Recent Advances in Materials, Synthesis, and Reaction Model of Particle Adsorbent for Flue Gas Desulfurization
Abstract
1. Introduction
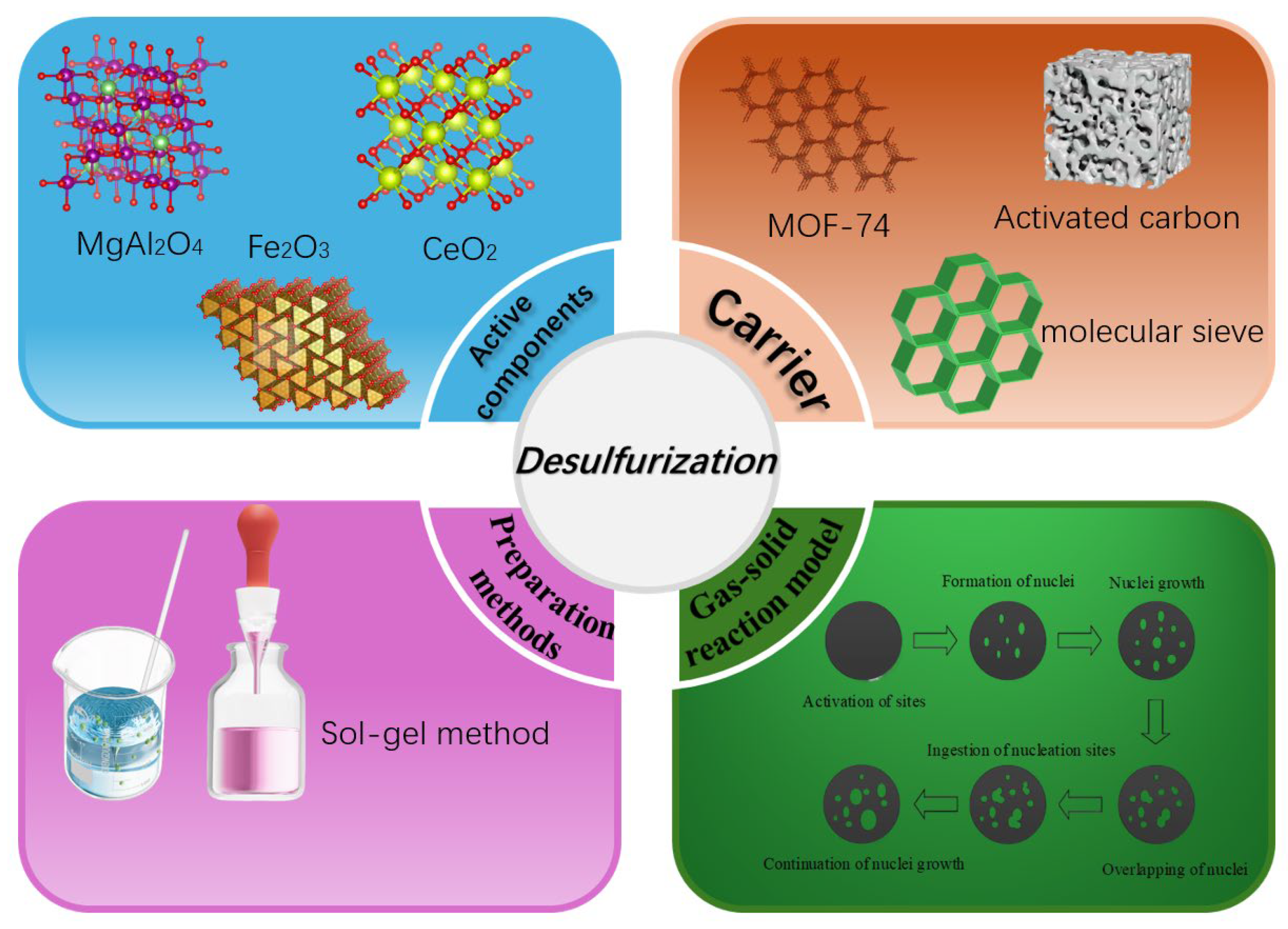
2. Active Components of Particle Adsorbents
2.1. Metal Oxides
2.2. Doped Metal Oxides
2.3. Special Metal Oxide Configurations
2.4. Non-Metallic Components
3. Carrier of Particle Adsorbents
3.1. Silicon-Based Carrier
3.2. Metal Oxide Carrier
