [Google Scholar]
- Elgayar, I.; Hill, R.; Chen, X.; Bubb, N.; Wood, D. Dielectric spectroscopy and dissolution studies of bioactive glasses. Int. J. Appl. Glass Sci. 2017, 8, 418–427. [Google Scholar] [CrossRef]
- Cody, G.D.; Mysen, B.; Sághi-Szabó, G.; Tossell, J.A. Silicate-phosphate interactions in silicate glasses and melts: I. A multinuclear (27 Al, 29 Si, 31 P) MAS NMR and ab initio chemical shielding (31 P) study of phosphorous speciation in silicate glasses. Geochim. Cosmochim. Acta 2001, 65, 2395–2411. [Google Scholar] [CrossRef]
- Mysen, B.O.; Cody, G.D. Silicate-phosphate interactions in silicate glasses and melts: II. quantitative, high-temperature structure of P-bearing alkali aluminosilicate melts. Geochim. Cosmochim. Acta 2001, 65, 2413–2431. [Google Scholar] [CrossRef]
- Maekawa, H.; Maekawa, T.; Kawamura, K.; Yokokawa, T. The structural groups of alkali silicate glasses determined from 29Si MAS-NMR. J. Non-Cryst. Solids 1991, 127, 53–64. [Google Scholar] [CrossRef]
- Dupree, R.; Holland, D.; McMillan, P.; Pettifer, R. The structure of soda-silica glasses: A mas NMR study. J. Non-Cryst. Solids 1984, 68, 399–410. [Google Scholar] [CrossRef]
- Mathew, R.; Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Direct Probing of the Phosphate-Ion Distribution in Bioactive Silicate Glasses by Solid-State NMR: Evidence for Transitions between Random/Clustered Scenarios. Chem. Mater. 2013, 25, 1877–1885. [Google Scholar] [CrossRef]
- Malfait, W.J.; Halter, W.E.; Morizet, Y.; Meier, B.H.; Verel, R. Structural control on bulk melt properties: Single and double quantum 29Si NMR spectroscopy on alkali-silicate glasses. Geochim. Cosmochim. Acta 2007, 71, 6002–6018. [Google Scholar] [CrossRef]
- Wacławska, I.; Szumera, M. Influence of MgO (CaO) on the structure of silicate-phosphate glasses. J. Therm. Anal. Calorim. 2006, 84, 185–190. [Google Scholar] [CrossRef]
- Yang, M.; Cai, X.; Wang, C.; Wang, Z.; Xue, F.; Chu, C.; Bai, J.; Liu, Q.; Ni, X. Highly Stable Amorphous (Pyro)phosphate Aggregates: Pyrophosphate as a Carrier for Bioactive Ions and Drugs in Bone Repair Applications. ACS Omega 2024, 9, 23724–23740. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, H.; Solla, E.; Serra, J.; González, P.; León, B.; Malz, F.; Jäger, C. Raman and NMR study of bioactive Na2O–MgO–CaO–P2O5–SiO2 glasses. J. Non-Cryst. Solids 2008, 354, 5004–5008. [Google Scholar] [CrossRef]
- Christie, J.K.; Ainsworth, R.I.; de Leeuw, N.H. Investigating structural features which control the dissolution of bioactive phosphate glasses: Beyond the network connectivity. J. Non-Cryst. Solids 2016, 432, 31–34. [Google Scholar] [CrossRef]
- Akunna, C.; Cerruti, M. Structural Connectivity and Bioactivity in Sol–Gel Silicate Glass Design. Acta Biomater 2024, 188, 374–392. [Google Scholar] [CrossRef]
- Eckert, H. Structural characterization of bioactive glasses by solid state NMR. J. Sol-Gel Sci. Technol. 2018, 88, 263–295. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Liu, L.; Sun, Y.; Wang, X. Roles of P2O5 addition on the viscosity and structure of CaO-SiO2-Al2O3-Na2O-P2O5 melts. ISIJ Int. 2018, 58, 1644–1649. [Google Scholar] [CrossRef]
- Tilocca, A.; Cormack, A.N. Structural Effects of Phosphorus Inclusion in Bioactive Silicate Glasses. J. Phys. Chem. B 2007, 111, 14256–14264. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.-H.; Lee, S.K. Pressure-Induced Structural Transitions in Na–Li Silicate Glasses under Compression. J. Phys. Chem. C 2019, 123, 26608–26622. [Google Scholar] [CrossRef]
- Abdullah, T.K. Study of the Redox and Acid-Base Properties of Soda-Lime Silicate Glass: Application to the High Temperature Corrosion of Ni-Based Alloys and Ceramic Materials. August 2018. Available online: http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm (accessed on 7 February 2025).
- Atila, A.; Ghardi, E.M.; Ouaskit, S.; Hasnaoui, A. Atomistic insights into the impact of charge balancing cations on the structure and properties of aluminosilicate glasses. Phys. Rev. B 2019, 100, 144109. [Google Scholar] [CrossRef]
- Watts, S.; Hill, R.; Onnell, O.; Law, R. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 2010, 356, 517–524. [Google Scholar] [CrossRef]
- O’donnell, M.D.; Watts, S.J.; Hill, R.G.; Law, R.V. The effect of phosphate content on the bioactivity of soda-lime-phosphosilicate glasses. J. Mater. Sci. Mater. Med. 2009, 20, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bing, H.; Wu, Y.; Sun, H.; Zhou, J. Low molecular weight organic acids regulate soil phosphorus availability in the soils of subalpine forests, eastern Tibetan Plateau. CATENA 2021, 203, 105328. [Google Scholar] [CrossRef]
- Sokolova, T.A. Low-Molecular-Weight Organic Acids in Soils: Sources, Composition, Concentrations, and Functions: A Review. Eurasian Soil Sci. 2020, 53, 580–594. [Google Scholar] [CrossRef]
- Othman, S.M.; Sarsam, S.I.; Ismail, S.A. Gypsum and limestone dissolution within fatha formation (Middle miocene) at various ph solutions: A laboratory study. Iraqi Geol. J. 2020, 53, 71–88. [Google Scholar] [CrossRef]
- Kiran-Yildirim, B.; Gencaslan, A.; Titiz-Sargut, S. Thermodynamic Models and Central Composite Design for Calcium Sulfate Dihydrate Solubility. Chem. Eng. Technol. 2020, 43, 1124–1136. [Google Scholar] [CrossRef]
