The Mechanism of a Novel Mitochondrial-Targeted Icaritin Derivative in Regulating Apoptosis of BEL-7402 Cells Based on the SIRT3 and CypD-Mediated ROS/p38 MAPK Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis
2.2. In Vitro Biological Activity
2.3. Aggregation and Distribution of Mito-ICT-4 in Mitochondria of BEL-7402 Cells
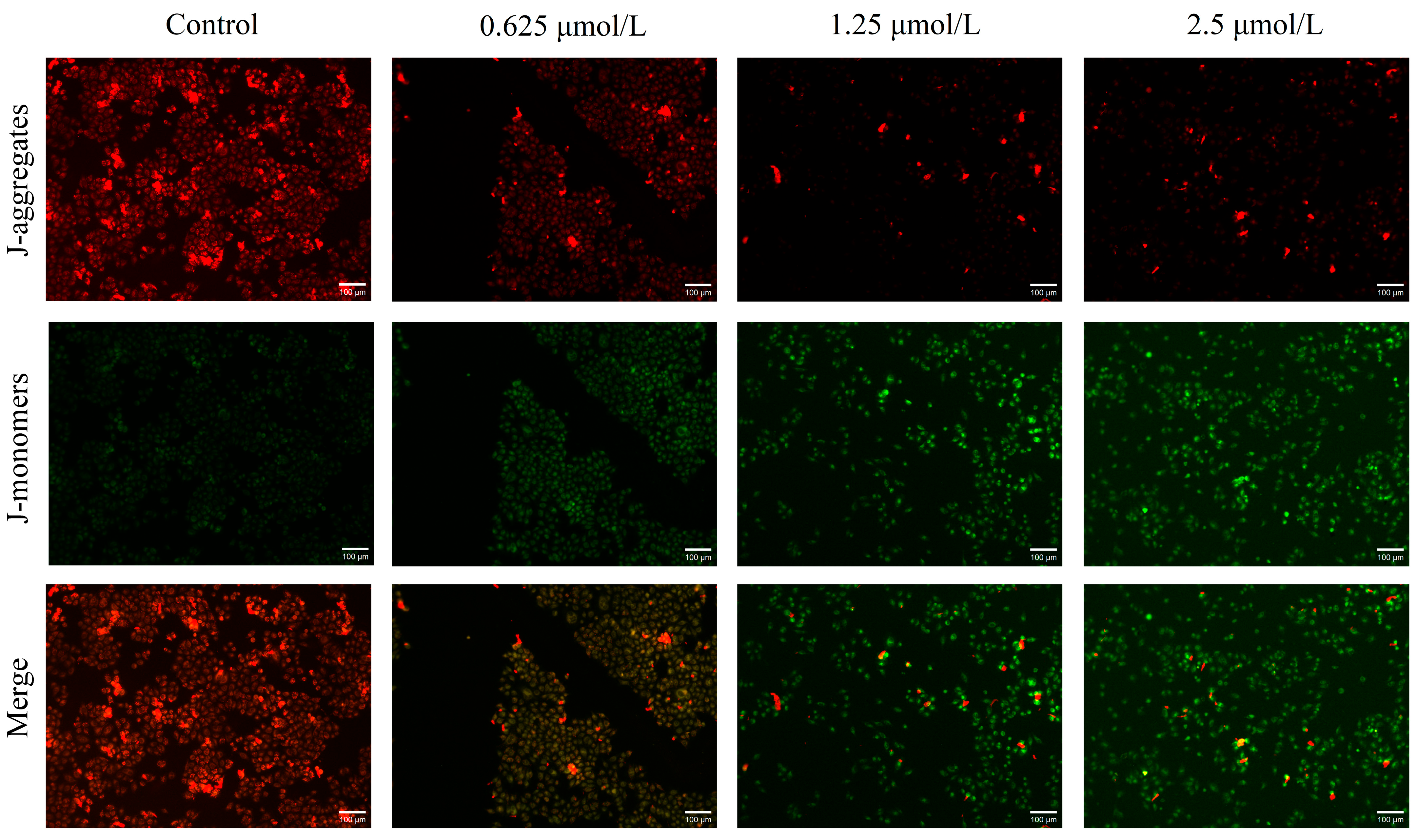
2.4. Effect of Mito-ICT-4 on Mitochondrial Membrane Potential in BEL-7402 Cells
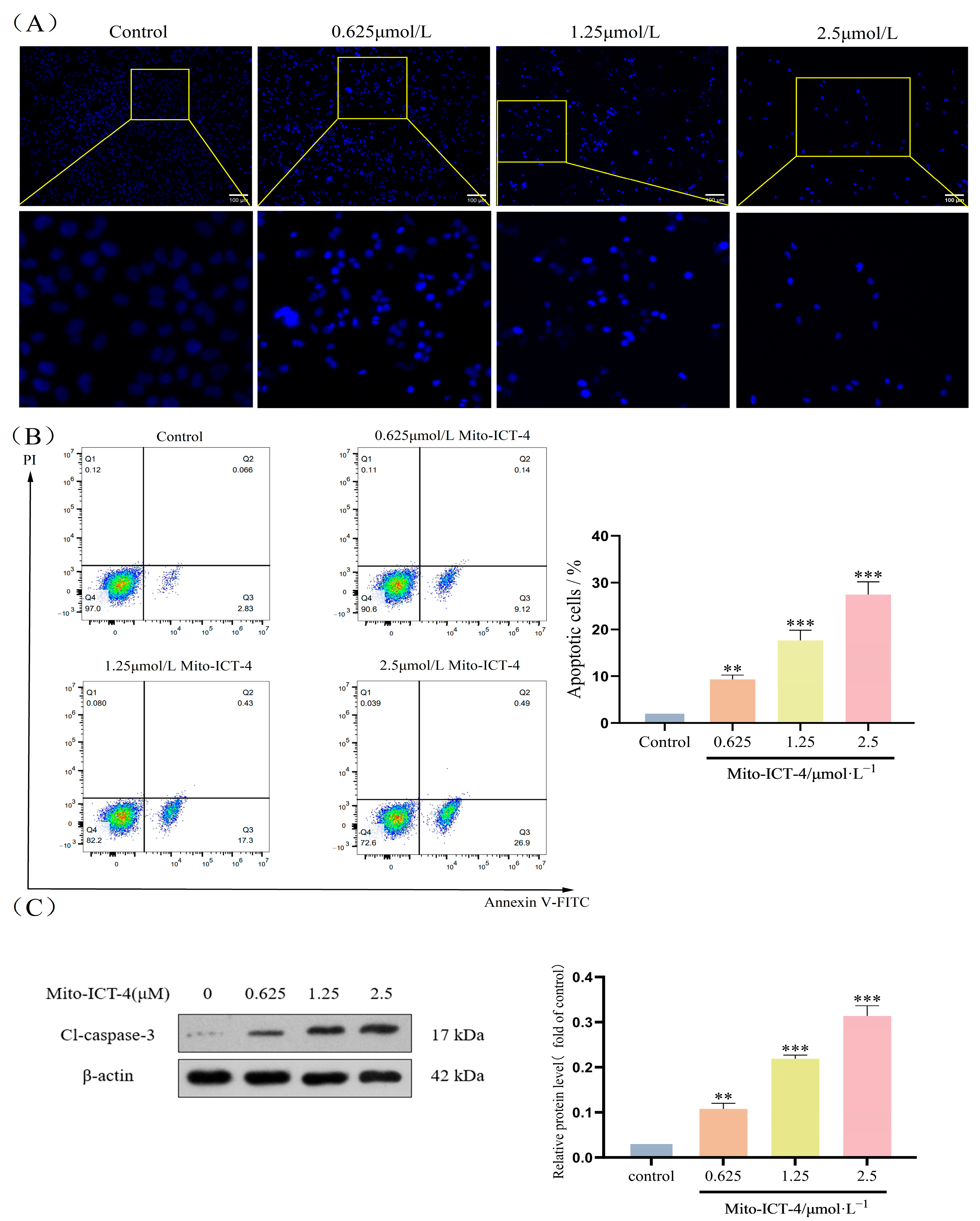
2.5. Mito-ICT-4 Induces Apoptosis in BEL-7402 Cells
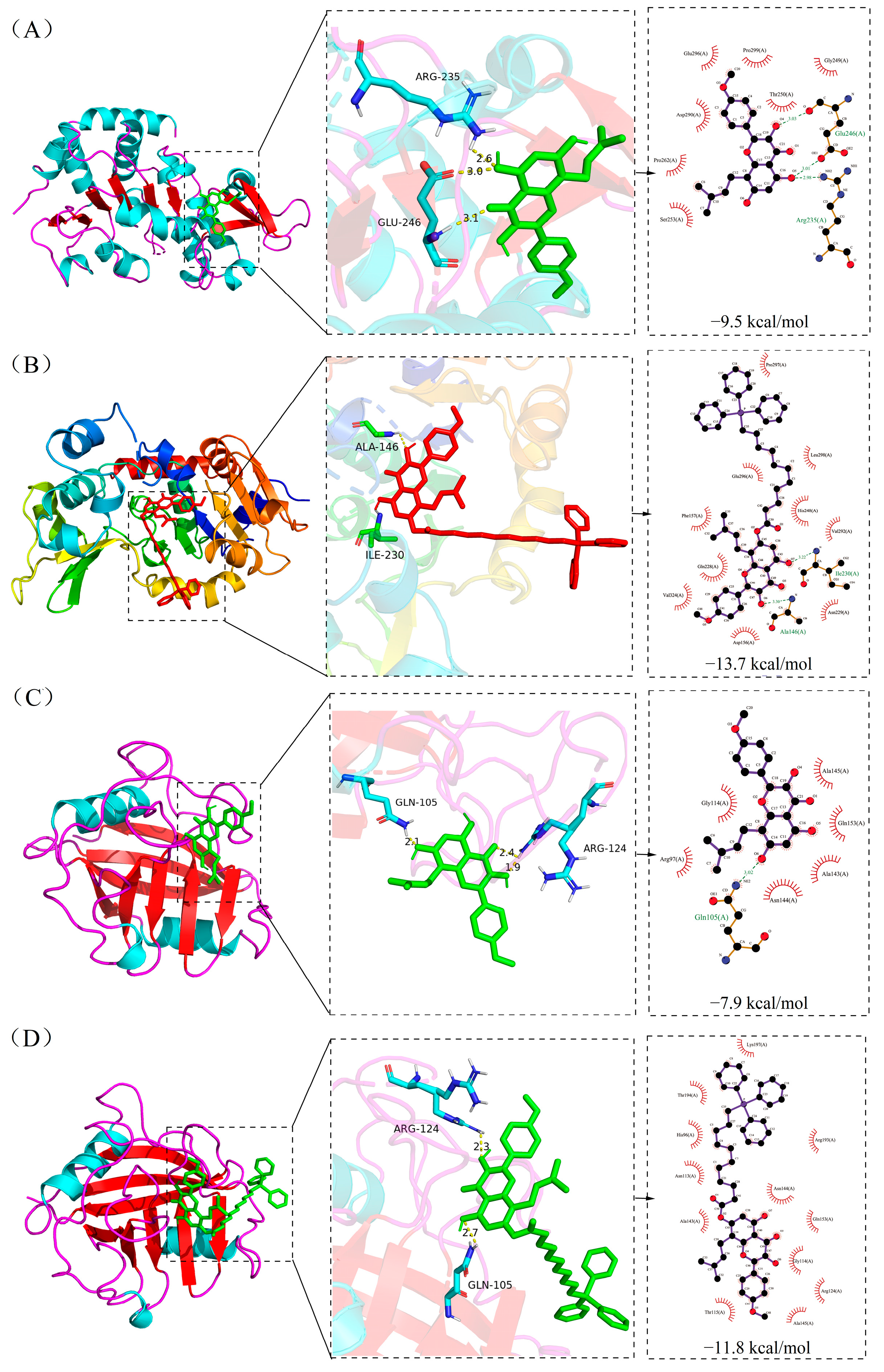
2.6. Molecular Docking Result
2.7. Effect of Mito-ICT-4 on Intracellular ROS in BEL-7402 Cells
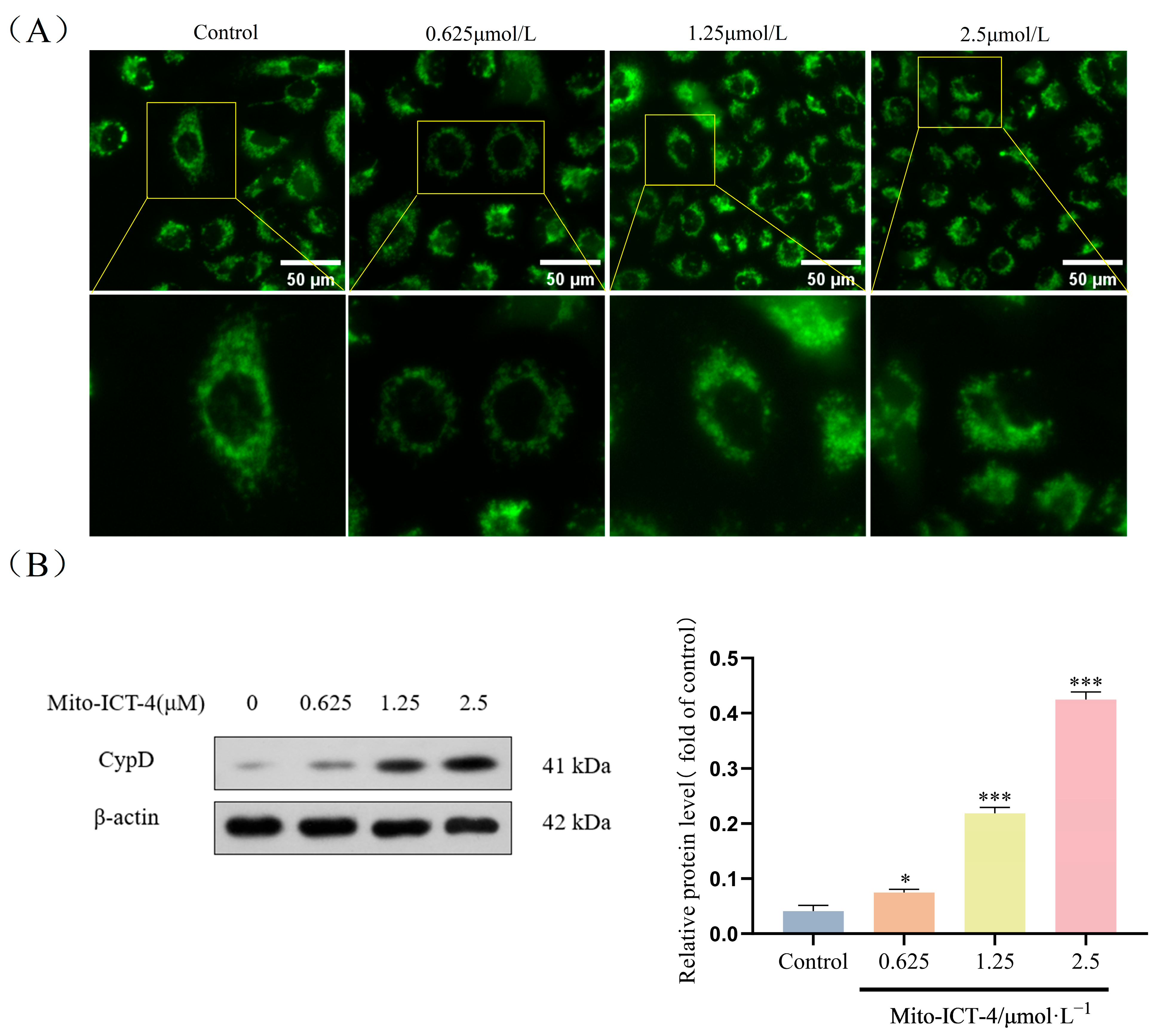
2.8. Effect of Mito-ICT-4 on Mitochondrial Membrane Permeability in BEL-7402 Cells
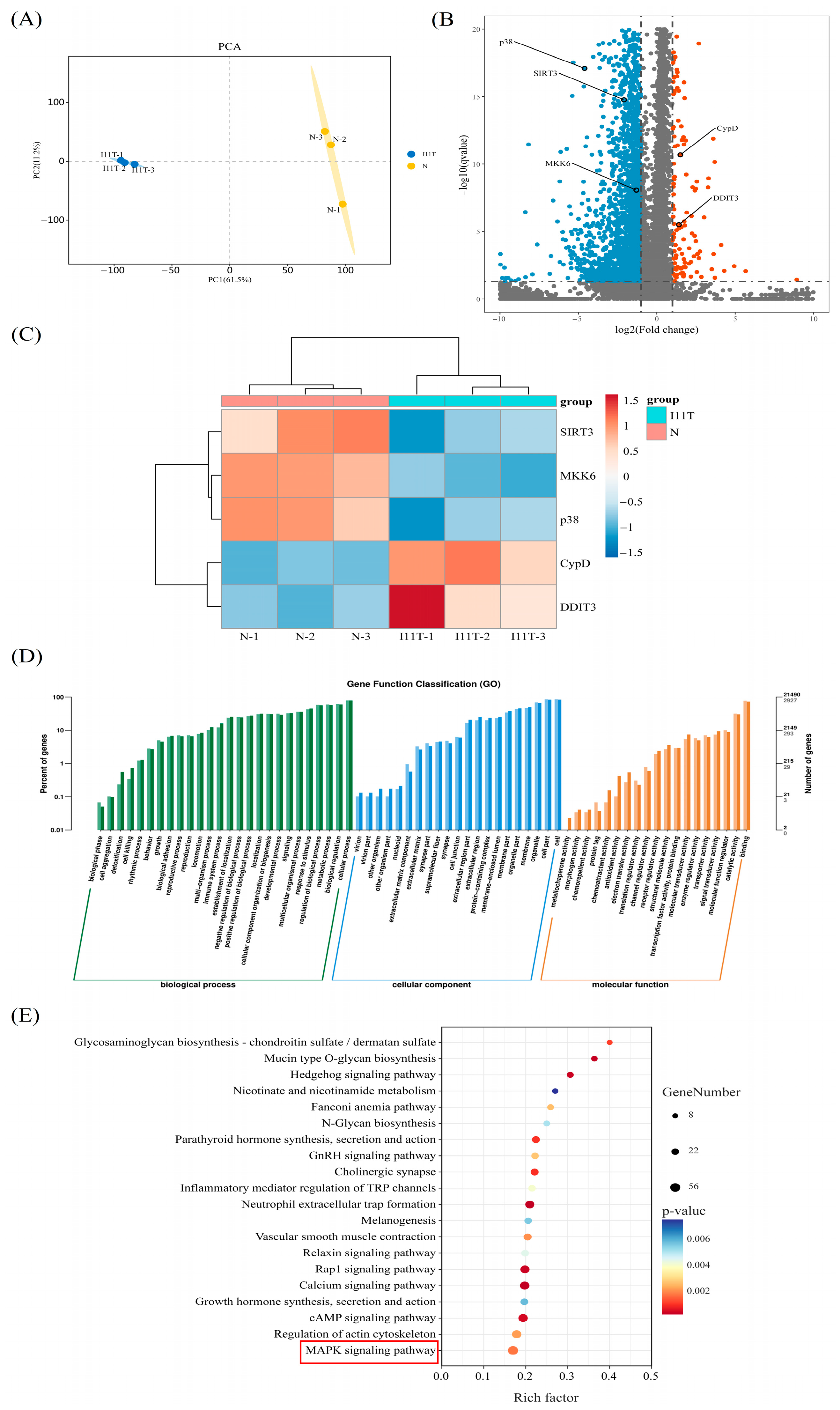
2.9. Transcriptome Sequencing Results
2.10. qRT-PCR Validation of Key Differential Genes
2.11. Western Blot Result
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Synthesis of Intermediates 1–4
4.3. Synthesis of Fatty Acid Analogs 5–9
4.4. Synthesis of Target Compounds Mito-ICT-1~Mito-ICT-4
4.5. Cell Culture
4.6. Detection of Cell Viability by MTT Assay
4.7. HPLC Analysis of Cellular Uptake of Compounds
4.8. Apoptosis Was Observed Using DAPI Staining
4.9. Apoptosis Was Detected Using Annexin V-FITC/PI Double Staining
4.10. Mitochondrial Membrane Potential Was Detected Using JC-1 Staining
4.11. Calcein AM Probe Detects MPTP Opening
4.12. Molecular Docking
4.13. DCFH-DA Fluorescent Probe to Detect ROS Levels
