Flavonoids and Furanocoumarins Involved in Drug Interactions
Abstract
1. Introduction
2. Results and Discussion
2.1. Grapefruit and the General Citrus Family
2.1.1. Orange
2.1.2. Lime
2.1.3. Pomelo
2.2. Fig
2.3. Blackberry
2.4. Apple
2.5. Pomegranate
2.6. Cranberry
2.7. Blueberry
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP | Cytochrome P450 |
| OATP | Organic Anion-transporting Polypeptides |
| P-gp | P-glycoprotein |
| CNS | Central Nervous System |
| FW | Fresh Weight |
| HPLC | High-performance Liquid Chromatography |
| UV | Ultraviolet radiation |
| PMAT | Plasma Membrane monoamine Transporter |
| SLC29 | Solute carrier 29 transporters |
References
- Seden, K.; Dickinson, L.; Khoo, S.; Back, D. Grapefruit-Drug Interactions. Drugs 2010, 70, 2373–2407. [Google Scholar] [CrossRef] [PubMed]
- Culm-Merdek, K.E.; von Moltke, L.L.; Gan, L.; Horan, K.A.; Reynolds, R.; Harmatz, J.S.; Court, M.H.; Greenblatt, D.J. Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: Comparison with ritonavir. Clin. Pharmacol. Ther. 2006, 79, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.; Arnold, J.M. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? CMAJ 2013, 185, 309–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roulet, L.; Asseray, N.; Mottier, M.L.; Chiffoleau, A.; Potel, G.; Lapeyre-Mestre, M.; Ballereau, F. Grapefruit consumption and food-drug interaction hazard. Therapie 2011, 66, 421–429. [Google Scholar] [CrossRef]
- Auten, A.A.; Beauchamp, L.N.; Taylor, J.; Hardinger, K.L. Hidden sources of grapefruit in beverages: Potential interactions with immunosuppressant medications. Hosp. Pharm. 2013, 48, 489–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaune, P. Human cytochromes P450. Applications in pharmacology. Therapie 1993, 48, 521–526. [Google Scholar] [PubMed]
- Lin, J.H.; Lu, A.Y. Inhibition and induction of cytochrome P450 and the clinical implications. Clin. Pharmacokinet. 1998, 35, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Stubbins, M.J.; Harries, L.W.; Wolf, C.R. Molecular genetics of the human cytochrome P450 monooxygenase superfamily. Xenobiotica 1998, 28, 1129–1165. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Di Carlo, F.J. Human cytochrome P450 enzymes: A status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab. Rev. 1997, 29, 413–580. [Google Scholar] [CrossRef] [PubMed]
- Kane, G.C.; Lipsky, J.J. Drug-grapefruit juice interactions. Mayo Clin. Proc. 2000, 75, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Lown, K.S.; Bailey, D.G.; Fontana, R.J.; Janardan, S.K.; Adair, C.H.; Fortlage, L.A.; Brown, M.B.; Guo, W.; Watkins, P.B. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J. Clin. Investig. 1997, 99, 2545–2553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petitet, F. Interactions pharmacocinétiques entre préparations à base de plantes et médicament: Une revue de l’importance clinique. Phytothérapie 2012, 10, 170–182. [Google Scholar] [CrossRef]
- Pirmohamed, M. Drug–grapefruit juice interactions: Two mechanisms are clear but individual responses vary. BMJ 2013, 346, f1. [Google Scholar] [CrossRef]
- Guo, L.Q.; Fukuda, K.; Ohta, T.; Yamazoe, Y. Role of furanocoumarin derivatives on grapefruit juice-mediated inhibition of human CYP3A activity. Drug Metab. Dispos. 2000, 28, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Q.; Yamazoe, Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol. Sin. 2004, 25, 129–136. [Google Scholar] [PubMed]