- Böschel, D.; Henning, S.; Michler, G.H.; Roggendorf, H.; Syrowatka, F.; Trempler, J. Characterisation of Gel Layers on Corroded Sodium Disilicate Glasses by Scanning Electron Microscopy and EDX Profiling. Eur. J. Glass Sci. Technol. Part B 2007, 48, 267–270. [Google Scholar]
- Jantzen, C.M.; Smith, M.E.; Peeler, D.K. Dependency of sulfate solubility on melt composition and melt polymerization. Ceram. Trans. 2005, 168, 141–152. [Google Scholar] [CrossRef]
- Bingham, P.; Hand, R. Sulphate incorporation and glass formation in phosphate systems for nuclear and toxic waste immobilization. Mater. Res. Bull. 2008, 43, 1679–1693. [Google Scholar] [CrossRef]
- Mahadevan, T.S.; Du, J. Evaluating Water Reactivity at Silica Surfaces Using Reactive Potentials. J. Phys. Chem. C 2018, 122, 9875–9885. [Google Scholar] [CrossRef]
- Feuston, B.P.; Garofalini, S.H. Topological and bonding defects in vitreous silica surfaces. J. Chem. Phys. 1989, 91, 564–570. [Google Scholar] [CrossRef]
- Zemek, J.; Jiricek, P.; Gedeon, O.; Lesiak, B.; Jozwik, A. Electron irradiated potassium-silicate glass surfaces investigated by XPS. J. Non-Cryst. Solids 2005, 351, 1665–1674. [Google Scholar] [CrossRef]
- Ashtekar, S.; Nguyen, D.; Zhao, K.; Lyding, J.; Wang, W.H.; Gruebele, M. Communication: An obligatory glass surface. J. Chem. Phys. 2012, 137, 141102. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.S.; de Groot, F.M.; Moulton, B.J. X-ray absorption near-edge structure (XANES) spectroscopy. Rev. Miner. Geochem. 2014, 78, 75–138. [Google Scholar] [CrossRef]
- Bingham, P.A.; Hyatt, N.C.; Hand, R.J.; Forder, S.D. Vitrification of UK intermediate level radioactive wastes arising from site decommissioning. Initial laboratory trials. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2013, 54, 1–19. [Google Scholar]
- Nienhuis, E.T.; Saleh, M.; Marcial, J.; Kriegsman, K.; Lonergan, J.; Lipton, A.S.; Guo, X.; McCloy, J.S. Structural characterization of ZnSO4-K2SO4-NaCl glasses. J. Non-Cryst. Solids 2019, 524, 119639. [Google Scholar] [CrossRef]
- Meng, Z.; Hong, W.; Wang, Y.; Jiang, H. Model of diffusion of sulfur in soda-lime-silicate glass: An experimental and comparative study. J. Wuhan Univ. Technol. Sci. Ed. 2017, 32, 76–79. [Google Scholar] [CrossRef]
- Al-Otaibi, F.A. Dissolution behavior of corrosive anions from sabkha soil southern kuwait under long term leaching. Int. J. GEOMATE 2020, 19, 138–144. [Google Scholar] [CrossRef]
- Li, S.; Sun, K.; Zhao, Y.; Nie, G.; Song, S. Purification and rapid dissolution of potassium sulfate in aqueous solutions. RSC Adv. 2019, 9, 2156–2161. [Google Scholar] [CrossRef]
- European Council. Regulation (EC) 2003/2003 Relating to Fertilisers; European Council: Brussels, Belgium, 2003. [Google Scholar]
- Fábián, M.; Kovács, Z.; Lábár, J.L.; Sulyok, A.; Horváth, Z.E.; Székács, I.; Kis, V.K. Network structure and thermal properties of bioactive (SiO2–CaO–Na2O–P2O5) glasses. J. Mater. Sci. 2020, 55, 2303–2320. [Google Scholar] [CrossRef]
- Gin, S.; Mir, A.H.; Jan, A.; Delaye, J.-M.; Chauvet, E.; De Puydt, Y.; Gourgiotis, A.; Kerisit, S.N. A General Mechanism for Gel Layer Formation on Borosilicate Glass under Aqueous Corrosion. J. Phys. Chem. C 2020, 124, 5132–5144. [Google Scholar] [CrossRef]
- Thorpe, C.L.; Neeway, J.J.; Pearce, C.I.; Hand, R.J.; Fisher, A.J.; Walling, S.A.; Hyatt, N.C.; Kruger, A.A.; Schweiger, M.; Kosson, D.S.; et al. Forty years of durability assessment of nuclear waste glass by standard methods. npj Mater. Degrad. 2021, 5, 61. [Google Scholar] [CrossRef]
- Hench, L.; Clark, D. Physical chemistry of glass surfaces. J. Non-Cryst. Solids 1978, 28, 83–105. [Google Scholar] [CrossRef]
- Rodrigues, A.; Fearn, S.; Palomar, T.; Vilarigues, M. Early stages of surface alteration of soda-rich-silicate glasses in the museum environment. Corros. Sci. 2018, 143, 362–375. [Google Scholar] [CrossRef]
- Delahaye, F.; Montagne, L.; Palavit, G.; Touray, J.C.; Baillif, P. Acid dissolution of sodium–calcium metaphosphate glasses. J. Non-Cryst. Solids 1998, 242, 25–32. [Google Scholar] [CrossRef]
- Vaishnav, S.; Hannon, A.C.; Barney, E.R.; Bingham, P.A. Neutron Diffraction and Raman Studies of the Incorporation of Sulfate in Silicate Glasses. J. Phys. Chem. C 2020, 124, 5409–5424. [Google Scholar] [CrossRef]
- Backhouse, D.J.; Fisher, A.J.; Neeway, J.J.; Corkhill, C.L.; Hyatt, N.C.; Hand, R.J. Corrosion of the International Simple Glass under acidic to hyperalkaline conditions. npj Mater. Degrad. 2018, 2, 29. [Google Scholar] [CrossRef]
- Chave, T.; Frugier, P.; Gin, S.; Ayral, A. Glass–water interphase reactivity with calcium rich solutions. Geochim. Cosmochim. Acta 2011, 75, 4125–4139. [Google Scholar] [CrossRef]
- Verney-Carron, A.; Sessegolo, L.; Chabas, A.; Lombardo, T.; Rossano, S.; Perez, A.; Valbi, V.; Boutillez, C.; Muller, C.; Vaulot, C.; et al. Alteration of medieval stained glass windows in atmospheric medium: Review and simplified alteration model. npj Mater. Degrad. 2023, 7, 49. [Google Scholar] [CrossRef]
- Bates, J.K.; Cunnane, J.C.; Bradley, C.R. High-Level Waste Borosilicate Glass, A Compendium of Corrosion Characteristics; United States Department of Energy Office of Waste Management: Washington, DC, USA, 1994; Volume I.