3.3. Metal–Organic Framework Carriers
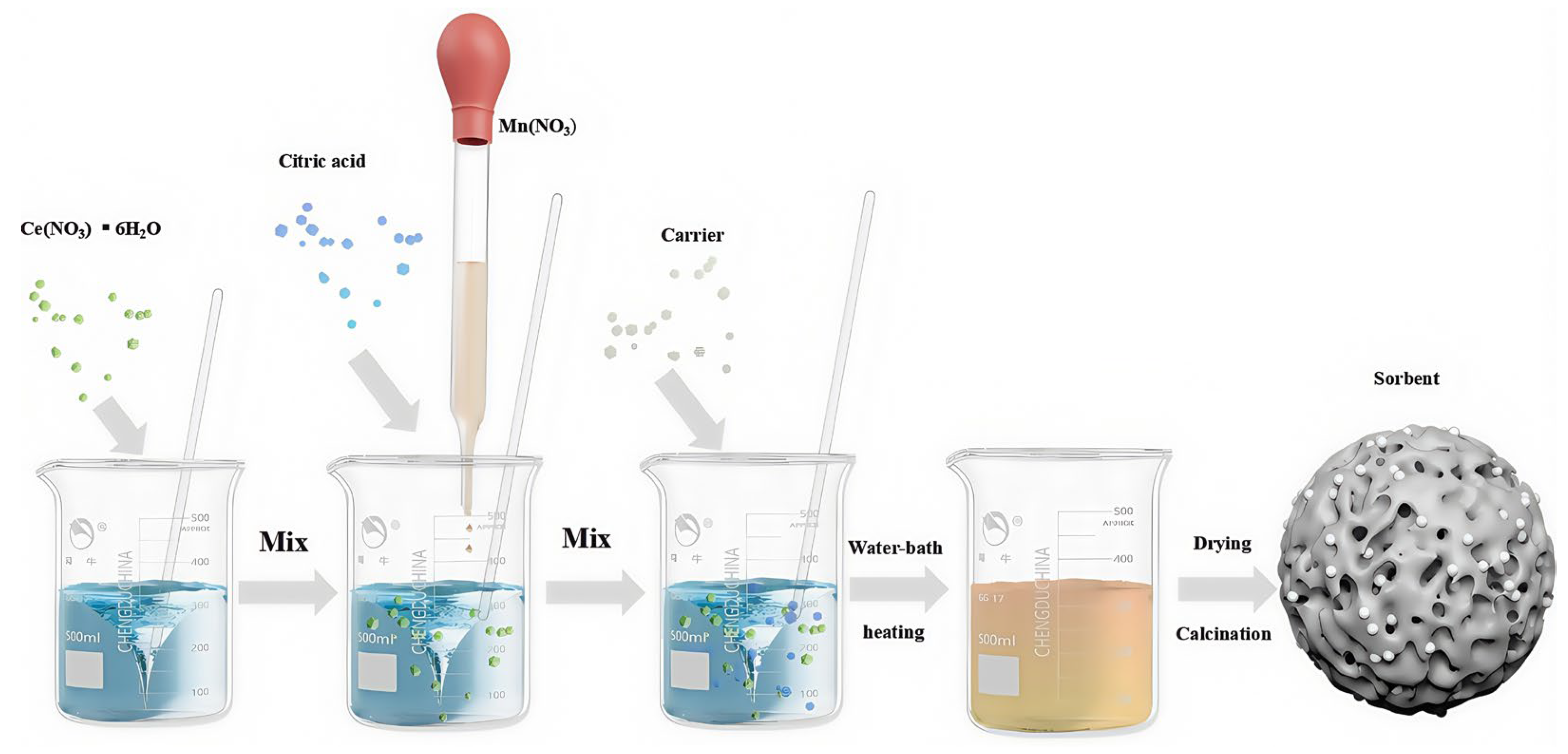
4. Preparation Methods of Particle Adsorbents
5. Gas–Solid Reaction Model
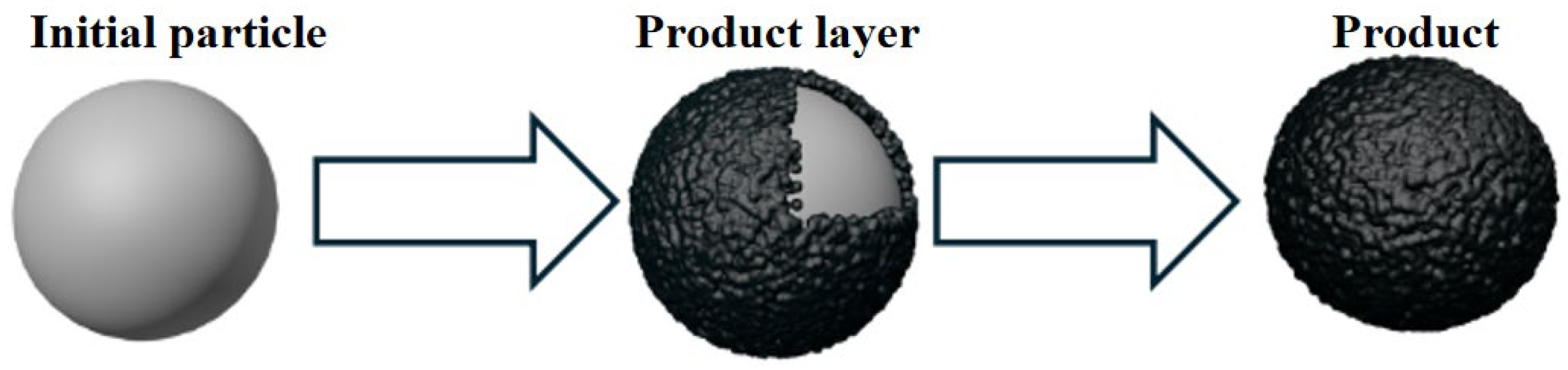
5.1. Shrinking Core Model
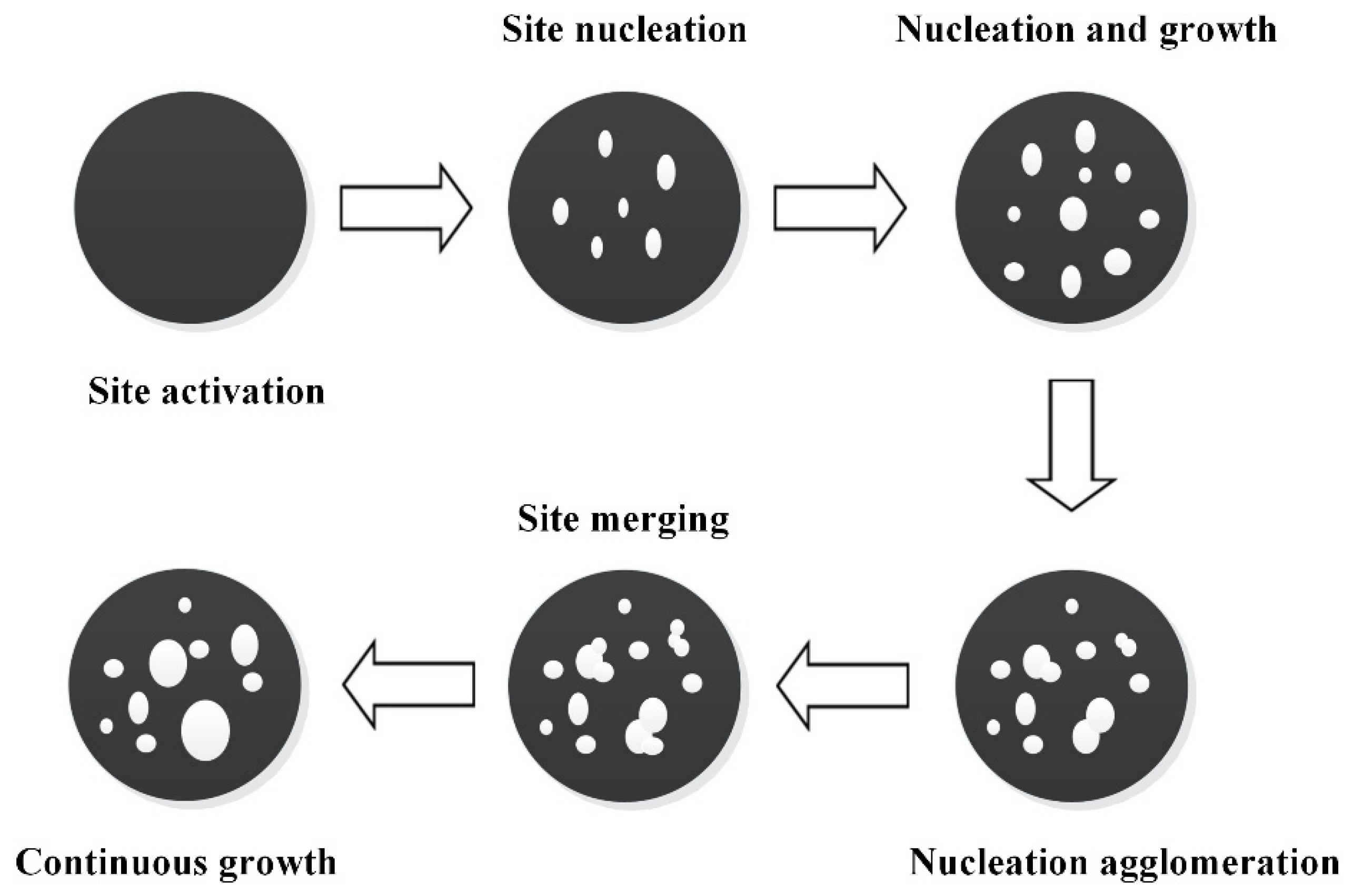
5.2. Nucleation and Nucleus Growth Model
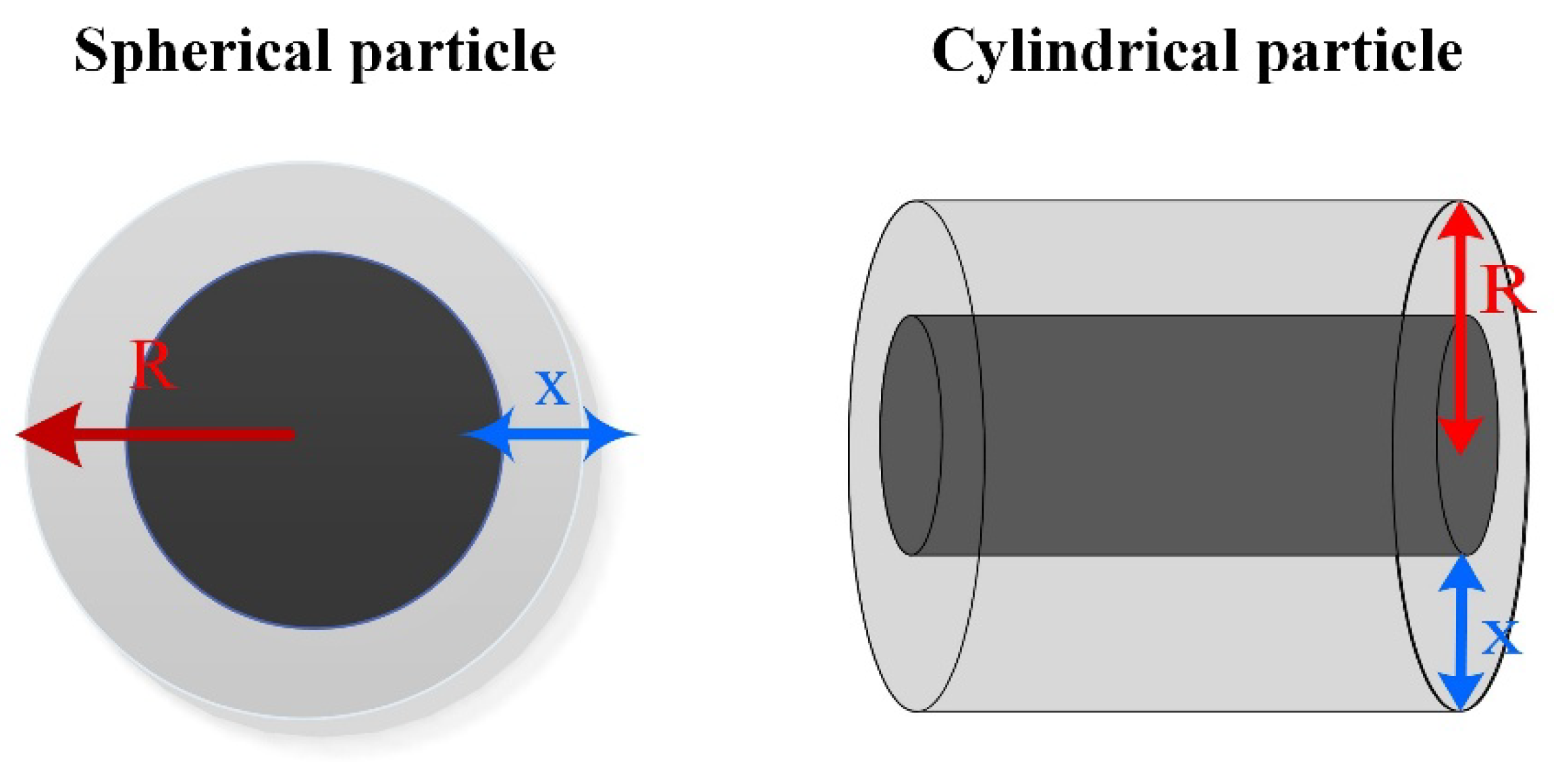
5.3. Diffusion Model
5.4. Chemical Reaction Model
6. Summary and Outlook
- 1.