4.14. Transcriptome Sequencing
4.15. qRT-PCR Validation of Differential Genes
4.16. Western Blot
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiri, S.; Ryba, T. Cancer, Metastasis, and the Epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Ferraz, L.S. Therapeutic Potential of Targeting Mitochondrial Dynamics in Cancer. Biochem. Pharmacol. 2020, 182, 114282. [Google Scholar] [CrossRef]
- Begum, H.M.; Shen, K. Intracellular and Microenvironmental Regulation of Mitochondrial Membrane Potential in Cancer Cells. WIREs Mech. Dis. 2023, 15, e1595. [Google Scholar] [CrossRef] [PubMed]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anticancer Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Xing, W.; Liu, G.; Zhang, Y.; Zhang, T.; Lou, H.; Fan, P. Selective Antitumor Effect and Lower Toxicity of Mitochondrion-Targeting Derivatization of Triptolide. J. Med. Chem. 2024, 67, 1093–1114. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Yadava, N.; Chambers, J.J.; Thayumanavan, S. Anionic Polymers Promote Mitochondrial Targeting of Delocalized Lipophilic Cations. Bioconjug. Chem. 2020, 31, 1344–1353. [Google Scholar] [CrossRef]
- Yang, C.B. Quassinoids Against Pancreatic Cancer from Bruceae Fructus and Their Mitochondrial Targeting Derivatization. Master’s Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2022. [Google Scholar] [CrossRef]