- Zhang, Z.; Kob, W.; Ispas, S. First-principles study of the surface of silica and sodium silicate glasses. Phys. Rev. B 2021, 103, 184201. [Google Scholar] [CrossRef]
- Agnello, G.; Youngman, R.; Lamberson, L.; Smith, N.; LaCourse, W.; Cormack, A.N. Bulk structures of silica-rich calcium aluminosilicate (CAS) glasses along the molar CaO/Al2O3 = 1 join via molecular dynamics (MD) simulation. J. Non-Cryst. Solids 2019, 519, 119450. [Google Scholar] [CrossRef]
- Snellings, R. Surface chemistry of calcium aluminosilicate glasses. J. Am. Ceram. Soc. 2015, 98, 303–314. [Google Scholar] [CrossRef]
- De Bardi, M.; Hutter, H.; Schreiner, M.; Bertoncello, R. Potash-lime-silica glass: Protection from weathering. Herit. Sci. 2015, 3, 22. [Google Scholar] [CrossRef]
- Guadagnino, E.; Zuccato, D. Delamination propensity of pharmaceutical glass containers by accelerated testing with different extraction media. PDA J. Pharm. Sci. Technol. 2012, 66, 116–125. [Google Scholar] [CrossRef]
- Elalaily, N.A.; Zahran, A.H.; Saad, E.A.; Sallam, O.I.; Ezz-Eldin, F.M. Corrosion and Infrared Study of Some γ-Irradiated Lead-Phosphate Glasses Doped with MoO3. Silicon 2018, 10, 1613–1623. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Elaboration and characterization of vitreous fertilizers and study of