- There are two core problems in the preparation and service of particle adsorbents: the active components are easy to agglomerate in the synthesis stage, and the crystallization phase growth and performance decline during recycling. Optimizing the carrier structure can improve the dispersion of active components, increase active sites and inhibit agglomeration. Although the core–shell structure can inhibit the reaction between the active component and the carrier, it is necessary to control the structural uniformity to maintain cycle stability. In the future, it is necessary to break through the synergistic reinforcement of rare earth doping on structure and performance, analyze the dispersion mechanism of active components, and solve the bottleneck of preparation and application.
- 2.
- Although the current preparation technology for particle adsorbents has achieved significant progress, there still exist a series of technical challenges such as poor uniformity, limited loading capacity of active components, difficult control of microstructure, complex operation, high preparation cost, and long preparation cycle. Particularly, under the premise of the national ultra-low emission standard, various industries have put forward even higher requirements for the microstructure and properties of particle adsorbents. In general, the preparation methods of adsorbents should develop in the direction of high efficiency and refined control.
- 3.
- The research system of particle adsorbents encompasses multiple disciplinary domains, including professional knowledge in fields like chemistry, materials science, and engineering. Developing a gas–solid reaction model that integrates multiple disciplines is highly conducive to promoting the optimization and upgrading of particle adsorbent preparation technology.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.Q.; Wang, F.F.; Qin, W.R.; He, C.Q.; Wang, F.S.; Liang, X.; Li, X.P. A critical review on the use of flue gas desulfurization gypsum to ameliorate saline-alkali soils and its prospect for reducing carbon emissions. Sci. Total Environ. 2024, 945, 174053. [Google Scholar] [PubMed]
- Cheng, C.; Li, J.J.; Cao, J.Y.; Guo, J.X.; Cen, W.L. The absorption of SO2 by morpholine cyclic amines with sulfolane as the solvent for flue gas. J. Hazard. Mater. 2021, 408, 124462. [Google Scholar] [PubMed]
- GB3095-2012; National Technical Committee for Information and Documentation Standardization. China Standard Press: Beijing, China, 2012.
- Tao, L.; Wang, X.Q.; Ning, P.; Wang, L.L.; Fan, W.J. Mechanical and thermo-gravimetric properties of unsaturated polyester resin blended with FGD gypsum. Fuel Process. Technol. 2019, 192, 36–64. [Google Scholar]
- Tian, L.; Dou, J.X.; Yu, J.L. Wet desulfurization characteristics of magnesium oxide for flue gas cleaning. Energy Metall. Ind. 2018, 37, 59–64. [Google Scholar]
- Xu, C.X.; Fu, G.G. Review on ammonia flue gas desulfurization. Electr. Environ. Protect. 2005, 21, 17–20. [Google Scholar]
- Sun, Z.Z.; Tang, Z.Y.; Qu, S.J.; Shao, Z.C.; He, C.W.; He, S.F.; Jia, X.J. Research and engineering application of wet desulfurization technology with active ore slurry. Chem. Eng. 2017, 45, 7–11. [Google Scholar]
- Han, J.; Cheng, H.; Zhang, L.; Fu, H.; Chen, J. Trash to treasure: Use flue gas SO2 to produce H2 via a photoelectrochemical process. Chem. Eng. J. 2018, 335, 231–235. [Google Scholar]
- Sohn, H.Y.; Kim, B.S. A new process for converting SO2 to sulfur without generating secondary pollutants through reactions involving CaS and CaSO4. Environ. Sci. Technol. 2002, 36, 3020–3024. [Google Scholar]
- Ng, K.H. Reduction of hazardous SO2 into elemental sulphur over chicken eggshells-derived calcium-based redox agent: A systematic step-by-step thermodynamic analysis and process validations. J. Clean. Prod. 2021, 278, 123927. [Google Scholar] [CrossRef]
- Brunet, S.; Mey, D.; Pérot, G.; Bouchy, C.; Diehl, F. On the hydrodesulfurization of FCC gasoline: A review. Appl. Catal. A 2005, 278, 143–172. [Google Scholar]
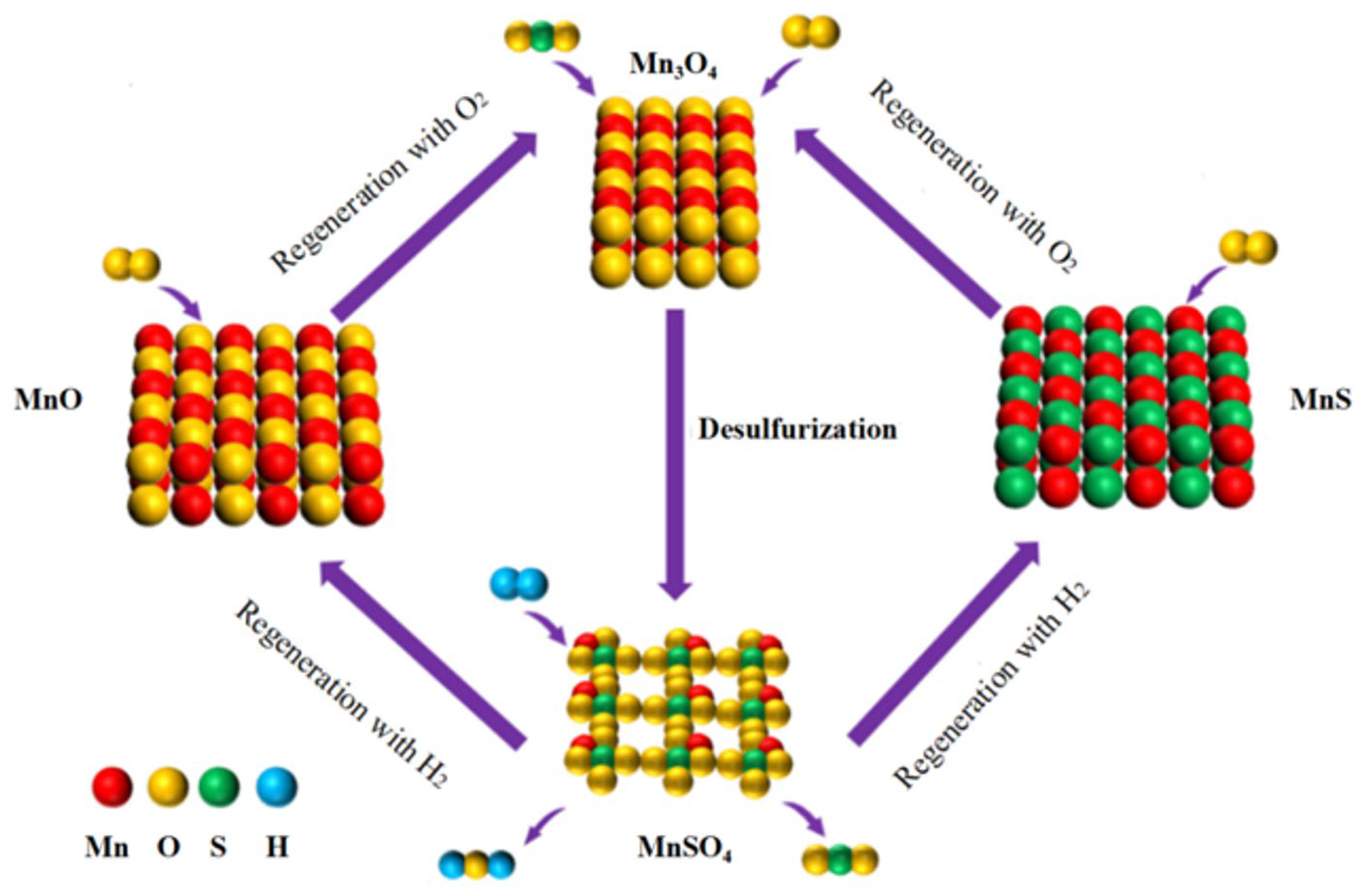
- Xuan, Y.N.; Yu, Q.B.; Wang, K.; Duan, W.J.; Li, Y.; Qin, Q.; Xue, Z.J. Evaluation of Mn-based sorbent for flue gas desulfurization through isothermal chemical-looping system. Chem. Eng. J. 2020, 379, 122283. [Google Scholar] [CrossRef]
- Ouyang, J.; Gu, W.; Zhang, Y.; Yang, H.M.; Jin, Y.L.; Chen, J.; Jiang, J.L. CO2 capturing performances of millimeter scale beads made by tetraethylenepentamine loaded ultra-fine palygorskite powders from jet pulverization. Chem. Eng. J. 2018, 341, 432–440. [Google Scholar]
- Duan, W.J.; Han, J.C.; Yang, S.; Wang, Z.M.; Yu, Q.B.; Zhan, Y.Q. Understanding CO2 adsorption in layered double oxides synthesized by slag through kinetic and modelling techniques. Energy 2024, 297, 131303. [Google Scholar]
- Duan, W.J.; Li, R.M.; Yang, S.; Han, J.C.; Lv, X.J.; Wang, Z.M.; Yu, Q.B. Theoretical study on coal gasification behavior in CO2 atmosphere driven by slag waste heat. Energy 2024, 305, 132269. [Google Scholar]
- Li, W.N.; Zhao, S.Y.; Yan, Y.C.; Biney, B.W.; Zhang, D.Q.; Al-shiaani, N.H.A.; Chen, K.; Guo, A.J.; Xia, W. High-efficiency desulfurization adsorbents loaded with uniformly dispersed nano-metal particles prepared from phytoremediation. J. Environ. Chem. Eng. 2023, 11, 110409. [Google Scholar] [CrossRef]
- Cai, Y.F.; Liu, W.; Sun, Z.; Yang, Y.; Li, P. Granulation of alkaline metal nitrate promoted MgO adsorbents and the low-concentration CO2 capture performance in the fixed bed adsorber. J. CO2 Util. 2022, 61, 102047. [Google Scholar]
- Scala, F.; Montagnaro, F.; Salatino, P. Attrition of limestone by impact loading in fluidized beds. Energy Fuels 2007, 21, 2566–2572. [Google Scholar]
- Materic, V.; Hyland, M.; Jones, M.I.; Holt, R. Investigation of the friability of Ca looping sorbents during and after hydration based reactivation. Fuel 2014, 127, 70–77. [Google Scholar]