- Wei, C.Y.; Wang, Y.P.; Wang, J.; Zhang, L. Advance in Chemical Synthesis and Structural Modification of lcaritin. Chem. Reag. 2023, 45, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Liu, Y.; Yang, F.; Zhang, H.; Kong, Y. Icaritin Inhibits Endometrial Carcinoma Cells by Suppressing O-GlcNAcylation of FOXC1. Phytomedicine 2023, 120, 155062. [Google Scholar] [CrossRef]
- Mou, Z.; Chen, Y.; Hu, J.; Hu, Y.; Zou, L.; Chen, X.; Liu, S.; Yin, Q.; Gong, J.; Li, S.; et al. Icaritin Inhibits the Progression of Urothelial Cancer by Suppressing PADI2-Mediated Neutrophil Infiltration and Neutrophil Extracellular Trap Formation. Acta Pharm. Sin. B 2024, 14, 3916–3930. [Google Scholar] [CrossRef]
- Luo, P.; An, Y.; He, J.; Xing, X.; Zhang, Q.; Liu, X.; Chen, Y.; Yuan, H.; Chen, J.; Wong, Y.-K.; et al. Icaritin with Autophagy/Mitophagy Inhibitors Synergistically Enhances Anticancer Efficacy and Apoptotic Effects through PINK1/Parkin-Mediated Mitophagy in Hepatocellular Carcinoma. Cancer Lett. 2024, 587, 216621. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Jia, X.; Tam, J.P.; Yang, H.; Shen, Y.; Li, X. Multidisciplinary Strategies to Enhance Therapeutic Effects of Flavonoids from Epimedii Folium: Integration of Herbal Medicine, Enzyme Engineering, and Nanotechnology. J. Pharm. Anal. 2023, 13, 239–254. [Google Scholar] [CrossRef]
- Szabó, R.; Rácz, C.P.; Dulf, F.V. Bioavailability Improvement Strategies for Icariin and Its Derivates: A Review. Int. J. Mol. Sci. 2022, 23, 7519. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, H. Precision Diagnosis and Treatment of Liver Cancer in China. Cancer Lett. 2018, 412, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Qian, S.; Wang, Y.; Zhao, C. Regio-Selective Synthesis and Activity Research on 7-Icaritin Norcantharidin Conjugates. Nat. Prod. Res. 2024, 38, 311–319. [Google Scholar] [CrossRef]