their impact on the growth, photosynthesis and yield of wheat (Triticum durum L.). Materials 2021, 14, 1295. [Google Scholar] [CrossRef]
- Labbilta, T.; Mesnaoui, M.; Aouad, H.; Abouliatim, Y.; Khouloud, M.; Abielaala, L. Study of the effect of calcium substitution by magnesium in the vitreous system 3P2O5-2K2O-(1 − x) CaO-x MgO. Materials 2020, 13, 2637. [Google Scholar] [CrossRef]
- Esquivel-Upshaw, J.; Dieng, F.; Clark, A.; Neal, D.; Anusavice, K. Surface degradation of dental ceramics as a function of environmental pH. J. Dent. Res. 2013, 92, 467–471. [Google Scholar] [CrossRef]
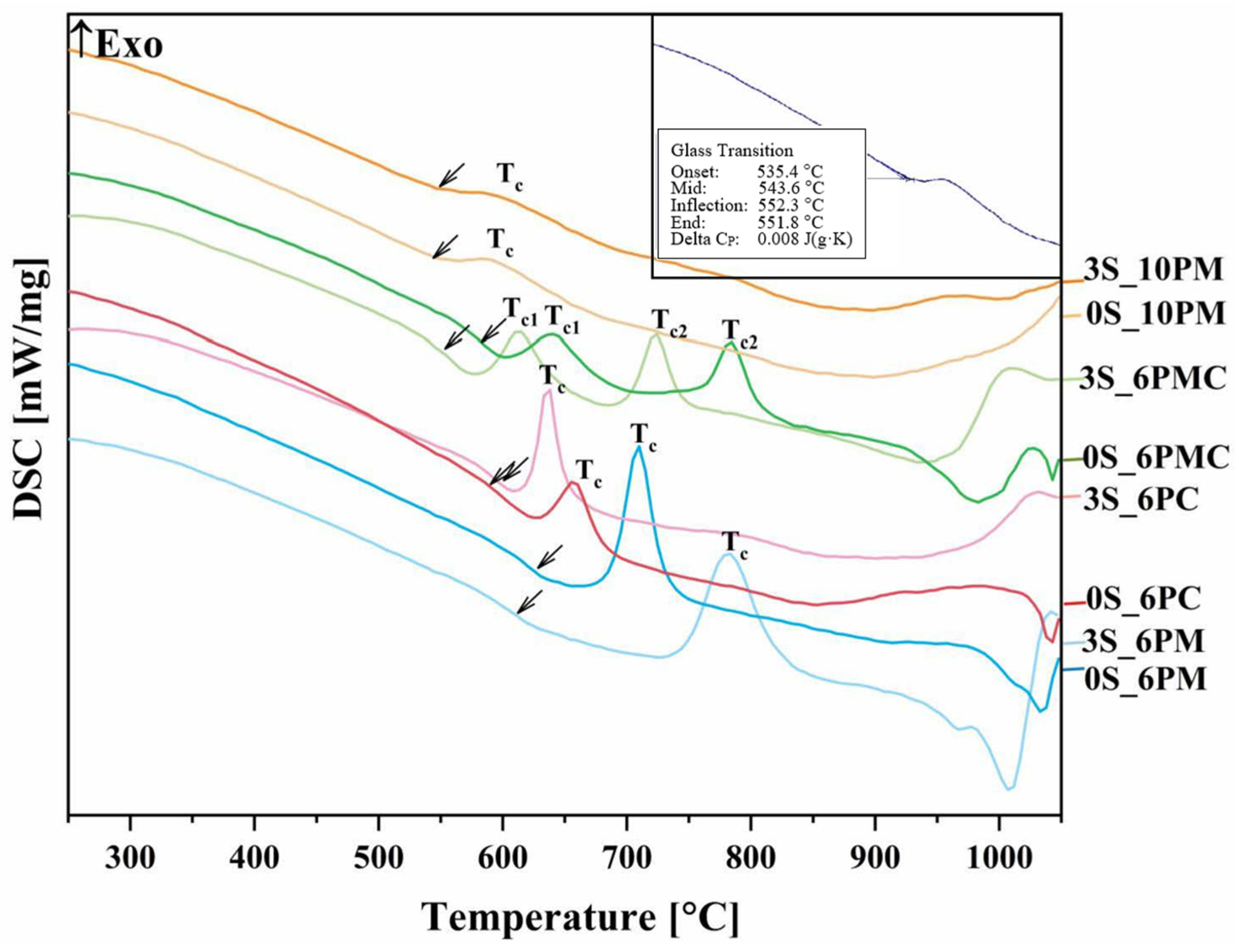