- Zhu, L.H.; Liu, C.; Zheng, C.H.; Zhang, S.H.; Fan, H.D.; Luo, K.; Gao, X. Numerical simulations of CO2 absorption by MgO-based sorbent in a gas-solid fluidized bed. Sep. Purif. Technol. 2024, 346, 127381. [Google Scholar]
- Hu, Y.C.; Liu, W.Q.; Peng, Y.; Yang, Y.D.; Sun, J.; Chen, H.Q.; Zhou, Z.J.; Xu, M.H. One-step synthesis of highly efficient CaO-based CO2 sorbent pellets via gel-casting technique. Fuel Process. Technol. 2017, 160, 70–77. [Google Scholar]
- Du, J.X.; Yang, W.; Xu, L.L.; Bei, L.; Lei, S.Y.; Li, W.; Liu, H.T.; Wang, B.; Sun, L.S. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia. Chem. Eng. J. 2024, 488, 150954. [Google Scholar]
- Qi, Z.; Chen, X.P.; Xu, Z.L.; Liu, Z.; Ma, J.L.; Liang, C.; Liu, D.Y. Investigation of the decarbonization performance of K2CO3/ZrO2 adsorbent for direct air capture. Chem. Eng. J. 2025, 509, 161420. [Google Scholar]
- Liu, X.; Mao, F.F.; Li, Z.M.; Xu, Z.H.; Shu, X.J.; Mi, J.P.; Zhou, Y.; Tao, D.J. Solidothermal synthesis of nitrogen-decorated, ordered mesoporous carbons with large surface areas for efficient selective capture and separation of SO2. Chem. Eng. J. 2022, 431, 134142. [Google Scholar]
- Zhang, G.Q.; Liu, F.Q.; Zhu, Q.L.; Qian, H.; Zhong, S.C.; Tan, J.Z.; Zheng, A.M.; Liu, F.J.; Jiang, L.L. Triple templates directed synthesis of nitrogen-doped hierarchically porous carbons from pyridine rich monomer as efficient and reversible SO2 adsorbents. Small 2024, 20, 2404548. [Google Scholar]
- Balasubramaniam, B.M.; Thierry, P.; Lethier, S.; Pugnet, V.; Llewellyn, P.; Rajendran, A. Process-performance of solid sorbents for Direct Air Capture (DAC) of CO2 in optimized temperature-vacuum swing adsorption (TVSA) cycles. Chem. Eng. J. 2024, 485, 149568. [Google Scholar]
- Xuan, Y.N.; Yu, Q.B.; Gao, H.T.; Wang, K.; Duan, W.J. Modular manganese/diatomite-Santa Barbara Amorphous-15 sorbent for moderate-temperature flue gas desulfurization. Chem. Eng. J. 2020, 395, 124984. [Google Scholar]
- Wen, C.; Liu, T.Y.; Wang, D.P.; Wang, Y.; Chen, H.P.; Luo, G.Q.; Zhou, Z.J.; Li, C.K.; Xu, M.H. Biochar as the effective adsorbent to combustion gaseous pollutants: Preparation, activation, functionalization and the adsorption mechanisms. Prog. Energy Combust. Sci. 2023, 99, 101098. [Google Scholar]
- Duan, W.J.; Li, R.M.; Wang, Z.M.; Ji, J.Y.; Liu, J.X.; Yu, Q.B. Optimizing sustainability and profitability: A multi-step approach to the synthesis of x-zeolite from blast furnace slag. Process Saf. Environ. 2024, 189, 1527–1537. [Google Scholar]
- Zheng, D.Y.; Liu, K.J.; Zhang, Z.S.; Fu, Q.; Bian, M.Y.; Han, X.Y.; Shen, X.; Chen, X.H.; Xie, H.J.; Wang, X.; et al. Essential features of weak current for excellent enhancement of NOx reduction over monoatomic V-based catalyst. Nat. Commun. 2024, 15, 6688. [Google Scholar]
- Xuan, Y.N.; Gao, H.T.; Tian, H.; Hu, Z.M.; Ma, J.J.; Yu, Q.B. Enhancement effect of Ce addition on Mn3O4/diatomite sorbent for moderate-temperature flue gas desulfurization. Chem. Eng. J. 2023, 460, 141592. [Google Scholar]
- Xing, G.S.; Wang, W.; Zhao, S.; Qi, L.Q. Application of Ca-based adsorbents in fixed-bed dry flue gas desulfurization (FGD): A critical review. Environ. Sci. Pollut. Res. 2023, 30, 76471–76490. [Google Scholar]
- Yu, H.Y.; Lyu, J.; Song, W.C.; Jiang, D.X.; Ma, X.H.; Chen, H.Y.; Lin, L.Y. Effects of modified sink beads on the efficiency of SO2 removal by adsorption. China Powder Sci. Technol. 2023, 29, 110–116. [Google Scholar]
- Andrzej, K.; Arkadiusz, T.S.; Artur, M.; Anatol, J. Fly ash and sorbent particles agglomeration and removal in system of electrostatic agglomerator and electrostatic precipitator. Powder Technol. 2024, 431, 119075. [Google Scholar]
- Zhang, Z.N.; Jin, B.; Liao, J.W.; Luo, X.; Liang, Z.W. Effect of loading strategy between promoter and calcium oxide on CO2 capture performance for metal-organic framework derived sorbents. Chem. Eng. J. 2022, 431, 133855. [Google Scholar]
- Kim, W.K.; Verma, S.; Ahmadi, Y.; Cho, M.S.; Kim, K.H. The effects of metal-oxide content in MnO2-activated carbon composites on reactive adsorption and catalytic oxidation of formaldehyde and toluene in air. Sci. Total Environ. 2024, 926, 172137. [Google Scholar]
- Zhang, A.J.; Liu, J.; Yang, Y.J.; Li, Y.M. Insights into chromium removal mechanism by Ca-based sorbents from flue gas. Sci. Total Environ. 2024, 912, 168928. [Google Scholar]
- Mehri, I.; Maryam, T.; Pedro, E.S. Metal-based eggshell particles prepared via successive incipient wetness impregnation method as a promoted sorbent for CO2 capturing in the calcium looping process. J. Environ. Chem. Eng. 2023, 11, 110584. [Google Scholar]
- Cui, S.; Niu, F.; Wang, N.J.; Zhou, J.M.; Wang, J.P.; Li, J.S. Study on desulfurization mechanism of adsorbents prepared by high ratio circulating fly ash and lime. Fuel 2020, 277, 118051. [Google Scholar]
- Liu, Q.Y.; Liu, Z.Y. Carbon supported vanadia for multi-pollutants removal from flue gas. Fuel 2013, 108, 149–158. [Google Scholar]
- Rahmaninejad, F.; Gavaskar, V.S.; Abbasian, J. Dry regenerable CuO/γ-Al2O3 catalyst for simultaneous removal of SOx and NOx from flue gas. Appl. Catal. B 2012, 119–120, 297–303. [Google Scholar]
- Wang, L.M.; Gao, Q.; Pan, Y.F.; Xu, X.F. Destructive sorption of NF3 as a novel greenhouse gas on Mn2O3/Al2O3 sorbents derived from calcination of MnOx/AlOOH/CS precursors. Mater. Chem. Phys. 2022, 280, 125810. [Google Scholar]
- Guo, J.X.; Liang, J.; Chu, Y.H.; Sun, M.C.; Yin, H.Q. Desulfurization activity of nickel supported on acid-treated activated carbons. Appl. Catal. A 2012, 421, 142–147. [Google Scholar]
- Li, Y.T.; Yi, H.H.; Tang, X.L.; Liu, X.; Wang, Y.E.; Cui, B.C.; Zhao, S.Z. Study on the performance of simultaneous desulfurization and denitrification of Fe3O4-TiO2 composites. Chem. Eng. J. 2016, 304, 89–97. [Google Scholar]