- Wang, C.; Wu, P.; Shi, J.-F.; Jiang, Z.-H.; Wei, X.-Y. Synthesis and Cancer Cell Growth Inhibitory Activity of Icaritin Derivatives. Eur. J. Med. Chem. 2015, 100, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xing, W.; Shi, X.; Zhang, T.; Lou, H.; Fan, P. Antitumor and Toxicity Study of Mitochondria-Targeted Triptolide Derivatives Using Triphenylphosphine (TPP+) as a Carrier. Bioorg. Med. Chem. 2021, 50, 116466. [Google Scholar] [CrossRef]
- Shu, H. Design and Synthesis of β-Anhydroicartin Derivatives and Preliminary Study on Its Anti-Tumor Mechanism. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2024. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Li, W.; Li, T.; Xiao, S.; Lu, J.; Xu, J.; Zhao, Y. Rational Design, Synthesis and Biological Evaluation of Triphenylphosphonium-Ginsenoside Conjugates as Mitochondria-Targeting Anti-Cancer Agents. Bioorg. Chem. 2020, 103, 104150. [Google Scholar] [CrossRef]
- Dumbrepatil, A.B.; Zegalia, K.A.; Sajja, K.; Kennedy, R.T.; Marsh, E.N.G. Targeting Viperin to the Mitochondrion Inhibits the Thiolase Activity of the Trifunctional Enzyme Complex. J. Biol. Chem. 2020, 295, 2839–2849. [Google Scholar] [CrossRef]