- Veronese, M.L.; Gillen, L.P.; Burke, J.P.; Dorval, E.P.; Hauck, W.W.; Pequignot, E.; Waldman, S.A.; Greenberg, H.E. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J. Clin. Pharmacol. 2003, 43, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; von Moltke, L.L.; Harmatz, J.S.; Chen, G.; Weemhoff, J.L.; Jen, C.; Kelley, C.J.; LeDuc, B.W.; Zinny, M.A. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin. Pharmacol. Ther. 2003, 74, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Ohnishi, A.; Murakami, H.; Matsuo, H.; Higuchi, S.; Urae, A.; Irie, S.; Furuie, H.; Matsukuma, K.; Kimura, M.; et al. Relationship between time after intake of grapefruit juice and the effect on pharmacokinetics and pharmacodynamics of nisoldipine in healthy subjects. Clin. Pharmacol. Ther. 2000, 67, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, J.; Regårdh, C.G.; Edgar, B.; Johnsson, G. Relationship between time of intake of grapefruit juice and its effect on pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur. J. Clin. Pharmacol. 1995, 49, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cancalon, P.F.; Barros, S.M.; Haun, C.; Widmer, W.W. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J. Food Sci. 2011, 76, C543–C548. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, Y.; Shichiri, M.; Mori, T.; Nakanishi, T.; Tamai, I. Major active components in grapefruit, orange, and apple juices responsible for OATP2B1-mediated drug interactions. J. Pharm. Sci. 2013, 102, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Dresser, G.K.; Bailey, D.G.; Leake, B.F.; Schwarz, U.I.; Dawson, P.A.; Freeman, D.J.; Kim, R.B. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin. Pharmacol. Ther. 2002, 71, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, H.; Bailey, D.G.; Dresser, G.K.; Gregor, J.C.; Schwarz, U.I.; McGrath, J.S.; Jolicoeur, E.; Lee, W.; Leake, B.F.; Tirona, R.G.; et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin. Pharmacol. Ther. 2007, 81, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dresser, G.K.; Kim, R.B.; Bailey, D.G. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: Possible role of organic anion transporting polypeptides. Clin. Pharmacol. Ther. 2005, 77, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J. Analysis of drug interactions involving fruit beverages and organic anion-transporting polypeptides. J. Clin. Pharmacol. 2009, 49, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.J.; Cancalon, P.; Widmer, W.W.; Greenblatt, D.J. The effect of grapefruit juice on drug disposition. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.; Yoon, Y.J.; Shon, J.H.; Cha, I.J.; Shin, J.G.; Liu, K.H. Inhibitory effects of fruit juices on CYP3A activity. Drug Metab. Dispos. 2006, 34, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.K.; Leake, B.F.; Kim, R.B. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin. Pharmacol. Ther. 2007, 81, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Colalto, C. Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol. Res. 2010, 62, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.H.; Mendez, J.; Brown, R.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; Wiley and Sons: Chichester, UK, 1982. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Estévez-Braun, A.; González, A.G. Coumarins. Nat. Prod. Rep. 1997, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Munakata, R.; Kitajima, S.; Nuttens, A.; Tatsumi, K.; Takemura, T.; Ichino, T.; Galati, G.; Vautrin, S.; Bergès, H.; Grosjean, J.; et al. Convergent evolution of the UbiA prenyltransferase family underlies the independent acquisition of furanocoumarins in plants. New Phytol. 