- Gao, L.; Li, Y.; He, J. Intensifying effects of zinc oxide wet flue gas desulfurization process with citric acid. J. Environ. Chem. Eng. 2019, 7, 102831. [Google Scholar]
- Liu, X.L.; Chen, J.; Ma, Y.X.; Liu, C.S.; Huang, A.Q.; He, J.J.; Wang, M.R.; Tang, H.D.; Zuo, W.D.; Li, Y.S. Synergistic effects of CeO2 and Al2O3 on reactivity of CaO-based sorbents for CO2 capture. Sep. Purif. Technol. 2024, 347, 127660. [Google Scholar]
- Feng, Y.N. Research on Desulfurization Technology Using Manganese Dioxide Adsorbent. Master’s Thesis, North China Electric Power University, Beijing, China, 2002. [Google Scholar]
- Li, Y.; Song, C.X.; You, C.F. Experimental study on abrasion characteristics of rapidly hydrated sorbent for moderate temperature dry flue gas desulfurization. Energy Fuels 2010, 24, 1682–1686. [Google Scholar]
- Gavaskar, V.S.; Abbasian, J. Dry regenerable metal oxide sorbents for SO2 removal from flue gases.1. Development and evaluation of copper oxide sorbents. Ind. Eng. Chem. Res. 2006, 45, 5859–5869. [Google Scholar]
- Li, C.R.; Liu, S.B.; Han, Z.H.; Wu, J.X. Life testing of composite metal oxide desulfurizers. J. Taiyuan Univ. Technol. 1996, 27, 100–102. [Google Scholar]
- Liu, P.J. Research on Desulfurization and Denitrification of Magnesium Based Catalytic Adsorbents. Ph.D. Thesis, Northeastern University, Shenyang, China, 2008. [Google Scholar]
- Zhang, X.L. Experimental Study on Catalytic Reduction of Desulfurization and Denitration by Rare Earth Oxides. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2007. [Google Scholar]
- Li, L.Y.; King, D.L. High-capacity sulfur dioxide absorbents for diesel emissions control. Ind. Eng. Chem. Res. 2005, 44, 168–177. [Google Scholar]
- Xing, G.S.; Li, S.L.; Wang, Y.G.; Zhang, Y.T.; Wang, W.B.; Qi, L.Q. Experimental study on the preparation of desulfurizer for low temperature dry desulfurization by digesting CaO in urea solution. J. Environ. Chem. Eng. 2024, 12, 113865. [Google Scholar]
- Xia, H.; Chang, X.Q.; Liu, B.S. High-temperature H2S removal performance over ordered mesoporous La-Mn-supported Al2O3-CaO sorbents. Chem. Eng. J. 2017, 321, 277–285. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Zheng, X.R.; Tian, G.Y.; Li, Y.J. Mechanism of SO2 removal by Mn-Ce high-content iron ash. Clean Coal Technol. 2023, 29, 89–97. [Google Scholar]
- Liu, D.J.; Zhou, W.G.; Wu, J. CeO2-MnOx/ZSM-5 sorbents for H2S removal at high temperature. Chem. Eng. J. 2016, 284, 862–871. [Google Scholar] [CrossRef]
- Jae, L.S.; Jun, H.K.; Jung, S.Y.; Lee, T.J.; Ryu, C.K.; Kim, J.C. Regenerable MgO-Based SOx removal sorbents promoted with cerium and iron oxide in RFCC. Ind. Eng. Chem. Res. 2005, 44, 9973–9978. [Google Scholar] [CrossRef]
- Mathieu, Y.; Soulard, M.; Patarin, J.; Molière, M. Mesoporous materials for the removal of SO2 from gas streams. Fuel Process Technol. 2012, 99, 35–42. [Google Scholar] [CrossRef]
- Li, X.; Osaka, Y.G.; Huang, H.Y.; Tsujiguchi, T.; Kodama, A. Effect of doped alkali metal ions on the SO2 capture performance of MnO2 desulfurization materials at low temperature. J. Renew. Mater. 2021, 9, 1541–1553. [Google Scholar] [CrossRef]
- Lv, P.J.; Chang, S.; Hong, Q.Q.; Mei, J.; Yang, S.J. Outstanding performance of reproducible sulfureted Fe-Ti spinel for the centralized control of Hg (both gaseous Hg0 and aqueous Hg2+) emitted from coal-fired power plants with seawater flue gas desulfurization. Chem. Eng. J. 2024, 483, 148955. [Google Scholar]
- Eseva, E.; Dunko, A.; Latypova, S.; Grafov, O.; Cherednickenko, K.; Motyakin, M.V.; Anisimov, A.; Akopyan, A. Cobalt-manganese spinel structure catalysts for aerobic oxidative desulfurization. Fuel 2024, 357, 129689. [Google Scholar] [CrossRef]
- Qi, T.Y.; Zhang, J.Z.; Li, T.; An, S.L.; Li, Q.W.; Wang, L.D. Facilitating calcium sulfite oxidation during flue-gas desulfurization for efficient sulfur utilization using a robust MnTiO3 perovskite. Appl. Surf. Sci. 2024, 648, 158981. [Google Scholar] [CrossRef]
- Zhao, X.L.; Li, J.J.; Yang, Z.P.; Li, D.K.; Liu, Z.S.; Zhu, R.H.; Wang, C.; Zhou, C.G.; Ma, K.; Song, L.; et al. Study of the kinetics of SO2 adsorption and regeneration over the spinel-type adsorbent. Chem. Eng. Sci. 2025, 306, 121148. [Google Scholar] [CrossRef]
- Song, W.M.; Fan, X.H.; Gan, M.; Ji, Z.; Sun, Z.Q. A novel and clean utilization of flue gas desulfurization ash coupled with converter sludge to produce calcium ferrate. Chem. Eng. J. 2024, 498, 154919. [Google Scholar] [CrossRef]
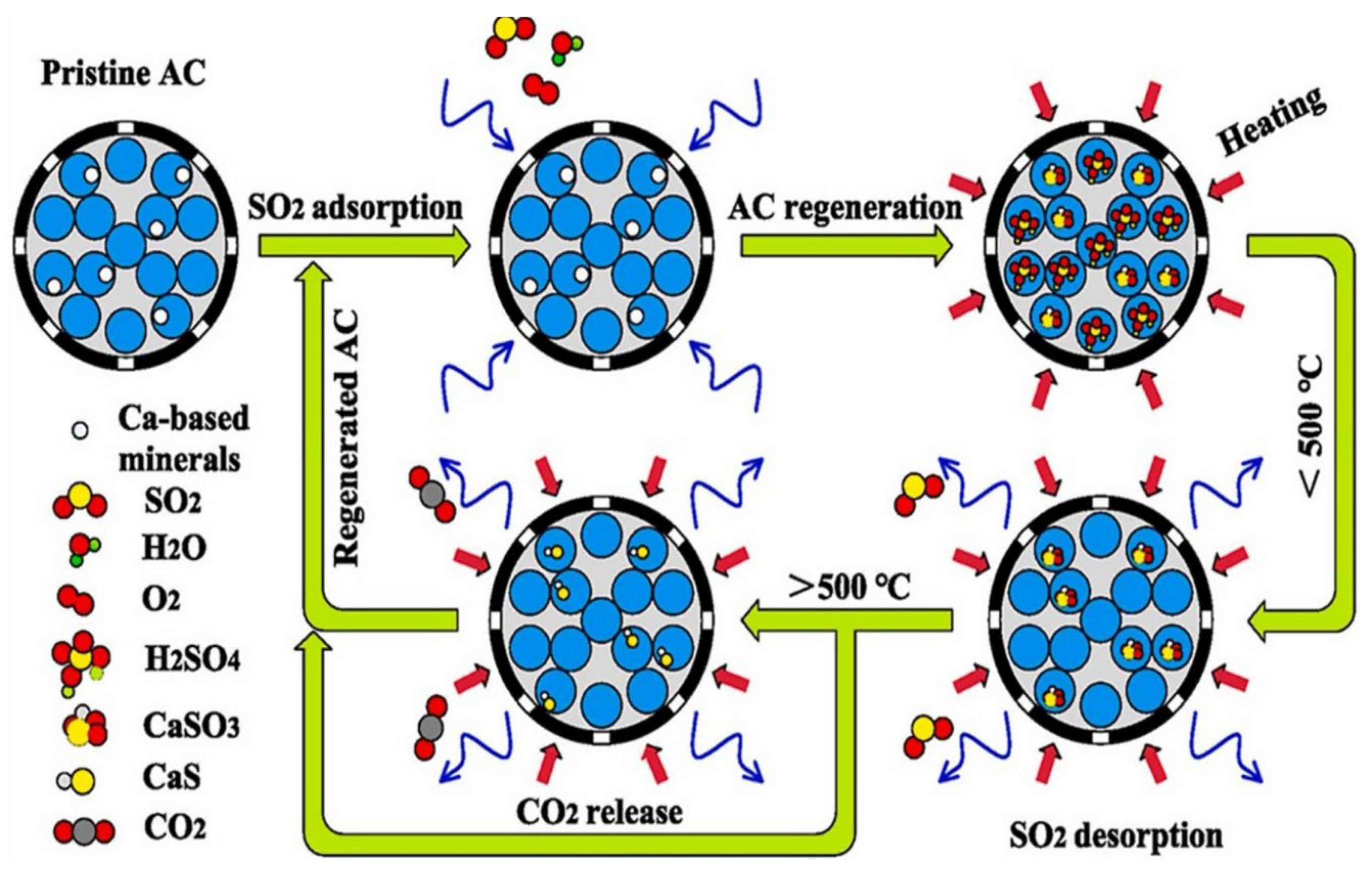
- Li, J.; Chen, G.F.; Chang, J.C.; Zhang, L.Q.; Wang, T.; Song, Z.L.; Ma, C.Y. Study on sulfur migration and carbon consumption during regeneration of SO2-loaded activated carbon: Effect of alkali/alkaline earth metals. J. Energy Inst. 2022, 102, 118–127. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Liu, G.D.; Wei, G.H.; Gao, J.H.; Lu, H.L. Analysis of SO2 physisorption by edge-functionalized nanoporous carbons using grand canonical monte carlo methods and density functional theory: Implications for SO2 removal. ACS Omega 2021, 6, 33735–33746. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.L.; Zhao, X.B.; Wang, S.N.; He, T.; Zhang, J.F.; He, S.; Huang, Y.J. Nitrogen-Doped high-surface-area activated carbon: Innovative adsorbent for enhanced SO2 and benzene removal. J. Ind. Eng. Chem. 2025, 144, 463–475. [Google Scholar]
- Quddus, M.R.; Hossain, M.M.; De, L.H.I. Ni based oxygen carrier over γ-Al2O3 for chemical looping combustion: Effect of preparation method on metal support interaction. Catal. Today 2013, 210, 124–134. [Google Scholar]
- Tian, X.; Wei, Y.J.; Zhao, H.B. Using a hierarchically-structured CuO@TiO2-Al2O3 oxygen carrier for chemical looping air separation in a paralleled fluidized bed reactor. Chem. Eng. J. 2018, 334, 611–618. [Google Scholar]