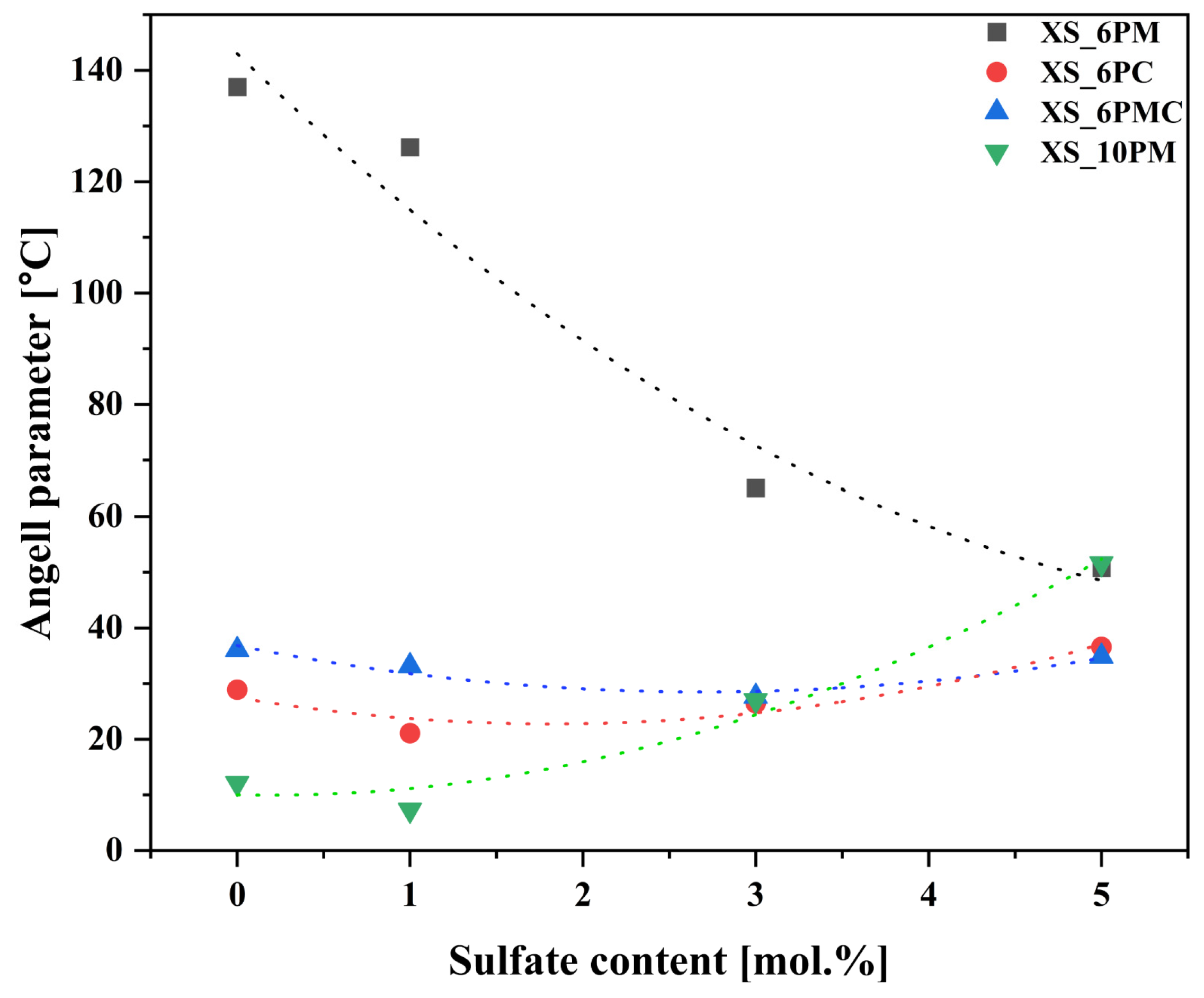


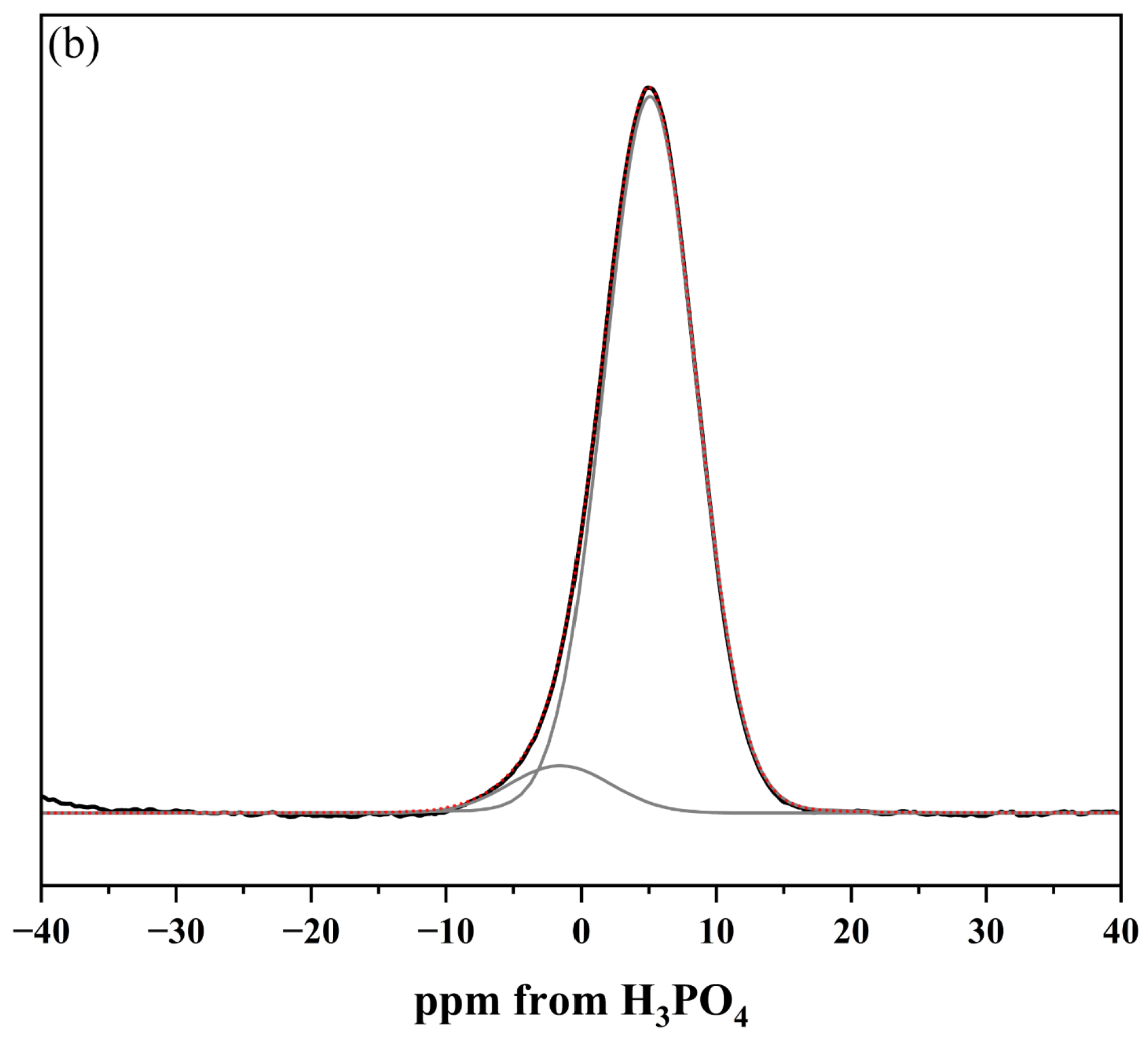

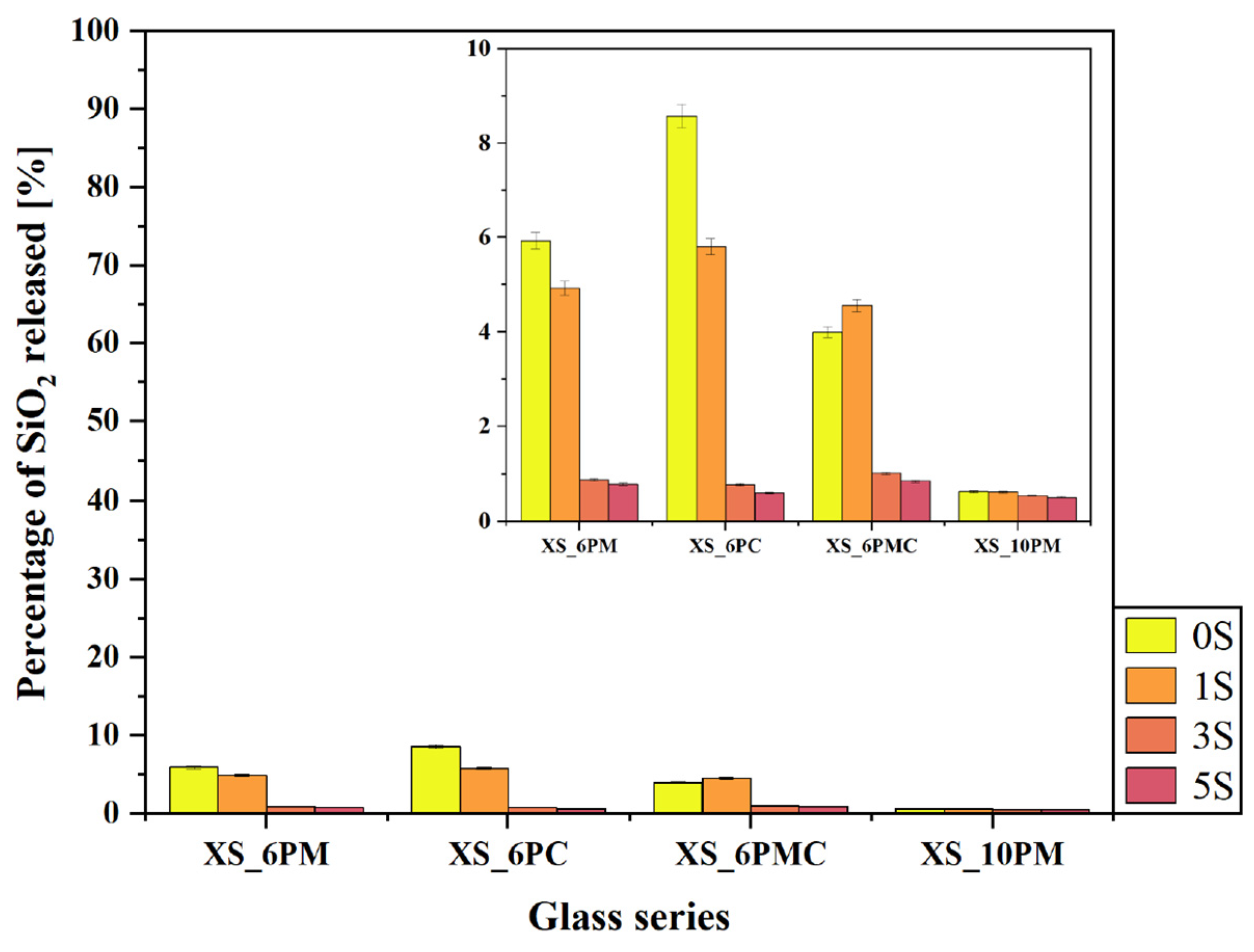

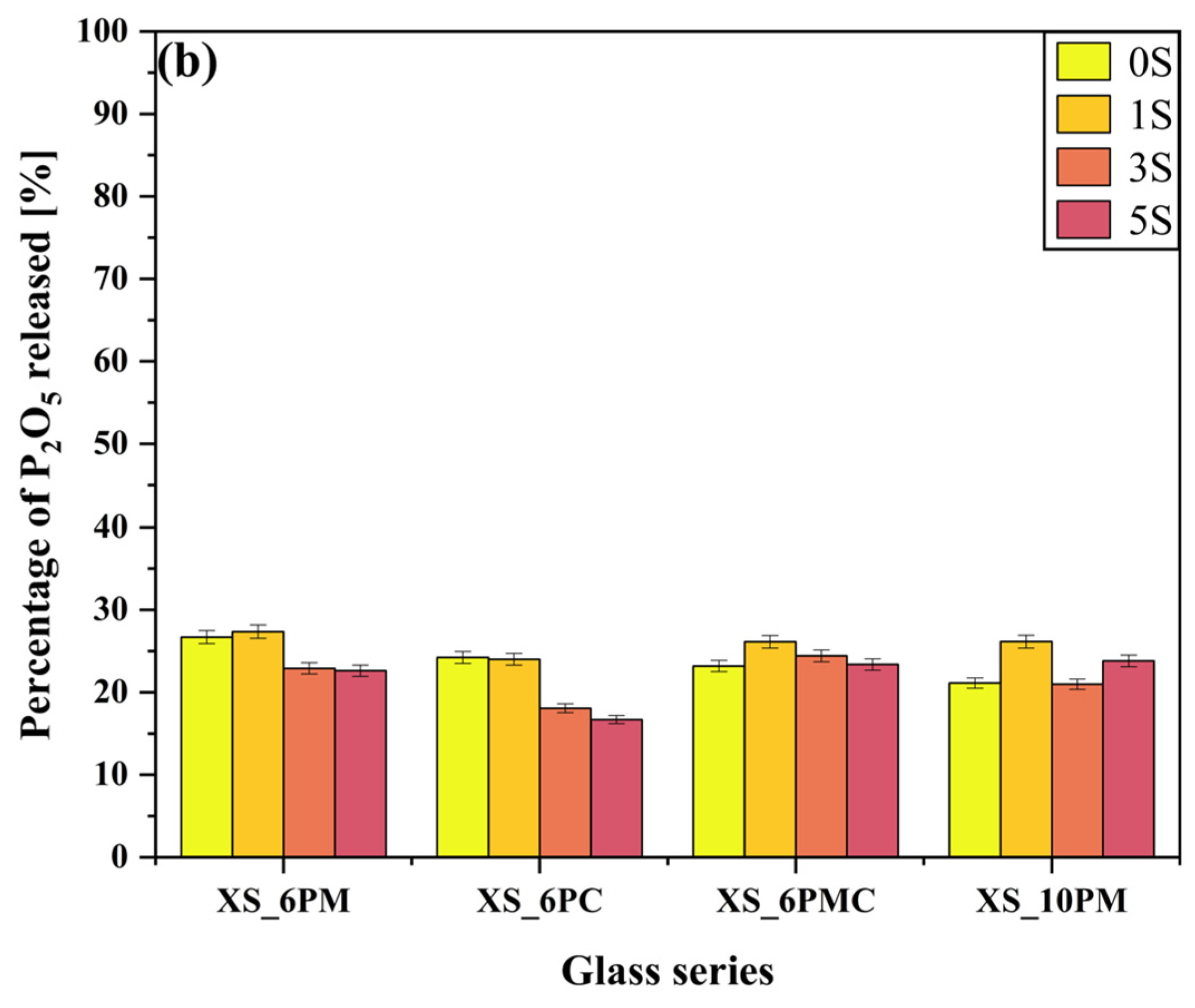
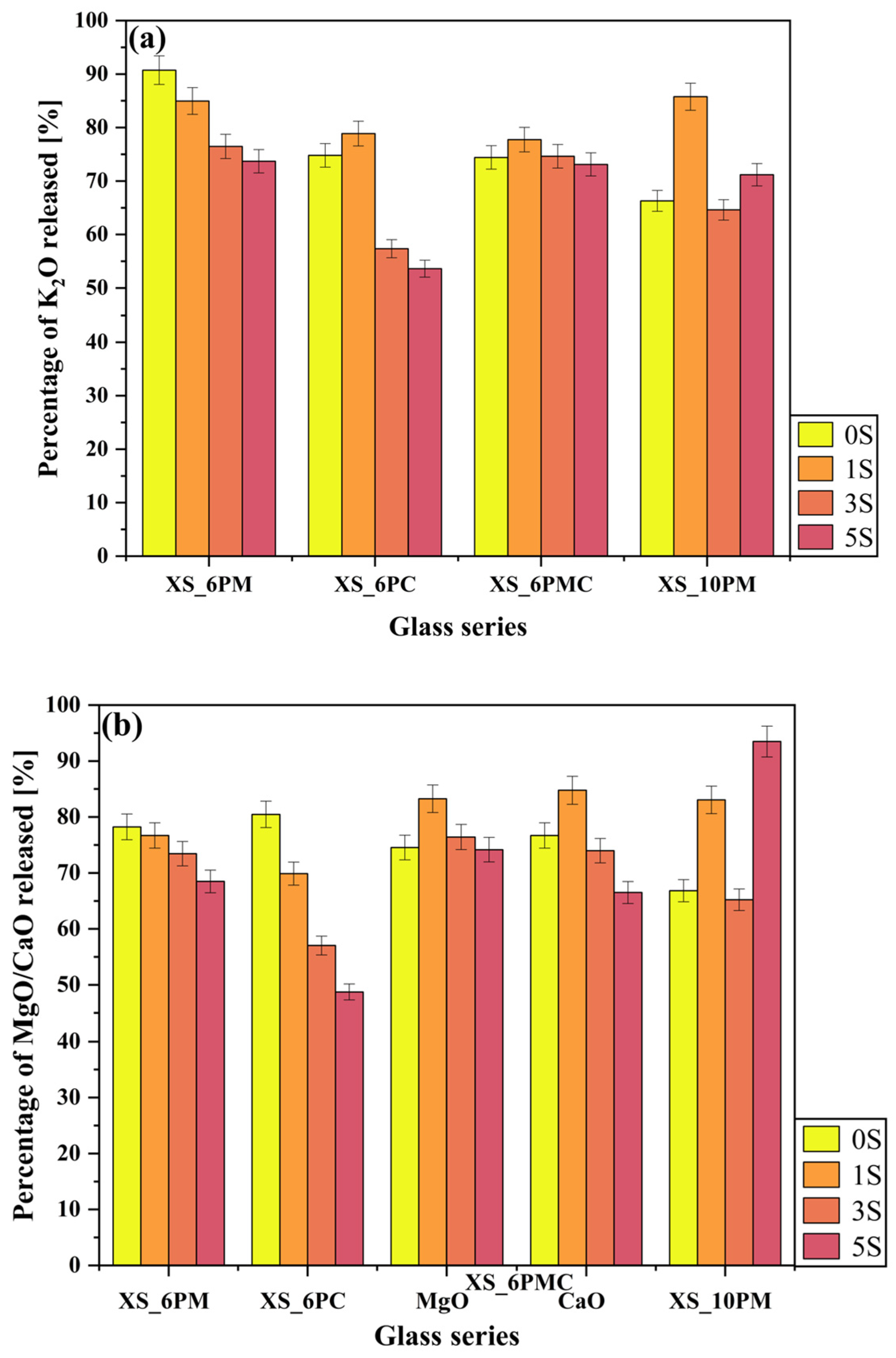
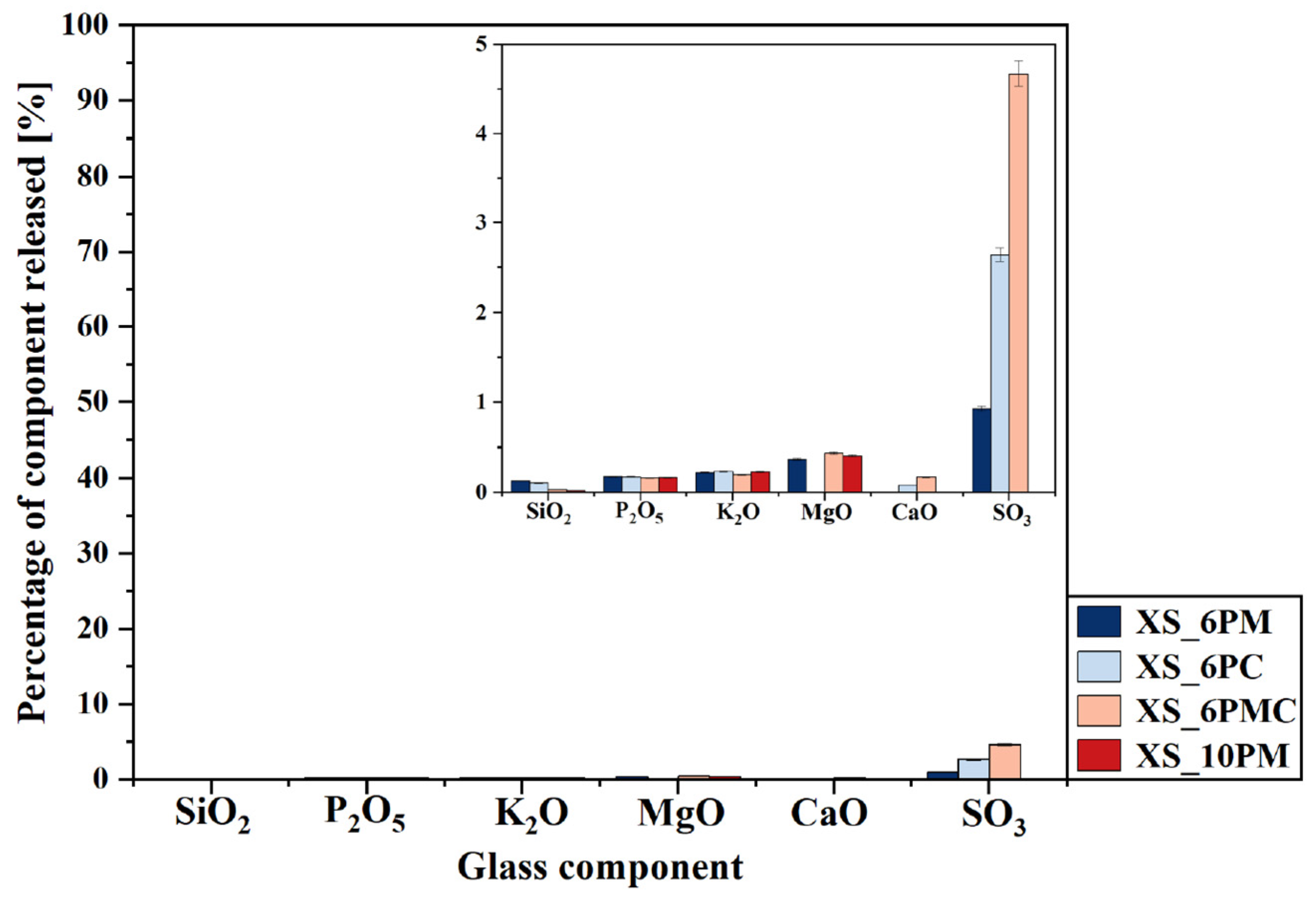

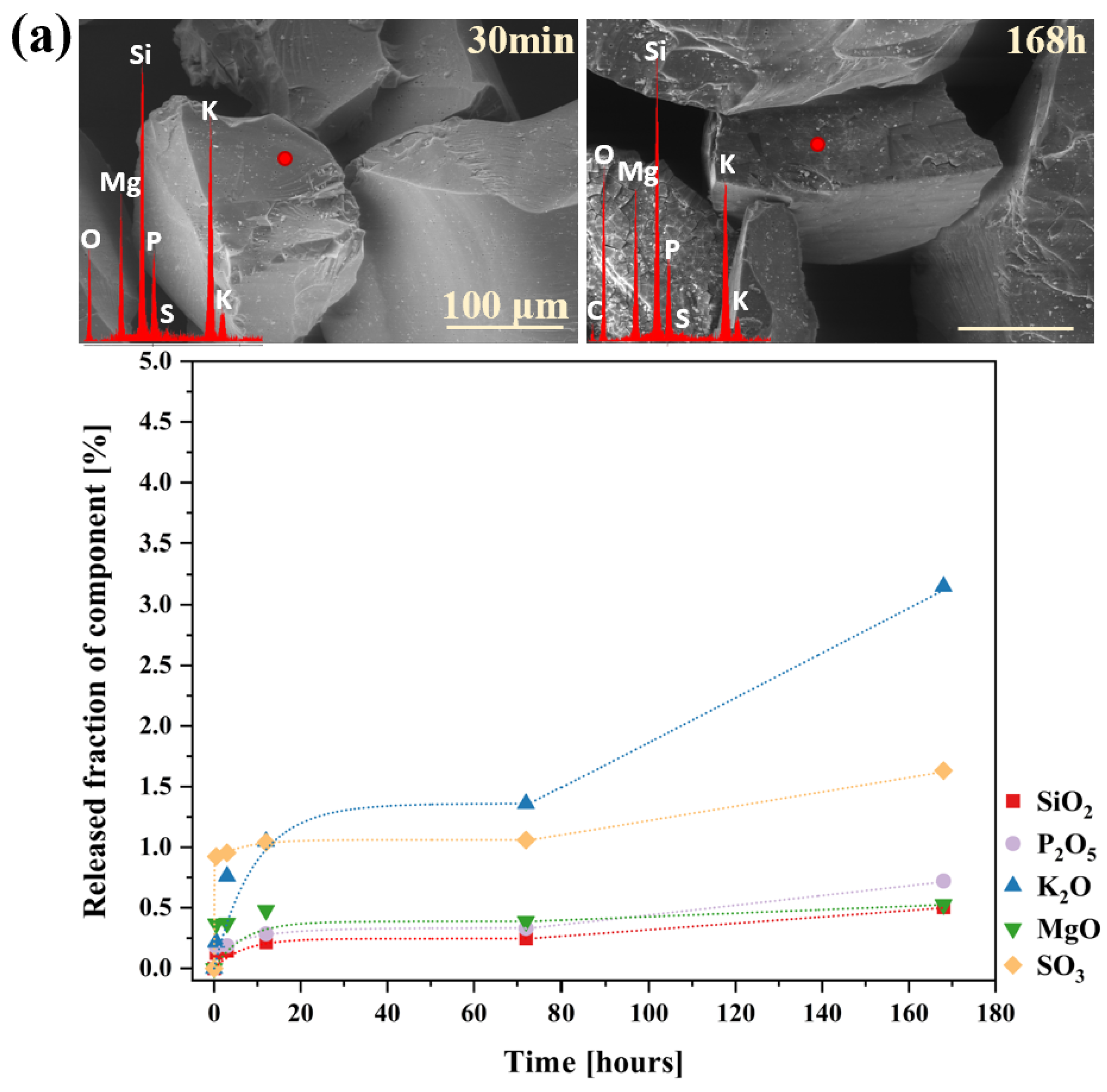
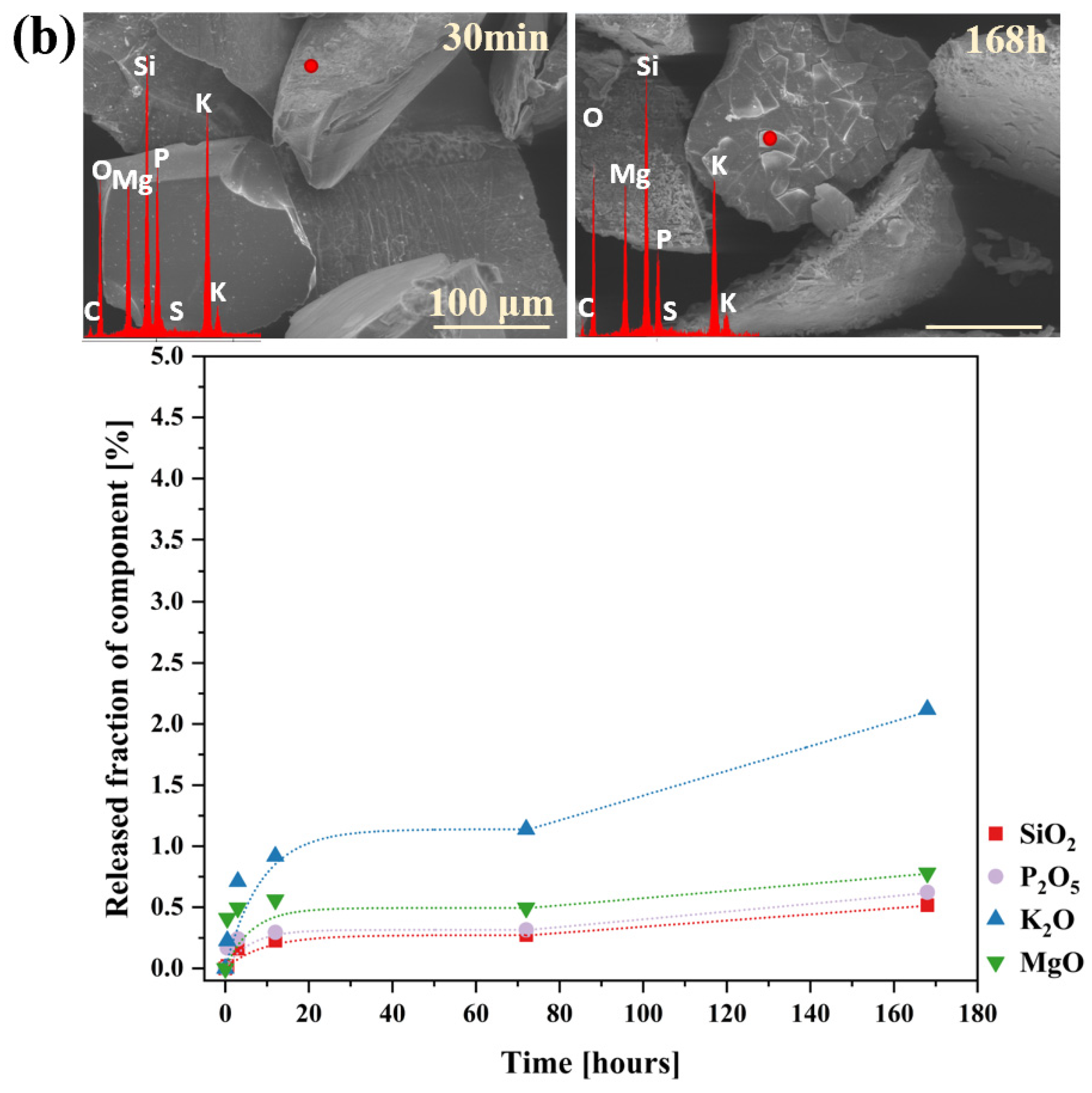
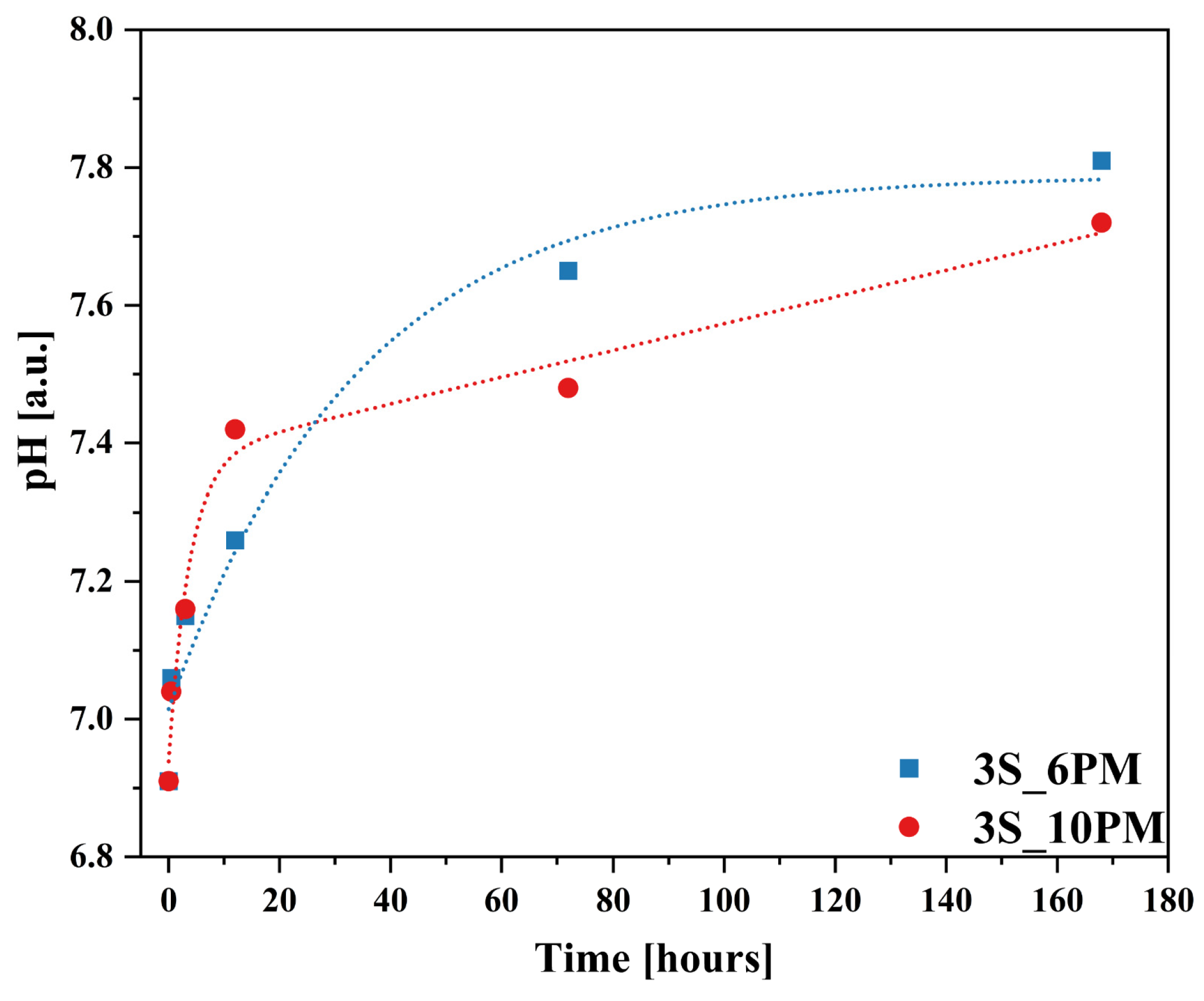
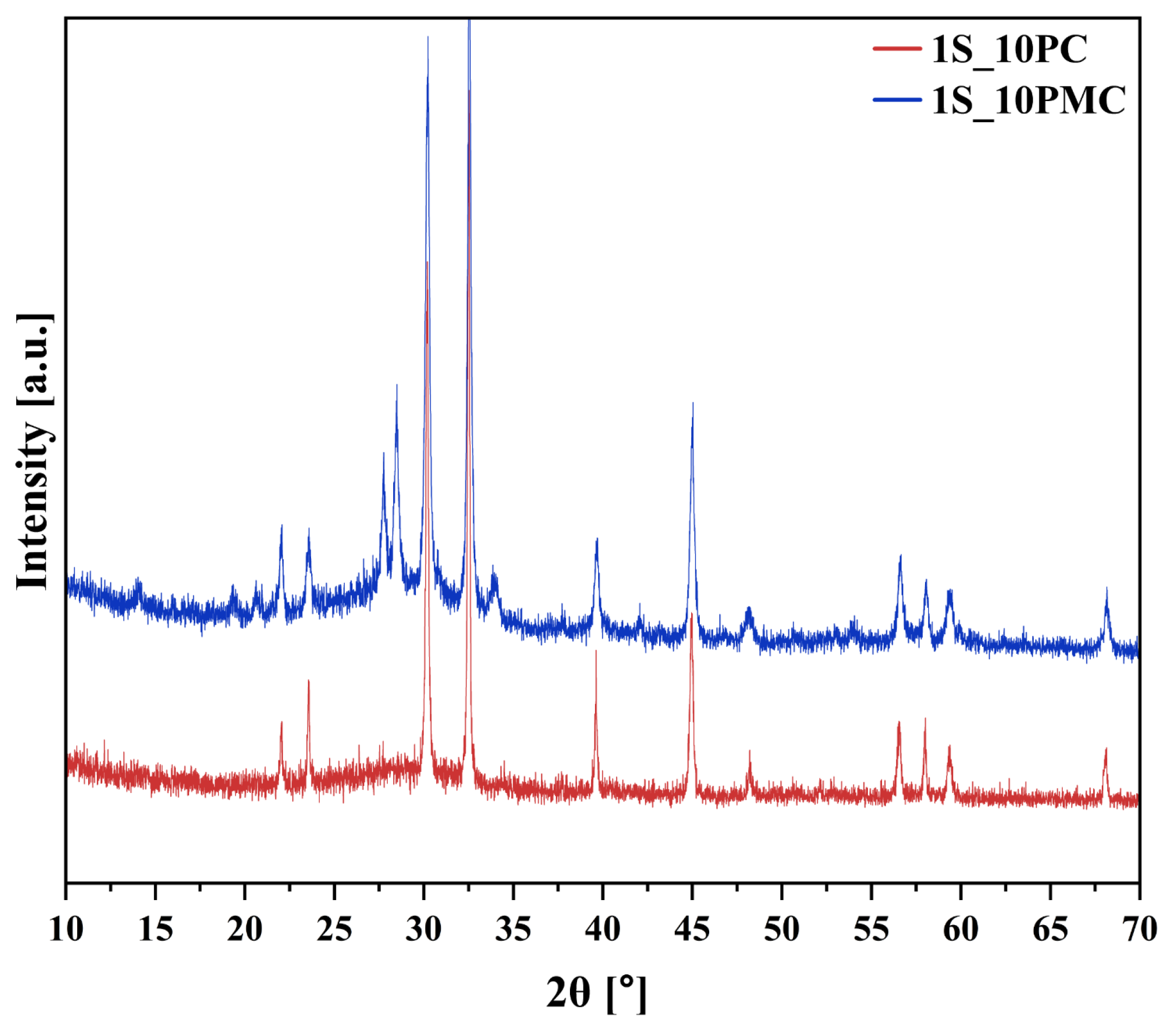
| No | Thermal Parameter | ||
|---|---|---|---|
| Tg | Tx | Angell (KA) | |
| [°C] | [a.u.] | ||
| 0S_6PM | 613 | 750 | 137.0 |
| 1S_6PM | 615 | 741 | 126.2 |
| 3S_6PM | 624 | 689 | 65.1 |
| 5S_6PM | 636 | 687 | 50.8 |
| 0S_6PC | 595 | 624 | 28.9 |