2020, 225, 2166–2182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathak, M.A.; Daniels, F., Jr.; Fitzpatrick, T.B. The presently known distribution of furocoumarins (psoralens) in plants. J. Investig. Dermatol. 1962, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Allard, N.; Guckert, A.; Forlot, P. Natural Sources for Furocoumarins; John Libbey Eurotext, Ltd.: Paris, France, 1989; pp. 301–306. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Springer Science & Business Media: New York, NY, USA, 1998. [Google Scholar]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Berenbaum, M. Coumarins and caterpillars: A case for coevolution. Evolution 1983, 37, 163–179. [Google Scholar] [CrossRef]
- Peroutka, R.; Schulzová, V.; Botek, P.; Hajšlová, J. Analysis of furanocoumarins in vegetables (Apiaceae) and citrus fruits (Rutaceae). J. Sci. Food Agric. 2007, 87, 2152–2163. [Google Scholar] [CrossRef]
- Karamat, F.; Olry, A.; Munakata, R.; Koeduka, T.; Sugiyama, A.; Paris, C.; Hehn, A.; Bourgaud, F.; Yazaki, K. A coumarinspecific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 2014, 77, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and furanocoumarin quantitation in Citrus peel via Ultraperformance Liquid Chromatography coupled with Mass Spectrometry (UPLC-MS). J. Agric. Food. Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. (JFDA) 2017, 25, 71–83. [Google Scholar] [CrossRef]
- Mallhi, T.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H.; Qadir, M.I.; Hamzah, A.A.; Khan, A.H. Effect of Fruit/Vegetable-Drug Interactions on CYP450, OATP and p-Glycoprotein: A Systematic Review. Trop. J. Pharm. Res. 2015, 14, 1927–1935. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, S.Y.; Kang, N.G.; Jin, M.H. A multi-faceted comparison of phytochemicals in seven citrus peels and improvement of chemical composition and antioxidant ativity by steaming. LWT 2022, 160, 113297. [Google Scholar] [CrossRef]
- Lilja, J.J.; Juntti-Patinen, L.; Neuvonen, P.J. Orange juice substantially reduces the bioavailability of the betaadrenergic-blocking agent celiprolol. Clin. Pharmacol. Ther. 2004, 75, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ieiri, I.; Doi, Y.; Maeda, K.; Sasaki, T.; Kimura, M.; Hirota, T.; Chiyoda, T.; Miyagawa, M.; Irie, S.; Iwasaki, K.; et al. Microdosing clinical study: Pharmacokinetic, pharmacogenomic (SLCO2B1), and interaction (grapefruit juice) profiles of celiprolol following the oral microdose and therapeutic dose. J. Clin. Pharmacol. 2012, 52, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Bailey, D.G.; Paine, M.F.; Watkins, P.B. Seville orange juice-felodipine interaction: Comparison with dilute grapefruit juice and involvement of furocoumarins. Clin. Pharmacol. Ther. 2001, 69, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Penzak, S.R.; Jann, M.W.; Cold, J.A.; Hon, Y.Y.; Desai, H.D.; Gurley, B.J. Seville (sour) Orange Juice: Synephrine Content and Cardiovascular Effects in Normotensive Adults. J. Clin. Pharmacol. 2001, 41, 1059–1063. [Google Scholar] [CrossRef]
- Adegoke, S.A.; Oyelami, O.A.; Olatunya, O.S.; Adeyemi, L.A. Effects of lime juice on malaria parasite clearance. Phytother. Res. 2011, 25, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Biswas, S.; Bera, R.; Mondal, S.; Kundu, A.; Ali, M.A.; Sen, T. Beverage-induced enhanced bioavailability of carbamazepine and its consequent effect on antiepileptic activity and toxicity. J. Food Drug Anal. 2015, 23, 327–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, R.; Russo, M.; De Grazia, S.; Grasso, E.; Dugo, P.; Mondello, L. Thorough investigation of the oxygen heterocyclic fraction of lime (Citrus aurantifolia (Christm.) Swingle) juice. J. Sep. Sci. 2014, 37, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazawi, M.A.; Tutunji, M.S.; AbuRuz, S.M. The effects of pummelo juice on pharmacokinetics of sildenafil in healthy adult male Jordanian volunteers. Eur. J. Clin. Pharmacol. 