- Ksepko, E.; Sciazko, M.; Babinski, P. Studies on the redox reaction kinetics of Fe2O3-CuO/Al2O3 and Fe2O3/TiO2 oxygen carriers. Appl. Energ. 2014, 115, 374–383. [Google Scholar] [CrossRef]
- Guo, L.L.; Liu, L.; Zhu, X.L.; Zhang, Q.; Li, C.Y. Effect of Mg/Al molar ratios on NO reduction activity of CO using Ce-La/MgAl2O4-x catalysts. J. Fuel Chem. Technol. 2017, 45, 723–730. [Google Scholar] [CrossRef]
- Elmhamdi, A.; Castañeda, R.; Kubacka, A.; Pascual, L.; Nahdi, K.; Martínez-Arias, A. Characterization and catalytic properties of CuO/CeO2/MgAl2O4 for preferential oxidation of CO in H2-rich streams. Appl. Catal. B 2016, 188, 292–304. [Google Scholar] [CrossRef]
- Liu, X.P.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar]
- Yu, J.; Liu, S.; Cardoso, A.; Han, Y.; Bikane, K.; Sun, L.S. Catalytic pyrolysis of rubbers and vulcanized rubbers using modified zeolites and mesoporous catalysts with Zn and Cu. Energy 2019, 188, 116117. [Google Scholar]
- Wang, X.W.; Zhang, R.; Li, Q.C.; Mi, J.; Wu, M.M. Insights into H2S-absorption and oxidation-regeneration behavior of Ni-doped ZnO-based sorbents supported on SBA-15 for desulfurization of hot coal gas. Fuel 2023, 332, 126052. [Google Scholar]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Anis Hamza Fakeeha, A.H.; Armbuster, U.; Abaseed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA-15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A 2018, 567, 102–111. [Google Scholar]
- Wang, H.; Chen, Y.D.; Liu, X.G.; Xu, H.L.; Yang, D.H.; Hua, Y.; Dai, X.H. Diatomite powder carrier improved nitrogen and phosphorus removal from real municipal wastewater: Insights into micro-granule formation and enhancement mechanism. Chem. Eng. J. 2024, 484, 149482. [Google Scholar]
- Chen, Y.; Liu, K.R. Preparation of granulated N-doped TiO2/diatomite composite and its applications of visible light degradation and disinfection. Powder Technol. 2016, 303, 176–191. [Google Scholar]
- Khatamian, M.; Divband, B.; Shahi, R. Ultrasound assisted co-precipitation synthesis of Fe3O4/bentonite nanocomposite: Performance for nitrate, BOD and COD water treatment. J. Water Process Eng. 2019, 31, 100870. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M.; Kohsrai, I. Biosynthesis, characterization and catalytic activity of the Pd/bentonite nanocomposite for base- and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of chromium (VI) and nitro compounds. Microporous Mesoporous Mater. 2018, 271, 128–137. [Google Scholar]
- Aivalioti, M.; Papoulias, P.; Kousaiti, A.; Gidarakos, E. Adsorption of BTEX, MTBE and TAME on natural and modified diatomite. J. Hazard. Mater. 2012, 207, 117–127. [Google Scholar]
- Weng, X.L.; Sun, P.F.; Long, Y.; Meng, Q.J.; Wu, Z.B. Catalytic oxidation of chlorobenzene over MnxCe1-xO2/HZSM-5 catalysts: A study with practical implications. Environ. Sci. Technol. 2017, 51, 8057–8066. [Google Scholar]
- Li, Q.C.; Wang, X.W.; Zhang, R.; MI, J.; Wu, M.M. Insights into the effects of metal-ion doping on the structure and hot-coal-gas desulfurization properties of Zn-based sorbents supported on SBA-15. Fuel 2022, 315, 123198. [Google Scholar]
- Liu, B.S.; Wei, X.N.; Zhan, Y.P.; Chang, R.Z.; Au, C.T. Preparation and desulfurization performance of LaMeOx/SBA-15 for hot coal gas. Appl. Catal. B 2011, 102, 27–36. [Google Scholar]
- Xuan, Y.N. Experimental Research of Mn-Based Sorbent for Flue Gas Desulfurization by Chemical Looping Technology. Ph.D. Thesis, Northeastern University, Shenyang, China, 2020. [Google Scholar]
- Zhang, F.M.; Liu, B.S.; Zhang, Y.; Guo, Y.H.; Wan, Z.Y.; Subhan, F. Highly stable and regenerable Mn-based/SBA-15 sorbents for desulfurization of hot coal gas. J. Hazard. Mater. 2012, 233, 219–227. [Google Scholar]
- Yuan, Y.; Huang, L.J.; Yılmaz, M.; Zhang, T.C.; Wang, Y. MgFe2O4-loaded N-doped biochar derived from waste cooked rice for efficient low-temperature desulfurization of H2S. Fuel 2023, 339, 127385. [Google Scholar]
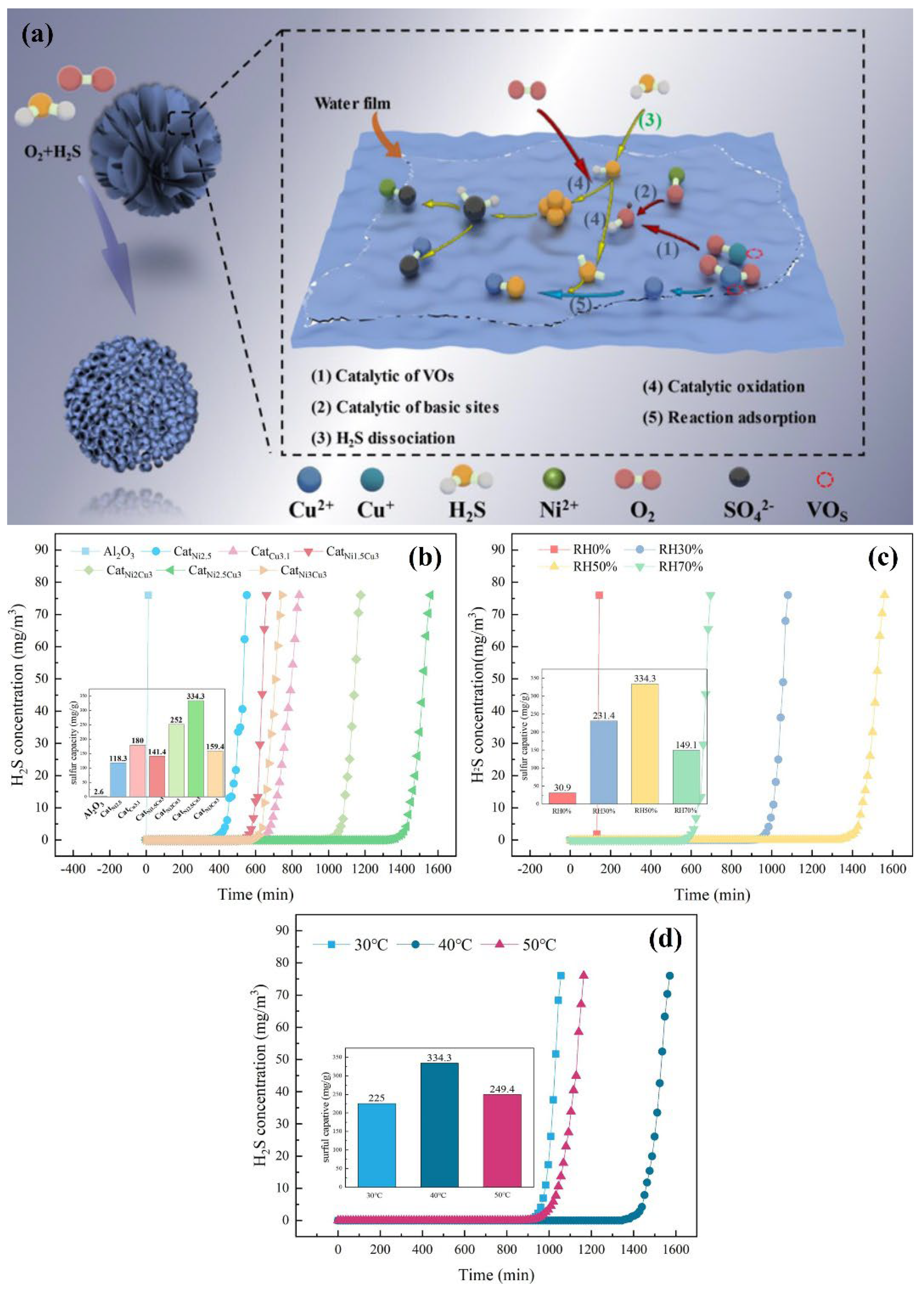
- Yin, M.X.; Wu, Z.H.; Yun, Z.C.; Zheng, Y.; Zhao, L.; Fan, F.Y.; Hou, H. Nickel-doped CuxO/Al2O3 catalyzes oxidation of H2S at near ambient temperature: Performance evaluation and mechanism exploration. Appl. Surf. Sci. 2023, 638, 158138. [Google Scholar]
- Xu, J.X.; Zheng, Y.J.; Tian, J.Z.; Zhao, Y.P.; Zheng, H.S. Enhanced desulfurization performance of model fuel by Cu-ZnO/TiO2 heterostructure. RSC Adv. 2024, 14, 36733. [Google Scholar]
- Song, Y.J.; Wang, T.; Cheng, L.; Li, C.Q.; Wang, H.; Wang, X.C. Simultaneous removal of SO2 and NO by CO reduction over prevulcanized Fe2O3/AC catalysts. Can. J. Chem. Eng. 2019, 97, 2015–2020. [Google Scholar]
- Peng, W.W.; Xu, Z.W.; Luo, C.; Zhao, H.B. Tailor-made core-shell CaO/TiO2-Al2O3 architecture as a high-capacity and long-life CO2 sorbent. Environ. Sci. Technol. 2015, 49, 8237–8245. [Google Scholar]
- Hu, S.Y.; Guo, G.J.; Zhang, J.X.; Khan, M.N.; Xu, S.H.; Yang, F.; Schwandt, B.W.; Hu, Z.G.; Zou, J.X. Advanced porous MOF materials and technologies for high-efficiency ppm-level toxic gas separation. Mat. Sci. Eng. R. 2024, 161, 100874. [Google Scholar]
- Ahmed, I.; Jhung, S.H. Room-temperature oxidative desulfurization with tungsten oxide supported on NU-1000 metal-organic framework. Fuel 2025, 393, 135043. [Google Scholar]
- Zhang, Z.H.; Yang, B.L.; Wu, Y.; Zhang, W.X.; Ma, H.P. Post modification of Oxo-clusters in robust Zirconium-Based metal organic framework for durable SO2 capture from flue gas. Sep. Purif. Technol. 2021, 276, 119349. [Google Scholar]
- Li, X.R.; Xing, G.S.; Yang, Q.; Zhou, J.F.; Yao, G.R.; Qi, L.Q. The synergistic effect of phosphotungstic acid as catalyst and Cu-based metal-organic framework in dry desulfurization enhances the ability to capture SO2: Synergistic effect of catalytic action and CO2 selectivity performance. J. Environ. Chem. Eng. 2025, 13, 115385. [Google Scholar]