- Wang, S.-H.; Zhang, Y.-W.; Wang, X.-D.; Zan, Q.; Yu, X.; Fan, L. An Esterase-Sensitive AIEgen Probe Targeting Mitochondria and Lipid Droplets for Assessing Cell Viability. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 122122. [Google Scholar] [CrossRef]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a Natural Flavonol Glycoside, Induces Apoptosis in Human Hepatoma SMMC-7721 Cells via a ROS/JNK-Dependent Mitochondrial Pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F. Design, Synthesis and Preliminary Study on Antitumor Activity of Arctium Derivatives. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2024. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Peng, X.; Gandhi, V. ROS-Activated Anticancer Prodrugs: A New Strategy for Tumor-Specific Damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. SIRT3: Oncogene and Tumor Suppressor in Cancer. Cancers 2017, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, Y.; Hu, Y.; Sun, Y.; Du, Z.; Xie, Z.; Zhou, T.; Kong, W. Age-Related Decrease in the Mitochondrial Sirtuin Deacetylase Sirt3 Expression Associated with ROS Accumulation in the Auditory Cortex of the Mimetic Aging Rat Model. PLoS ONE 2014, 9, e88019. [Google Scholar] [CrossRef]
- Fayaz, S.M.; Raj, Y.V.; Krishnamurthy, R.G. CypD: The Key to the Death Door. CNS Neurol. Disord.-Drug Targets. 2015, 14, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Luis-García, E.R.; Becerril, C.; Salgado-Aguayo, A.; Aparicio-Trejo, O.E.; Romero, Y.; Flores-Soto, E.; Mendoza-Milla, C.; Montaño, M.; Chagoya, V.; Pedraza-Chaverri, J.; et al. Mitochondrial Dysfunction and Alterations in Mitochondrial Permeability Transition Pore (mPTP) Contribute to Apoptosis Resistance in Idiopathic Pulmonary Fibrosis Fibroblasts. Int. J. Mol. Sci. 2021, 22, 7870. [Google Scholar] [CrossRef]