2010, 66, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.Y.; Xu, W.J.; Tao, J.; Zhang, J.H.; Zhou, X.R.; Yuan, G.; Yang, Y.; Zhang, J.; Zhang, H.Y.; Xu, Q.; et al. Glycemic index, glycemic load, and glycemic response to pomelo in patients with type 2 diabetes. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Anmol, R.J.; Marium, S.; Hiew, F.T.; Han, W.C.; Kwan, L.K.; Wong, A.K.Y.; Khan, F.; Sarker, M.M.R.; Chan, S.Y.; Kifli, N.; et al. Phytochemical and Therapeutic Potential of Citrus grandis (L.) Osbeck: A Review. J. Evid. Based. Integr. Med. 2021, 26, 2515690X211043741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messire, G.; Serreau, R.; Berteina-Raboin, S. Antioxidant Effects of Catechins (EGCG),Andrographolide, and Curcuminoids Compounds for Skin Protection, Cosmetics, and Dermatological Uses: An Update. Antioxidants 2023, 12, 1317. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, B.; Erdelmeier, C.A.J.; Wright, A.D.; Rali, T.; Sticher, O. An antimicrobial alkaloid from Ficus septica. Phytochemistry 1990, 29, 3327–3330. [Google Scholar] [CrossRef]
- Al-Khdhairawi, A.A.Q.; Krishnan, P.; Mai, C.W.; Chung, F.F.; Leong, C.O.; Yong, K.T.; Chong, K.W.; Low, Y.Y.; Kam, T.S.; Lim, K.H. A Bis-benzopyrroloisoquinoline Alkaloid Incorporating a Cyclobutane Core and a Chlorophenanthroindolizidine Alkaloid with Cytotoxic Activity from Ficus fistulosa var. tengerensis. J. Nat. Prod. 2017, 80, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.P.; Ramachandran, V.; Brooks, P.; Smyth, W.F. Investigation of antibacterial phytochemicals in the bark and leaves of Ficus coronata by high-performance liquid chromatography-electrospray ionization-ion trap mass spectrometry (HPLC-ESI-MSn) and ESI-MSn. Electrophoresis 2012, 33, 713–718. [Google Scholar] [CrossRef]
- Yap, V.A.; Loong, B.J.; Ting, K.N.; Loh, S.H.; Yong, K.T.; Low, Y.Y.; Kam, T.S.; Lim, K.H. Hispidacine, an unusual 8,4’-oxyneolignan-alkaloid with vasorelaxant activity, and hispiloscine, an antiproliferative phenanthroindolizidine alkaloid, from Ficus hispida Linn. Phytochemistry 2015, 109, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yap, V.A.; Qazzaz, M.E.; Raja, V.J.; Bradshaw, T.D.; Loh, H.S.; Sim, K.S.; Yong, K.T.; Low, Y.Y.; Lim, K.H. Fistulopsines A and B antiproliferative septicine-type alkaloids from Ficus fistulosa. Phytochem. Lett. 2016, 15, 136–141. [Google Scholar] [CrossRef]
- Rubnov, S.; Kashman, Y.; Rabinowitz, R.; Schlesinger, M.; Mechoulam, R. Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J. Nat. Prod. 2001, 64, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Sabir, A.W. Irritant potential of triterpenoids from Ficus carica leaves. Fitoterapia 2002, 73, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Ajiboye, B.O.; Ojo, A.B.; Onikanni, S.A.; Olarewaju, O.I. Phytochemical, proximate analysis and mineral composition of aqueous crude extract of Ficus asperifolia Miq. J. Adv. Med. Life. Sci. 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Baptista, P.; Andrade, P.B.; Martins, F.; Pereira, J.; Silva, B.M.; Valentao, P. Characterization of Ficus carica L. cultivars by DNA and secondary metabolite analysis: Is genetic diversity reflected in the chemical composition? Food Res. Int. 2012, 49, 710–719. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okiura, A.; Saito, K.; Kohno, M. Identification of phenylpropanoids in fig (Ficus carica L.) leaves. J. Agric. Food Chem. 2014, 62, 10076–10083. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okiura, A.; Kohno, M. Phenylpropanoid composition in fig (Ficus carica L.) leaves. J. Nat. Med. 2017, 71, 770–775. [Google Scholar] [CrossRef]