- Xing, P.S.; Li, C.; Chen, Y.X.; Zhang, R.L. Preparation of 30%PMoV2@MOF@mSiO2 (MOF = MIL-101, HKUST-1, UiO-67, ZIF-8) catalysts and their oxidative desulfurization performance. Mol. Catal. 2024, 569, 114613. [Google Scholar]
- Wang, K. Research of Chemical Looping Air Separation Technology Based on Cu-Based Oxygen Carrier. Ph.D. Thesis, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- Liu, F.; Li, Y.; Song, C. Chemical looping hydrogen production using activated carbon and carbon black as multi-function carriers. Int. J. Hydrogen Energy 2018, 43, 5501–5511. [Google Scholar]
- Yang, C.C.; Yang, Q.H.; Sun, Q. Research progress on preparation methods of hydrodesulfurization catalysts. Ind. Catal. 2021, 29, 1–6. [Google Scholar]
- Yang, C.; Su, Z.L.; Wang, Y.S.; Fan, H.L.; Liang, M.S.; Chen, Z.H. Insight into the effect of gel drying temperature on the structure and desulfurization performance of ZnO/SiO2 adsorbents. Chin. J. Chem. Eng. 2023, 56, 233–241. [Google Scholar]
- Subhan, F.; Aslam, S.; Yan, Z.F.; Yaseen, M. Facile fabrication of La3+ sites in confined spaces for adsorptive desulfurization. Fuel Process. Technol. 2022, 236, 107423. [Google Scholar]
- Baboukani, Z.K.; Chermahini, A.N.; Farrokhpour, H. Photocatalytic oxidative desulfurization of a model fuel using S-doped TiO2/BiVO4 composites: A combination of experimental and theoretical study. J. Alloys Compd. 2024, 1002, 175478. [Google Scholar]
- Santos, L.F.S.; de Jesus, R.A.; Costa, J.A.S.; Gouveia, L.G.T.; de Mesquita, M.E..; Navickiene, S. Evaluation of MCM-41 and MCM-48 mesoporous materials as sorbents in matrix solid phase dispersion method for the determination of pesticides in soursop fruit (Annona muricata). Inorg. Chem. Commun. 2019, 101, 45–51. [Google Scholar]
- Mahmoud, R.M.; Sayed, F.N.; Shehata, M.R.; El-Naggar, A.M.; Mohamed, G.G.; Abdelaal, A.M.; Morshedy, A.S. Sonochemical synthesis of heterostructured ZnO/Bi2O3 for photocatalytic desulfurization. Sci. Rep. 2023, 13, 19391. [Google Scholar]
- Hua, L.; Yang, F.S.; Meng, X.Y.; Bao, Z.W.; Zhang, Z.X. Progress in kinetic models for hydrogenation/dehydrogenation of metal hydrides. Chem. Ind. Eng. Prog. 2010, 29, 413–418. [Google Scholar]
- Yang, J.; Ma, L.P.; Yang, J.; Liu, H.P.; Liu, S.Y.; Yang, Y.C.; Mu, L.S.; Wei, Y.; Ao, R.; Guo, Z.Y.; et al. Thermodynamic and kinetic analysis of CuO-CaSO4 oxygen carrier in chemical looping gasification. Energy 2019, 188, 116109. [Google Scholar]
- Ishida, M.; Jin, H.; Okamoto, T. A fundamental study of a new kind of medium material for chemical-looping combustion. Energy Fuel 1996, 10, 958–963. [Google Scholar]
- Lu, C.; Li, K.; Wang, H.; Zhu, X.; Wei, Y.G.; Zheng, M.; Zeng, C.H. Chemical looping reforming of methane using magnetite as oxygen carrier: Structure evolution and reduction kinetics. Appl. Energy 2018, 211, 1–14. [Google Scholar]
- Mei, D.F.; Zhao, H.B.; Yan, S.P. Kinetics model for the reduction of Fe2O3/Al2O3 by CO in chemical looping combustion. Chem. Eng. Process 2018, 124, 137–146. [Google Scholar]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Reduction kinetics of Fe2O3/Al2O3 by ultralow concentration methane under conditions pertinent to chemical looping combustion. Energy Fuel 2015, 29, 337–345. [Google Scholar]
- Alalwanh, A.; Cwiertny, D.M.; Grassian, V.H. Co3O4 nanoparticles as oxygen carriers for chemical looping combustion: A materials characterization approach to understanding oxygen carrier performance. Chem. Eng. J. 2017, 319, 279–287. [Google Scholar]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar]
- Lopes, W.S.; Morais, C.R.S.; Souza, A.G.; Vd, L. Synthesis and non-isothermal kinetic study of the thermal decomposition of gadolinium (III) complexes. J. Therm. Anal. Calorim. 2005, 79, 343–347. [Google Scholar] [CrossRef]
- Pap, A.E.; Kordás, K.; George, T.F.; Leppävuori, S. Thermal oxidation of porous silicon: Study on reaction kinetics. J. Phys. Chem. B 2004, 108, 12744–12747. [Google Scholar]
- Yang, Y.S. Study on the Gas-Solid Reaction of Sintering Flue Gas Dry Desulfurization by Low Grade Pyrolusite. Master’s Thesis, Guizhou University, Guiyang, China, 2016. [Google Scholar]
- Chang, J.Y.; Liu, M.; Wan, J.; Shi, G.W.; Li, T. Preparation of high-reactivity Ca-based SO2 adsorbent and experimental study for simulated flue gas dry desulfurization. Energy Rep. 2023, 9 (Suppl. S6), 85–95. [Google Scholar]
- Parandin, M.S.; Ebrahim, H.A.; Norouzi, H.R. Flue gas desulfurization by natural recyclable manganese ore in packed bed reactor and its performance prediction by random pore model. J. Ind. Eng. Chem. 2025, 141, 243–259. [Google Scholar] [CrossRef]
- Liu, W.Z.; Yu, J.; Zhang, J.W.; Gao, S.Q.; Xu, G.W. Study on the kinetics of gas-solid reaction of porous materials. Sci. China Chem. 2012, 4, 1210–1216. [Google Scholar]
- Chu, M.S.; Wang, Z.C.; Liu, Z.G.; Lv, J.P. Experimental study on the controlling step of self-reduction process of carbon composite iron ore hot briquette. Chin. J. Process Eng. 2010, 10, 121–126. [Google Scholar]
- Yang, Y.S.; Wu, F.Z.; Wang, K.K. Study on controlling step of gas-solid reaction of sintering flue gas dry desulfurization with low grade pyrolusite. Nonferr. Metal. 2016, 5, 57–60. [Google Scholar]
- Wang, K.K.; Wu, F.Z.; Jin, H.X.; Li, S.E. Dynamics analysis of dry-type desulfurization from sintering flue gas of pyrolusite. Nonferr. Metal. 2015, 6, 76–79. [Google Scholar]


| Metal Oxides | Desulfurization Efficiency/% | Experimental Temperature/°C | SO2 Concentration/vol.% | Flue Gas Flow/m3·h−1 | Ref. |
|---|---|---|---|---|---|
| MnO2 | 95 | 200–250 | 0.07–0.17 | 0.12–0.15 | [47] |
| CaO | 83 | 600–800 | 0.2 | 0.06 | [48] |
| CuO | 96 | 350–450 | 0.12–0.5 | 0.05 | [49] |
| Fe2O3 | 90 | 420–460 | 0.5–1 | 0.06 | [50] |