- Feng, B.; Ye, C.; Qiu, L.; Chen, L.; Fu, Y.; Sun, W. Mitochondrial ROS Release and Subsequent Akt Activation Potentially Mediated the Anti-Apoptotic Effect of a 50-Hz Magnetic Field on FL Cells. Cell Physiol. Biochem. 2016, 38, 2489–2499. [Google Scholar] [CrossRef]
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic Antimicrobial Peptide NRC-03 Induces Oral Squamous Cell Carcinoma Cell Apoptosis via CypD-mPTP Axis-Mediated Mitochondrial Oxidative Stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef]
- Zhang, G.; He, J.; Ye, X.; Zhu, J.; Hu, X.; Shen, M.; Ma, Y.; Mao, Z.; Song, H.; Chen, F. β-Thujaplicin Induces Autophagic Cell Death, Apoptosis, and Cell Cycle Arrest through ROS-Mediated Akt and P38/ERK MAPK Signaling in Human Hepatocellular Carcinoma. Cell Death Dis. 2019, 10, 255. [Google Scholar] [CrossRef]
- Wang, L.-J.; Lee, Y.-C.; Huang, C.-H.; Shi, Y.-J.; Chen, Y.-J.; Pei, S.-N.; Chou, Y.-W.; Chang, L.-S. Non-Mitotic Effect of Albendazole Triggers Apoptosis of Human Leukemia Cells via SIRT3/ROS/P38 MAPK/TTP Axis-Mediated TNF-α Upregulation. Biochem. Pharmacol. 2019, 162, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Chen, B.L.; Wu, H.Y.; Yang, D.L.; Chen, X.M.; Lv, J.Y. Research Progress on Mechanism of Apoptosis Induced by Yinyanghuo(Epimedium brevicornu Maxim.) and lts Derivatives in Cancer Cells. Chin. Arch. Tradit. Chin. Med. 2022, 40, 167–172. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Khan, A.A.; Babiker, A.Y.; Alanezi, M.; Allemailem, K.S. The Multifaceted Role of Baicalein in Cancer Management through Modulation of Cell Signalling Pathways. Molecules 2022, 27, 8023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Preliminary Study on the Mechanism of Apoptosis Induction by Benzoisoselenazolone Derivatives in Lung Cancer Cells. Master’s Thesis, Tianjin University of Technology, Tianjin, China, 2023. [Google Scholar] [CrossRef]





| Compound | Compounds IC50 Values (μM) a | ||||
|---|---|---|---|---|---|
| BEL-7402 | MCF-7 | A549 | PC-3M | HEK293 | |
| 1 | 47.83 ± 2.65 | 25.78 ± 1.07 | 35.84 ± 3.57 | 44.52 ± 2.15 | 46.82 ± 1.82 |
| 2 | 40.59 ± 5.50 | 42.05 ± 5.47 | >100 | 76.13 ± 4.44 | >100 |
| 3 | >100 | >100 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 | 76.04 ± 4.69 | >100 |
| 5 | 39.88 ± 2.06 | 48.92 ± 1.42 | 97.81 ± 6.40 | 60.25 ± 3.88 | 77.17 ± 5.13 |
| 6 | 40.56 ± 5.17 | 45.58 ± 3.02 | >100 | >100 | >100 |
| 7 | 74.57 ± 4.83 | >100 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 |
| Mito-ICT-1 | 2.85 ± 0.70 | 10.70 ± 0.35 | 21.11 ± 2.67 | 33.57 ± 2.53 | 47.01 ± 2.91 |
| Mito-ICT-2 | 3.43 ± 0.91 | 23.68 ± 2.24 | 10.72 ± 1.09 | 27.83 ± 1.74 | 53.72 ± 5.15 |
| Mito-ICT-3 | 3.85 ± 0.69 | 22.46 ± 1.78 | 13.68 ± 0.92 | 20.61 ± 2.06 | 54.99 ± 3.92 |
| Mito-ICT-4 | 0.73 ± 0.06 | 10.79 ± 1.20 | 5.15 ± 0.59 | 11.26 ± 1.55 | 67.11 ± 2.09 |
| ICT | 21.78 ± 2.42 | 25.72 ± 1.89 | 43.09 ± 1.93 | 63.61 ± 2.67 | 43.19 ± 3.05 |
| DOX | 3.23 ± 0.64 | 2.44 ± 0.81 | 6.88 ± 0.62 | 0.24 ± 0.11 | >100 |