- Debib, A.; Tir-Touil, A.; Mothana, R.A.A.; Meddah, B.; Sonnet, P. Phenolic content, antioxidant and antimicrobial activities of two fruit varieties of Algerian Ficus carica L. J. Food. Biochem. 2013, 38, 207–215. [Google Scholar] [CrossRef]
- Perez, C.; Dominguez, E.; Ramiro, J.M.; Romero, A.; Campillo, J.E.; Torres, M.D. A study on the glycaemic balance in streptozotocin diabetic rats treated with aqueous extract of Ficus carica (Fig tree) leaves. Phytother Res. 1996, 10, 82–83. [Google Scholar] [CrossRef]
- Irudayaraj, S.S.; Christudas, S.; Antony, S.; Duraipandiyan, V.; Naif Abdullah, A.D.; Ignacimuthu, S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharm. Biol. 2017, 55, 1074–1081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Liu, X.; Chen, S.; Wang, Q.; Jin, B.; Wang, L. Functions, accumulation, and biosynthesis of important secondary metabolites in the fig tree (Ficus carica). Front. Plant Sci. 2024, 15, 1397874. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.Y.; Wang, P.; Guo, N.; Niu, L.L.; Fu, Y.J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. .J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mamoucha, S.; Fokialakis, N.; Christodoulakis, N.S. Leaf structure and histochemistry of Ficus carica (Moraceae), the fig tree. Flora 2016, 218, 1–11. [Google Scholar] [CrossRef]
- Marrelli, M.; Menichini, F.; Statti, G.A.; Bonesi, M.; Duez, P.; Menichini, F.; Conforti, F. Changes in the phenolic and lipophilic composition, in the enzyme inhibition and antiproliferative activity of Ficus carica L. cultivar Dottato fruits during maturation. Food Chem. Toxicol. 2012, 50, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Jain, S.C.; Bhagchandani, T.; Yadav, N. New furanocoumarins and other chemical constituents from Ficus carica root heartwood. Z. Naturforsch. C J. Biosci. 2013, 68, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, V.K.; Srivastava, D.; Singh, P.; Kumar, U.; Kumar, D.; Gosipatala, S.B.; Saha, S.; Kumar, D.; Raj, R. Mulberries: A promising fruit for phytochemicals, nutracicals and biological activities. Int. J. Fruit Sci. 2020, 20, S1254–S1279. [Google Scholar] [CrossRef]
- Budiman, A.; Aulifa, D.L.; Kusuma, A.S.W.; Sulastri, A. Antibacterial and antioxidant activity of black mulberry (Morus nigra) extracts for acne treatment. Pharmacog. J. 2017, 9, 611–614. [Google Scholar] [CrossRef]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Ong, C.N.; Liu, X.; Huang, D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 2008, 56, 9410–9416. [Google Scholar] [CrossRef] [PubMed]
- Deniz, G.Y.; Laloglu, E.; Koc, K.; Nadaroglu, H.; Geyikoglu, F. The effect of black mulberry (Morus nigra) extracts on carbon tetrachloride-induced liver damage. Arch. Biol. Sci. 2018, 70, 371–378. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The mulberry (Morus alba L.) fruit: A review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.W.; Shia, C.S.; Lin, S.P.; Chao, P.D.L.; Juang, S.H.; Hou, Y.C. Potential Risk of Mulberry–Drug Interaction: Modulation on P-Glycoprotein and Cytochrome P450 3A. J. Agric. Food Chem. 2013, 61, 4464–4469. [Google Scholar] [CrossRef]