| MgO | 92 | 30–150 | 0.05–0.1 | 0.065 | [51] |
| NiO | 94 | 500–600 | 0.25 | 0.06 | [52] |
| ZrO2 | 99 | 325 | 0.025 | 0.494 | [53] |
| CeO2 | 82 | 500–600 | 0.25 | 0.06 | [52] |
| Ni-Ce oxides | 85 | 500–600 | 0.25 | 0.06 | [52] |
| Carrier Name | Molecular Formula | The Characteristics of the Carrier | Ref. |
|---|---|---|---|
| Activated alumina | γ-Al2O3 | The specific surface area is larger than 100 m2/g, the average pore diameter is 4–10 nm, the hydrothermal stability is relatively good, the applicable temperature range is wide, and it is prone to forming a spinel structure with metal components, which is not conducive to the regeneration of the adsorbent. | [41,69] |
| Titanium oxide | TiO2 | The specific surface area is within 10 m2/g, the mechanical strength is high, and TiO2 has good resistance to SO2. | [70,71] |
| Spinel | MgAl2O4 | The specific surface area ranges from 70 to 180 m2/g. The mechanical strength is higher than that of γ-Al2O3. It has stable properties, anti-sintering, wear-resistant, corrosion-resistant, impact-resistant, and has high strength and high hardness. | [72,73] |
| Zeolite molecular sieve | Na12Al12Si12O48 | It has a three-dimensional framework structure. The specific surface area is larger than that of γ-Al2O3. The thermal stability and hydrothermal stability are high. However, it is not conducive to the preparation of adsorbents with high loading capacity, and the efficiency of the adsorbent is relatively low. | [74,75] |
| Mesoporous Silicon | SBA-15 | It has hexagonal through-holes, and the pore size is adjustable. The relatively good thermal stability and large specific surface area increase the loading amount of metal oxides and result in a high utilization rate of the adsorbent. | [76,77] |
| Diatomite | SiO2 | It has a macroporous porous structure (50–800 nm), a stable framework structure, high thermal stability, and the material is inexpensive. As an adsorbent carrier, it has great economic advantages. | [78,79] |
| Bentonite | Al2O3.4(SiO2) | It has a 2:1 type crystal structure composed of two silicon–oxygen tetrahedrons sandwiching one layer of aluminum–oxygen octahedron. It has strong hygroscopicity and expansibility and has an adsorption capacity for gases up to five times its own weight. It is a good adsorbent carrier. | [80,81] |
| Method | Preparation Methods | Advantage | Disadvantage |
|---|---|---|---|
| Solid-phase method | Mechanical Mixing Method | The operation is simple, the conditions are easily controllable, and it is suitable for large-scale production. | The mixture is not uniform and the active components are prone to agglomeration. |
| Freezing Granulation Method | The adsorbent has high activity and uniform particle size. | The preparation process is cumbersome with high cost and large energy consumption. | |
| Liquid-phase method | Impregnation Method | The components are controllable. The content of the active components can be controlled by regulating the amount of the precursor solution. | The loading amount of the active components is controlled by the pore volume of the carrier. |
| Sol–Gel Method | The dispersion of the active components is good; the microstructure is controllable; the specific surface area is high. | The raw materials are expensive and the preparation period is long. | |
| Dispersion Method | The dosage of the active components is precise; the mixture is uniform. | The demand for preparation raw materials is large and the process is complex. | |
| Coprecipitation Method | The particles are uniform and highly active; it is easy to obtain nano-scale adsorbents. | High local concentration causes the agglomeration of active components. |
| Number | Gas–Solid Reaction Model | Differential Function | Integral Function |
|---|---|---|---|
| A2 | Nucleus contraction (h = 2) | 2 (1 − α)1/2 | 1 − (1 − α)1/2 |
| A3 | Nucleus contraction (h = 3) | 3 (1 − α)2/3 | 1 − (1 − α)1/3 |
| D1 | One-dimensional diffusion | (1/2) α−1 | α2 |
| D2 | Two-dimensional diffusion | [−ln(1 − α)]−1 | α + (1 − α) ln(1 − α) |
| D3 | Three-dimensional cylindrical diffusion | (3/2) [(1 − α)−1/3 − 1]−1 | 1 − (2/3) α − (1 − α)2/3 |
| D4 | Three-dimensional spherical diffusion | (3/2) (1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]2 |
| R1 | Nucleation and growth (h = 1) | 1 − α | −1n(1 − α) |
| R2 | Nucleation and growth (h = 2) | 2 (1 − α)[−ln(1 − α)]1/2 | [−1n(1 − α)]1/2 |
| R3 | Nucleation and growth (h = 3) | 3 (1 − α)[−ln(1 − α)]2/3 | [−1n(1 − α)]1/3 |
| C3/2 | Chemical reaction (h = 1.5) | 2 (1 − α)3/2 | (1 − α)−1/2 |
| C2 | Chemical reaction (h = 2) | (1 − α)2 | (1 − α)−1−1 |
| C3 | Chemical reaction (h = 3) | (1 − α)3 | (1/2) [(1 − α)−2 − 1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Y.; Yu, K.; Tian, H.; Hu, Z.; Zhang, W.; Yin, Y.; Gao, H.; Yu, Q. Recent Advances in Materials, Synthesis, and Reaction Model of Particle Adsorbent for Flue Gas Desulfurization. Molecules 2025, 30, 1653. https://doi.org/10.3390/molecules30081653
Xuan Y, Yu K, Tian H, Hu Z, Zhang W, Yin Y, Gao H, Yu Q. Recent Advances in Materials, Synthesis, and Reaction Model of Particle Adsorbent for Flue Gas Desulfurization. Molecules. 2025; 30(8):1653. https://doi.org/10.3390/molecules30081653
Chicago/Turabian StyleXuan, Yanni, Kun Yu, Hong Tian, Zhangmao Hu, Wei Zhang, Yanshan Yin, Haitao Gao, and Qingbo Yu. 2025. "Recent Advances in Materials, Synthesis, and Reaction Model of Particle Adsorbent for Flue Gas Desulfurization" Molecules 30, no. 8: 1653. https://doi.org/10.3390/molecules30081653
APA StyleXuan, Y., Yu, K., Tian, H., Hu, Z., Zhang, W., Yin, Y., Gao, H., & Yu, Q. (2025). Recent Advances in Materials, Synthesis, and Reaction Model of Particle Adsorbent for Flue Gas Desulfurization. Molecules, 30(8), 1653. https://doi.org/10.3390/molecules30081653