| 5-FU | 38.96 ± 1.32 | 13.45 ± 2.78 | 86.21 ± 7.94 | 58.67 ± 2.64 | >100 |
| Compound | Affinity/kcal·mol−1 | ||||
|---|---|---|---|---|---|
| SIRT3 | CypD | MKK6 | P-38 | DDIT3 | |
| ICT | −9.5 | −7.9 | −10.3 | −9.0 | −7.0 |
| Mito-ICT-4 | −13.7 | −11.8 | −5.7 | −7.9 | −5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, W.; Zhao, Y.; Liu, D.; Han, J.; Cai, E. The Mechanism of a Novel Mitochondrial-Targeted Icaritin Derivative in Regulating Apoptosis of BEL-7402 Cells Based on the SIRT3 and CypD-Mediated ROS/p38 MAPK Signaling Pathway. Molecules 2025, 30, 1667. https://doi.org/10.3390/molecules30081667
Chen Z, Li W, Zhao Y, Liu D, Han J, Cai E. The Mechanism of a Novel Mitochondrial-Targeted Icaritin Derivative in Regulating Apoptosis of BEL-7402 Cells Based on the SIRT3 and CypD-Mediated ROS/p38 MAPK Signaling Pathway. Molecules. 2025; 30(8):1667. https://doi.org/10.3390/molecules30081667
Chicago/Turabian StyleChen, Zenan, Wei Li, Yan Zhao, Dingrui Liu, Jiahong Han, and Enbo Cai. 2025. "The Mechanism of a Novel Mitochondrial-Targeted Icaritin Derivative in Regulating Apoptosis of BEL-7402 Cells Based on the SIRT3 and CypD-Mediated ROS/p38 MAPK Signaling Pathway" Molecules 30, no. 8: 1667. https://doi.org/10.3390/molecules30081667
APA StyleChen, Z., Li, W., Zhao, Y., Liu, D., Han, J., & Cai, E. (2025). The Mechanism of a Novel Mitochondrial-Targeted Icaritin Derivative in Regulating Apoptosis of BEL-7402 Cells Based on the SIRT3 and CypD-Mediated ROS/p38 MAPK Signaling Pathway. Molecules, 30(8), 1667. https://doi.org/10.3390/molecules30081667