- Fredericks, S.; Holt, D.W. Pharmacogenomics of immunosuppressive drug metabolism. Curr. Opin. Nephrol. Hypertens. 2003, 12, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Lown, K.S.; Mayo, R.R.; Leichtman, A.B.; Hsiao, H.L.; Turgeon, D.K.; Schmiedlin-Ren, P.; Brown, M.B.; Guo, W.; Rossi, S.J.; Benet, L.Z.; et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 1997, 62, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.T.; Liou, Y.B.; Kao, Y.H.; Lin, Y.K.; Ho, H.O. A quantitative structure-activity relationship for the modulation effects of flavonoids on p-glycoprotein-mediated transport. Chem. Pharm. Bull. 2010, 58, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Dupont, I.; Van der Heiden, E.; Scippo, M.L.; Pussemier, L.; Larondelle, Y.; Schneider, Y.J. CYP1A1 and CYP3A4 modulation by dietary flavonoids in human intestinal Caco-2 cells. Toxicol. Lett. 2009, 191, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mitra, A.K. MDR- and CYP3A4-mediated drug−herbal interactions. Life Sci. 2006, 78, 2131–2145. [Google Scholar] [CrossRef]
- Choi, J.S.; Piao, Y.J.; Kang, K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharm. Res. 2011, 34, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Wu, P.P.; Hou, Y.C.; Lin, S.P.; Tsai, S.Y.; Chen, C.T.; Chao, P.D. Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. J. Agric. Food. Chem. 2011, 59, 4644–4648. [Google Scholar] [CrossRef] [PubMed]
- Akamine, Y.; Miura, M.; Komori, H.; Saito, S.; Kusuhara, H.; Tamai, I.; Ieiri, I.; Uno, T.; Yasui-Furukori, N. Effects of one-time apple juice ingestion on the pharmacokinetics of fexofenadine enantiomers. Eur. J. Clin. Pharmacol. 2014, 70, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanover, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica) var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Zessner, H.; Pan, L.; Will, F.; Klimo, K.; Knauft, J.; Niewohner, R.; Hummer, W.; Owen, R.; Richling, E.; Frank, N.; et al. Fractionation of polyphenol-enriched apple juice extracts to identify constituents with cancer chemopreventive potential. Mol. Nutr. Food Res. 2008, 52, S28–S44. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Yasujima, T.; Ohta, K.; Inoue, K.; Yuasa, H. Functional Identification of Plasma Membrane Monoamine Transporter (PMAT/SLC29A4) as an Atenolol Transporter Sensitive to Flavonoids Contained in Apple Juice. J. Pharm. Sci. 2017, 106, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, J.; Kotegawa, T.; Imai, H.; Tsutsumi, K.; Yoshizato, T.; Ohyama, T.; Shirasaka, Y.; Tamai, I.; Tateishi, T.; Ohashi, K. The effects of the SLCO2B1 c.1457C > T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet. Genom. 2011, 21, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Melgarejo, P.; Tomas-Barberan, F.A.; Artes, F. Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. Eur. Food Res. Technol. 1999, 210, 39–42. [Google Scholar] [CrossRef]
- Qian, F.; Wei, D.; Zhang, Q.; Yang, S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (−)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005, 59, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef]
- Hanley, M.J.; Masse, G.; Harmatz, J.S.; Court, M.H.; Greenblatt, D.J. Pomegranate juice and pomegranate extract do not impair oral clearance of flurbiprofen in human volunteers: Divergence from in vitro results. Clin. Pharmacol. Ther. 2012, 92, 651–657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misaka, S.; Nakamura, R.; Uchida, S.; Takeuchi, K.; Takahashi, N.; Inui, N.; Kosuge, K.; Yamada, S.; Watanabe, H. Effect of 2 weeks’ consumption of pomegranate juice on the pharmacokinetics of a single dose of midazolam: An open-label, randomized, single-center, 2-period crossover study in healthy Japanese volunteers. Clin. Ther. 2011, 33, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.; Oleson, L.E.; Zhao, Y.; Harmatz, J.S.; Zinny, M.A.; Court, M.H.; Greenblatt, D.J. Pomegranate juice does not impair clearance of oral or intravenous midazolam, a probe for cytochrome P450-3A activity: Comparison with grapefruit juice. J. Clin. Pharmacol. 