| 1S_6PC | 594 | 615 | 21.1 |
| 3S_6PC | 608 | 635 | 26.5 |
| 5S_6PC | 626 | 663 | 36.6 |
| 0S_6PMC | 550 | 586 | 36.1 |
| 1S_6PMC | 564 | 594 | 29.5 |
| 3S_6PMC | 584 | 613 | 29.4 |
| 5S_6PMC | 589 | 625 | 36.3 |
| 0S_10PM | 544 | 556 | 12.1 |
| 1S_10PM | 544 | 552 | 7.3 |
| 3S_10PM | 546 | 573 | 27.0 |
| 5S_10PM | 549 | 601 | 51.6 |
| Glass | ||
|---|---|---|
| 3S_6PM | 3S_10PM | |
| [g/h] | ||
| Citric acid, 2% | 0.432 | 0.576 |
| Distilled water | 0.018 | 0.024 |
| Si initial release rate (citric acid) | 0.006 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezicka, A.; Sułowska, J.; Szumera, M. Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies. Molecules 2025, 30, 1684. https://doi.org/10.3390/molecules30081684
Berezicka A, Sułowska J, Szumera M. Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies. Molecules. 2025; 30(8):1684. https://doi.org/10.3390/molecules30081684
Chicago/Turabian StyleBerezicka, Anna, Justyna Sułowska, and Magdalena Szumera. 2025. "Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies" Molecules 30, no. 8: 1684. https://doi.org/10.3390/molecules30081684
APA StyleBerezicka, A., Sułowska, J., & Szumera, M. (2025). Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies. Molecules, 30(8), 1684. https://doi.org/10.3390/molecules30081684