2007, 47, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Shema-Didi, L.; Kristal, B.; Ore, L.; Shapiro, G.; Geron, R.; Sela, S. Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during hemodialysis. Nutr. Res. 2013, 33, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Lilja, J.J.; Backman, J.T.; Neuvonen, P.J. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam–probes of CYP2C9, CYP1A2, and CYP3A4. Clin. Pharmacol. Ther. 2007, 81, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; McDonough, M.; Zhao, Y.; Harmatz, J.S.; Greenblatt, D.J. The absence of an interaction between warfarin and cranberry juice: A randomized, double-blind trial. J. Clin. Pharmacol. 2009, 49, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Seeram, N.P.; Carpenter, C.L.; Thames, G.; Minutti, C.; Bowerman, S. Cranberry does not affect prothrombin time in male subjects on warfarin. J. Am. Diet. Assoc. 2006, 106, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; von Moltke, L.L.; Perloff, E.S.; Luo, Y.; Harmatz, J.S.; Zinny, M.A. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: In vitro and clinical studies. Clin. Pharmacol. Ther. 2006, 79, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Shmuely, H.; Yahav, J.; Samra, Z.; Chodick, G.; Koren, R.; Niv, Y.; Ofek, I. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antibiotics and a proton pump inhibitor. Mol. Nutr. Food Res. 2007, 51, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanley, M.J.; Masse, G.; Harmatz, J.S.; Cancalon, P.F.; Dolnikowski, G.G.; Court, M.H.; Greenblatt, D.J. Effect of blueberry juice on clearance of buspirone and flurbiprofen in human volunteers. Br. J. Clin. Pharmacol. 2012, 75, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; McDonald, J.E.; Forney, C.F. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Guo, J.; Chu, H.; Gao, Y.; Pang, L. Blueberry Improves the Therapeutic Effect of Etanercept on Patients with Juvenile Idiopathic Arthritis: Phase III Study. Tohoku. J. Exp. Med. 2015, 237, 183–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
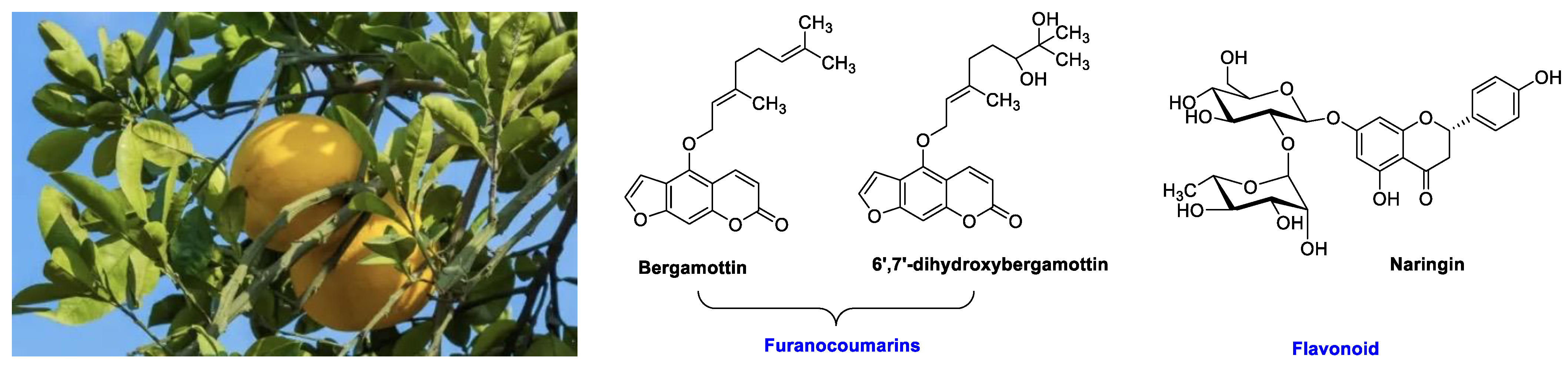

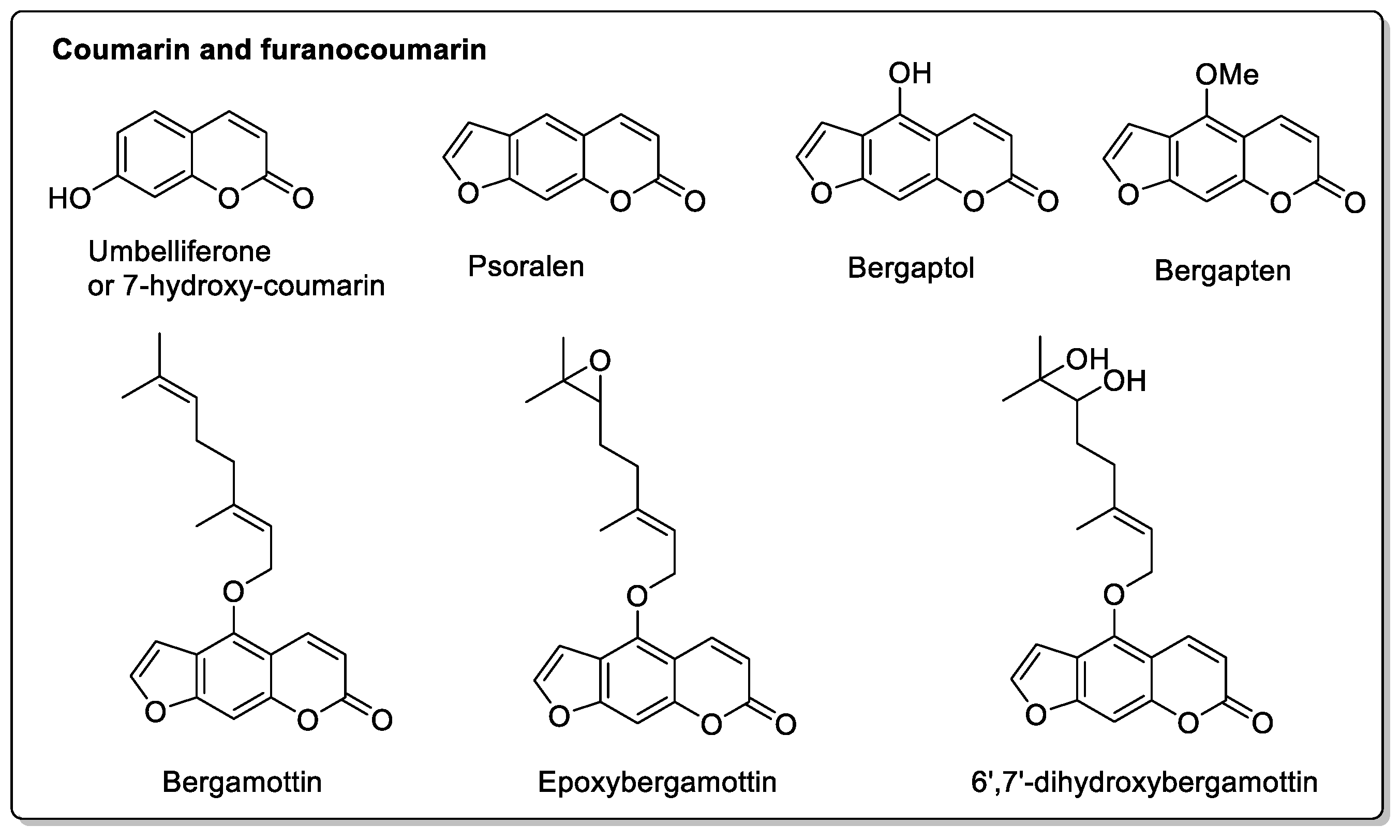

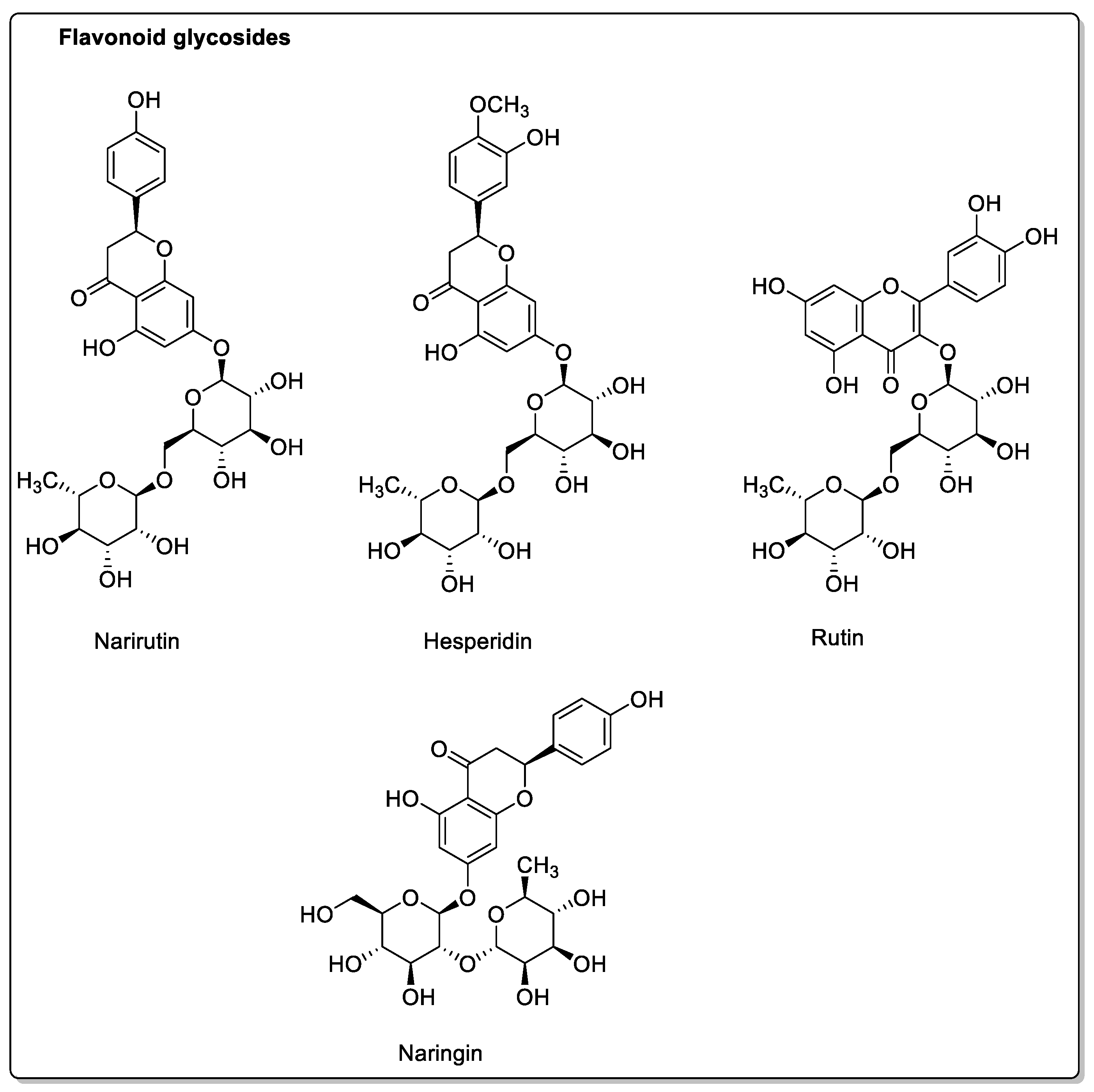
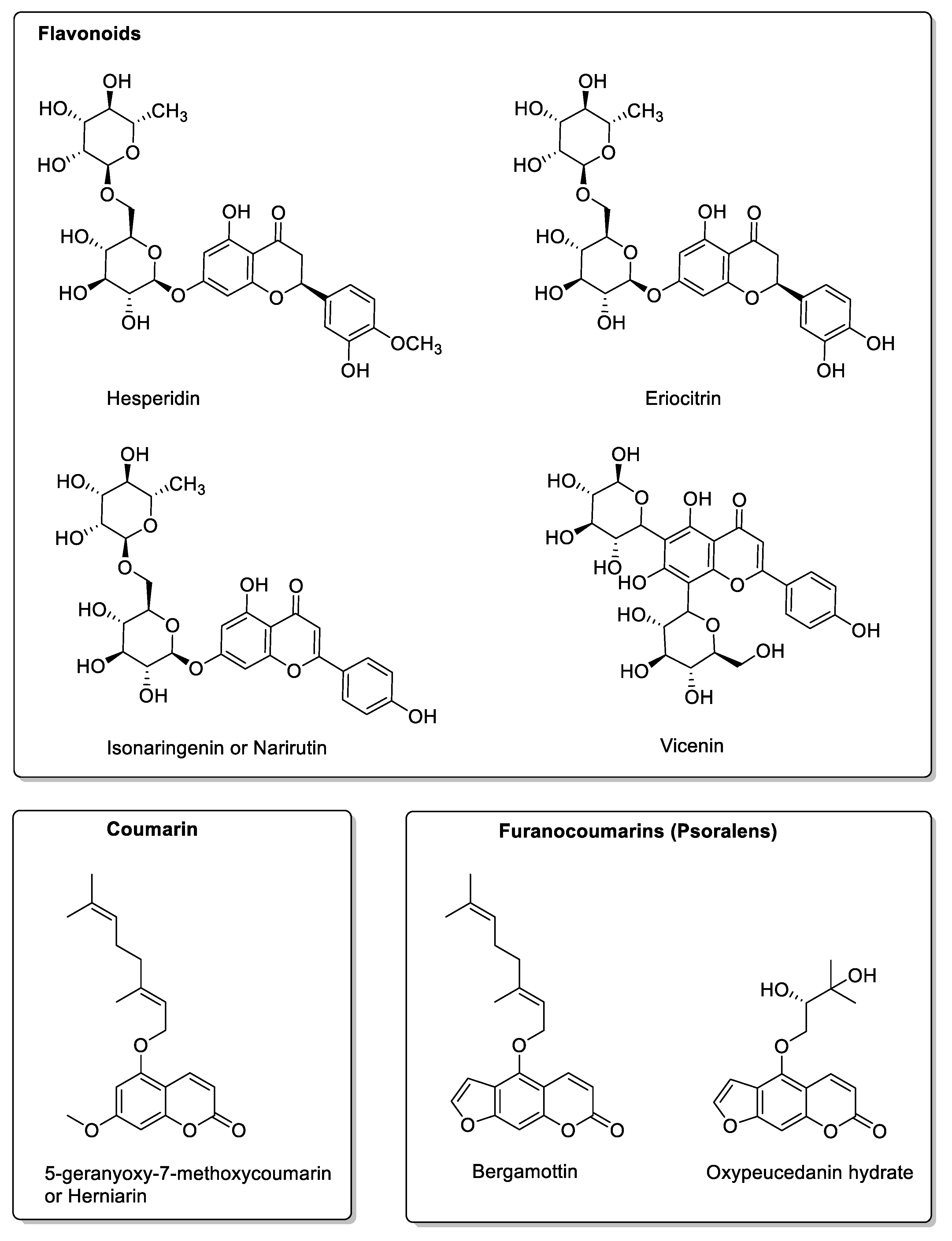
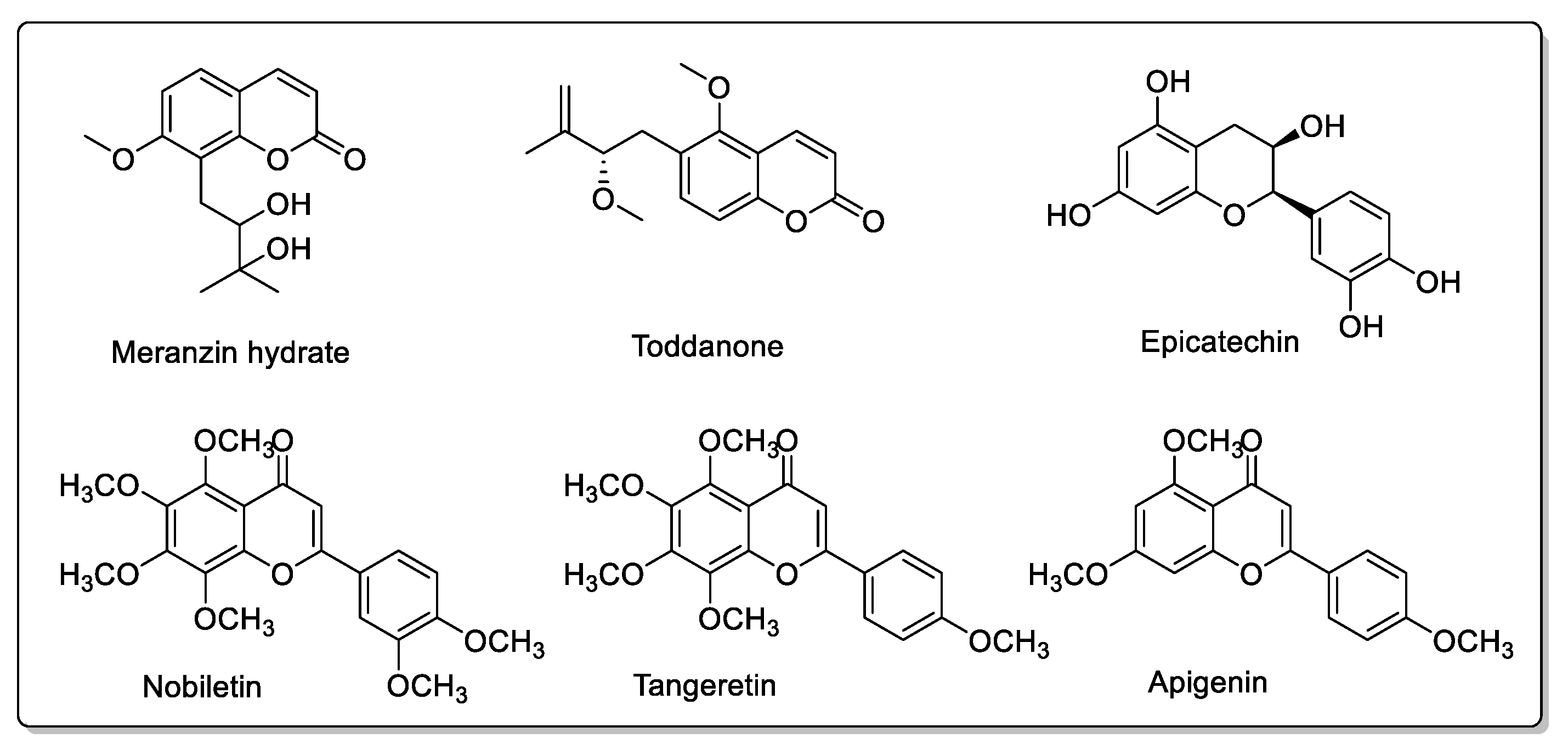
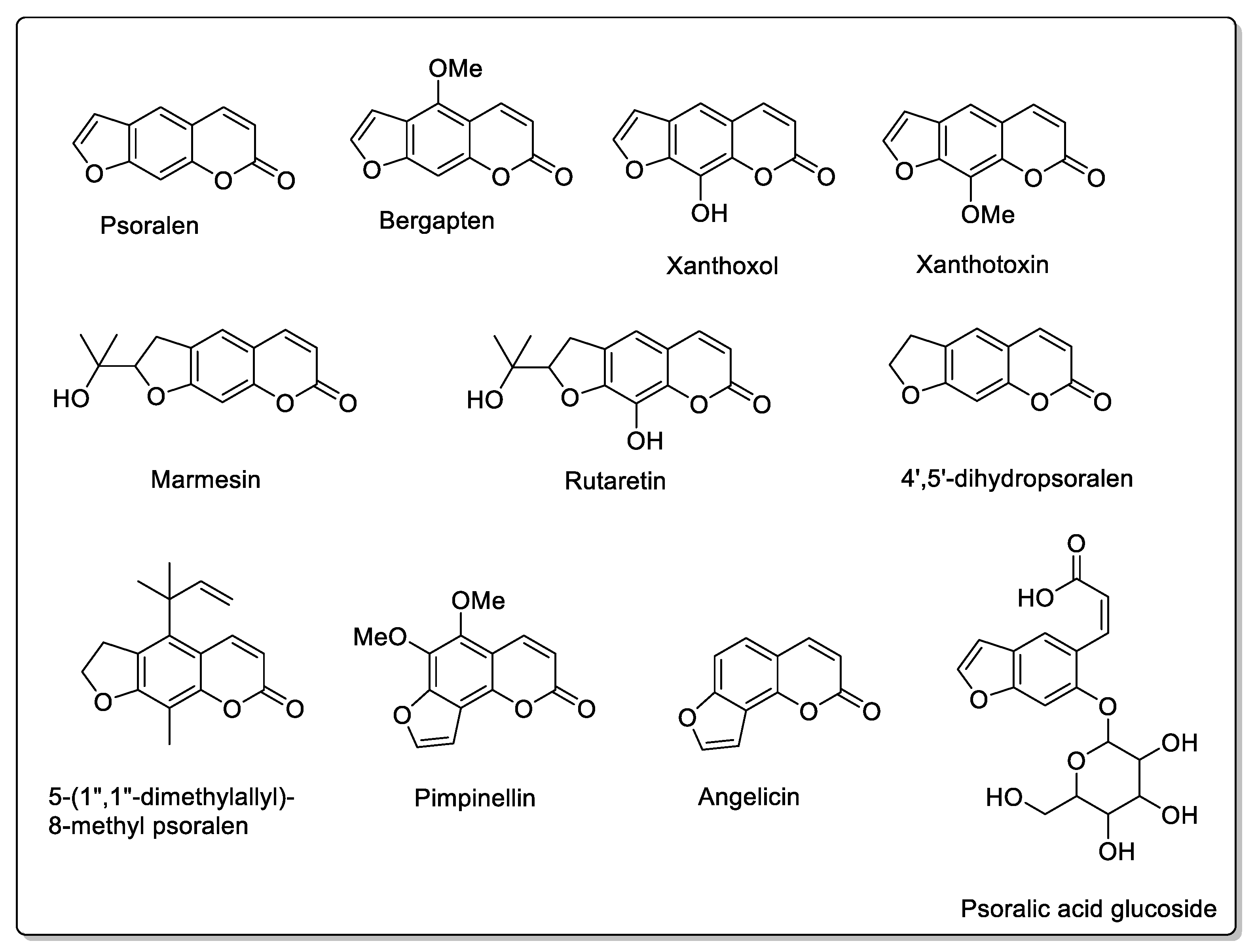
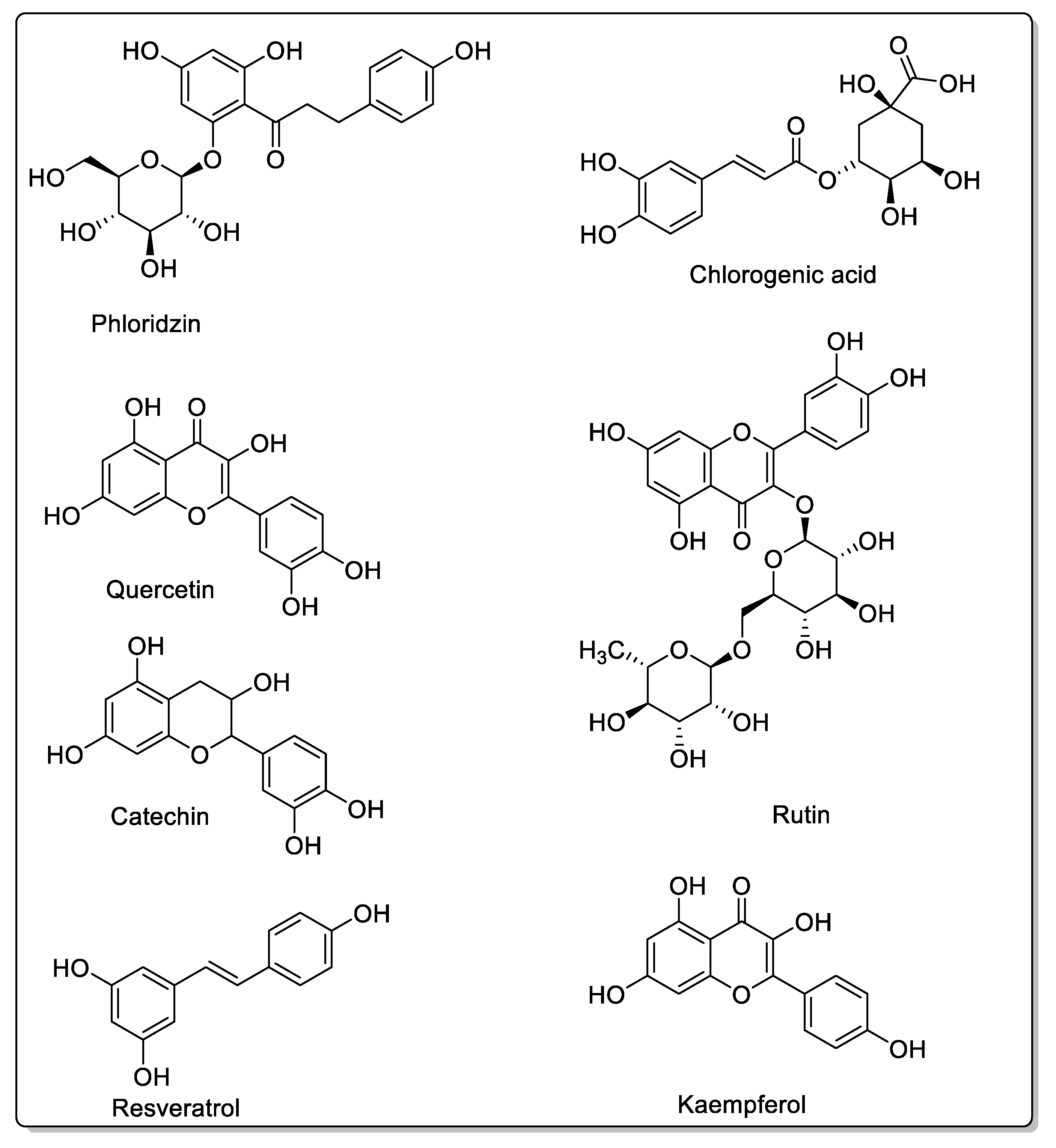
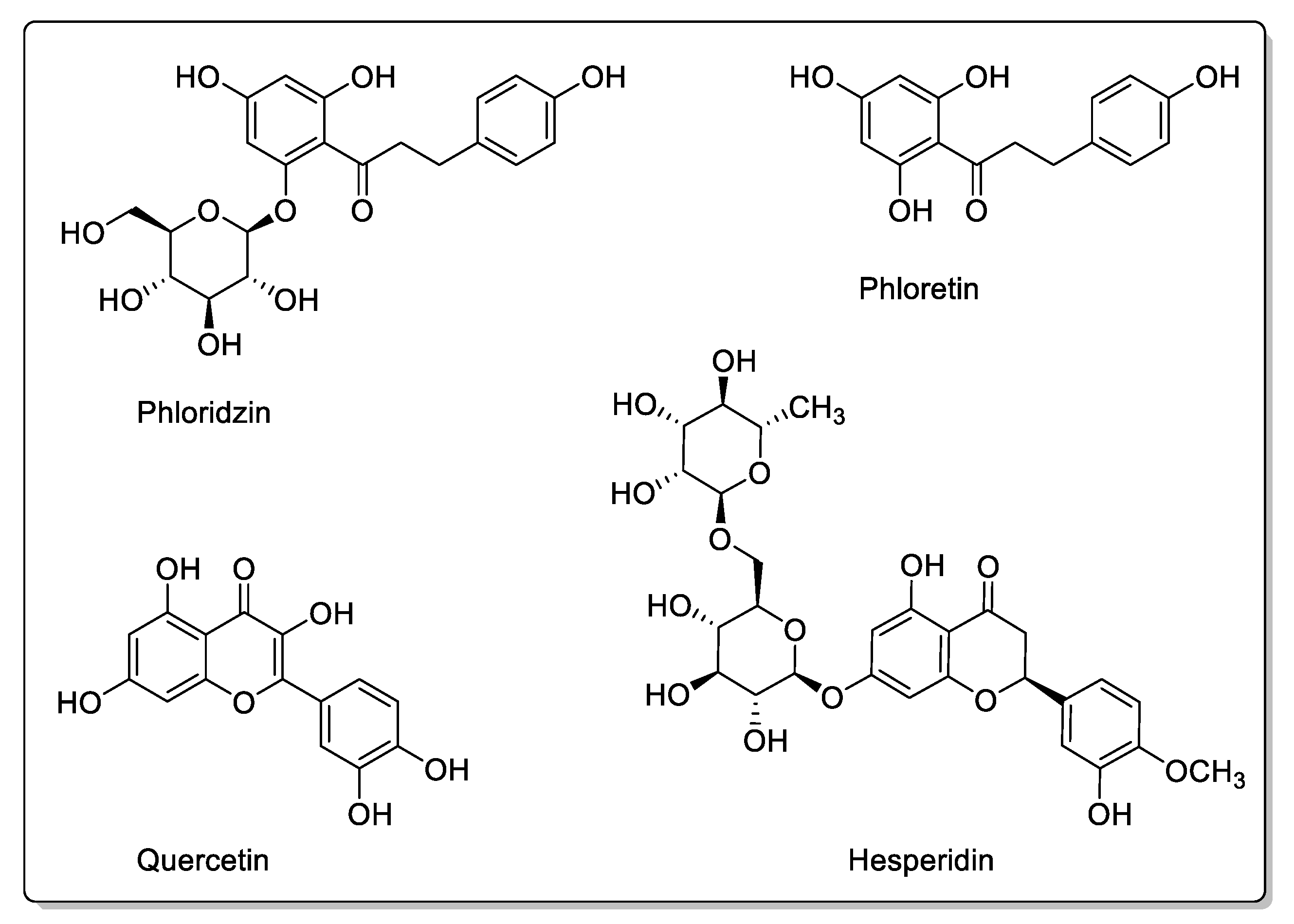
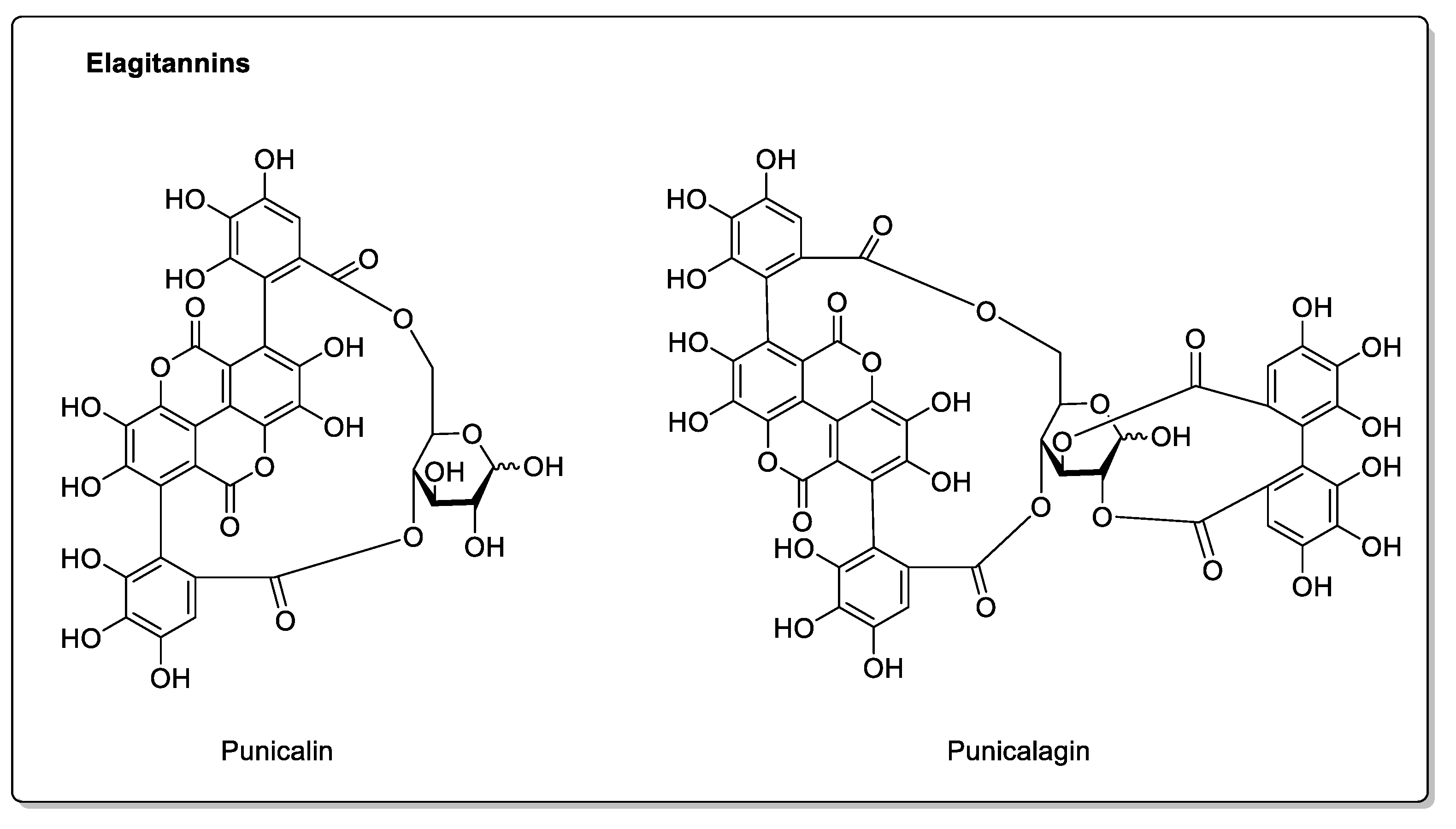

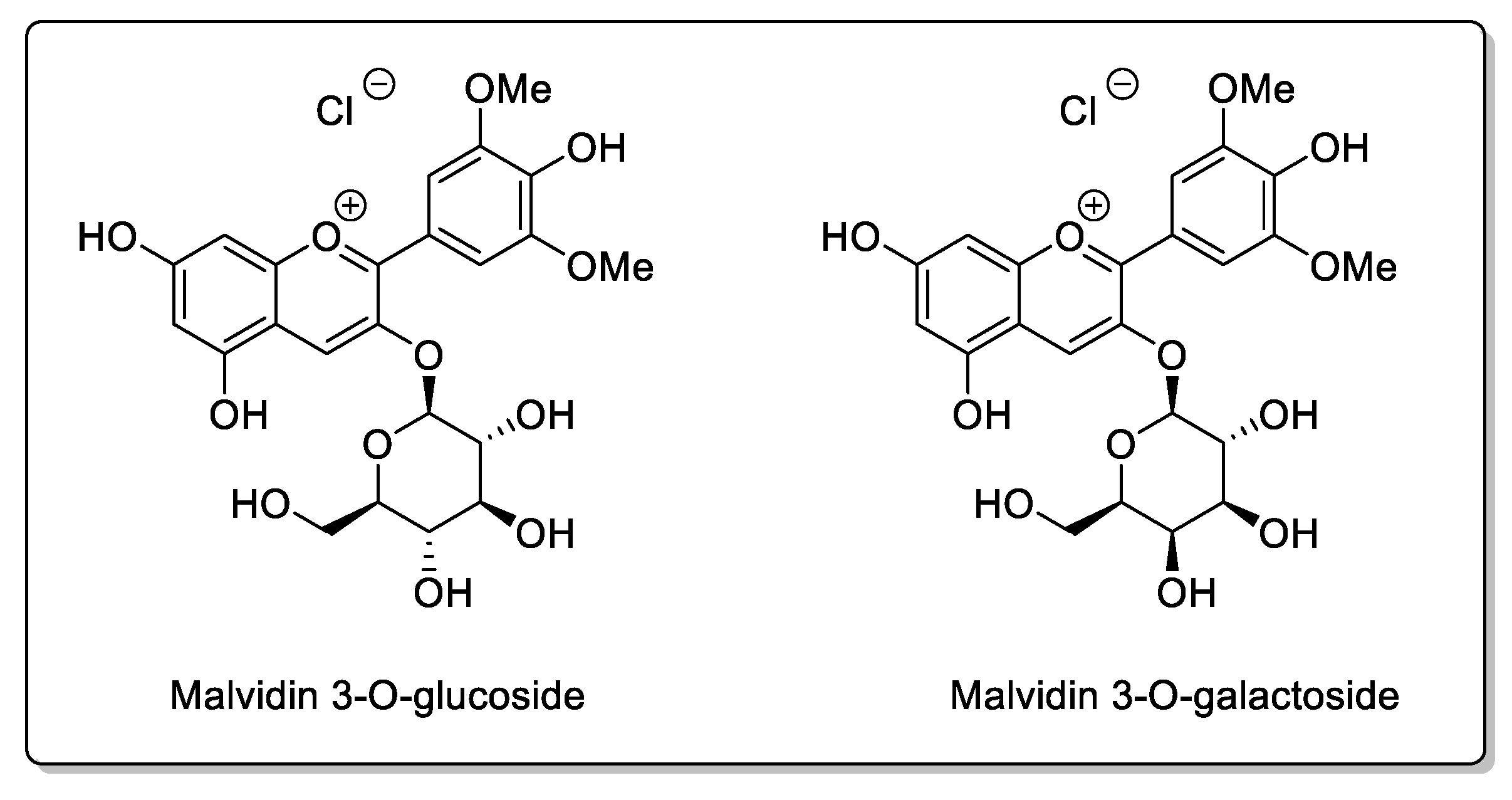
| Fruit | Family | Biological Interactions | Types of Constituents and Examples | Results in Terms of Drug Interactions |
|---|---|---|---|---|
| Grapefruit (Citrus × paradise) | Rutaceae | Irreversible inhibition of CYP3A4. Inhibition of P-gp. | Furanocoumarins: Psoralen; Bergaptol; Bergapten; Bergamottin; Epoxybergamottin; 6′,7′-dihydroxybergamottin; Oxypeucedanin. | Reduced absorption; decreased bioavailability. |
| OATP and P-gp inhibitions. | Flavonoids: Narirutin; Hesperidin; Rutin; Narengenin; Naringin. | Decreased bioavailability. | ||
| Orange (Valencia) | Rutaceae | Incomplete inhibition of OATP1A2. Inhibition of OATP2B1. | No furanocoumarins. Flavonoids: Narirutin; Hesperidin; Phenolic acids: p-Coumaric acid; Ferulic acid. | No furanocoumarins. Fewer interactions generated. |
| Seville orange (Citrus aurantium) | Rutaceae | CYP3A4, CYP 2C9, and OATP inhibitions. P-gp not affected. | Furanocoumarins: Bergapten. Flavonoids: Narirutin; Naringin. Phenolic acids: p-Coumaric acid; Ferulic acid Caffeic acid; Chlorogenic acid. | Decreased bioavailability. |
| Lime (Citrus aurantiifolia, Citrus latifolia) | Rutaceae | CYP3A4, CYP2C9, and OATP2B1 inhibitions. | Same Furanocoumarins, flavonoids, and polyphenols as in grapefruits. Bergapten; Bergamottin; Oxypeucedanin; Narirutin; Naringin; Naringenin; Hesperetin; Rutin; Eriocitrin; Isonaringenin; Vicenin; Lucenin; p-Coumaric acid; Ferulic acid; Caffeic acid. | Reduced absorption; decreased bioavailability. |
| Pomelo (Citrus grandis L. Osbeck or Citrus maxima) | Rutaceae | Inhibition of CYP2C9, CYP3A4, and OATP2B1. P-gp not affected. | Coumarins: Rutin; Meranzin; Taddanone Flavonoids: Naringin; Hesperidin; Apigenin Tangeretin; Nobiletin; Taddanone Naringin; Epicatechin. Phenolic acids: p-Coumaric acid; Ferulic acid; Caffeic acid. | Reduced absorption; decreased bioavailability. |
| Fig (Ficus carica L.) | Moraceae | CYP3A4 inhibition. | Furanocoumarins Psoralen; Bergapten; Marmesin; Xanthotoxol; Xanthotoxin; Rutaretin; Angelicin; Pimpinellin. | Interactions have been reported with anticoagulant and antibacterial treatments. |
| Blackberry (Morus alba, Morus nigra, Morus rubra) | Moraceae | CYP3A4, CYP3A1, and OATP-B1 inhibitions. P-gp and CYP3A4 modulations. | Coumarins: Rutin. Flavonoids: Phloridzin; Quercetin; Kaempferol; Resveratrol. Phenolic acids: Chlorogenic acid. Anthocyanins. | Decreased bioavailability. |
| Apple (Malus domestica) | Rosaceae | PMAT/SLC29A4, CYP1A1, CYP2C9, OATP2B1, OATP1, and OATP3 inhibtions. | Flavonoids and polyphenols: Phloridzin; Phloretin; Hesperidin; Quercetin; Rutin. | Potential reduction in absorption. No effect on CYP3A. |
| Pomegranate (Punica granatum L.) | Lythraceae | CYP3A4 and CYP2C9 inhibitions in vitro. | Flavonoids: Catechins; Epicatechins; Quercetin. Phenolic acids: p-Coumaric acid; Ferulic acid; Caffeic acid; Chlorogenic acid. Proanthocyanidins. Eligatannins: Punicalin; Punicalagins. | Bioavailability unaffected in vivo. |
| Cranberry (Vaccinium macrocarpon) | Ericaceae | CYP3A4, CYP1A2, and CYP2C9 inhibitions in vitro. | Flavonols: Catechins; Epicatechin; Epigallocatechin; Quercetin; Kaempferol; Myricetin. Phenolic acids: p-Coumaric acid; Ferulic acid; Caffeic acid; Chlorogenic acid. Proanthocyanidins: Procyanidin A2; Procyanidin B2. | Bioavailability unaffected in vivo. |
| Blueberry (Vaccinium angustifolium Aiton and Vaccinium corymbosum L.) | Ericaceae | CYP3A inhibition in vitro. | Flavonols: Catechins; Epicatechin; Epigallocatechin; Quercetin; Kaempferol. Phenolic acid: p-Coumaric acid; Ferulic acidl Caffeic acid; Chlorogenic acid. Proanthocyanidins: Malvidin 3-glucoside; Malvidin 3-galactoside. | Bioavailability unaffected in vivo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berteina-Raboin, S. Flavonoids and Furanocoumarins Involved in Drug Interactions. Molecules 2025, 30, 1676. https://doi.org/10.3390/molecules30081676
Berteina-Raboin S. Flavonoids and Furanocoumarins Involved in Drug Interactions. Molecules. 2025; 30(8):1676. https://doi.org/10.3390/molecules30081676
Chicago/Turabian StyleBerteina-Raboin, Sabine. 2025. "Flavonoids and Furanocoumarins Involved in Drug Interactions" Molecules 30, no. 8: 1676. https://doi.org/10.3390/molecules30081676
APA StyleBerteina-Raboin, S. (2025). Flavonoids and Furanocoumarins Involved in Drug Interactions. Molecules, 30(8), 1676. https://doi.org/10.3390/molecules30081676






